Abstract
Environmental control in greenhouse horticulture is essential for providing optimal conditions for plant growth and achieving greater productivity and quality. To develop appropriate environmental management practices for greenhouse horticulture through sensing technologies for monitoring the environmental stress responses of plants in real time, we evaluated the relative value of the stomatal opening to develop a technology that continuously monitors stomatal aperture to determine the moisture status of plants. When plants suffer from water stress, the stomatal conductance of leaves decreases, and transpiration and photosynthesis are suppressed. Therefore, monitoring stomatal behavior is important for controlling plant growth. In this study, a method for simply monitoring stomatal conductance was developed based on the heat balance method. The stomatal opening index (SOI) was derived from heat balance equations on intact tomato leaves, wet reference leaves, and dry reference leaves by measuring their temperatures in a growth chamber and a greenhouse. The SOI can be approximated as the ratio of the conductance of the intact leaf to the conductance of the wet reference leaf, which varies from 0 to 1. Leaf temperatures were measured with infrared thermometry. The theoretically and experimentally established SOI was verified with tomato plants grown hydroponically in a greenhouse. The SOI derived by this method was consistent with the leaf conductance measured via the porometer method, which is a standard method for evaluating actual leaf conductance that mainly consists of stomatal conductance. In conclusion, the SOI for the continuous monitoring of stomatal behavior will be useful not only for studies on interactions between plants and the environment but also for environmental management, such as watering at plant production sites.
1. Introduction
In greenhouse horticulture, various environmental control techniques are used to provide an optimal environment for plant growth to improve plant yield and quality. In particular, in advanced greenhouse horticulture, there is a trend toward more sophisticated environmental control using cutting-edge technologies such as information technologies. As production systems become highly optimized, low-cost technology, which uses high-temporal-resolution biological information to predict future productivity with high precision and avoids or reduces the risks of poor productivity, is emerging [1]. For example, deep learning approaches for imaging plant stress status in greenhouse horticulture have been introduced [2,3]. This trend makes it possible to efficiently maintain the optimal environmental conditions necessary for plant growth, such as rooting medium moisture, temperature, humidity, light intensity, and CO2 concentration. By making full use of advanced environmental control technology, we can increase the yield of high-quality agricultural products, leading to a stable supply of these products. In addition, it is possible to optimize the amount of water and fertilizer used, preventing the runoff of excess water and fertilizer that become wasted resources and contributing to reducing production costs. Thus, environmental control techniques in greenhouse horticulture are essential for providing optimal conditions for plant growth and achieving greater productivity and quality. Advances in these technologies are expected to further develop greenhouse horticulture and contribute to sustainable food production. Resource use efficiency, cost performance, and the vulnerability of value yield and quality of production are three key indices for assessing and improving the sustainability of plant production [4].
Recent integrated environmental control systems utilize computers to comprehensively and centrally manage equipment within greenhouses, allowing users to check the environment inside the greenhouse and remotely control equipment from their smartphones. The introduction of environmental control technology greatly contributes to improving agricultural productivity but requires initial investment. Costs vary greatly depending on the type and scale of the technology being introduced and the facility’s conditions. Additionally, even after implementation, operational costs related to system maintenance and updates will be incurred. This includes electricity consumption costs and regular maintenance costs.
In recent years, progress has been made in the development of environmental control technologies that can be introduced at a low cost, and to improve productivity [5]. It is important to provide technologies at a price range that can be easily introduced by small-scale farmers.
In greenhouse horticulture, more precise environmental control is now possible through the use of technology that monitors plant responses to provide optimal conditions for plant growth [6,7]. This technology allows us to understand the physiologically active state of plants, such as photosynthesis and transpiration, in real time and the resulting growth process and to use this feedback to automatically control environmental conditions. Recently, sensing technologies that can visualize the environmental stress responses of plants in real time [8,9,10,11,12], as well as compact, low-power, and low-cost sensing technologies, have been developed to measure environmental elements.
Traditionally, skilled growers have used experience and visual information to obtain information from plants and control the environment optimally to produce high-quality, high-yield plants. To scientifically optimize such plant production, it is important to use sensors to measure the physiological responses of plants and use that information for control. Considering environmental factors as input variables and plant responses as output variables, we must optimize environmental factors by feeding back the results of measurements of plant responses affected by environmental factors.
The stomatal opening is important for understanding the dynamics of transpiration and photosynthesis [13,14]. By opening their stomata, plants absorb CO2 and perform photosynthesis. When there is no stress and the stomata are opening, transpiration occurs actively through the stomata, facilitating the absorption of water and nutrients from the roots. This allows plants to grow healthily. The stomatal aperture is regulated by sensitive environmental conditions such as light intensity, drought stress, and CO2 concentration [15,16]. This allows plants to adapt to changing environmental conditions. Especially under dry conditions, the stomata close to prevent water loss by sensing the internal moisture status of leaves and adjusting stomatal apertures accordingly. This allows for optimal gas exchange and water retention to respond to environmental stress. In this way, plants can grow healthily by adjusting their stomatal opening according to environmental conditions. Conversely, understanding the behavior of the stomatal opening leads to understanding the environmental response of the plant’s physiological activity, which is important for environmental control in greenhouse horticulture.
Vialet-Chabrand and Lawson evaluated the stomatal conductance of wheat leaves theoretically and experimentally using the temperatures of intact leaves and reference aluminum plates with tiny holes for evaporation and without evaporation [17] and assessed stomatal behavior in a dynamic environment [18]. In the present study, a method for simply monitoring stomatal aperture as relative stomatal conductance at plant production sites was developed based on the heat balance method. This method is expected to continuously evaluate the relative value of the stomatal opening at a low cost to contribute to appropriate environmental management, such as watering, based on the moisture status of plants in greenhouse horticulture.
2. Materials and Methods
2.1. Theoretical Outline for Determining the Stomatal Opening Index (SOI)
In general, when plants are exposed to water stress due to water absorption suppression, the stomatal aperture of leaves decreases, and transpiration and photosynthesis are suppressed. Therefore, monitoring stomatal behavior is important for controlling plant growth, especially through environmental control based on understanding plant responses.
The transpiration rate is well known as a function of stomatal conductance [19], and the leaf temperature is also well known as a function of stomatal conductance [20]. Leaf temperatures measured with the infrared thermometry method are often used to estimate stomatal conductance [21,22,23,24,25]. Thermography, often used in conjunction with other image sensors and data mining techniques, is critical for enabling more automated, accurate, and sustainable agriculture. Pineda et al. reviewed the state of the art in thermography from the perspective of biotic stress detection [26].
As an index of stomatal conductance, the stomatal opening index (SOI), which is the relative stomatal aperture, was determined. The SOI was derived from heat balance equations on intact, wet reference, and dry reference leaves.
The heat balance of a leaf is generally calculated via the following equation:
The temperatures of the intact (TL), wet reference (TW), and dry reference (TD) leaves were obtained from Equation (1), as shown below.
Using Equations (2)–(4), the ratios of surface temperature differences are expressed by Equations (5) and (6). Here, it is assumed that heat transfer is mainly carried out by convection and that the boundary layer conductance gHa values of intact leaves, dry reference leaves, and wet reference leaves are equal.
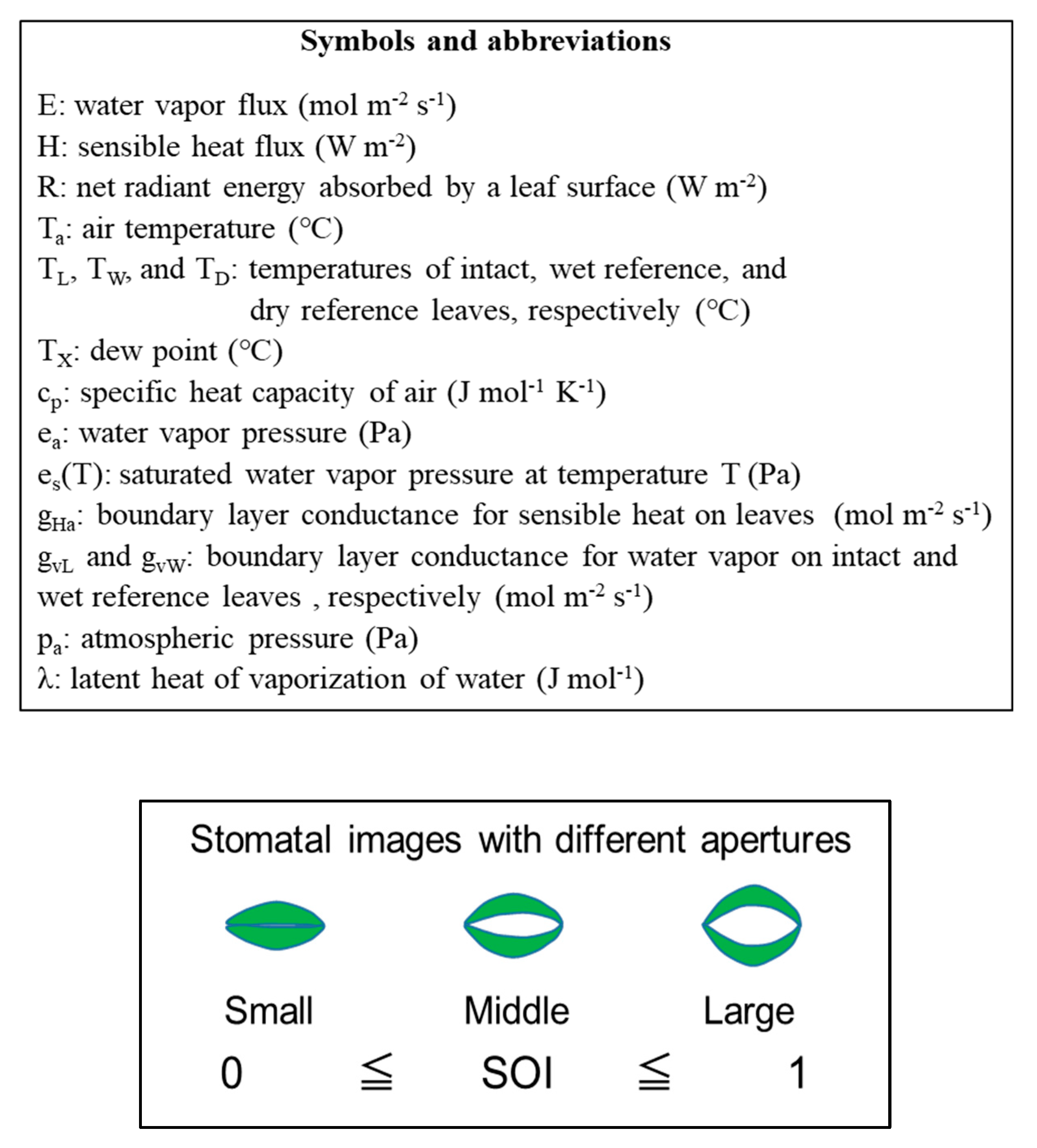
Finally, the value obtained from Equation (7) is the SOI. The SOI, which is the ratio of the temperature difference between the intact and reference leaves, is the ratio of the conductance of the intact leaf to the conductance of the wet reference leaf, and it varies from 0 to 1. A larger value of the SOI indicates a greater degree of stomatal aperture, as shown in Figure 1.
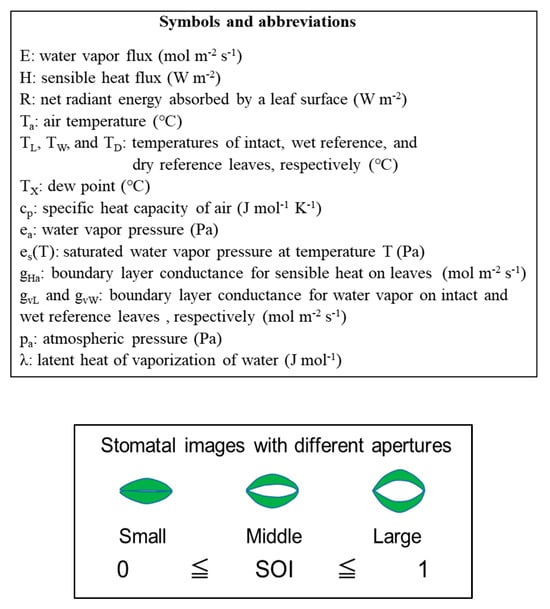
Figure 1.
Conceptual diagram of the relationship between the SOI and stomatal opening.
2.2. Verification of the Thermal Image Camera including Setting Values of Emissivity
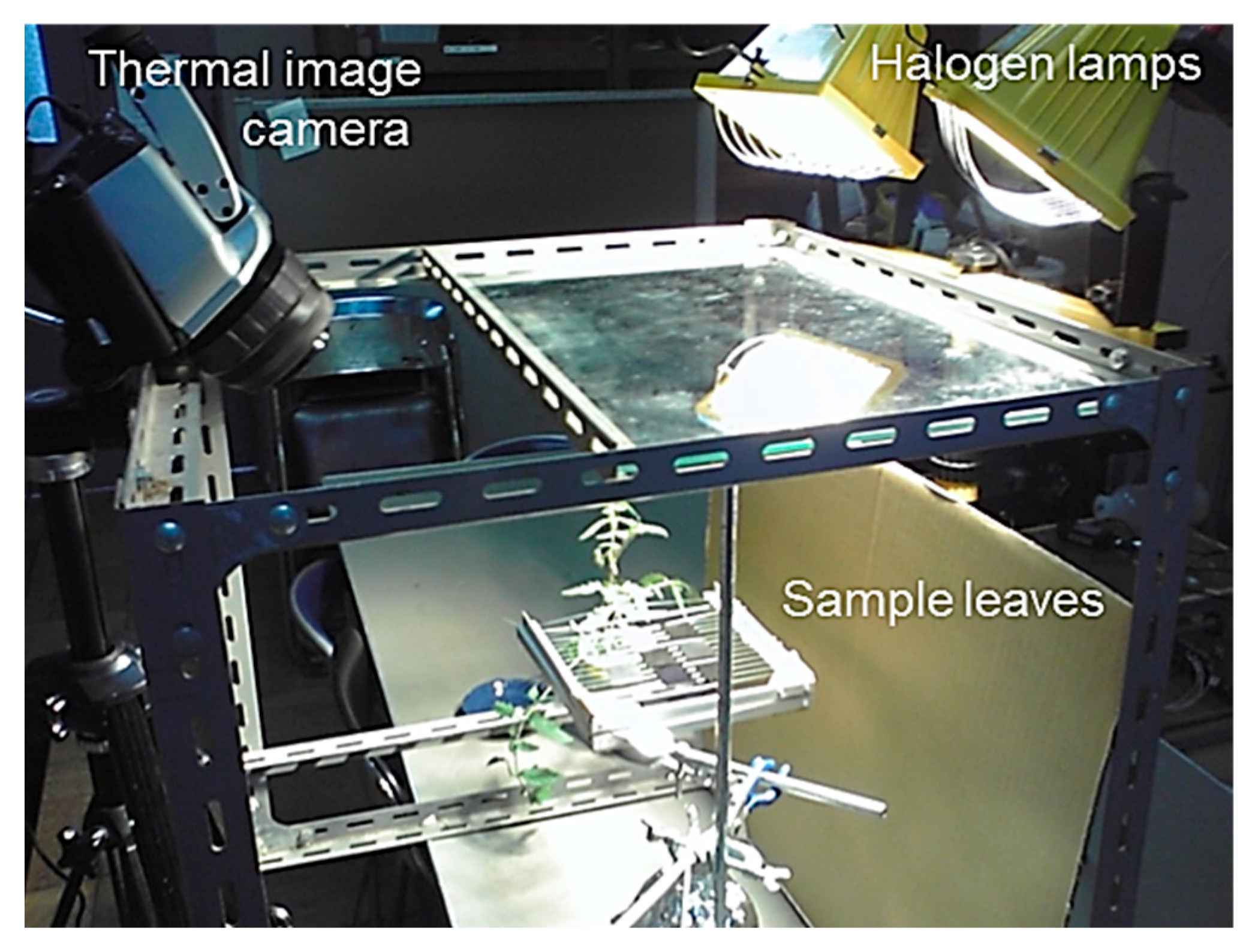
In this study, a thermal image camera (TH9100, Nippon Avionics Co., Ltd., Yokohama, Japan) was used to measure the surface temperature (Figure 2). However, the correct interpretation of thermal data requires corrections related to the environmental and measurement conditions. Before using a thermal image camera, which is more susceptible to the effects of factors other than the measurement object compared to thermocouples, we conducted a comparison test with thermocouples. When the water temperature was changed by adding cold or hot water containing black paint to a Styrofoam container (450 mL), the water temperature was measured using a thermal image camera and compared with the measured value using thermocouples (0.1 mm in diameter), which are considered to indicate the correct temperature.
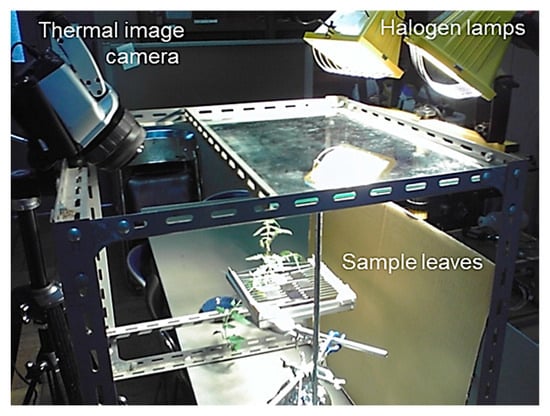
Figure 2.
Overview of the measurement experiment with intact leaves, dry reference leaves, and wet reference leaves.
Next, the emissivity must be set when obtaining the surface temperature from thermal images via a thermal image camera, but the actual emissivity varies somewhat depending on the type of target surface. Therefore, we examined the surface temperature of each type of leaf by changing the emissivity set value of the thermal image camera in the range of 0.6 to 1.0 in steps of 0.05. The emissivity was adjusted by comparing the measured temperature with the value obtained using thermocouples.
2.3. Examination of Materials Used for Measurement as Reference Leaves
The wet reference leaf was made of paper (0.26 mm in thickness), and each leaf was cut into 4 × 4 cm pieces. The emissivity of the reference leaves was close to that of the intact leaves (approximately 0.95). Since black body paint is hydrophobic and cannot absorb water if the same treatment is applied to filter paper as a wet reference leaf, we tested the method of coloring filter paper through the following experiment.
Five types of paper sheets colored black with watercolor paint, Indian ink, or water-based dye (Cold A52, Dylon Japan Co., Ltd., Tokyo, Japan), water-based ink, and commercially available black Japanese paper were examined. The surface temperatures of seven specimens were measured 20 min after irradiation at 400 W m−2 under halogen lamps. The specimens were placed on nylon threads (0.1 mm in diameter) that were stretched at 10 mm intervals. Wet reference leaves were moistened with ion exchange water (W29-A1, TRUSCO NAKAYAMA Co., Tokyo, Japan) 20 min before the experiment started.
The surface temperatures of dry reference leaves and intact leaves of tomato plants (Lycopesolicon esculentum Mill., cultivar ‘Momotaro’) grown in pots in a greenhouse were also measured and compared. The dry reference leaf was an aluminum plate (0.4 mm in thickness). The surface of the plate was painted with black body paint (THI-1B, Okitsumo Co., Ltd., Nabari, Japan: emissivity 0.96).
2.4. Radiation Reflectance, Transmittance, and Absorption of Reference and Intact Leaves
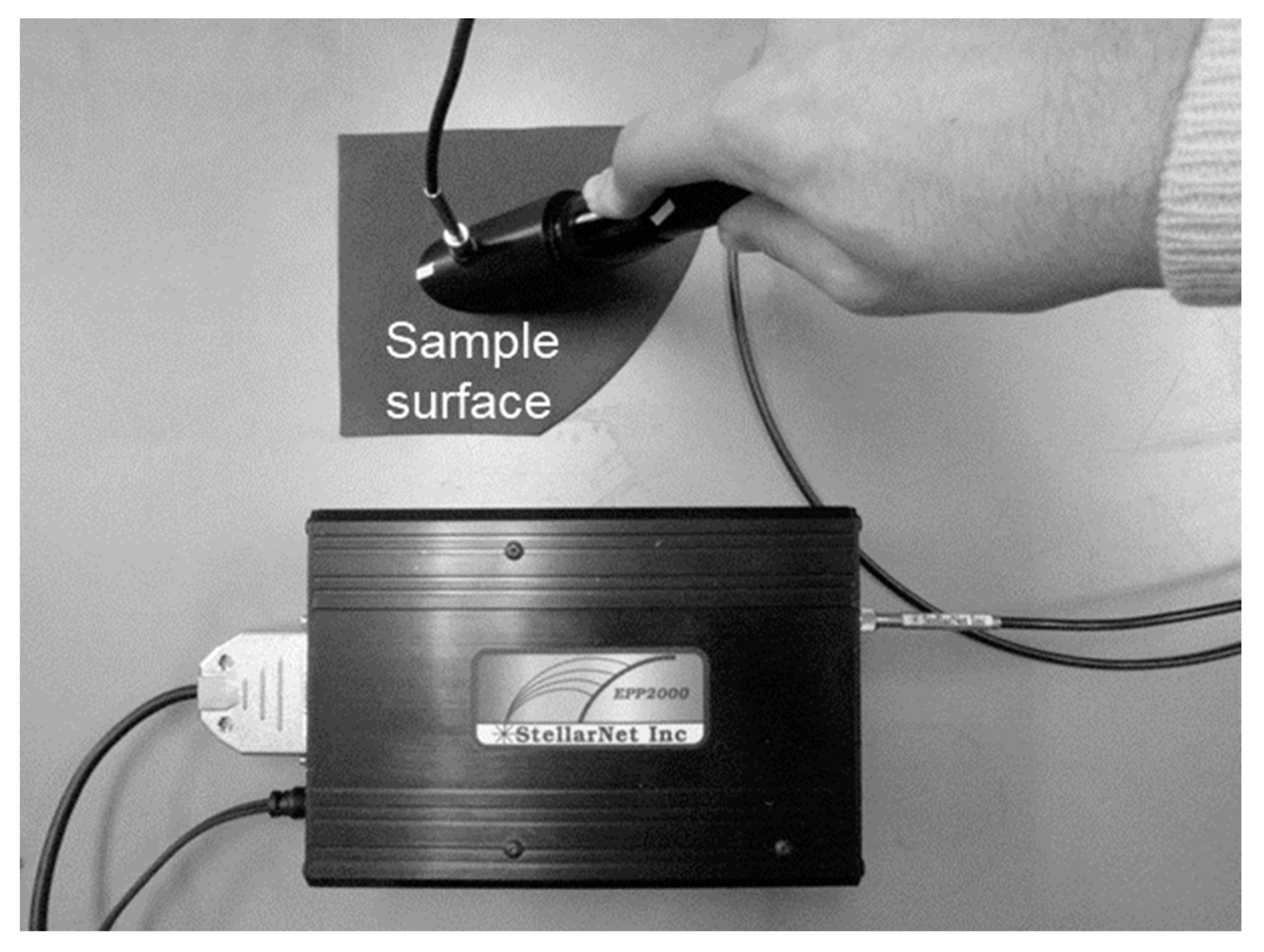
The index used in this study was calculated based on the heat balance on the surfaces of the materials, so their absorption rates must be confirmed. The reflectance of each material was measured using a spectrophotometer (PS-300-S, Apogee Instruments Inc., North Logan, UT, USA), as shown in Figure 3. The transmittance was measured by placing the sensor on the lower surface of the leaf. The values obtained by subtracting the reflectance and transmittance values from 1.0 were taken as the absorptance.
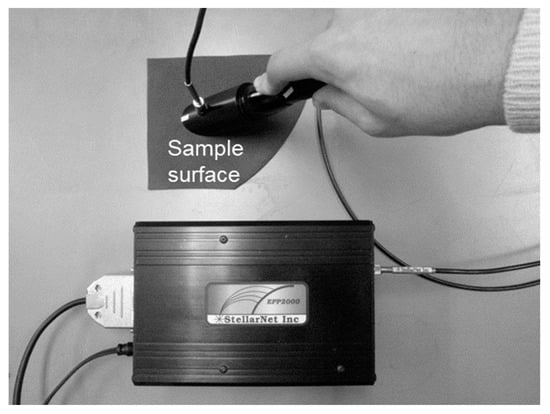
Figure 3.
Overview of reflectance measurements.
2.5. Effects of Environmental Factors on SOI Determination
The radiation energy input to leaves is an important factor influencing leaf temperature. Leaf conductance also greatly influences leaf temperature through sensible heat and latent heat exchanges between the leaf and the surrounding atmosphere, and this conductance is greatly influenced by wind speed [27,28]. To evaluate the effectiveness of the SOI, we compared the transpiration rate measured simultaneously with the SOI when changing the radiation flux and wind speed.
Tomato plants were used as the plant material. Plants were grown in a growth chamber for one week after cutting and then hydroponically cultured for one month using Otsuka House A formula half-strength nutrient solution (EC: 1.5 dS m−1). The experimental measurements were conducted in a wind tunnel-type growth chamber. In the experiments, the roots of the plants were placed in a polypropylene container (510 mL) containing the culture solution, a hole was made to allow the stem to pass through, and the lid was sealed to prevent evaporation from the water surface.
The SOI and transpiration rate were simultaneously measured and compared. The temperature of each leaf used to calculate the SOI was measured using a thermal image camera. The wet reference leaves were maintained in a moist state by continuously supplying water via the capillary action of a nonwoven fabric connected to a small water reservoir. The transpiration rate was determined using the weighing method and was derived from the change in weight of the whole system, including the plant and nutrient solution per unit of time due to transpirational water loss divided by the leaf area.
2.5.1. Radiation Flux
We used halogen lamps to vary the radiation flux at the SOI measurement surface to 30, 330, and 740 W m−2. The SOI and transpiration rates were measured simultaneously 10 min after reaching a steady state.
2.5.2. Wind Speeds
In the wind tunnel-type growth chamber, after the transpiration rate of the plant body stabilized, the wind speed decreased in the order of 0.3, 0.4, 0.6, 1.0, 1.5, and 2.0 m s−1. The SOI and transpiration rates were measured simultaneously 15 min after reaching a steady state.
2.6. Evaluation to Confirm the Effectiveness of the SOI in a Greenhouse
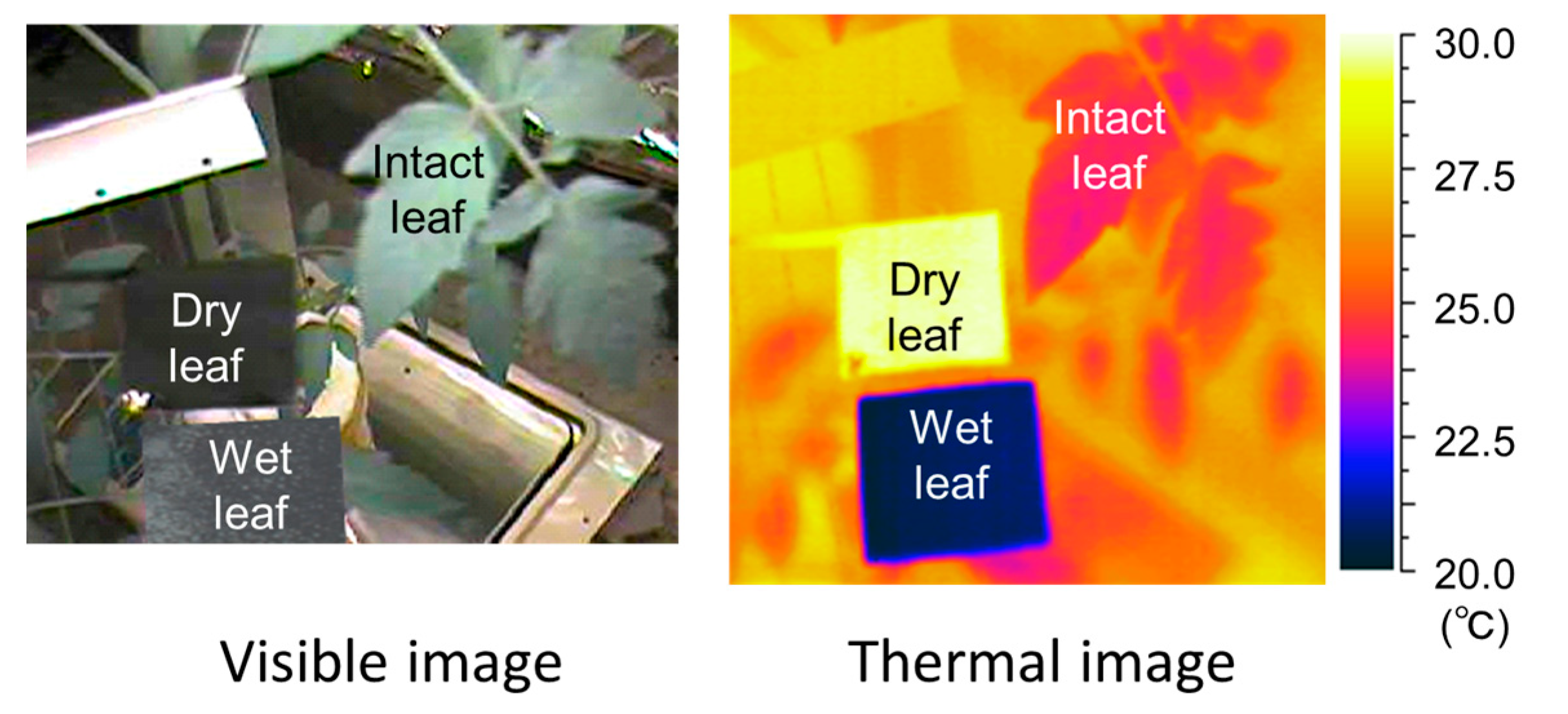
To confirm the effectiveness of the SOI in various environments at the production site, the SOI was verified experimentally with tomato plants grown hydroponically in a greenhouse. The wet reference leaf made of a black paper sheet and the dry reference leaf made of a black aluminum plate were placed near an intact representative tomato leaf, as shown in Figure 4, so that the incident irradiance and other environmental conditions on each leaf were mostly the same. Leaf temperatures were measured with a thermal image camera. The measurement day was clear, the temperature inside the greenhouse was 25–37 °C, the water vapor pressure deficit was 5–50 hPa, and the irradiance varied from 0 to 700 W m−2. The wind speed ranged from 0.1 to 0.8 m s−1.
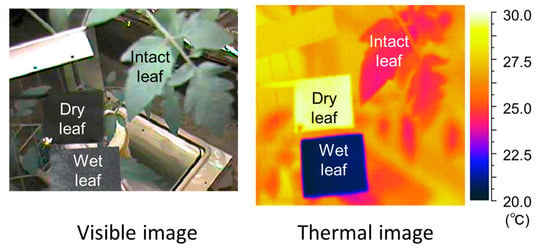
Figure 4.
Examples of visible and thermal images of the intact leaf, dry reference leaf, and wet reference leaf.
Leaf conductance of the intact leaf was measured just after the measurement of leaf temperature. Leaf conductance was measured via the ventilated leaf chamber method with a porometer (Li-1600, LI-COR Co., Lincoln, NE, USA).
3. Results and Discussion
3.1. Verification of the Thermal Image Camera including Setting Values of Emissivity
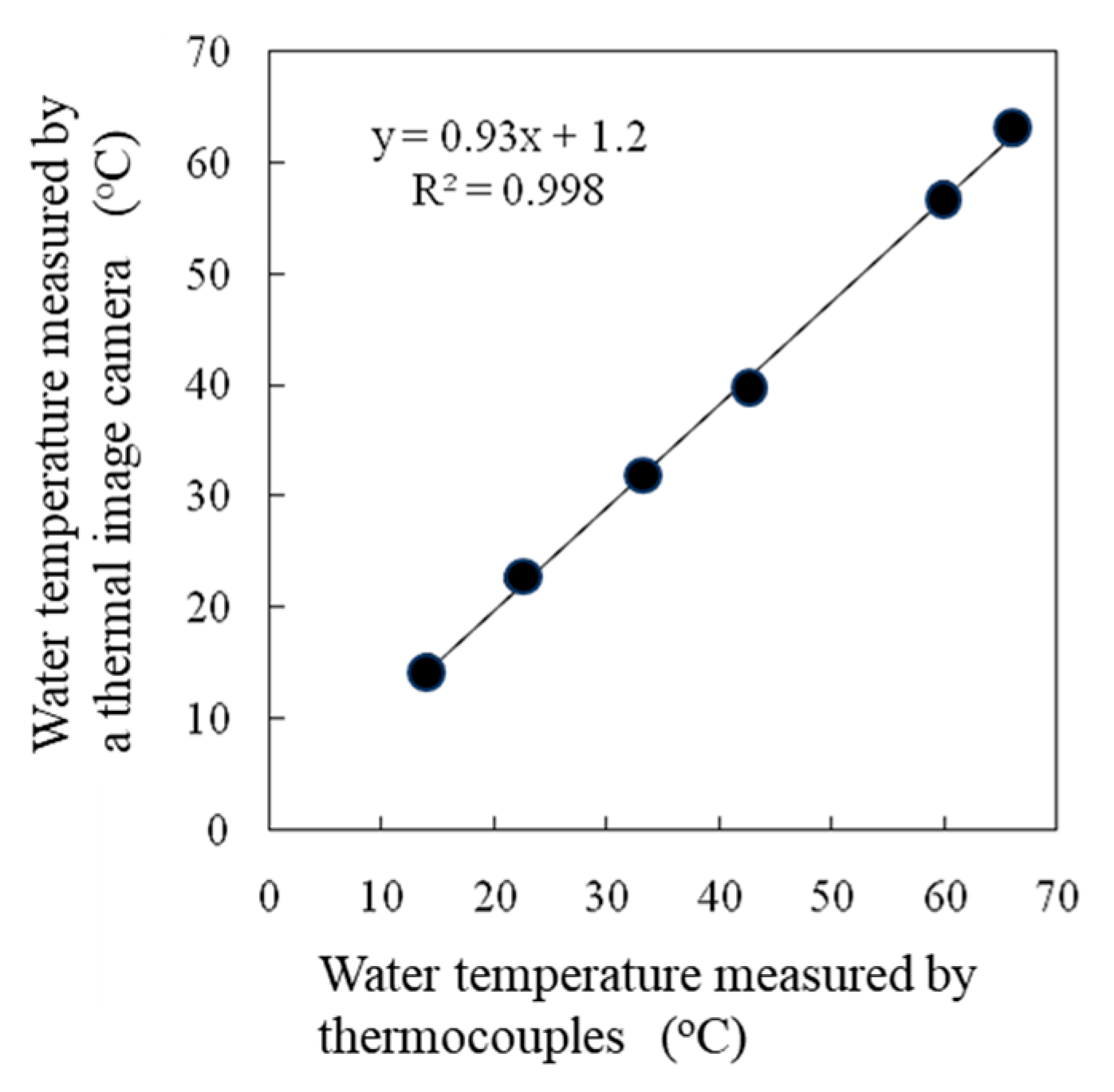
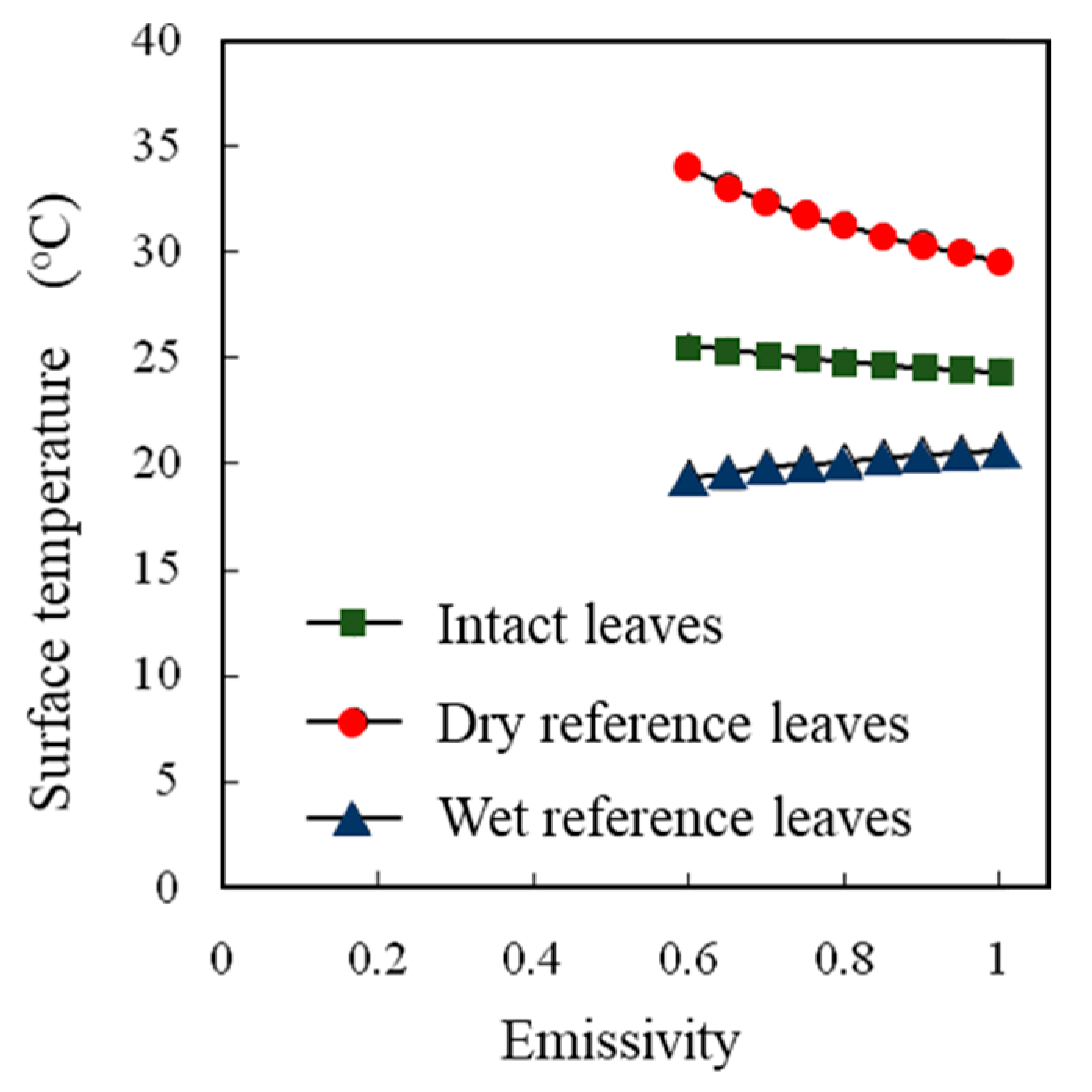
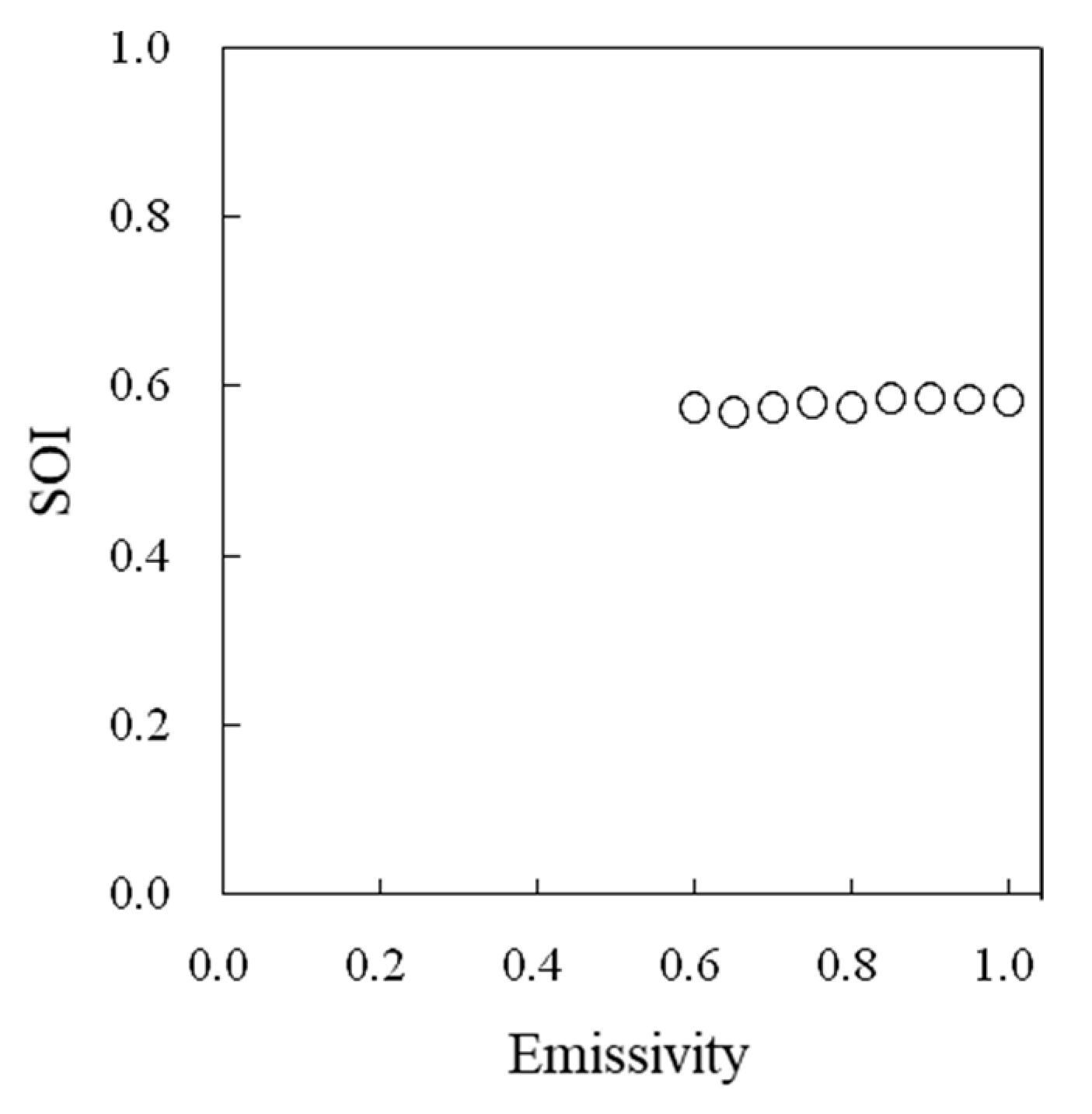
A high correlation was observed between the water temperatures measured by thermocouples and those measured by thermal image cameras (Figure 5). The effects of the emissivity values set in a thermal image camera on the surface temperature readings differed among the intact leaves, dry reference leaves, and wet reference leaves (Figure 6), but no effect on the SOI was detected (Figure 7).
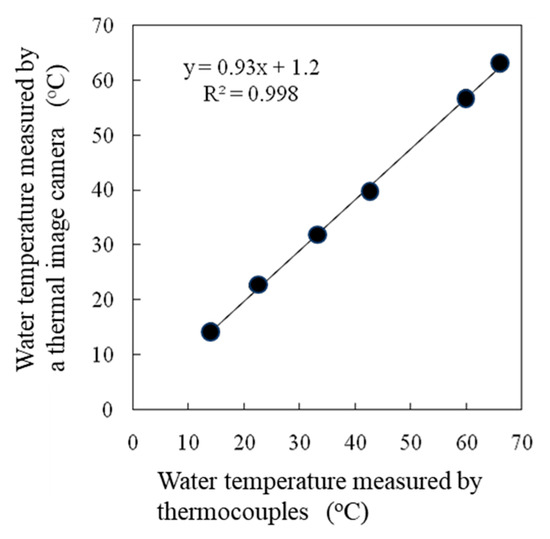
Figure 5.
Correlation between the water temperature measured by a thermocouple and the water temperature measured by a thermal image camera. < Moist → Wet >.
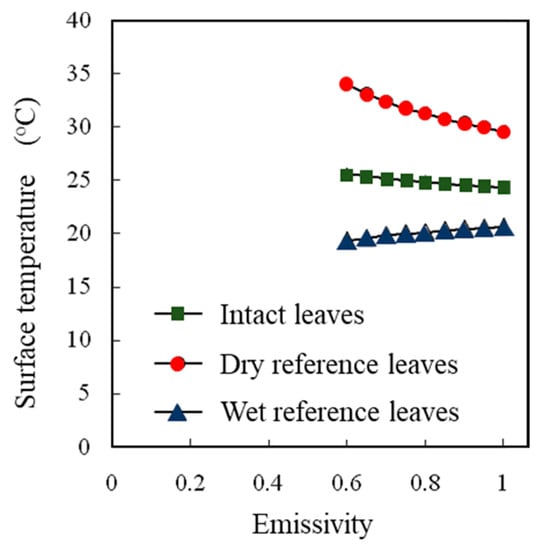
Figure 6.
Effect of the emissivity set values set in a thermal image camera on the surface temperatures of intact leaves, dry reference leaves, and wet reference leaves.
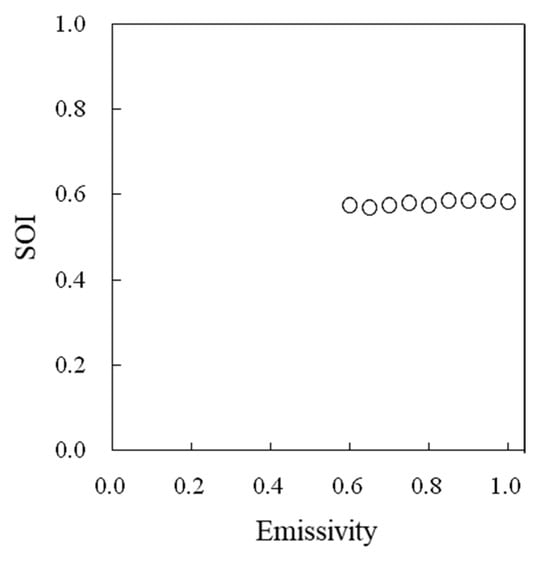
Figure 7.
Effect of the emissivity values set in a thermal image camera on the SOI.
When the actual surface temperature is measured by using a thermal image camera, the emissivity must be set carefully. However, since the SOI is a relative value, it is not considered necessary to accurately adjust the set value of emissivity when determining the SOI. It was confirmed that there was no problem in using a thermal image camera for SOI measurements.
3.2. Examination of Materials Used for Measurement as Reference Leaves
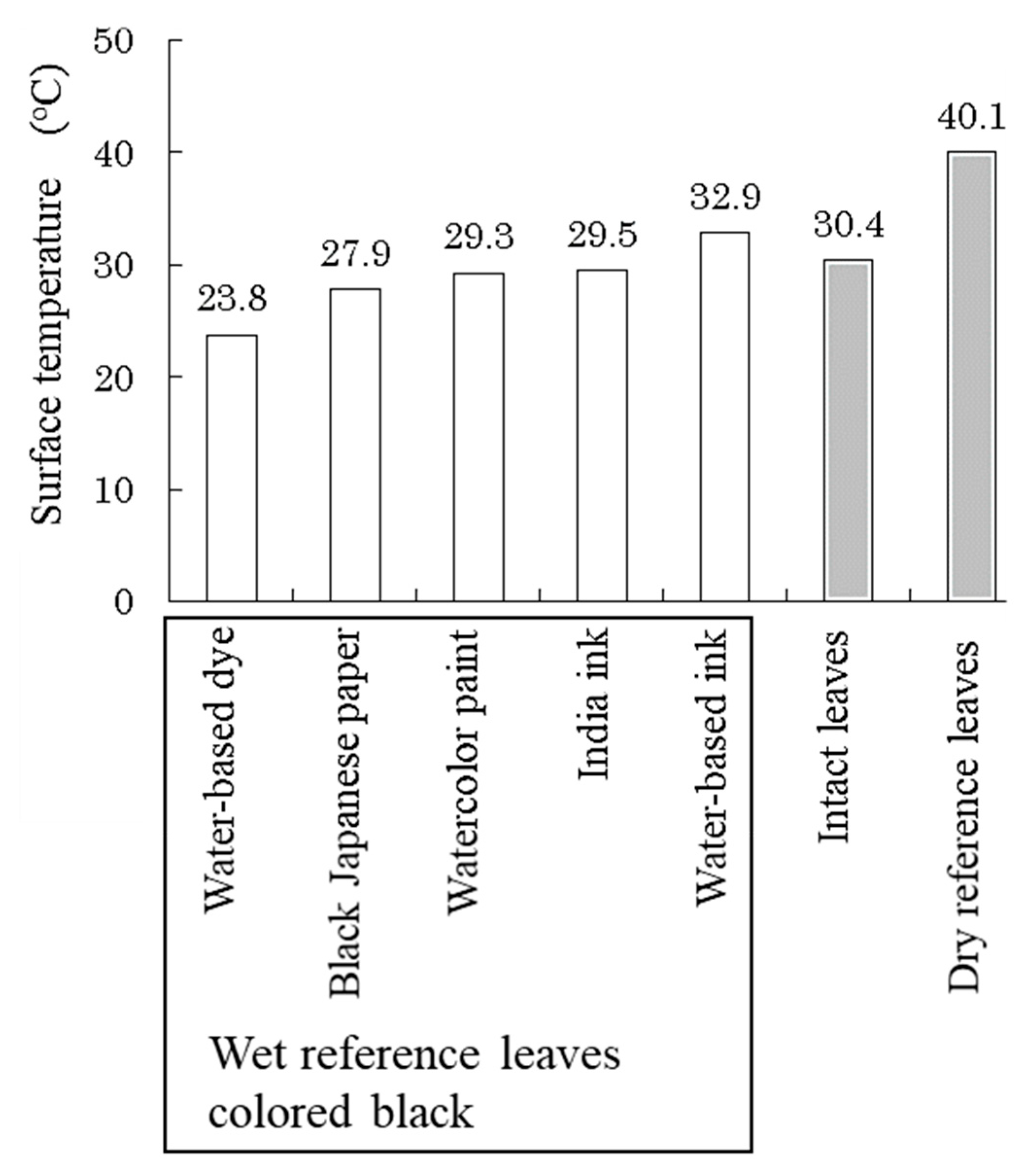
Paper sheets colored with the water-based dye showed the lowest temperature (Figure 8). The mechanism of dyeing using dyes is to chemically bind the dye to the fibers, so it is possible to dye even the inside of the fibers while maintaining the spaces between the fibers. The other colored materials used in this experiment were solid pigment particles that partially filled the spaces between the fibers, which reduced the water holding capacity among the fibers and suppressed evaporation. It was confirmed that water-based dyes are suitable for coloring wet reference leaves for SOI measurements. Therefore, we used paper sheets colored black with water-based dye for wet reference leaves to simulate well-transpired leaves.
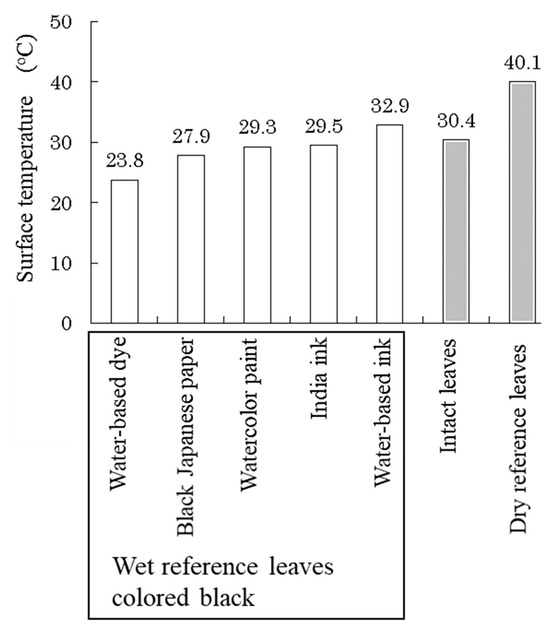
Figure 8.
Surface temperatures of different types of wet reference leaves made of paper, intact leaves, and dry reference leaves after 20 min under light irradiation conditions.
3.3. Radiation Reflectance, Transmittance, and Absorption of Reference and Intact Leaves
The results are summarized in Table 1. Since the absorption rate of dry reference leaves was greater and thus the temperature was higher than that of tomato leaves and wet reference leaves, the SOI might be overestimated compared with the ideal conditions. By using reference leaves that have radiation characteristics closer to those of intact leaves, the degree of stomatal aperture could be estimated more accurately.

Table 1.
Radiation reflectance, transmittance, and absorption of intact leaves, dry reference leaves, and wet reference leaves.
3.4. Effects of Environmental Factors on SOI Determination
3.4.1. Radiation Flux
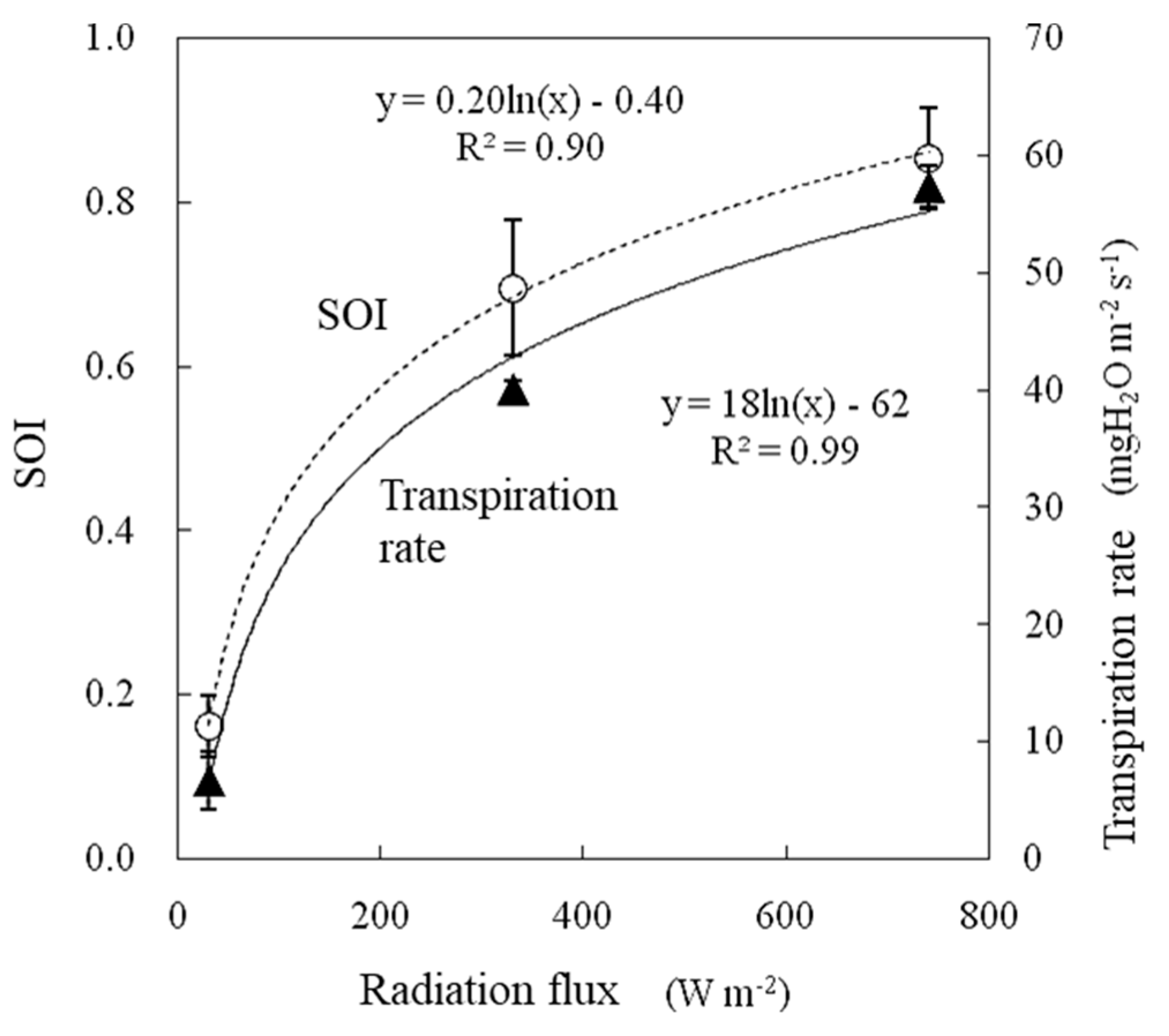
The air temperature during the experiment was 25 °C, and the water vapor saturation deficit was 15.0 hPa. The wind speed was 1.0 m s−1. The SOI increased logarithmically with respect to the radiation flux between 0.16 and 0.85, and the transpiration rate also changed similarly (Figure 9). The SOI and transpiration rate were strongly correlated when measured under variable radiation flux conditions, as shown in Figure 10. It should be noted that as the radiation flux increased from 30 to 330 W m−2, the SOI increased significant by approximately four times. This method is primarily aimed at application inside greenhouses. The radiation flux varies temporally and spatially inside greenhouses especially due to structural flames and plant stands. When monitoring the SOI, it is necessary to ensure that each sample leaf is evenly irradiated. The response of the SOI to radiation flux must be further investigated especially under conditions in which solar radiation fluctuates significantly over short periods of time.
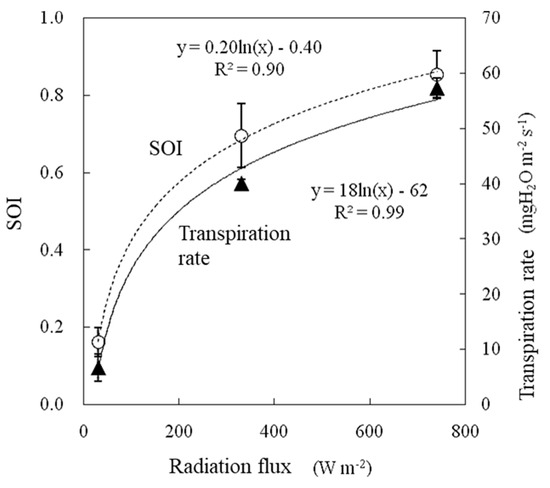
Figure 9.
Effect of radiation flux on the SOI and transpiration rate measured in a growth chamber.
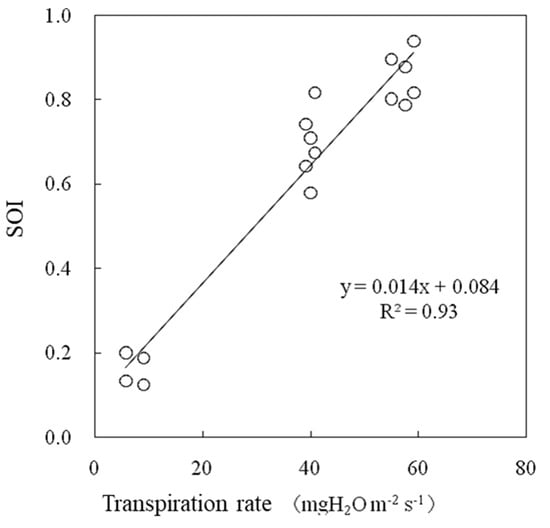
Figure 10.
Relationships between transpiration rates and the SOI measured in a growth chamber.
3.4.2. Wind Speeds
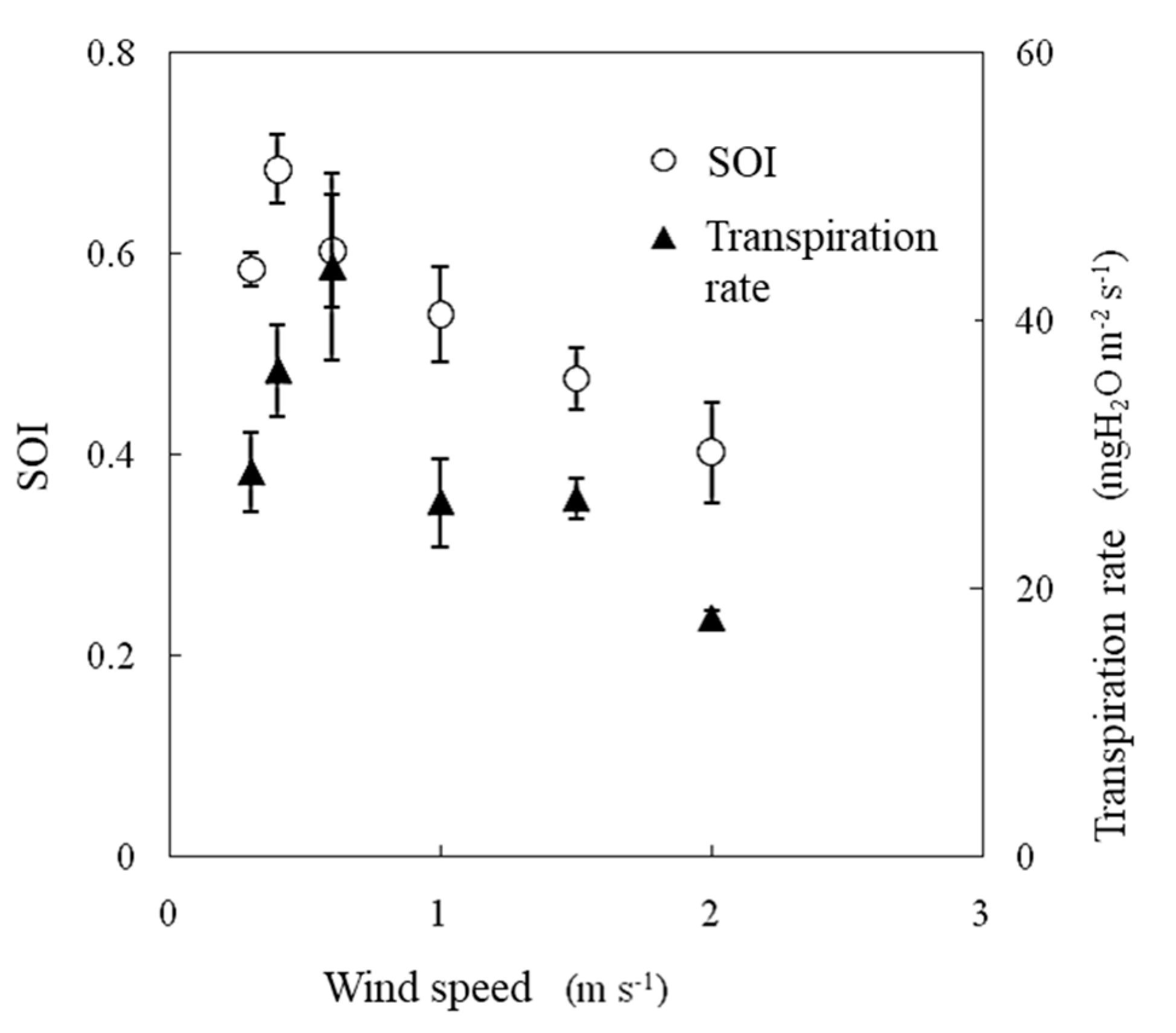
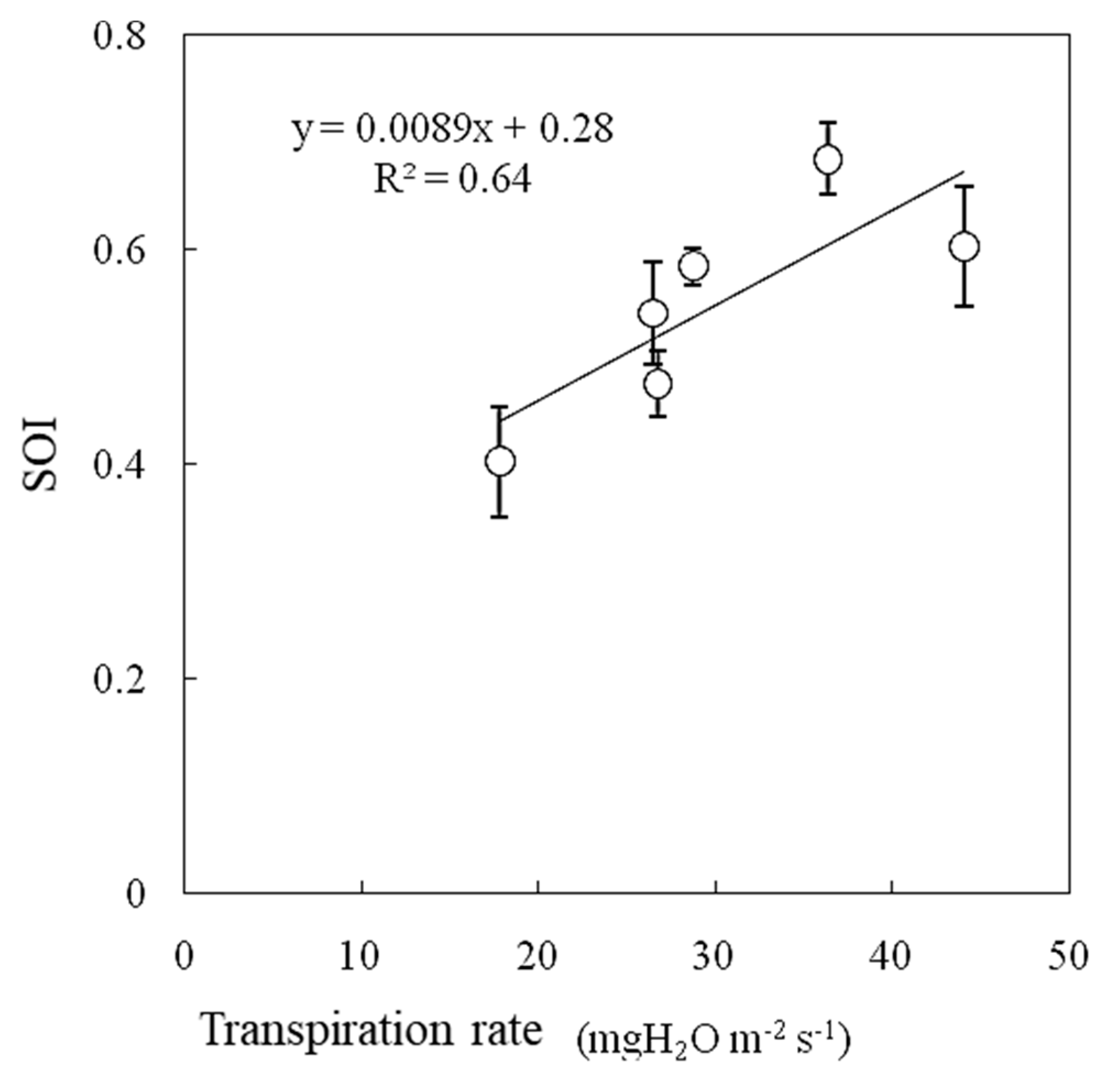
During the experiment, the air temperature was 25 °C, the water vapor saturation deficit was 15.4 hPa, and the irradiance was 600 W m−2. In this experiment, the transpiration rate increased between wind speeds of 0.3 m s−1 and 0.6 m s−1 and then decreased as the wind speed increased (Figure 11). Photosynthesis was promoted with increasing wind speed, reaching a maximum at wind speeds of 0.5–0.6 m s−1, and then it gradually decreased, especially under higher water vapor saturation deficit and higher irradiance conditions [14]. The SOI and transpiration rate were strongly correlated when measured under variable wind speed conditions, as shown in Figure 12.
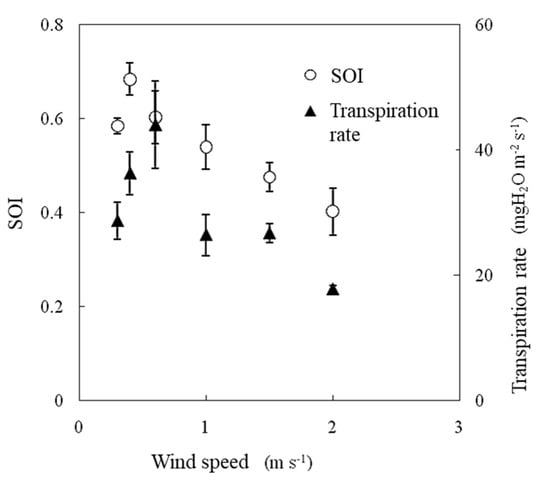
Figure 11.
Effect of wind speed on the SOI and transpiration rates measured in a growth chamber. Error bars indicate standard deviations (n = 3).
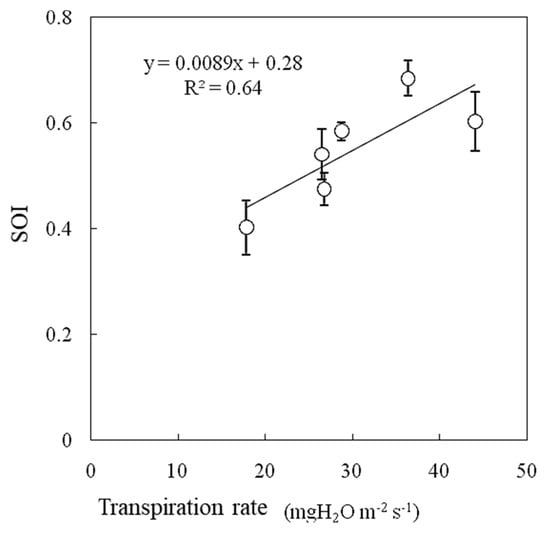
Figure 12.
Relationships between transpiration rate and SOI measured at variable wind speeds measured in a growth chamber. Error bars indicate standard deviations (n = 3).
The airflow is mostly stable in closed greenhouses. However, there will be airflow fluctuations in greenhouses having side or roof windows opening, or air circulation fans working. In such an environment, it is necessary to ensure that each sample leaf is in the same airflow condition.
3.5. Evaluation to Confirm the Effectiveness of the SOI in a Greenhouse
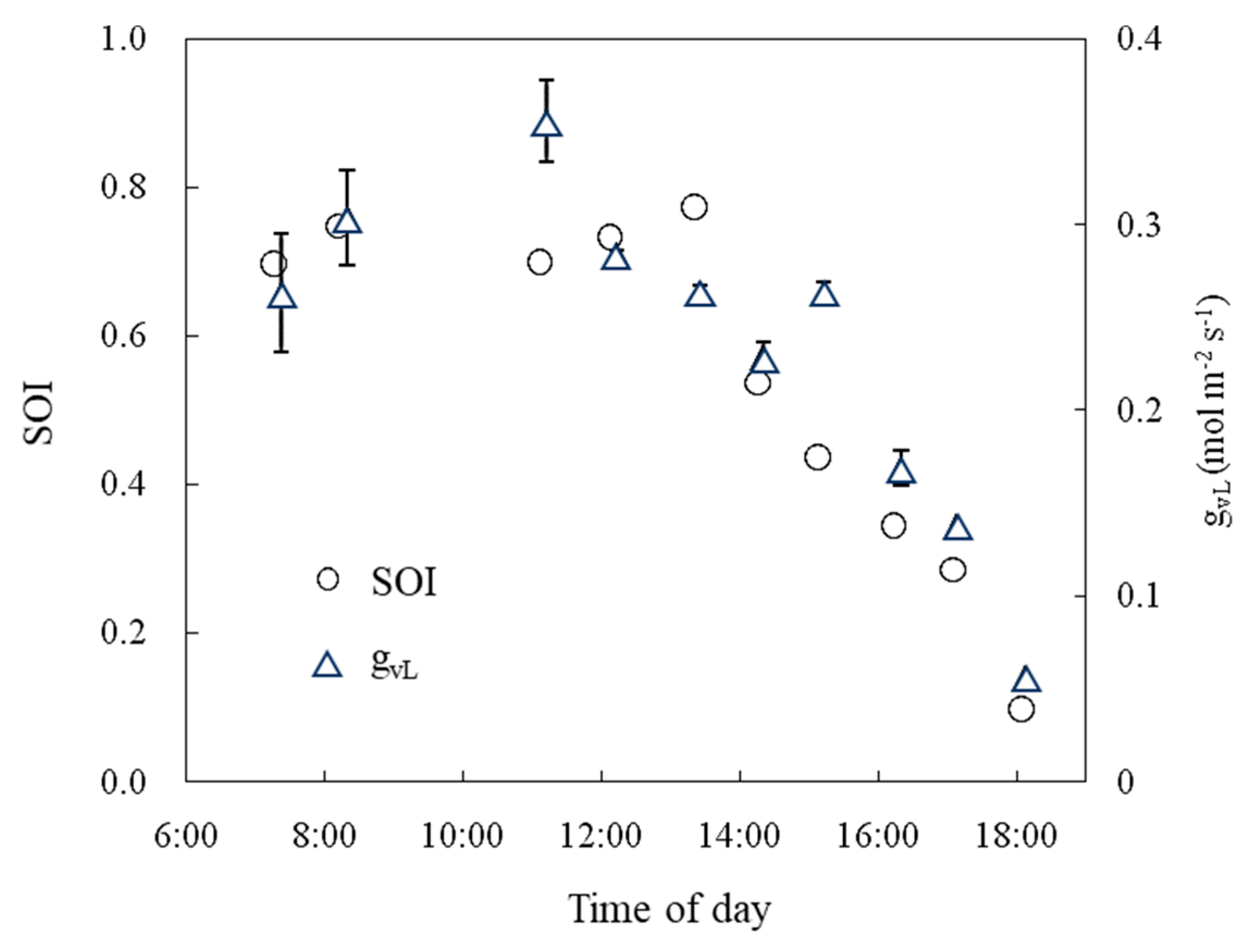
Diurnal changes in the SOI were measured in the tomato culture greenhouse. The SOI ranged from 0.7 to 0.8 from 06:00 to 13:00, then decreased, and finally approached 0.1 at sunset (Figure 13). Leaf conductance showed a similar diurnal change.
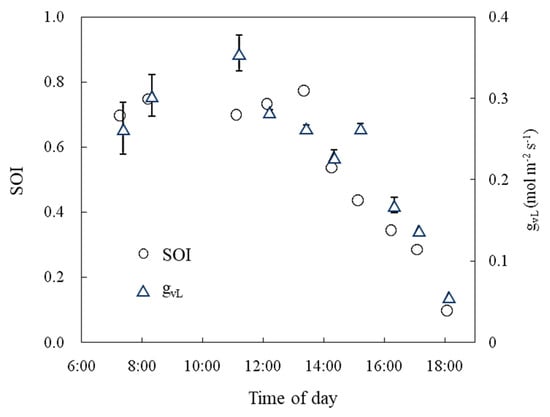
Figure 13.
Daily variation in the SOI and leaf conductance (gVL) measured in a greenhouse. The error bars indicate the S.E. (n = 3).
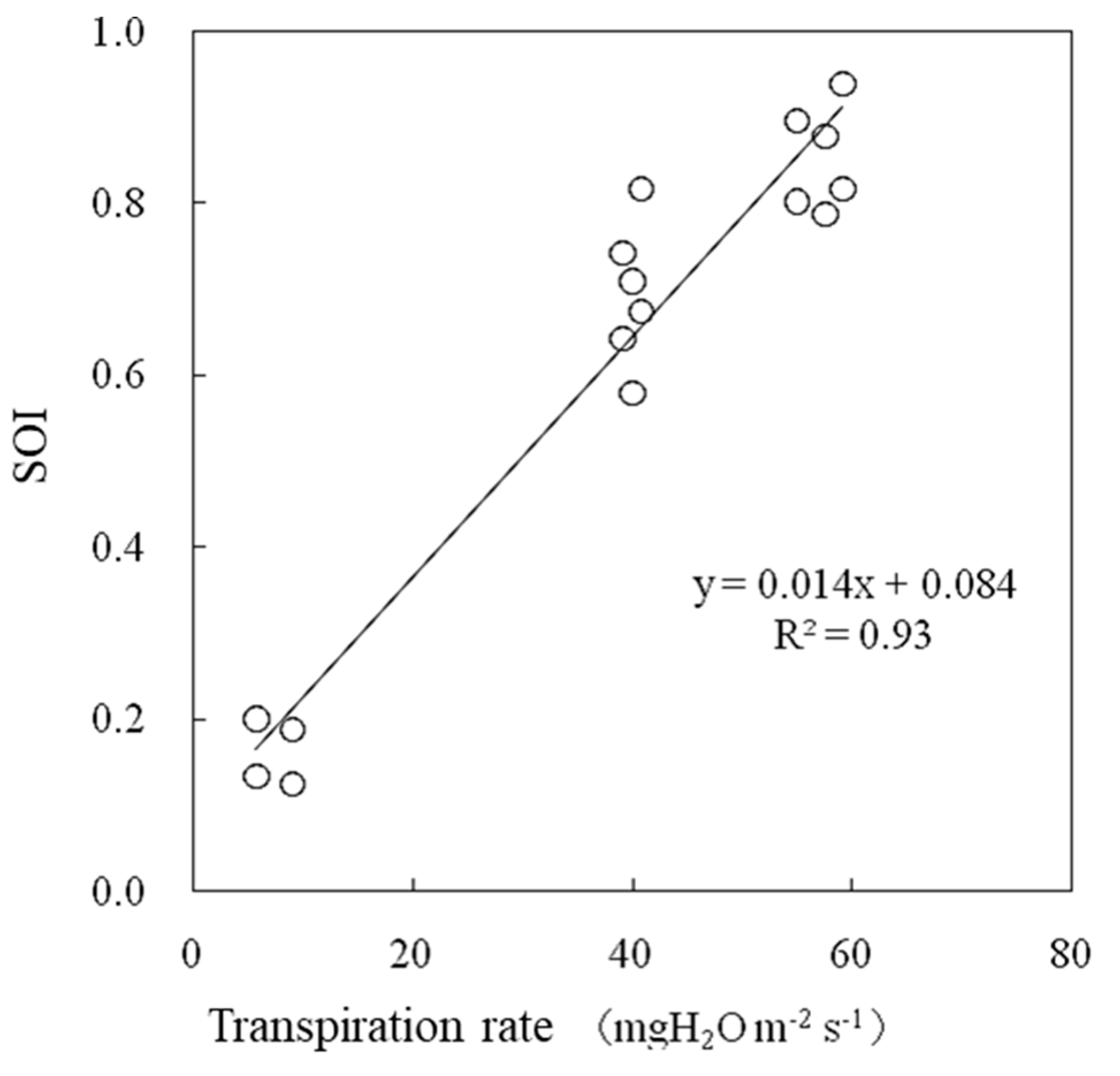
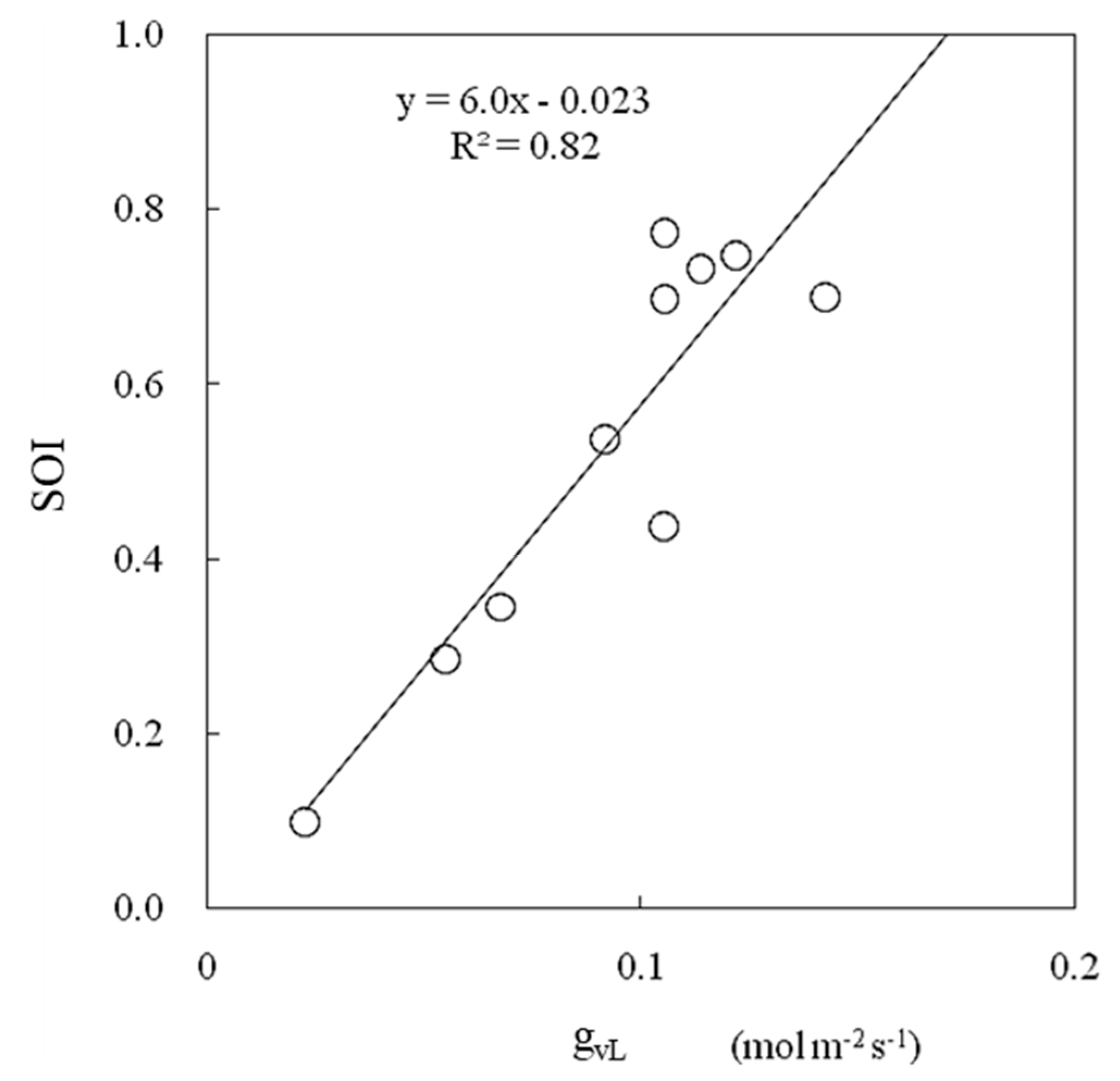
The SOI and leaf conductance were strongly correlated (Figure 14). The SOI estimated via this method was consistent with the leaf conductance measured via the porometer method, which is a standard method for evaluating leaf conductance that mainly consists of stomatal conductance.
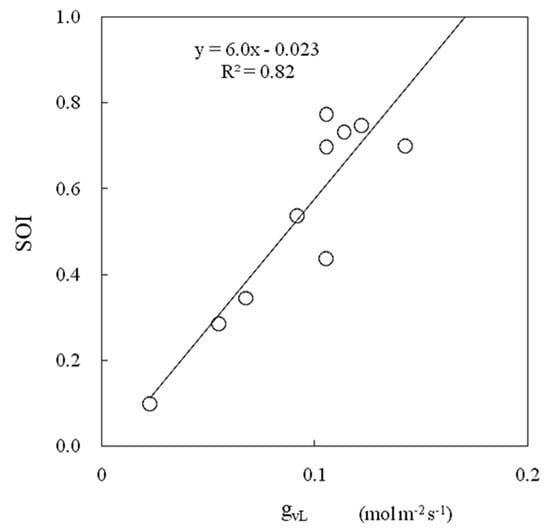
Figure 14.
Relationships between leaf conductance (gVL) and the SOI measured in a greenhouse.
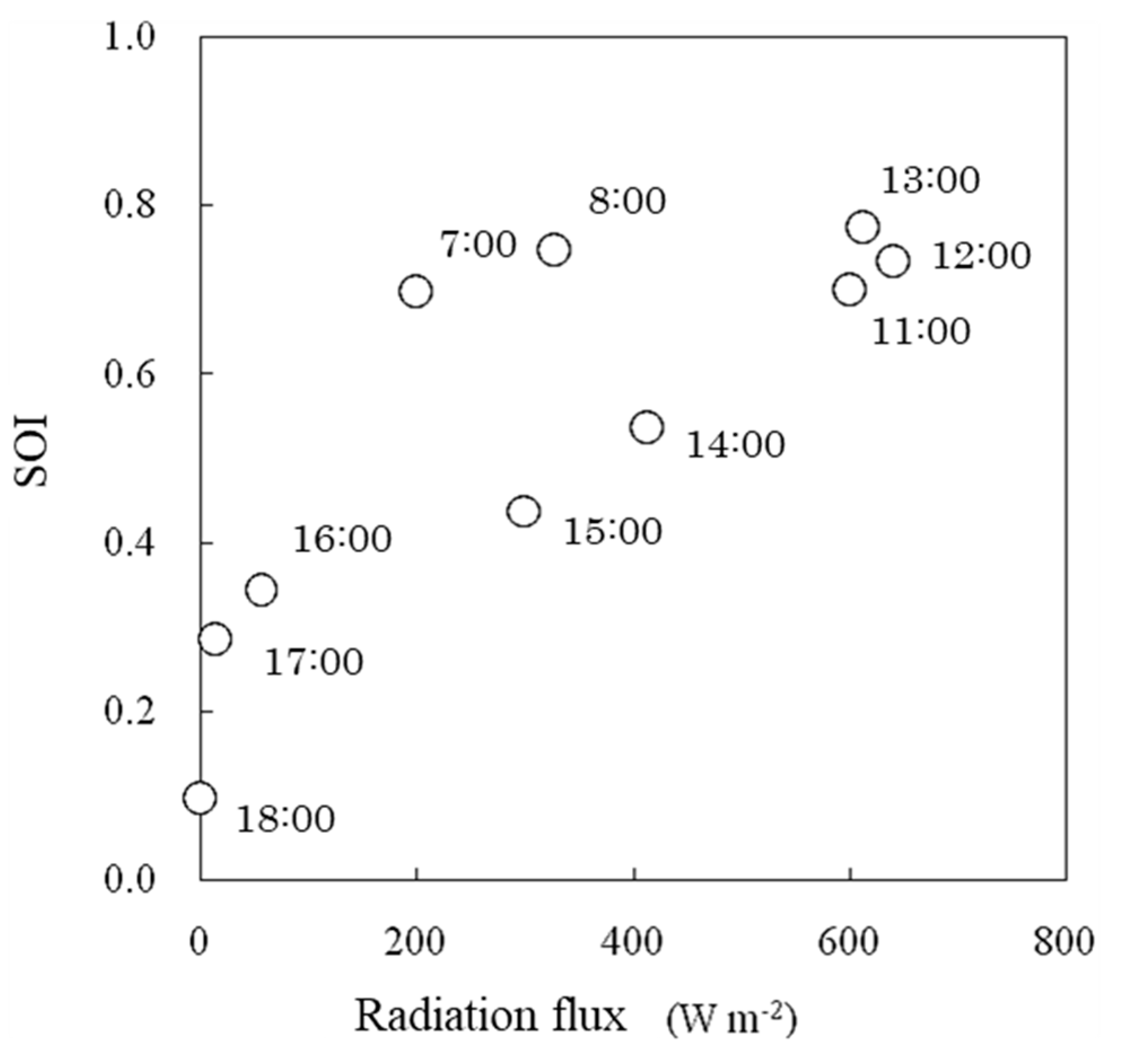
The SOI tended to increase logarithmically with increasing solar radiation flux (Figure 15). This tendency was similar to the results of the growth chamber experiment (Figure 9). Under the same solar radiation flux, the SOI was lower in the afternoon than in the morning (Figure 15). This finding was consistent with the usual phenomenon in which the stomatal aperture decreases and thus leaf conductance decreases in the afternoon [29].
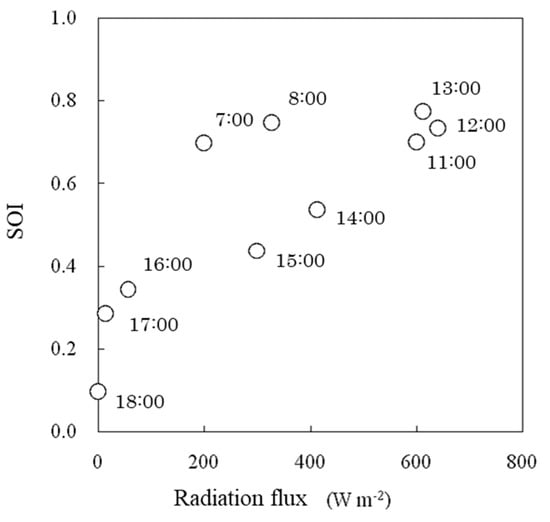
Figure 15.
Relationships between solar radiation flux and the SOI measured in a greenhouse. The number beside each symbol indicates the time.
4. Conclusions
There was no problem in using a thermal image camera for SOI measurements. It was found that water-based dyes are suitable for coloring wet reference leaves. The SOI was confirmed to be an indicator of leaf conductance. The SOI estimated by this method was consistent with the leaf conductance measured by the standard porometer method.
It was confirmed that the SOI is an indicator of stomatal conductance and thus transpiration and photosynthesis ability. Since this method is primarily aimed at application inside greenhouses, the airflow is mostly stable in closed greenhouses, but the radiation flux fluctuates significantly. The response of the SOI to radiation flux fluctuations must be further investigated.
Thermography is a powerful tool for detecting water stress in plants suffering from water shortage in various cases [30,31,32,33]. Our method is also expected to use continuous monitoring of the stomatal behavior of plants for detecting water stress in greenhouses and fields. The monitored data will be useful not only for studies on interactions between plants and the environment but also for controlling the environment, such as watering in plant production sites. The SOI, including leaf temperature data, is also a valuable indicator of the physiological state of plants in response to both biotic and abiotic stressors.
By using thermocouples instead of thermal image cameras for surface temperature measurements, a simpler and lower-cost system can be established. By arranging them at multiple points, it is possible to address the environmental responses of plants that differ depending on their location inside the greenhouse.
Advances in plant response monitoring technology have made environmental control in greenhouse horticulture more precise and efficient. These technologies can contribute to improving yields and stabilizing the quality of produced plants through understanding the health status and growth process of plants in real time and providing optimal environmental conditions. It is expected that further advances in technology will enable even more precise and lower-cost environmental control by using this method.
Author Contributions
Conceptualization, Y.K.; methodology, Y.K.; validation, Y.K., N.I., R.E. and T.S.; formal analysis, Y.K.; investigation, Y.K., N.I., R.E. and T.S.; data curation, Y.K. and N.I.; writing—original draft preparation, Y.K.; writing—review and editing, R.E. and T.S.; project administration, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kozai, T. (Ed.) Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; p. 441. [Google Scholar]
- Gao, Z.; Luo, Z.; Zhang, W.; Lv, Z.; Xu, Y. Deep learning application in plant stress imaging: A review. AgriEngineering 2020, 2, 29. [Google Scholar] [CrossRef]
- Zhou, Z.; Majeed, Y.; Naranjo, G.D.; Gambacorta, E.M. Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications. Comput. Electron. Agric. 2020, 182, 106019. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C.; Takagaki, M.; Maruo, T. Greenhouse environment control technologies for improving the sustainability of food production. Acta Hortic. 2014, 1107, 1–14. [Google Scholar] [CrossRef]
- Kitić, G.; Tagarakis, A.; Cselyuszka, N.; Panić, M.; Birgermajer, S.; Sakulski, D.; Matović, J. A new low-cost portable multispectral optical device for precise plant status assessment. Comput. Electron. Agric. 2019, 162, 300–308. [Google Scholar] [CrossRef]
- Achour, Y.; Ouammi, A.; Zejli, D. Technological progresses in modern sustainable greenhouses cultivation as the path toward precision agriculture. Renew. Sustain. Energy Rev. 2021, 147, 111251. [Google Scholar] [CrossRef]
- Pekkerieta, E.J.; Van Henten, E.J.; Campen, J.B. Contribution of innovative technologies to new developments in horticulture. Acta Hortic. 2015, 1099, 45–54. [Google Scholar] [CrossRef]
- Carrasco-Benavides, M.; Espinoza-Meza, S.; Umemura, K.; Ortega-Farías, S.; Baffico-Hernández, A.; Neira-Román, J.; Ávila-Sánchez, S.; Fuentes, S. Evaluation of thermal-based physiological indicators for determining water-stress thresholds in drip-irrigated ‘Regina’cherry trees. Irrig. Sci. 2024, 42, 1–15. [Google Scholar] [CrossRef]
- Poirier-Pocovi, M.; Bailey, B.N. Sensitivity analysis of four crop water stress indices to ambient environmental conditions and stomatal conductance. Sci. Hortic. 2020, 259, 108825. [Google Scholar] [CrossRef]
- Savvides, A.M.; Velez-Ramirez, A.I.; Fotopoulos, V. Challenging the water stress index concept: Thermographic assessment of Arabidopsis transpiration. Physiol. Plant. 2022, 174, e13762. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Patra, H.; Bajpai, V.; Das, B.; Kumar, S.; Dhandapani, R.; Vishwakarma, C.; Pal, M. Application of thermal imaging and hyperspectral remote sensing for crop water deficit stress monitoring. Geocarto Int. 2021, 36, 481–498. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Lawson, T. Dynamic leaf energy balance: Deriving stomatal conductance from thermal imaging in a dynamic environment. J. Exp. Bot. 2019, 70, 2839–2855. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Lawson, T. Thermography methods to assess stomatal behavior in a dynamic environment. J. Exp. Bot. 2020, 71, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Monteith, J.L. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995, 18, 357–364. [Google Scholar] [CrossRef]
- Guilioni, L.; Leinonen, I.; Lhomme, J.P. On the relationships between stomatal resistance and leaf temperatures in thermography. Agric. For. Meteology 2008, 148, 1908–1912. [Google Scholar] [CrossRef]
- Jones, H.G. The use of infrared thermometry for estimation of stomatal conductance is a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Struthers, R.; Ivanova, A.; Tits, L.; Swennen, R.; Coppin, P. Thermal infrared imaging of the temporal variability in stomatal conductance for fruit trees. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 9–17. [Google Scholar] [CrossRef]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.L. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Yabuki, K. Photosynthetic Rate and Dynamic Environment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; p. 136. [Google Scholar]
- Kitaya, Y.; Tsuruyama, J.; Shibuya, T.; Yoshida, M.; Kiyota, M. Effects of air current speed on gas exchange in plant leaves and plant canopies. Adv. Space Res. 2003, 31, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Suwannarut, W.; Vialet-Chabrand, S.; Kaiser, E. Diurnal decline in photosynthesis and stomatal conductance in several tropical species. Front. Plant Sci. 2023, 14, 1273802. [Google Scholar] [CrossRef] [PubMed]
- Gontia, N.K.; Tiwari, K.N. Development of crop water stress index of wheat crop for scheduling irrigation using infrared thermometry. Agric. Water Manag. 2008, 95, 1144–1152. [Google Scholar] [CrossRef]
- Maes, W.; Achten, W.M.J.; Reubens, B.; Muys, B. Monitoring stomatal conductance of Jatropha curcas seedlings under different levels of water shortage with infrared thermography. Agric. For. Meteorol. 2011, 151, 554–564. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in superhigh density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and future perspectives of multi-/hyperspectral thermal infrared remote sensing for crop water-stress detection: A review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).