Abstract
The need to use environmentally friendly substances in agriculture for pest control has become increasingly urgent in recent years. This was generated by humanity’s awareness of the harmful effects of chemicals with increased persistence, which accumulated in nature and harmed living beings. Essential oils are among the most important biopesticides and could significantly contribute to the expansion of ecological agriculture, replacing traditional methods. However, for judicious use, it is necessary to have a thorough knowledge of the mechanisms by which these oils act on both harmful and useful insects. An important step in transitioning from theory to practice is adapting essential oil application technologies for open fields, overcoming the difficulties created by their high volatility and low remanence, which results in a rapid reduction in the toxic effect. The review proposes an in-depth, up-to-date analysis of the existing literature on these subjects, aiming to provide researchers with some potential future study directions and practitioners with a solid base of information regarding the interaction between insects and essential oils.
1. Introduction
The essential oils produced by plants as secondary metabolites have influenced the modulation of the interaction between them and insects over millions of years of evolution. They have served as attractors for pollinators and other insects useful to plants, or as a defense against harmful insects [1]. The coexistence between plants and other organisms (insects, herbivorous mammals, fungi, bacteria, etc.) has determined the appearance of numerous subtle mechanisms of adaptation and resistance to those and consequently to various secondary metabolites.
People recognized the potential of substances extracted from plants and the help they could give in the fight against agricultural pests long before understanding their structure and properties. The first historical data showing the use of plant extracts against insects date back to over 3000 years ago [2]. The progress of knowledge, on the one hand, and the damage caused to the environment by the most recently introduced synthetic pesticides have determined in recent years the appearance of numerous studies on essential oils used as biopesticides.
Despite their similar anatomical structures, not all insect species respond uniformly to essential oils; different components of the volatile oils can have completely opposite effects on related insect species. For example, geraniol acts as a repellent for flies [3] or mosquitoes [4], but it is attractive for pollinators [5], including bees [3].
Considering the wide range of insect responses to certain essential oils, some defense mechanisms of plant species producing these oils can serve as indicators of the degree of toxicity of the oil against a specific harmful insect species. For example, citrus species (lemon, orange, grapefruit) are almost never affected by infestation by the fruit fly Anastrepha fraterculus Wiedemann (Diptera: Tephritidae) [6]; even when females lay eggs on these plants, the larvae are found dead near the place of oviposition due to the insecticidal effects of the essential oils stored in the secretory cavities present both in the leaves and in the pericarp of the fruits of these species.
An increasing number of studies on the effects of essential oils on insects and their results outline more and more clearly the idea that these effects are varied and cannot be generalized even to all species within a family once they have been found in relation to a pest.
At the same time, another problem emerges from the results of researchers who dealt with this subject: the application technology of the oil significantly influences the obtained results. Since most studies are conducted under laboratory conditions, extrapolating the effects to open-field administration is not always possible [7,8].
In this paper, we aim to investigate in depth the mechanisms by which essential oils generally act on insects. Understanding the interaction between pests and the active substances in essential oils is crucial in choosing the most effective solutions for their combat. Many studies focused on the toxic effects of essential oils on different species of insects are limited to specifying the LC50 (lethal concentration that kills 50% of the individuals tested) or LD50 (the amount of substance that causes the death of half of the individuals in a group), without delving into the study of the mechanisms by which this death is caused.
In order to gain an overview of the studies carried out in the last 25 years, a bibliometric analysis on this subject was performed. This analysis aimed to highlight trends in authors’ interest in researching the mechanisms of action of essential oils on insects, the evolution of approaches on successive stages over time, and the contribution of various countries to obtaining practical and theoretical data.
Additionally, modern technologies for applying essential oils that can be successfully used in agricultural practice were investigated and systematized. This is important because different formulations of the oils before application lead to obtaining quite varied results, highlighting the fact that not only the active substance is important but also the ”vehicle” through which it reaches the target species [9].
2. Methodology
To conduct this study, we undertook a comprehensive exploration of specialized paper databases. This involved thorough research and analysis of the available literature relevant to our investigation.
The bibliometric analysis was carried out using the following programs Bibliometrix (University of Naples Federico II, Naples, Italy) (version 4.3.1) https://www.bibliometrix.org/home/index.php (accessed on 18 February 2024) [10,11] and VOSviewer (Leiden University, Leiden, The Netherlands) (version 1.6.20) https://www.vosviewer.com (accessed on 18 February 2024).
To gather essential data, we accessed the Web of Science core collection (WoS) databases on 16 February 2024. WoS is a database that collects papers published in peer-reviewed journals, with an impact factor, widely accepted and used by specialists in various fields [12]. This includes over 21700 journals and over 91 million records (February 2024, source https://clarivate.libguides.com/librarianresources/coverage) (accessed on 16 February 2024). The objective was to discern the current trend in researchers’ investigations concerning the mechanisms of essential oils in insects. The search was carried out under the following conditions: all types of documents for the period 1975–2024 were considered; the search terms were “essential oil” (All Fields) AND “insects” (All Fields) AND “mechanism” (All Fields).
Using these data saved in BibTex and Ris formats (including full records and cited references), a bibliometric analysis was conducted. Following the search, 300 papers were identified, of which 240 were retained to carry out the proposed study. Papers that do not refer to essential oils, those that focused on their antimicrobial activity, the insecticidal activity of other plant-derived compounds, as well as papers referring only to the composition of the essential oils, were excluded from the analysis. The abstract of each paper was analyzed in order to establish whether the content was correlated with the investigated subject. Also, papers published in 2024 were excluded, given the short time that has passed since the beginning of the year, making these results irrelevant for the entire calendar year. A diagram showing the methods of collection, selection and processing of data from WoS [13] is presented in Figure 1.
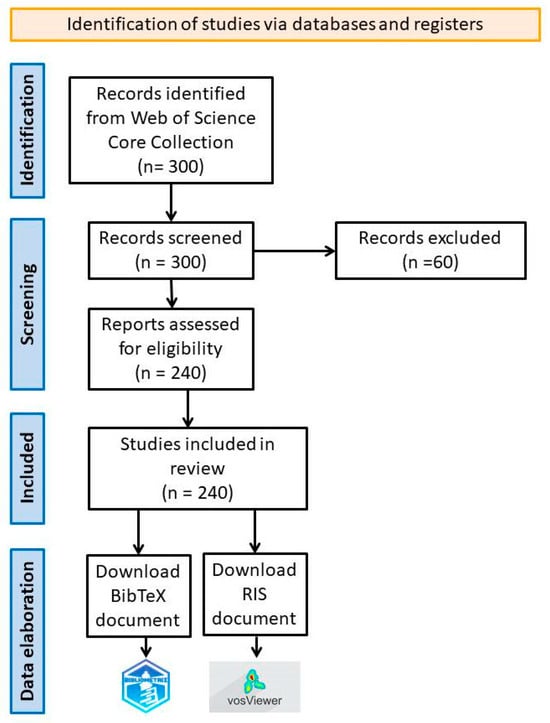
Figure 1.
Flow diagram for data collection, selection and processing.
In parallel, both Web of Science and other databases (Scopus and Google Academic) were queried for keywords such as “biopesticides”, “field experiments”, “nanoemulsions”, “nanoparticles”, “NaDES”, “acetylcholinesterases”, “octopamine receptors”, “tyramine receptors”, “GABA receptors”, “GluCls”, “superoxide dismutase”, “catalase”, “peroxidases”, “glutathione-S-transferase”, “Insect Growth Regulators”, “ cuticle”, “repellent”, “synergistic”, in conjunction with “insects”, “essential oil” or “mechanism” in order to identify as many studies as possible that are related to the subject of the current paper.
3. Results and Discussions
3.1. Bibliometric Analysis Regarding the Mechanisms of Action of the Essential Oils on Insects
In order to analyze trends in the study of the mechanisms of action of essential oils on insects, we used the following bibliometric indicators: number of publications and their total citations/year, the most relevant sources (the top 15 journals in which selected papers were published), author’s production and country production overtime, co-occurrence network of terms from the title and abstract of the papers, word cloud diagram representing the major research highlights (Keywords Plus) and thematic evolution plot.
The structure of entries by document type was as follows: out of 240 works, 138 are articles (57.5%), 56 early access articles (23.33%); 7 proceedings papers (2.91%), 30 reviews (12.5%), 8 early access reviews (3.33%), and 1 book chapter (0.41%). The language of the papers was English.
From the analysis of the temporal distribution of papers published in connection with the analyzed subject, we observed a low interest from researchers between 1999 and 2012 (with only 1–2 papers published annually). Between the years 2013 and 2019, there was a moderate increase in the number of works (ranging between 6 and 18), while in the last 4 years (2020–2023), there has been a significant increase in the number of publications (ranging between 22 and 39/year) (Figure 2).
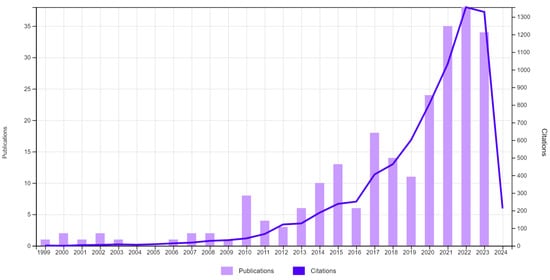
Figure 2.
Number of publications/year and their total citations/year (source—Web of Science).
The number of citations for the 240 selected papers is high: 7363 citations (7013 without self-citations), with an average of 30.68 citations/article and an h-index of 40. A directly proportional increase in citations compared to the number of published papers can be observed, which is particularly remarkable after 2021.
It follows that in the last 4 years, 55% of the papers on this topic from the last 25 years were published. The growing trend of researchers’ interest in the use of essential oils in the fight against insect pests is combined with the need to understand the mechanisms of their action and the implications that various methods of administration have on both pests and non-target species.
The results are in accordance with those obtained by the authors in a recent study on use of the essential oils as biopesticides [14]: papers in WoS in the last 4 years represent 62.61% (in correlation with harmful insect species) and 65.21% (in correlation with non-target species) of the total number of indexed publications referring to these subjects.
The most relevant sources (top 15 journals) were Pesticide Biochemistry and Physiology, Insects, Pest Management Science, Molecules, Industrial Crops and Products, Journal of Pest Science, Journal of Stored Products Research, Journal of Chemical Ecology, Plants-Basel, Journal of Economic Entomology, Plos One, Scientific Reports, Acs Omega, Crop Protection, Entomologia Experimentalis et Applicata (Figure 3). Of these, seven journals are classifiedin Q1, six journals in Q2 and one in Q3.
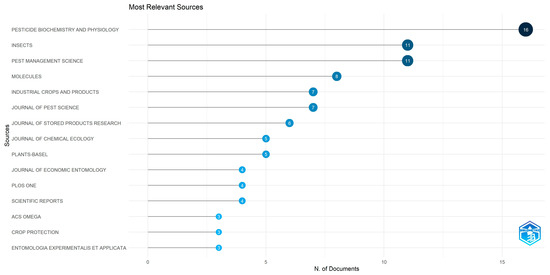
Figure 3.
Most relevant sources—top 15 journals (source: Bibliometrix ®R package, version 4.3.1. software).
There were 14,805 references to the 240 documents analyzed; among these papers the most cited are from the following journals: Pest Management Science (376 citations), Industrial Crops and Products (360 citations), Journal of Economic Entomology (337 citations), Journal of Agricultural and Food Chemistry (331 citations), Journal of Stored Products Research (326 citations), Pesticide Biochemistry and Physiology (294 citations), Parasitology Research (264 citations), Journal of Chemical Ecology (259 citations), Plos One (245 citations) and Annual Review of Entomology (220 citations).
We notice that the top 3 journals in terms of papers cited in the group of documents analyzed are among the top 10 journals that publish works related to this subject. This fact denotes a concentration of interest in some journals to publish studies on the use of environmentally friendly pesticides in accordance with the general increased interest in green agriculture in recent years.
In the group of investigated papers, 1158 authors were identified. Among these, the top 10 authors can be found in Figure 4. This also illustrates the production of the first 10 authors from 2010 (when the author Coats J.R started his work) until now. We notice that 8 out of 10 authors continue their publishing activity. There is an overlap between the top 10 authors and the most prolific countries: 7 authors are institutionally affiliated in China, 1 in Italy (the first author in the top 10), 1 in Brazil and 1 in the USA.
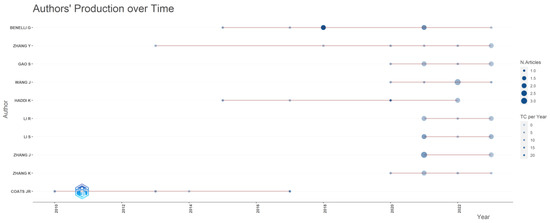
Figure 4.
Authors production overtime (plot created with VOS viewer 1.6.20 software).
The most prolific countries are China (202 appearances), followed by Brazil (150), USA (107), Italy (44) and South Korea (40). Figure 5 also shows the production of the top 5 countries during the years 1999 and now. If the production of the five countries was initially low and almost equal, after 2009, we can see the detachment of the USA compared to the other four countries. Its dominance lasts until 2021, because starting in 2020, the rapid ascent of China begins, which in the following year overtakes the existing leader at that time.
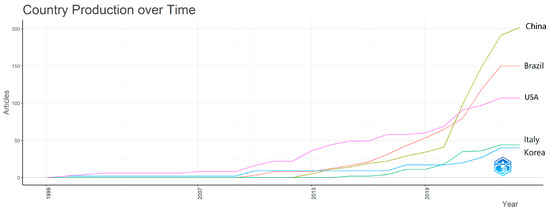
Figure 5.
Country production over time (plot created with VOS viewer 1.6.20 software).
The analysis of keywords extracted from the title and abstract was carried out with Vos Viewer using the full counting method. In the co-occurrence network, links between keywords are highlighted, with the size of the nodes directly proportional to the frequency of their appearance in the analyzed group of papers.
The results obtained were the following: out of 7654 terms with at least 20 occurrences/terms (n = 20), 60 met the conditions. Among these, the most relevant 60% were selected (36 items of which 35 were analyzed), and grouped in three clusters (Figure 6).
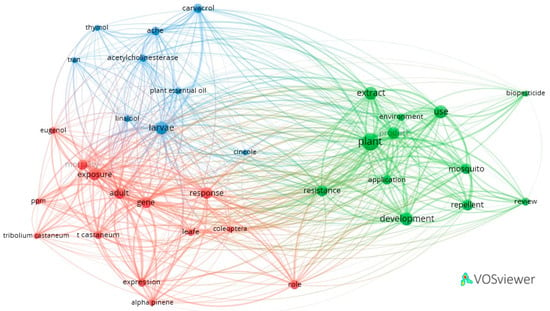
Figure 6.
Co-occurrence network of terms from the title and abstract of the papers: three clusters can be observed: cluster 1 (red) with 14 items, cluster 2 (green) with 12 items and cluster 3 (blue) with 9 items. (plot created with VOS viewer 1.6.20 software).
Cluster 1 (red) contains 14 items, the most relevant of which are “adult”, “mortality”, “exposure”, “response” and “gene”. It is focused on the mortality induced by the essential oils in tests performed on insects (one of the secondary terms being “ppm”). Cluster 2 (green) contains 12 items, of which “plant”, “extract”, “development”, “resistance” and “biopesticides” appear more frequently. This cluster predominantly refers to the role of plant extracts used as biopesticides in the fight against harmful insects, as well as their natural or induced resistance to potentially toxic substances they encounter. Cluster 3 (blue) contains nine items that refer, in particular, to components of essential oils, “carvacrol”, “cineole”, “linalool” and “thymol” and their effect on insects. Other keywords represented include “acetylcholinesterase” and “larvae”.
The absence from the three clusters of some important terms in the analysis of the mechanisms of action of the essential oils on insects, such as “nanoemulsions”, “octopamine receptors”, “tyramine receptors”, “GABA receptors”, “GluCls”, “superoxide dismutase”, “catalase”, “peroxidases”, “glutathione-S-transferase”, and “Insect Growth Regulators”, reveals their low frequency in the analyzed documents and implicitly the limited number of studies in which they currently appear.
Figure 7 depicts the word cloud diagram; this was conducted using KeyWords Plus, a distinctive algorithm exclusive to Clarivate databases. It enhances cited-reference searching by scouring across disciplines to identify articles that share commonly cited references.
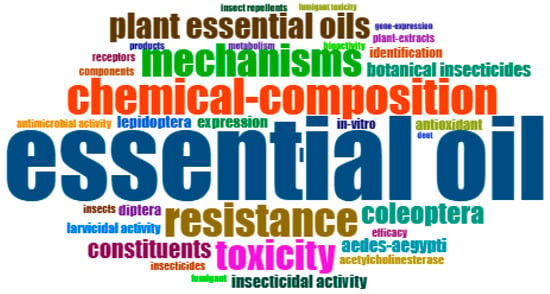
Figure 7.
Word cloud diagram representing the major research highlights (35 Keywords Plus) (source: Bibliometrix ®R package, version 4.3.1. software).
The top five keywords were as follows: essential oil (90), chemical composition (39), resistance (37), mechanisms (35), and toxicity (33); the frequency of each word is specified in parentheses. As expected, the primary term is “essential oil”, and the following words express research directions and associations made in the studies regarding the action mechanisms of essential oils. Establishing the chemical composition of the tested oils is crucial due to the significant variations observed depending on the environment in which the oil-providing plants grow [14,15]. Investigations on the mechanism of action of the essential oils are correlated with the degree of toxicity that they (or their components) exhibit on insects [16,17], as well as with resistance phenomena that reduce the effectiveness of biopesticides on target species [18].
The evolution of research on the mechanisms of action of essential oils on insects can be observed by following the plot in Figure 8. In the first time interval 1999–2010, the number of keywords is lower, in accordance with the reduced number of papers published in that period (Figure 2). Most studies refer to the effects of essential oils on harmful pests in storage [19], where they were easier to apply because closed spaces enhanced the effectiveness of volatile substances compared to open fields. In the period 2011–2016, the emphasis of investigations shifted to the toxicity of essential oils as well as their repellent effect.
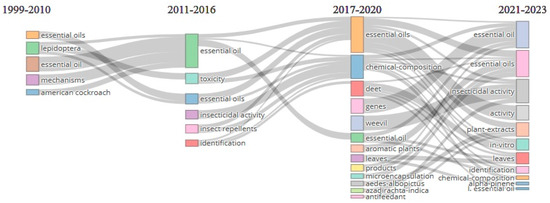
Figure 8.
Thematic evolution plot illustrating the evolution of the research trends and their convergence as well as divergence under four different time intervals: 1999–2010, 2011–2066, 2017–2020, and 2021–2023 (plot created with Bibliometrix®R package, version 4.3.1 software).
In the intervals of 2017–2020 and 2021–2023, the number of publications is increasing and with them, the topics covered are also diversifying. Microencapsulation techniques (used for the open field distribution of the essential oils used as biopesticides) and their insecticidal activity in in vitro experiments are highlighted.
3.2. Application Technologies of the Essential Oils: From Laboratory Experiments to Field Results
Until now, the vast majority of the experimental studies on the insecticidal effects of essential oils from different plant species have been carried out in laboratories, with only a few continuing to establish the effectiveness of their application in open-field conditions [20,21].
In the case of laboratory experiments, the application of the essential oils (pure or in various types of formulations) is carried out by several methods:
- −
- Direct contact/topical application methods, by applying a known amount of essential oils directly on the insect’s body [7,22];
- −
- By fumigation [22,23,24].
- −
- Administration in food [21,25,26].
In all these situations, the exposure of the insects to the action of the essential oils is enforced, as they are kept in cages or boxes throughout the experiment [27]. This approach eliminates, the possibility for insects to evade the harmful effects of the essential oils, which also exert repellent effects, as is well known [28,29]. For these reasons, the simple extrapolation of the results obtained in the laboratory to applications in the open field is nearly impossible in these types of studies.
Due to their high volatility at temperatures frequently encountered in the atmosphere, as well as rapid degradation under the influence of environmental factors such as light and air contact [30], essential oils are often applied by fumigation in greenhouses or in grain storages [31].
That is why it is necessary to continuously seek out the most effective methods of applying essential oils used as biopesticides, ensuring their longer persistence in the environment and maintaining maximum efficiency. For a long time, emulsions/microemulsions were used because they were cheap and easy to make, but this technology does not have the ability to maintain the effectiveness of the active substance for too long [32]. However, in order to achieve increased efficiency in the bioavailability of the active substance, nanotechnologies represent an option that needs to be studied much more intensively to find the most effective methods of application in pest management.
3.2.1. Nanostructured Formulations of the Essential Oil—Advantages and Disadvantages
The administration method of essential oils can significantly impact toxic doses, affecting both harmful species and non-target species alike.
Traditional methods of nanoformulation of various lipid substances often involve substances toxic to the environment and detrimental to human health [33]. Therefore, special attention must be paid to the use of these technologies in manufacturing products intended to be biopesticides to ensure the environmentally friendly effect of the active substances even after they have been processed to prolong persistence, reduce volatility, or increase solubility. Nanoemulsions are commonly prepared by mixing essential oils with Tween® 80, a dispersing agent in the form of a nonionic detergent and surfactant, typically in concentrations ranging from 3% to 5% [34,35]. The emulsion is subsequently stirred at many rotations per minute (RPM), optionally followed by ultrasonication. If these values are increased, the process occurs with cooling to prevent the evaporation of the active substance. The diameter of the obtained droplets typically ranges between 20 and 200 nm, with variations depending on the type of essential oils used and the method employed [27,34,35,36]. The stability of bioactive substances in nanoencapsulation with Tween 80 or 20 is enhanced, often without the need for a co-surfactant [37].
Other substances that have been tested for the creation of nanoemulsions include alginate and glycerol [38], as well as saponin [36], which itself has insecticidal properties and can act synergistically with the essential oils used [39].
The chemical nature of the component elements in the essential oils influences the size of the particles obtained: when oxygenated monoterpenes and sesquiterpene hydrocarbons predominate in the oil component, smaller particles are obtained [36,40]; at the opposite pole are organosulfur compounds from garlic oil [40], phenylpropanoids and alkane hydrocarbon from clove oil [41].
Even in the case of nanoemulsions, the problem of stability over time and maintaining the reduced size of the particles with the active substance is of great importance. For example, in the case of citronella oil (Cymbopogon nardus (L.) Rendle (Poaceae)), the nanoemulsions obtained using a mixture of Tween 60 and Span 60 were stable when stored at 4 °C for 28 days. However, the particles increased considerably in size, leading to phase separation when the mixture was stored at 45 °C [42].
In addition to the immediate effect of nanoemulsions, studies have also shown a cumulative effect over time due to the progressive release of the active substance from the solutions formulated in this manner [40].
For instance, in the case of citronella oils, their application in the form of nanoemulsions significantly increased the mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) larvae [43]. At concentrations reduced by half, the nanoemulsified oil was 12–18% more efficient than bulk oil.
Nanoemulsification of the essential oils does not always increase the insecticidal effects on the target species. Mahran and collaborators [41] showed that this technique did not improve the performance of clove (Syzygium aromaticum (L.) Merr. & L. M. Perry (Myrtaceae)) and ginger (Zingiber officinalis Roscoe (Zingiberaceae)) oils on the larvae of Culex pipens L. (Diptera: Culicidae).
Nanoparticles containing essential oils can be prepared using various materials, including metals, semiconductors or polymers (chitosan, cyclodextrin, pectin, starch cellulose, polycaprolactone, alginate and polyethylene glycol (PEG)), [44]. Among these, PEG is most frequently used in agriculture for different purposes [45,46] (Ibrahim, 2020, Antofie and Sava, 2021).
Polymer-based nanoparticles represent an efficient method of administering essential oils, which has been tested against both harmful insects in storage (Rajkumar et al., 2020) and some phytophagous species [47].
The effectiveness of nanoparticles with chitosan was demonstrated regarding the essential oil of Trachyspermum ammi (L.) Sprague ex Turrill (Apiaceae) (syn Carum copticum (L.)) on Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae) and Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) [48]. However, the authors warn of the need for further studies to confirm the effectiveness of the use of nanoparticles in open-field treatments. Nanoparticles with polyethylene glycol, using lemon peel essential oil were significantly more effective in combating the black cutworm Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae) [47]. Additionally, nanoparticles with citronella essential oil and chitosan–cellulose increased the insecticide effect against the cotton leafworm Spodoptera littoralis [49].
Not all studies have shown a higher efficiency of nanoparticles with essential oils compared to bulk oils. Ikawati and collaborators [17] found that nanoparticles with PEGs of clove (Syzygium aromaticum) essential oil did not exhibit increased efficiency in activity against red flour beetle (Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae)); however, their persistence, due to the encapsulation process, increases up to 16 weeks of high contact toxicity. Similar results were obtained in the case of garlic oil included in nanoparticles with PEG: its persistence in the environment increased for up to 5 months, although the initial toxicity was lower [50].
Atomized powders are obtained through spray-drying techniques, which involve drying a previously prepared emulsion with hot gas, typically an inert one. This process causes the solvent to evaporate leaving behind fine, solid drops containing the active substance [30] (Yammine et al., 2023). Both micro- and nanometric particles can be obtained through this technique [51]. For instance, Lippia sidoides Cham. (Verbenaceae) oils (in chitosan) [52], rosemary, and Artemisia oils (with gum arabic and maltodextrin) have been encapsulated in nanoparticles using this method [34].
Due to the high temperatures used, well above the evaporation point of the essential oils, important quantities of oil can be lost in this process [53], and it is necessary to evaluate the economic efficiency in the case of mass production.
One of the major obstacles to the use of essential oils as biopesticides is their poor solubility in water. For this reason, finding non-toxic solvents to facilitate the application of these compounds in agriculture has been the concern of many researchers. The development of Natural Deep Eutectic Solvents (NaDES) and testing for their use as vectors of the essential oils to combat pests is still in its infancy, and very few studies are available: Choi and collaborators [54] described for the first time this class of green solvents, which consist of substances normally found in the cellular environment, such as citric acid, choline chloride, maleic acid, choline tartrate, betaine, carnitine, etc. [55].
NaDES acts as a solvent for dissolving essential oils creating a suitable reaction environment for nanoparticle synthesis. These solvents have important characteristics that favor their use as nano-carriers for active substances with increased volatility such as essential oils: they are in liquid form at the temperatures frequently found in the environment; they are not toxic, non-flammable, weakly volatile and chemically stable [56].
Dunan and collaborators [34] investigated the insecticidal effects of commercially obtained Artemisia and Rosemary essential oil on silverleaf whiteflies Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), one of the most important invasive insect pests in the world. They compared the insecticidal effects of these oils administered in different forms. The NaEDS formula proved to be less toxic for the harmful species compared to the oil administered in the form of nanoemulsions, but this was also less toxic than the host plant (tomato), for which it is recommended for pest control.
Although they are not yet widely used in agricultural practice, nanoformulations of essential oils represent a promising option for expanding organic agriculture. These formulations have the ability to overcome the main shortcomings of essential oils that prevent them from being effective as biopesticides. In the form of nanoemulsions or nanoparticles, they become more effective, presenting a considerably increased contact surface relative to volume [57].
In the use of nanoformulated essential oils, an important obstacle is the production costs, which in some situations can be quite high. Spray drying techniques involve significant costs for purchasing the necessary equipment to obtain nanoparticles [58].
3.2.2. Mechanisms of Action of Essential Oils in Insect Species
Most of the pesticides used in agriculture, whether synthetic or biopesticides, rely on the neurotoxic action of the different compounds on the target species. However, when the target species and the useful species in the agricultural ecosystem, especially the pollinators, are phylogenetically related and therefore structurally and functionally similar, it becomes challenging to identify the active substances that affect the pest without harming the non-target species.
In other words, the origin of the bioactive molecule cannot guarantee higher selectivity. Concerning bioinsecticides, the source of the compound does not dictate its toxicity, and the perceived safety of these substances is once again a misconception [59]. Among the components of essential oils, monoterpenes are known to have the strongest insecticidal action [60].
Virtual screening methods, also known as chemoinformatics, have revolutionized the discovery of new bioactive compounds by efficiently assessing large structural libraries against biological systems. These methods streamline the discovery process, saving time and resources [61].
Used mainly in pharmacological studies to identify active substances useful in the treatment of various diseases, chemoinformatics is beginning to gain ground in studies related to the effectiveness of some biopesticides. There are several methods to investigate in silico the interactions between biomolecules and various receptors [61]. Ligand-Based Virtual Screening (LBVS) analyzes compound structures to predict activity (Garcia-Hernandez et al., 2019), while Structure-Based Virtual Screening (SBVS) uses ligand recognition and binding in bio-receptor structures [62]. LBVS relies on similarity search, machine learning, and pharmacophore modeling, while SBVS employs molecular docking and dynamics simulations. Both methods are crucial for discovering new bioactive molecules (including essential oils components) for medicine, pharmacy, and agriculture.
- Changes in the activity of acetylcholinesterases (AChEs)
Acetylcholinesterase (AChE) is an enzyme that plays a crucial role in the nervous system of most animals, including insects [63]. It hydrolyzes the neurotransmitter acetylcholine (ACh) into its components, acetate and choline [64]. This process is vital in preventing continuous stimulation of the postsynaptic neuron and allows for proper nerve signal regulation. This was the target for most synthetic insecticides [65].
The components of the essential oils, including terpenes terpenoids, and phenylpropanoids, interact with AChE activity. Their insecticidal/miticidal effect is manifested by the inhibition of enzymatic activity. This inhibition leads to the accumulation of ACh at the synapses [66], resulting in increased nervous excitement and ultimately causing the death of the insect.
Classical pesticides are known to strongly inhibit AChE in insects [67]. However, their toxic effects are equally manifested in both insects and vertebrates, including humans. Nonetheless, there are slight structural differences at the ligand-binding sites in AChE between insects and mammals [68]. These differences can be exploited by selecting specific inhibitors for AChE in insects that do not affect the enzyme’s activity in mammals.
Oregano oil inhibited the AChE activity of Aedes aegypti after 12 h. A concentration of 40 µg/mL of nanoemulsion caused complete mortality for both adults and larvae [69].
AChE inhibition by Artemisia nakaii Pamp. (Asteraceae) essential oil was observed on Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) [70]. Among the components of the essential oil, analyzed separately, β-selinene had the strongest AChE inhibition, almost 70%, at a concentration of 10 μg/mL.
Carvacrol and α-pinene showed a high inhibition rate of AChE in the German Cockroach (Blattella germanica), ranging between 55.1% and 86.3% [71]. Carvacrol has been demonstrated to affect the nervous system of insects in a manner similar to that of classic pesticides [72], specifically by inhibiting acetylcholinesterases (AChE) [73].
Lemongrass oil [74], and citrus oil [75] significantly decrease AChE activity in Callosobruchus maculatus (Coleoptera: Chrysomelidae). Among the most effective components of the essential oils that inhibit enzymatic activity are α-pinene β-pinene, β-phellandrene, carvacrol, limonene, menthol, menthone, 1,8-cineole, etc. [65].
The interaction mechanism of the main components of Eucalyptus camaldulensis essential oil (among which trans-myrtanol, carvacrol and β-caryophyllene) with acetylcholinesterase was modeled in silico by Beyaoui and collaborators [76]. Molecular docking analyses serve the purpose of assessing the compatibility of chemical compounds with the binding site of a receptor and determining specific interactions.
Hymenoptera species from the genera Apis, Bombus, Camponotus, Scolia, Rhynchium, Polistes, Vespula, and Vespa, known for their crucial role as pollinators in various ecosystems, especially agricultural ones, possess AChE2-type acetylcholinesterases. Insects from other genera, such as Megachile, Chalicodoma, Xylocopa, Crocisa, and Anthophora, have AChE1-type acetylcholinesterases [63]. Many conventional pesticides aim to inhibit AChE by binding to its active site in harmful insects. Considering the presence of AChE in both useful (pollinating) and pest insects, we can infer that organic pesticides containing essential oils with an AChE-inhibiting effect may equally affect pests and useful insects, which are non-target species.
Under the pressure of pesticides administered in agriculture, especially in the last hundred years, some insect species have developed resistance to them. This resistance, including point mutations in the gene encoding AChE, has been observed in species such as the melon aphid (Aphis gossypii Glover, Hemiptera: Aphididae)) [77].
An increase in AChE activity was recorded in Tuta absoluta (Lepidoptera: Gelechiidae) treated with oregano oil, mint oil, and the individual component elements of these oils [78]. This reaction serves as a defense mechanism for lepidopteran insects against the action of the essential oils.
- Inhibition of octopamine and tyramine receptors (OARs/TARs)
Octopamine and tyramine are important biogenic amines [79], synthesized from tyrosine [80]. They serve as neurotransmitters, neurohormones, and neuromodulators [81,82] and are present in large quantities in the nervous system of arthropods, including insects [83]. Comparable to stress hormones, their levels increase under specific conditions, similar to adrenaline and noradrenaline in vertebrates [80]. Octopamine influences memory and learning processes in honeybees, such as Apis mellifera [79], and modulates muscle performance, fat metabolism, heart rate, and respiration in insects [80]. OA/TA neurons extensively branch out throughout the insect body [84], reaching skeletal muscles, reproductive organs, the heart, antennae, legs, wings and halteres (based on anatomical studies conducted on Drosophila melanogaster Meigen (Diptera: Drosophilidae)). This justifies the relevance of modulating complex behaviors.
Since octopamine and tyramine are absent in vertebrates, pesticides formulated to interfere with these compounds are harmless to them [85].
There are relatively few studies investigating the interaction of essential oils and their components with the octopamine receptors system (OAR) or tyramine receptors (TAR), as well as the mechanisms of action. Generally, these substances mimic the effects of octopamine [86], leading to increased levels of intracellular calcium and cAMP [86], primarily functioning as agonists of these receptors.
The experimental studies conducted by Enan [87] revealed that the insecticidal effect of eugenol is mediated through the octopamine receptor system (OAR), and phenolic compounds are more toxic than monoterpenoids. The presence and location of a hydroxyl group and spacing on the benzene ring determine the degree of toxicity of a compound from the essential oils via the OAR system. Clove oil, with an increased content of eugenol and b-caryophyllene, interacts with this and blocks OAR in both the corn leaf aphid Rhopalosiphum maidis (Fitch) (Hemiptera: Aphididae) and the non-target ladybeetle Coleomegilla maculata De Geer (Coleoptera: Coccinellidae) [59].
Cinnamon oil and its primary component, cinnamaldehyde, have been found to disrupt the structure of antennal receptors (damage to sensilla was observed under the scanning electron microscope) in red fire worker ants of the genus Solenopsis (Hymenoptera: Formicidae). This disruption leads to a decrease in octopamine content and, consequently, impairs the ants’ social behavior and the ability to recognize their nest mates [88].
The monoterpenoids α-terpineol and cinnamic alcohol induced the blockade of octopamine receptors in the species Camponotus pennsylvanicus (De Geer) (Hymenoptera: Formicidae), Periplaneta americana (L.) (Blattodea: Blattidae), and Blattella germanica (Blattodea: Blattellidae) at an approximate inhibitory concentration (IC50) of 9 nmol/mL [87]. Ants were found to be more sensitive to eugenol and α-terpineol [89]. However, monoterpenoids may serve another role in interacting with OAR; for instance, menthol potentiates the toxic action of carbamate (a synthetic pesticide) by activating OAR, ensuring increased efficiency at a lower dose than usual [86]. In this way, the adverse environmental effects of synthetic pesticides can be mitigated.
Type 1 tyramine receptors (TAR1s) in Apis mellifera have a different structural configuration compared to that of Diptera or Coleoptera [82]. This particularity could explain the low sensitivity of bees to phenolic monoterpenoids that inhibit TAR, such as carvacrol [90].
- Inhibition of GABA (Gamma-amminobutyric acid) receptors
GABA is an inhibitory neurotransmitter present in the nervous systems of nearly all insect species [91]. It stands as one of the primary targets for pesticides, alongside AChE (acetylcholinesterase) and OARs/TARs. This includes not only traditional pesticides but also, more recently, certain biopesticides.
When GABA binds to its receptors, it triggers the opening of chloride (Cl−) channels, enabling chloride ions to enter neurons, ultimately leading to the inhibition of the nervous system [92]. GABA receptors can either bind to or be inhibited by the active substances found in various pesticides. In both scenarios, this leads to the insect’s death, either through hyperexcitation or the inhibition of neuronal activity [93].
The monoterpenoids carvacrol, pulegone, and thymol were all identified as positive allosteric modulators for the insect’s GABA receptor [94], while linalool and α-terpineol did not exhibit any effects on chloride ion uptake in the ventral nerve cords of the American cockroach Periplaneta americana [93].
The effects of essential oils or some of their components have been studied extensively in mammals [65,93], with less emphasis on insects [92]. However, the interaction between these receptors and some biopesticides opens the perspective of their use in combating harmful arthropods.
Recent studies [95] indicate that numerous insect species, especially those belonging to Lepidoptera and Coleoptera, have evolved alongside plants that produce essential oils, particularly those rich in terpenes. These insects have developed various mechanisms of resistance to the neurotoxic effects of the essential oils, including adaptations in GABA receptors.
- Glutamate-gated chloride channels (GluCls)
GluCls are an important neurotransmitter receptor in the nervous system of invertebrates in general (including insects) [96]. GluCls are generally expressed in the zone area of the head and neural ganglia, but can also be found in the legs, intestine, or reproductive system of different species of insects.
Because GluCls are not present in vertebrates, insecticides that target them are considered safe for humans. GluCls were identified in Apis mellifera [97] in the brain (being involved in the processes of olfactory learning and memory), muscles, and antennae.
Among synthetic insecticides, fipronil (from the phenylpyrazole family) inhibits GluCls [98], causing hyperexcitability in insects. This mechanism is similar to that exerted on GABA receptors, as both are related in the Cys-loop ligand-gated ion channels (cysLGICs) family [94,96].
Although studies on the effects of essential oils on GluCls in insects are very limited, their future exploration may provide interesting perspectives for the use of this class of biopesticides. It has already been shown that in worms (Haemonchus contortus Cobb (Nematoda: Trichostrongylidae)), thymol and menthol inhibit GluCls [99].
- Antioxidant defense enzymatic systems
Reactive oxygen species (ROS) are generated during the oxidative metabolism process in living organisms. They have evolved antioxidant defense mechanisms to protect against the damages caused by ROS, which comprise both enzymatic and non-enzymatic components.
The antioxidant enzyme system serves as a vital physiological mechanism for combating oxidative stress in insects. This system includes enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidases (POD), and glutathione-S-transferase (GST). SOD facilitates the conversion of superoxide anions into hydrogen peroxide and molecular oxygen. Meanwhile, CAT and POD collaborate to break down hydrogen peroxide into water and oxygen. Additionally, GST plays a role in eliminating toxic compounds resulting from lipid peroxidation within cells [100,101]. A decrease in the level of these enzymes (under the influence of the essential oils) is associated with a sensitivity of insects to their action, while increased levels indicate resistance.
Insects possess enzymatic and nonenzymatic defense mechanisms against chemical stress, potentially mitigating the adverse impact of reactive oxygen species (ROS) on cells [102]. Increased oxidative stress may contribute to insect mortality.
Neem oil is one of the essential oils most intensively used as a biopesticide [103]. Following the treatment applied to Apis cerana cerana adults, azadirachtin (AZA) does not have an immediate effect on the activity of two antioxidant enzymes, SOD and CAT, at 24 h and 48 h. However, there are fluctuating changes observed in the activity of another enzyme, phenoloxidase (PO), with 10 mg·L−1 AZA increasing PO activity at 48 h, while both concentrations of AZA (5 mg·L−1 and 10 mg·L−1) inhibited PO activity at 24 h [5].
Some studies have reported a decrease in the concentration of protective enzymes against oxidative stress when certain essential oils are applied at LD50 levels. For instance, the application of lemongrass oil to Spodoptera littoralis resulted in a reduction in the concentration of cytochrome P-450 and GST (LD50 = 2664 mg·L−1) [104]. Interestingly, the application of citral alone did not yield a similar effect. This observation suggests that the synergistic action between the components of the oil enhances the insecticidal effect, potentially by impacting the enzymatic system involved in defense [104]. In studies conducted on mammals, citral has demonstrated antioxidant activity in various investigations, suggesting a potential ability to suppress oxidative stress [105]. Stimulating effects of SOD and GST were also observed [106] in the cowpea weevil Callosobruchus maculatus (Coleoptera: Chrysomelidae) after the application of lemongrass.
The essential oil of peppermint (and its component elements, tested separately—menthone and menthol) on the harmful insects Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium castaneum led to an increase in the SOD level and a decrease in the CAT level [107]. In this case, a significant increase in SOD activity was observed, surpassing the levels observed in the control group. This heightened SOD activity effectively countered the adaptive response aimed at generating reactive oxygen species (ROS) and resulted in a reduction in CAT activity.
A great variability in the responses of insects’ antioxidant enzyme systems can be observed. The increase in the level of enzymes involved in reducing oxidative stress is, on the one hand, a natural response to the increase in ROS at the cellular level under the influence of essential oils [108]. On the other hand, the ability of some oils to reduce the levels of these enzymes facilitates the insecticidal effect by decreasing the defense capacity of the insects against the administered substances. The modulation of antioxidant systems serves as a response to cytotoxicity induced by oxidative stress from both bioinsecticides and conventional insecticides [108]. Nevertheless, the reactions are particular and different depending on the essential oil administered and the targeted species.
- Dysregulation of insect development in different stages of the life cycle
Essential oils (or certain of their components) sometimes act as Insect Growth Regulators (IGR), interfering with insect development processes. Their use is a convenient option because they are specific for insects and harmless for other non-target species [19]. They interact with the methoprene-tolerant receptor (Met), which is the intracellular receptor for juvenile hormones (JH) involved in insect metamorphosis [16], and also with the ecdysone hormone receptor. Considering that JH has a sesquiterpenoid structure [109], substances that are also found in the composition of many essential oils, interference with it is to be expected.
The computational protocol used by Corrêa and collaborators [16] reveals how compounds in essential oils can affect insect metamorphosis. By acting as agonists, they suppress the production of juvenile hormones by insects, leading to abnormalities in development and increased mortality. Low concentrations of Baccharis dracunculifolia DC (Asteraceae) extract and high levels of E-Nerolidol extend the larval stage of Chrysomia albiceps (Wiedemann) (Diptera: Calliphoridae), as predicted by the computational model.
Nerolidol, a squiterpene alcohol found as a component of essential oils in several plants including Stevia rebaudiana [110], jasmine, lavender, ginger, and cannabis [111], has the effect of inhibiting the larval development of some species of insects. It prolongs the development period of the larva or pupa, leading to the interruption of metamorphosis and the death of the individual in higher concentrations. On the Egyptian cotton leafworm, Spodoptera littoralis, nerolidol significantly affects the development process, leading to the appearance of malformed individuals in different stages and blocking the emergence of adults [16].
Ecdysone, a polyhydroxysteroid, is the major ecdysteroid secreted by insects in the prothoracic glands [112]; its active form is ecdysteroid 20-hydroxyecdysone (20E). Its secretion is stimulated by the prothoracicotropic hormone (PTTH) produced by neurons in the insect’s brain [113]. Some components of essential oils interact with ecdysone, which plays a key role in the molting phenomenon in insects.
Azadirachtin, an oxygenated sesquiterpene and the main component of neem oil, interacts with ecdysteroids in the bodies of insects, reducing their concentration [114]. Studies have shown that larval development is inhibited in Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). The authors demonstrated the specific effect of this component by administering ecdysone: it reverses these effects of azadirachtin. Azadirachtin also causes morphological changes in insects, such as delays in growth, malformations, and inhibition of molting [115].
- Cuticular permeability and its role in sensitivity/resistance of the insects to the essential oils
The insect cuticle consists of chitin (a polysaccharide), lipids, and proteins. It comprises two layers: an internal layer called the procuticle, which contains chitin, and an external layer known as the epicuticle, where chitin is absent [116]. Lipids, synthesized in oenocytes, are deposited on the outer layer. The cuticle plays a vital role in an insect’s life by shielding it from dehydration and protecting it against the harmful effects of toxins in the environment.
Cuticular permeability plays an important role in the sensitivity/resistance of insects to pesticides in general and biopesticides/essential oils in particular [117]. The penetration of the essential oil into the body of the larva/adult is conditioned by the thickness of the cuticle and surface tension of the oil [118].
Some components of essential oils, such as limonene, a monoterpene found in the composition of oils not only from Citrus sp. but also from other plant species, have the effect of dislocating the lipids that make up the cuticle. This results in dehydration and, ultimately, the death of the insect [25].
Icerya aegyptiaca (Douglas) (Hemiptera: Monophlebidae) is a highly polyphagous insect, attacking over 120 plant species, and is widely distributed. They possess a well-developed wax scale, which prevents pesticides from penetrating inside their bodies. Zhou and collaborators [119] showed that rosehip seed oil and its separate components, cineole and camphor, in combination with the matrine alkaloid effectively dissolved and broke the wax shell of the insect. In this way, the body is left unprotected and easily accessed by the applied insecticides. Additionally, by dissolving the wax filaments on the insect’s body, the hydrophobic character of the scale was greatly reduced, leaving it defenseless.
The increased efficiency of the essential oils (compared to synthetic insecticides) in partially dissolving and destroying the structure of the cuticle is even more important, as its thickening is a mechanism developed by insects to acquire resistance to pesticides [116]. The enzymes that catalyze the synthesis of cuticular hydrocarbons appear to be overexpressed in insects that develop resistance to pesticides over time.
Moreover, reducing the degree of hydrophobicity and increasing the wettability of the cuticle by administering essential oils can have a harmful effect on the target insects in two ways: firstly, by allowing the entrance of the oils (or other substances with insecticidal action whose activity is potentiated) into their body where they can exert their toxic effects; secondly, the destruction of the cuticle leads to the dehydration of the insect and ultimately to its death [120].
Addressing insecticide resistance through novel pest management technologies requires understanding the complex role of the insect cuticle as both a target and a barrier for chemical insecticides. This understanding offers insights for developing effective solutions in pest management [121].
- Repellent and deterrent effects on insects
Numerous components of essential oils have been reported to have repellent action on some species of harmful insects, including eucalyptol (1,8-cineole), eugenol, limonene, menthol, thymol, etc. [122].
The repellent effect can be utilized to keep pests at a distance, especially in grain storage, where their persistence is longer due to the enclosed space compared to open fields. The essential oil from a species of mint (Mentha haplocalyx Briq. (Lamiaceae)), which contains significant amounts of menthol (59.71%), menthyl acetate (7.83%) and limonene (6.98%)), exhibits a strong repellent effect on the cigarette beetle (a stored product pest that affects tobacco) Lasioderma serricorne (Fabricius) (Coleoptera: Ptinidae) [123]. Furthermore, when tested separately, the main component of the oil, menthol, showed an even stronger repellent effect than DEET at 2 h.
The essential oils from other species of Lamiaceae have demonstrated repellent effects on some species of harmful insects, indicating their increased potential for use in this regard. For example, Ocimum kenyensea Ayob. ex A.J.Paton. (Lamiaceae) oil exhibited repellent effects on Thrips tabaci Lindeman (Thysanoptera: Thripidae), Bemisia tabaci, and Aphis gosypii [124]. Similarly, Salvia officinalis L. (Lamiaceae) and S. rosmarinus Spenn. (Lamiaceae) oil demonstrated a repellent effect on the pomegranate aphid Aphis punicae Passerini (Hemiptera: Aphididae), without significantly affecting an important natural enemy of insect pests, Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) [125].
The lemongrass essential oil (Cymbopogon citratus (DC.) Stapf) demonstrated strong repellency (Class IV) against adults of three prevalent stored product pests: Sitophilus oryzae, Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) and Tribolium castaneum, when used in quantities varying between 0.05% and 0.5%. However, when administered in a quantity exceeding 0.5%, it exhibits extreme repellency (Class V) against A. obtectus and T. castaneum. Simultaneously, at the same concentration, it showed a moderate repellency against the larvae of the species Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) [126].
The variability of obtained results suggests the need to extend the studies to identify the oils to which the harmful insects are sensitive when using the lowest concentrations.
The repellent effect of the essential oils must be considered from several aspects when applied to an ecosystem. While an essential oil or component may have a negative impact on a pest, it can simultaneously repel beneficial insects. For example, ginger oil (Zingiber officinale), has been found to repel adults of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae), a valuable parasitoid in controlling numerous species of harmful insects [127]. Similarly, oils from Allium sativum L. (Amaryllidaceae), Carapa guianensis Aubl. (Meliaceae), and Mentha x piperita L. (Lamiaceae) also demonstrated moderate repellent effects against this species. Understanding such correlations is crucial because, in cases where combined pest management strategies are employed, one of the active substances used may inadvertently combat even the biological agents introduced into the ecosystem for pest control purposes.
3.2.3. The Synergistic Action of Essential Oils and Their Components on Insects
The effects of essential oil mixtures on harmful insects have been relatively under investigated, with studies being carried out mainly in the last 5–10 years. By combining essential oils, three types of effects can be obtained: synergistic, additive, and antagonistic.
Rarely do the separate components of the essential oils demonstrate a stronger insecticidal effect than the whole oil. Most of the time, the action of some components potentiates the action of others. Even the compounds found in small quantities in oil can have significant effects on its degree of toxicity [120].
The synergistic action of the essential oils or their various components is specific, as the results of the studies undertaken in this regard have shown. The reactions vary for different insect species when the same oils are used, and the selectivity sometimes also decreases at the level of the development stage. For instance, in the case of Musca domestica L. (Diptera: Muscidae) larvae exposed to thymol and p-cymene, which are the major components of Thymus vulgaris L. (Lamiaceae) oil, their synergistic effect was not manifested, but it appeared in the case of the adults [120].
For example, the simultaneous application of basil and mandarin oils on Spodoptera litura shows a significantly increased synergistic effect compared to the separate application of the two oils [118].
The mixtures of the essential oil of Dysphania ambrosioides with oils from Pelargonium graveolens, Piper arboreum, P. diospyrifolium, P. gaudichaudianum, and P. tuberculatum) proved to be very toxic to the maize weevil Sitophilus zeamais Motsch (Coleoptera: Curculionidae) [128]. These mixtures exhibited a more toxic effect than the essential oils tested separately, indicating a synergistic effect. Specifically, D. ambrosioides oil significantly potentiates the action of the other oils with which it was tested. This finding holds potential significance for pest management strategies targeting this pest. Indeed, essential oils can exhibit synergistic effects when combined with other plant-derived substances. For instance, rose oil used in combination with matrine, an alkaloid extracted from species of the Sophora genus, demonstrated increased toxicity towards the Egyptian mealybug Icerya aegyptiaca. This combination facilitated the penetration of oil through the thick layer of protective wax that covers the insect, thereby enhancing its toxic effect on acetylcholinesterase [119].
Components of the essential oil are also utilized in the production of pheromones The synthetic Nasonov pheromone composed of citral, geraniol, and nerol has been shown to reduce the time spent by honey bees in feeding areas to a similar extent as the synthetic pesticide DEET. Electroantennogram (EAG) recordings of bee antennae exposed to the synthetic pheromone and its separate components revealed a more intense response to the synthetic Nasonov application than to citral, geraniol and nerol (in that order). In all cases, the response was superior to that induced by the application of DEET. Honeybee antennae contain placoidea sensilla, which contain neuronal receptors (5–35) with increased sensitivity to citral and geraniol [129]. In this case, the synergistic effect of terpenoids in their action on insects is evident.
4. Conclusions
Of the almost 3000 essential oils produced by plants across various botanical families that have been described so far, only approximately 300 of these are marketed and used in various fields such as medicine/pharmacy, food, cosmetics, agriculture, etc. and of these only 11 are marketed as biopesticides, being registered at the level of the U.S. Environmental Protection Agency (EPA) and/or which are included in the EU Pesticides Database So, the possibilities of future exploration of the use of the essential oils in pest control are very promising.
Also, according to the bibliometric analysis, the number of studies on the mechanisms of action of essential oils on insects has experienced a significant increase in the last four years. These approaches will help in a more precise choice of the oils used in pest management, depending on the target species and protecting, at the same time, the useful species.
After the study carried out in this review, the following needs emerge in the future approaches of researchers:
- First, research is required to investigate the effects of essential oils that have shown insecticidal properties in laboratory tests when applied in open-field conditions.
- The impact of the essential oils on non-target species must be studied in parallel with their effect against harmful insects; although they are considered green insecticides, they are not without action on other species, besides those they directly target. Insects, having similar anatomical structures, are assumed to be susceptible to the effects of the same bioactive substances.
- It is necessary to carry out studies on the economic efficiency of the use of nanoformulations in the distribution of essential oils in the open field. Although many studies show their increased effectiveness compared to bulk oils, the cost/efficiency ratio is important to ascertain.
- The mechanisms of action of essential oils on insects, although they have experienced a significant increase in recent years, are still insufficiently known. Chemoinformatics can be used to predict the effects of essential oil compounds on the molecular targets of insecticides.
Author Contributions
Conceptualization, I.E.P. and I.N.G.; methodology, I.N.G., C.F.B. and I.E.P.; software, I.N.G.; validation, I.E.P., I.N.G. and C.F.B.; formal analysis, I.E.P., I.N.G. and C.F.B.; investigation, I.N.G. and I.E.P.; data curation, I.N.G.; writing—original draft preparation, I.E.P., I.N.G. and C.F.B.; writing—review and editing I.N.G., I.E.P. and C.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Maria Mihaela Antofie for checking the English language in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B.; et al. Essential Oils for a Sustainable Control of Honeybee Varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the Botanical Repellents Geraniol, Linalool, and Citronella against Mosquitoes. J. Vector Ecol. 2009, 34, 2–8. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, H.; Hou, R.; Wu, J.; Wang, Y.; Huang, S.; Cheng, D.; Xu, H.; Zhang, Z. Effects of Sublethal Azadirachtin on the Immune Response and Midgut Microbiome of Apis cerana cerana (Hymenoptera: Apidae). Ecotoxicol. Environ. Saf. 2022, 229, 113089. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Juárez, M.L.; Alzogaray, R.A.; Arrighi, F.; Arroyo, L.; Gastaminza, G.; Willink, E.; Bardón, A.; Vera, M.T. Oviposition Behaviour and Larval Development of Anastrepha fraterculus from Argentina in Citrus. Entomol. Exp Appl. 2015, 157, 198–213. [Google Scholar] [CrossRef]
- Da Silva, I.M.; Zanuncio, J.C.; Brügger, B.P.; Soares, M.A.; Zanuncio, A.J.V.; Wilcken, C.F.; Tavares, W.D.S.; Serrão, J.E.; Sediyama, C.S. Selectivity of the Botanical Compounds to the Pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 2020, 10, 4820. [Google Scholar] [CrossRef]
- Andrade, L.H.D.; Oliveira, J.V.D.; Breda, M.O.; Marques, E.J.; Lima, I.M.D.M. Effects of Botanical Insecticides on the Instantaneous Population Growth Rate of Aphis gossypii Glover (Hemiptera: Aphididae) in Cotton. Acta Sci. Agron. 2012, 34, 119–214. [Google Scholar] [CrossRef][Green Version]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Xu, X.; Chen, Q.; Zhu, Z. Evolutionary Overview of Land Consolidation Based on Bibliometric Analysis in Web of Science from 2000 to 2020. Int. J. Environ. Res. Public Health 2022, 19, 3218. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gostin, I.N.; Popescu, I.E. Evaluation of the Essential Oils Used in the Production of Biopesticides: Assessing Their Toxicity toward Both Arthropod Target Species and Beneficial Pollinators. Agriculture 2023, 14, 81. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, E.J.A.; Carvalho, F.C.; De Castro Oliveira, J.A.; Bertolucci, S.K.V.; Scotti, M.T.; Silveira, C.H.; Guedes, F.C.; Melo, J.O.F.; De Melo-Minardi, R.C.; De Lima, L.H.F. Elucidating the Molecular Mechanisms of Essential Oils’ Insecticidal Action Using a Novel Cheminformatics Protocol. Sci. Rep. 2023, 13, 4598. [Google Scholar] [CrossRef]
- Ikawati, S.; Himawan, T.; Abadi, A.L.; Tarno, H. Toxicity Nanoinsecticide Based on Clove Essential Oil against Tribolium castaneum (Herbst). J. Pestic. Sci. 2021, 46, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Miresmailli, S.; Isman, M.B. Botanical Insecticides Inspired by Plant–Herbivore Chemical Interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E. Biochemical Mechanisms Conferring Cross-Resistance to Fumigant Toxicities of Essential Oils in a Chlorpyrifos-Methyl Resistant Strain of Oryzaephilus surinamensis L. (Coleoptera: Silvanidae). J. Stored Prod. Res. 2002, 38, 157–166. [Google Scholar] [CrossRef]
- Tia, V.E.; Gueu, S.; Cissé, M.; Tuo, Y.; Gnago, A.J.; Konan, E. Bio-insecticidal effects of essential oil nano-emulsion of Lippia multiflora Mold. on major cabbage pests. J. Plant Prot. Res. 2023, 61, 103–109. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Salem, N.Y.; Abd ElNaby, S.S.; Adel, M.M. Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity Against Phthorimaea operculella (Zeller) (PTM) (Lepidoptera: Gelechiidae). Int. J. Nanosci. Nanotechnol. 2021, 17, 147–160. [Google Scholar]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal Activity of Essential Oil from Cephalotaxus sinensis and Its Main Components against Various Agricultural Pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Ngongang, M.D.T.; Eke, P.; Sameza, M.L.; Mback, M.N.L.N.; Lordon, C.D.; Boyom, F.F. Chemical Constituents of Essential Oils from Thymus vulgaris and Cymbopogon citratus and Their Insecticidal Potential against the Tomato Borer, Tuta absoluta (Lepidoptera: Gelechiidae). Int. J. Trop. Insect Sci. 2022, 42, 31–43. [Google Scholar] [CrossRef]
- Krzyżowski, M.; Baran, B.; Łozowski, B.; Francikowski, J. The Effect of Rosmarinus officinalis Essential Oil Fumigation on Biochemical, Behavioral, and Physiological Parameters of Callosobruchus maculatus. Insects 2020, 11, 344. [Google Scholar] [CrossRef]
- De Souza, M.T.; De Souza, M.T.; Bernardi, D.; De Melo, D.J.; Zarbin, P.H.G.; Zawadneak, M.A.C. Insecticidal and Oviposition Deterrent Effects of Essential Oils of Baccharis spp. and Histological Assessment against Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2021, 11, 3944. [Google Scholar] [CrossRef]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Júnior, P.A.S.; Ramos, R.S.; Martins, J.C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci. 2015, 15, 137. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Zappalà, L.; Palmeri, V. Bioactivity of Essential Oil-Based Nano-Biopesticides toward Rhyzopertha dominica (Coleoptera: Bostrichidae). Ind. Crops Prod. 2021, 162, 113257. [Google Scholar] [CrossRef]
- El-Helaly, A.A.; EL-Masarawy, M.S.; El-Bendary, H.M. Using Citronella to Protect Bees (Honeybee Apis mellifera L.) from Certain Insecticides and Their Nano Formulations. Braz. J. Biol. 2021, 81, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Neupane, A.C.; Sapakuka, S.; Tao, P.; Kafle, L. Repellancy and Contact Toxicity of Clove Bud Oil and Its Constituents against German Cockroaches, Blatella germanica (Dictyoptera: Blattellidae), under Laboratory Conditions. Int. J. Pest Manag. 2020, 66, 289–297. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2023, 81, 3837–3882. [Google Scholar] [CrossRef]
- Mossa, A.-T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-pest Management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Lucia, A.; Guzmán, E. Emulsions Containing Essential Oils, Their Components or Volatile Semiochemicals as Promising Tools for Insect Pest and Pathogen Management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef] [PubMed]
- Antunes Filho, S.; Dos Santos, M.S.; Dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef] [PubMed]
- Dunan, L.; Malanga, T.; Benhamou, S.; Papaiconomou, N.; Desneux, N.; Lavoir, A.-V.; Michel, T. Effects of Essential Oil-Based Formulation on Biopesticide Activity. Ind. Crops Prod. 2023, 202, 117006. [Google Scholar] [CrossRef]
- Laudani, F.; Campolo, O.; Caridi, R.; Latella, I.; Modafferi, A.; Palmeri, V.; Sorgonà, A.; Zoccali, P.; Giunti, G. Aphicidal Activity and Phytotoxicity of Citrus sinensis Essential-Oil-Based Nano-Insecticide. Insects 2022, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Manjesh, K.; Kundu, A.; Dutta, A.; Saha, S.; Neelakanthaiah, B.S. Bio-Insecticidal Nanoemulsions of Essential Oil and Lipid-Soluble Fractions of Pogostemon cablin. Front. Plant Sci. 2022, 13, 874221. [Google Scholar] [CrossRef] [PubMed]
- Sediawan, W.B.; Hartati, I.; Sulistyo, H.; Azis, M.M. A Review and Bibliometric Analysis on Essential Oil Nanoencapsulation. In Proceedings of the 4th Borobudur International Symposium on Science and Technology 2022 (BIS-STE 2022); Setiyo, M., Pambuko, Z.B., Praja, C.B.E., Setiawan, A., Yuliastuti, F., Muliawanti, L., Dewi, V.S., Eds.; Advances in Engineering Research; Atlantis Press International BV: Dordrecht, The Netherlands, 2023; Volume 225, pp. 79–90. ISBN 978-94-6463-283-5. [Google Scholar]
- Pascual-Villalobos, M.J.; López, M.D.; Castañé, C.; Soler, A.; Riudavets, J. Encapsulated Essential Oils as an Alternative to Insecticides in Funnel Traps. J. Econ. Entomol. 2015, 108, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Dolma, S.K.; Suresh, P.S.; Singh, P.P.; Sharma, U.; Reddy, S.G.E. Insecticidal Activity of the Extract, Fractions, and Pure Steroidal Saponins of Trillium govanianum W All. Ex D. D on for the Control of Diamondback Moth (Plutella xylostella L.) and Aphid (Aphis craccivora K Och). Pest Manag. Sci. 2021, 77, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.B.; Cappellari, L.D.R.; Chiappero, J.; Meneguzzi, R.D.V.; Gil, S.; Giordano, W.; Banchio, E. How Do Plants Protect Themselves from Insects? A Practical Laboratory Exercise to Illustrate the Defence Mechanisms of the Plant through Secondary Metabolites. J. Biol. Educ. 2022, 1–17. [Google Scholar] [CrossRef]
- Mahran, H.A. Using Nanoemulsions of the Essential Oils of a Selection of Medicinal Plants from Jazan, Saudi Arabia, as a Green Larvicidal against Culex pipiens. PLoS ONE 2022, 17, e0267150. [Google Scholar] [CrossRef]
- Somala, N.; Laosinwattana, C.; Teerarak, M. Formulation Process, Physical Stability and Herbicidal Activities of Cymbopogon nardus Essential Oil-Based Nanoemulsion. Sci. Rep. 2022, 12, 10280. [Google Scholar] [CrossRef]
- Khalil, M.S.; Halawa, S.M.; Azab, M.M.; Morsy, A.R. Toxicity and Biochemical Effects of Citronella, Mustard and Sage Essential Oils and Their Nanoemulsions against Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Benha J. Appl. Sci. 2023, 8, 79–88. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Giraldo, J.D.; Schoebitz, M. Essential Oils and Their Formulations for the Control of Curculionidae Pests. Front. Agron. 2022, 4, 876687. [Google Scholar] [CrossRef]
- Ibrahim, S.S. Essential Oil Nanoformulations as a Novel Method for Insect Pest Control in Horticulture. In Horticultural Crops; Kossi Baimey, H., Hamamouch, N., Adjiguita Kolombia, Y., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-421-3. [Google Scholar]
- Antofie, M.-M.; Sava Sand, C. Drought Stress Study on Nicotiana tabacum L., “Baladi”, an In Vitro Experimental Model. Agriculture 2021, 11, 845. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Nassrallah, A.A.; Abdel-Raheem, M.A.; Elbehery, H.H. Lemon Peel Essential Oil and Its Nano-Formulation to Control Agrotis ipsilon (Lepidoptera: Noctuidae). Sci. Rep. 2023, 13, 17922. [Google Scholar] [CrossRef]
- Ziaee, M.; Sheikhzadeh Takabi, A.; Ebadollahi, A. Fabrication of Carum copticum Essential Oil–Loaded Chitosan Nanoparticles and Evaluation Its Insecticidal Activity for Controlling Rhyzopertha dominica and Tribolium confusum. Front. Plant Sci. 2023, 14, 1187616. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Abou-Elseoud, W.S.; Elbehery, H.H.; Hassan, M.L. Chitosan-Cellulose Nanoencapsulation Systems for Enhancing the Insecticidal Activity of Citronella Essential Oil against the Cotton Leafworm Spodoptera littoralis. Ind. Crops Prod. 2022, 184, 115089. [Google Scholar] [CrossRef]
- Yang, F.-L.; Li, X.-G.; Zhu, F.; Lei, C.-L. Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- De Matos, S.P.; Lucca, L.G.; Koester, L.S. Essential Oils in Nanostructured Systems: Challenges in Preparation and Analytical Methods. Talanta 2019, 195, 204–214. [Google Scholar] [CrossRef]
- Paula, H.C.B.; Sombra, F.M.; Abreu, F.O.M.S.; Paul, R.C.M.D. Lippia Sidoides Essential Oil Encapsulation by Angico Gum/Chitosan Nanoparticles. J. Braz. Chem. Soc. 2010, 21, 2359–2366. [Google Scholar] [CrossRef]
- Aguiar, M.C.S.; Das Graças Fernandes Da Silva, M.F.; Fernandes, J.B.; Forim, M.R. Evaluation of the Microencapsulation of Orange Essential Oil in Biopolymers by Using a Spray-Drying Process. Sci. Rep. 2020, 10, 11799. [Google Scholar] [CrossRef]
- Choi, Y.H.; Van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernández, E.; Peña-Chora, G.; Hernández-Velázquez, V.M.; Lormendez, C.C.; Toribio-Jiménez, J.; Romero-Ramírez, Y.; León-Rodríguez, R. The Stingless Bees (Hymenoptera: Apidae: Meliponini): A Review of the Current Threats to Their Survival. Apidologie 2022, 53, 8. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of Mechanisms and Uses of Biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Santana, K.; Do Nascimento, L.D.; Lima E Lima, A.; Damasceno, V.; Nahum, C.; Braga, R.C.; Lameira, J. Applications of Virtual Screening in Bioprospecting: Facts, Shifts, and Perspectives to Explore the Chemo-Structural Diversity of Natural Products. Front. Chem. 2021, 9, 662688. [Google Scholar] [CrossRef] [PubMed]
- Maia, E.H.B.; Assis, L.C.; De Oliveira, T.A.; Da Silva, A.M.; Taranto, A.G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020, 8, 343. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H. Which Acetylcholinesterase Functions as the Main Catalytic Enzyme in the Class Insecta? Insect Biochem. Mol. Biol. 2013, 43, 47–53. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Mohideen, H.S.; Raman, C.; Mohamad, S.B. Potential Acetylcholinesterase Inhibitor Acting on the Pesticide Resistant and Susceptible Cotton Pests. ACS Omega 2022, 7, 20515–20527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Classification and Uses of Organophosphates and Carbamates. In Toxicology of Organophosphate & Carbamate Compounds; Elsevier: Amsterdam, The Netherlands, 2006; pp. 5–24. ISBN 978-0-12-088523-7. [Google Scholar]
- Tong, F.; Islam, R.M.; Carlier, P.R.; Ma, M.; Ekström, F.; Bloomquist, J.R. Effects of Anticholinesterases on Catalysis and Induced Conformational Change of the Peripheral Anionic Site of Murine Acetylcholinesterase. Pestic. Biochem. Physiol. 2013, 106, 79–84. [Google Scholar] [CrossRef][Green Version]
- Almadiy, A.A.; Nenaah, G.E. Essential Oil of Origanum vulgare, Its Nanoemulsion and Bioactive Monoterpenes as Eco-Friendly Novel Green Pesticides for Controlling Aedes aegypti, the Common Vector of Dengue Virus. J. Essent. Oil Res. 2022, 34, 424–438. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal Terpenes from the Essential Oils of Artemisia nakaii and Their Inhibitory Effects on Acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef]
- Yeom, H.-J.; Kang, J.S.; Kim, G.-H.; Park, I.-K. Insecticidal and Acetylcholine Esterase Inhibition Activity of Apiaceae Plant Essential Oils and Their Constituents against Adults of German Cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Glavan, G.; Novak, S.; Božič, J.; Jemec Kokalj, A. Comparison of Sublethal Effects of Natural Acaricides Carvacrol and Thymol on Honeybees. Pestic. Biochem. Physiol. 2020, 166, 104567. [Google Scholar] [CrossRef]
- Alves, M.D.S.; Campos, I.M.; Brito, D.D.M.C.D.; Cardoso, C.M.; Pontes, E.G.; Souza, M.A.A.D. Efficacy of Lemongrass Essential Oil and Citral in Controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae), a Post-Harvest Cowpea Insect Pest. Crop Prot. 2019, 119, 191–196. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Olumuyiwa, T.A.; Olasehinde, T.A.; Ademiluyi, A.O.; Adeyemo, A.C. Insecticidal Activity of Essential Oil from Orange Peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and Its Inhibitory Effects on Acetylcholinesterase and Na+/K+-ATPase Activities. Phytoparasitica 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Beyaoui, A.; Jlizi, S.; Ascrizzi, R.; Flamini, G.; Harrath, A.H.; Ben Jannet, H. Chemical Profiling and Biological Assessment of Trunk Bark Essential Oil from Eucalyptus camaldulensis: In Vitro Study Coupled with Chemoinformatics Calculations. J. Mol. Struct. 2024, 1300, 137120. [Google Scholar] [CrossRef]
- Lokeshwari, D.; Krishna Kumar, N.K.; Manjunatha, H. Multiple Mutations on the Second Acetylcholinesterase Gene Associated with Dimethoate Resistance in the Melon Aphid, Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, N.R.; Jyothi, N.; Saroja, S.; Lokesha, A.N. Insecticidal Properties of Ocimum basilicum and Mentha piperita Essential Oils against South American Tomato Moth, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelichiidae). Pestic. Biochem. Physiol. 2023, 190, 105329. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.D.; Maqueira, B. Insect Octopamine Receptors: A New Classification Scheme Based on Studies of Cloned Drosophila G-Protein Coupled Receptors. Invert. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. Tyramine and Octopamine: Ruling Behavior and Metabolism. Annu. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of Octopaminergic Receptors by Essential Oil Constituents Isolated from Aromatic Plants: Possible Mode of Action against Insect Pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Finetti, L.; Roeder, T.; Calò, G.; Bernacchia, G. The Insect Type 1 Tyramine Receptors: From Structure to Behavior. Insects 2021, 12, 315. [Google Scholar] [CrossRef]
- Orchard, I. Octopamine in Insects: Neurotransmitter, Neurohormone, and Neuromodulator. Can. J. Zool. 1982, 60, 659–669. [Google Scholar] [CrossRef]
- Pauls, D.; Blechschmidt, C.; Frantzmann, F.; El Jundi, B.; Selcho, M. A Comprehensive Anatomical Map of the Peripheral Octopaminergic/Tyraminergic System of Drosophila melanogaster. Sci. Rep. 2018, 8, 15314. [Google Scholar] [CrossRef]
- Roeder, T.; Seifert, M.; Kähler, C.; Gewecke, M. Tyramine and Octopamine: Antagonistic Modulators of Behavior and Metabolism: Octopamine and Tyramine in Insects. Arch. Insect Biochem. Physiol. 2003, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Lapied, B.; Jankowski, W.; Stankiewicz, M. The Unusual Action of Essential Oil Component, Menthol, in Potentiating the Effect of the Carbamate Insecticide, Bendiocarb. Pestic. Biochem. Physiol. 2019, 158, 101–111. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and Pharmacological Analysis of an Octopamine Receptor from American Cockroach and Fruit Fly in Response to Plant Essential Oils. Arch. Insect. Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lin, J.; Li, X.; Huang, J.; Liang, X.; Li, Y.; Bai, M.; He, H.; Lin, F.; Xu, H.; et al. Changes in Dopamine and Octopamine Levels Caused Disordered Behaviour in Red Imported Fire Ants Exposed to Cinnamon Essential Oils. Ind. Crops Prod. 2023, 199, 116801. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal Activity of Essential Oils: Octopaminergic Sites of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.B.; Braza, M.K.E.; Nellas, R.B. The Interaction and Mechanism of Monoterpenes with Tyramine Receptor (SoTyrR) of Rice Weevil (Sitophilus oryzae). SN Appl. Sci. 2020, 2, 1592. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential Oils and Their Bioactive Compounds as Eco-Friendly Novel Green Pesticides for Management of Storage Insect Pests: Prospects and Retrospects. Env. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Devrnja, N.; Milutinović, M.; Savić, J. When Scent Becomes a Weapon—Plant Essential Oils as Potent Bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of Monoterpenoid Insecticides on [3H]-TBOB Binding in House Fly GABA Receptor and 36Cl− Uptake in American Cockroach Ventral Nerve Cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Ozoe, Y. γ-Aminobutyrate- and Glutamate-Gated Chloride Channels as Targets of Insecticides. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 44, pp. 211–286. ISBN 978-0-12-394389-7. [Google Scholar]
- Guo, L.; Qiao, X.; Haji, D.; Zhou, T.; Liu, Z.; Whiteman, N.K.; Huang, J. Convergent Resistance to GABA Receptor Neurotoxins through Plant–Insect Coevolution. Nat. Ecol. Evol. 2023, 7, 1444–1456. [Google Scholar] [CrossRef]
- Qian, K.; Jiang, C.; Guan, D.; Zhuang, A.; Meng, X.; Wang, J. Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum. Insects 2023, 14, 580. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Schuster, S.; Dyck, Y.; Demares, F.; Leboulle, G.; Armengaud, C. Identification, Localization and Function of Glutamate-gated Chloride Channel Receptors in the Honeybee Brain. Eur. J. Neurosci. 2012, 36, 2409–2420. [Google Scholar] [CrossRef]
- Abdel Rahman, K.M. Effects of Abamectin and Fipronil Insecticides on the Brain and Compound Eyes of the Embryo of Heteracris littoralis (Rambur) (Orthoptera: Acrididae). Int. J. Trop. Insect. Sci. 2023, 43, 1237–1241. [Google Scholar] [CrossRef]
- Lynagh, T.; Cromer, B.A.; Dufour, V.; Laube, B. Comparative Pharmacology of Flatworm and Roundworm Glutamate-Gated Chloride Channels: Implications for Potential Anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 244–255. [Google Scholar] [CrossRef]
- Yang, L.-H.; Huang, H.; Wang, J.-J. Antioxidant Responses of Citrus Red Mite, Panonychus citri (McGregor) (Acari: Tetranychidae), Exposed to Thermal Stress. J. Insect Physiol. 2010, 56, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, Z.-X.; Kang, Z.-W.; Li, H.; Liu, T.-X.; Wang, D. Identification and Characterization of Antioxidant Enzyme Genes in Parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) and Expression Profiling Analysis under Temperature Stress. Insects 2022, 13, 447. [Google Scholar] [CrossRef]
- Ercan, F.S.; Baş, H.; Azarkan, S.Y. In Silico Detection of Cucurbitacin-E on Antioxidant Enzymes of Model Organism Galleria mellonella L. (Lepidoptera: Pyralidae) and Variation of Antioxidant Enzyme Activities and Lipid Peroxidation in Treated Larvae. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 130. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial Development of Plant Essential Oils and Their Constituents as Active Ingredients in Bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Hassan, N.N.; Alfuhaid, N.A.; Amer, A.; Awad, M. Insights into the Toxicity, Biochemical Activity, and Molecular Docking of Cymbopogon citratus Essential Oils and Citral on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 1185–1195. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyamoto, M.; Murakami, A.; Ohigashi, H.; Osawa, T.; Uchida, K. A Phase II Detoxification Enzyme Inducer from Lemongrass: Identification of Citral and Involvement of Electrophilic Reaction in the Enzyme Induction. Biochem. Biophys. Res. Commun. 2003, 302, 593–600. [Google Scholar] [CrossRef]
- Omotoso, S.E.; Akinpelu, B.A.; Soyelu, O.J. Insecticidal Effect of Lemongrass Oil on Behavioural Responses and Biochemical Changes in Cowpea Weevil, Callosobruchus maculatus (Fabricius). J. Phytopathol. Pest Manag. 2020, 7, 14–30. [Google Scholar]
- Rajkumar, V.; Gunasekaran, C.; Christy, I.K.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A. Toxicity, Antifeedant and Biochemical Efficacy of Mentha piperita L. Essential Oil and Their Major Constituents against Stored Grain Pest. Pestic. Biochem. Physiol. 2019, 156, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, A.O.; Olajuyigbe, F.M.; Ajele, J.O.; Adedire, C.O. Activity of the Antioxidant Defense System in a Typical Bioinsecticide-and Synthetic Insecticide-Treated Cowpea Storage Beetle Callosobrochus maculatus F. (Coleoptera: Chrysomelidae). Int. J. Insect. Sci. 2014, 6, IJIS.S19434. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.O.; Nouzova, M.; Fruttero, L.L.; Leyria, J.; Ligabue-Braun, R.; Noriega, F.G.; Canavoso, L.E. Role of Methoprene-Tolerant in the Regulation of Oogenesis in Dipetalogaster maxima. Sci. Rep. 2022, 12, 14195. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Desneux, N.; Maggi, F. Phytol, (E)-Nerolidol and Spathulenol from Stevia rebaudiana Leaf Essential Oil as Effective and Eco-Friendly Botanical Insecticides against Metopolophium dirhodum. Ind. Crops Prod. 2020, 155, 112844. [Google Scholar] [CrossRef]
- Ghoneim, K.; Hamadah, K.; Selim, S.; Waheeb, H. Biopesticidal Potential of Nerolidol, a Sesquiterpene Compound, and Its Drastic Impact on Growth and Metamorphosis of the Cotton Leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Sch. Acad. J. Biosci. 2021, 9, 36–57. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Brown, M.R.; Belles, X. Hormonal Control of Reproductive Processes. In Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 433–491. ISBN 978-0-444-51924-5. [Google Scholar]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the Insect Metamorphic Transition by Ecdysteroid Production and Secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.A.A.; De Souza, N.A.; Feder, M.D.; Da Silva, C.E.; Garcia, E.D.S.; Azambuja, P.; Gonzalez, M.S.; Rangel, E.F. Effects of Azadirachtin on the Development and Mortality of Lutzomyia longipalpis Larvae (Diptera: Psychodidae: Phlebotominae). J. Med. Entomol. 2006, 43, 262–266. [Google Scholar] [CrossRef]
- Rembold, H. The Azadirachtins—Their Potential for Insect Control. Econ. Med. Plant Res. 1989, 3, 57–72. [Google Scholar]
- Bass, C.; Jones, C.M. Mosquitoes Boost Body Armor to Resist Insecticide Attack. Proc. Natl. Acad. Sci. USA 2016, 113, 9145–9147. [Google Scholar] [CrossRef]
- Gao, L.; Qiao, H.; Wei, P.; Moussian, B.; Wang, Y. Xenobiotic Responses in Insects. Arch. Insect Biochem. Physiol. 2022, 109, e21869. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, J.; Tak, J.-H. Synergistic Mechanism of Insecticidal Activity in Basil and Mandarin Essential Oils against the Tobacco Cutworm. J. Pest Sci. 2021, 94, 1119–1131. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Lin, S.; He, J.; Deng, Y.; He, J.; Cheng, D. The Synergistic Effects of Rosehip Oil and Matrine against Icerya aegyptiaca (Douglas) (Hemiptera: Coccoidea) and the Underlying Mechanisms. Pest Manag. Sci. 2022, 78, 3424–3432. [Google Scholar] [CrossRef]
- Yoon, J.; Tak, J.-H. Cuticular Property Affects the Insecticidal Synergy of Major Constituents in Thyme Oil against Houseflies, Musca domestica. Sci. Rep. 2023, 13, 12654. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect Cuticle and Insecticide Development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef]
- Karabörklü, S.; Ayvaz, A. A Comprehensive Review of Effective Essential Oil Components in Stored-Product Pest Management. J. Plant Dis. Prot. 2023, 130, 449–481. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, K.; You, C.; Wang, C.; Geng, Z.; Su, Y.; Wang, Y.; Du, S.; Deng, Z. Contact Toxicity and Repellency of the Essential Oil from Mentha haplocalyx BRIQ. against Lasioderma serricorne. Chem. Biodivers. 2015, 12, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, M.J.; Hutchins, M.M.; Karenga, S. Efficacy of Essential Oils from Ocimum kenyense as a Biopesticide against Aphis gosypii, Thrips tabaci and Bemisia tabaci. Int. J. Adv. Res. 2021, 4, 14–26. [Google Scholar] [CrossRef]
- Sayed, S.; Soliman, M.M.; Al-Otaibi, S.; Hassan, M.M.; Elarrnaouty, S.-A.; Abozeid, S.M.; El-Shehawi, A.M. Toxicity, Deterrent and Repellent Activities of Four Essential Oils on Aphis punicae (Hemiptera: Aphididae). Plants 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenac, S.; Kiprovski, B.; Aćimović, M.; Jeremić, J.S.; Cvetković, M.; Bursić, V.; Ovuka, J. Repellent Activity of Cymbopogon citratus Essential Oil Against Four Major Stored Product Pests: Plodia interpunctella, Sitophilus oryzae, Acanthoscelides obtectus and Tribolium castaneum. Contemp. Agric. 2021, 70, 140–148. [Google Scholar] [CrossRef]
- Parreira, D.S.; Alcántara-de La Cruz, R.; Rodrigues Dimaté, F.A.; Batista, L.D.; Ribeiro, R.C.; Rigueira Ferreira, G.A.; Zanuncio, J.C. Bioactivity of Ten Essential Oils on the Biological Parameters of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Adults. Ind. Crops Prod. 2019, 127, 11–15. [Google Scholar] [CrossRef]
- Santana, A.D.S.; Baldin, E.L.L.; Santos, T.L.B.D.; Baptista, Y.A.; Santos, M.C.D.; Lima, A.P.S.; Tanajura, L.S.; Vieira, T.M.; Crotti, A.E.M. Synergism between Essential Oils: A Promising Alternative to Control Sitophilus zeamais (Coleoptera: Curculionidae). Crop Prot. 2022, 153, 105882. [Google Scholar] [CrossRef]
- Akers, R.P.; Getz, W.M. A Test of Identified Response Classes among Olfactory Receptor Neurons in the Honey-Bee Worker. Chem. Senses 1992, 17, 191–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).