Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Preparation and Extraction
2.3. Instrumentation
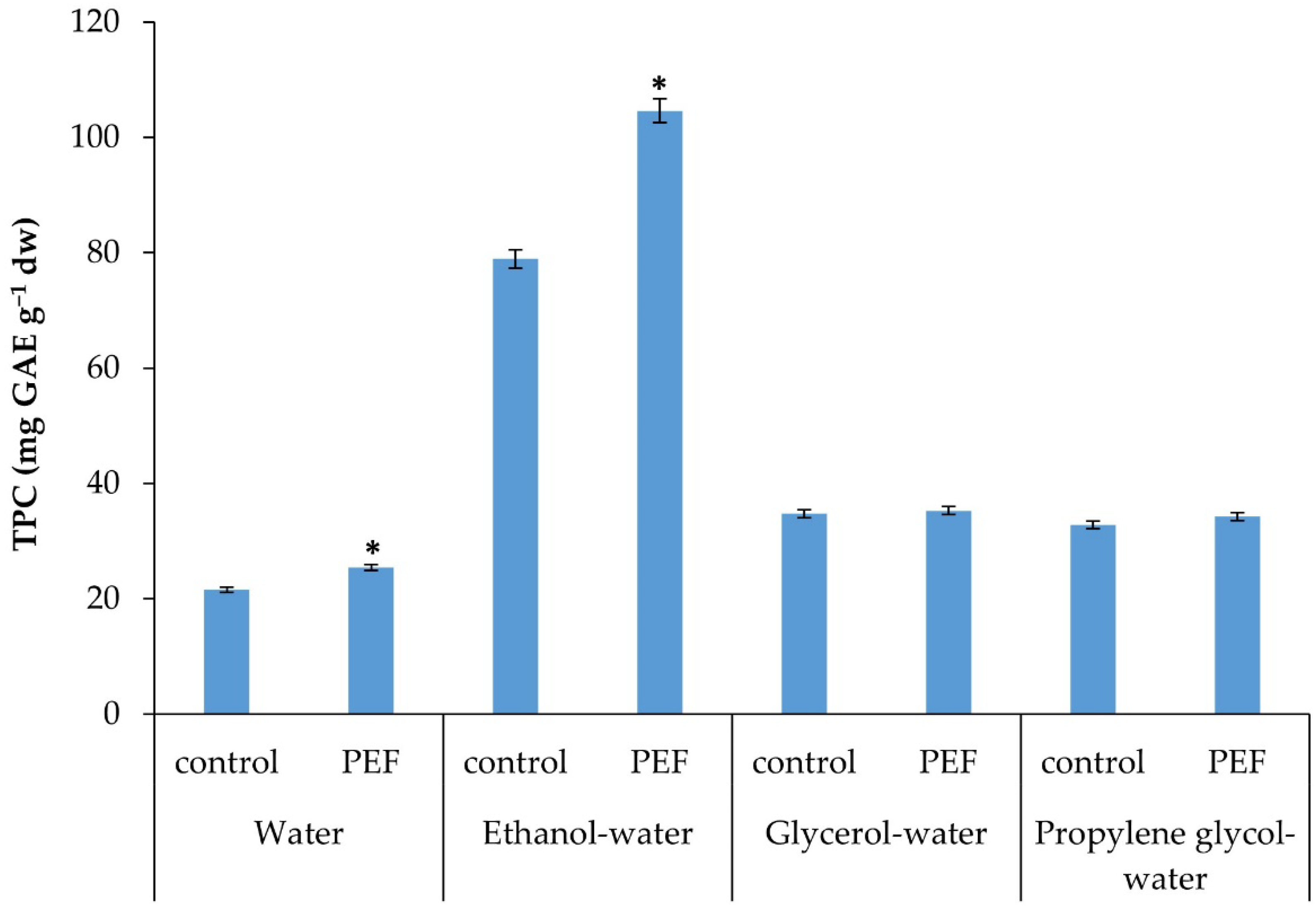
2.4. Comparative Analysis of Total Polyphenol Content (TPC) of the Extracts
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oszmiański, J.; Kalisz, S.; Aneta, W. The Content of Phenolic Compounds in Leaf Tissues of White (Aesculus hippocastanum L.) and Red Horse Chestnut (Aesculus carea H.) Colonized by the Horse Chestnut Leaf Miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar] [CrossRef]
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, ethanomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. Towards future medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.; Aadil, R.M. Effective valorization of food wastes and by-products through pulsed electric field: A systematic review. J. Food Process Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Makris, D.; Lalas, S. Evaluation of Pulsed Electric Field Polyphenol Extraction from Vitis vinifera, Sideritis scardica and Crocus sativus. ChemEngineering 2021, 5, 25. [Google Scholar] [CrossRef]
- EL Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. Technol. 2021, 69, 91–98. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; De Bruno, A.; Piscopo, A. Green-sustainable extraction techniques for the recovery of antioxidant compounds from “citrus Limon” by-products. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2022, 57, 220–232. [Google Scholar] [CrossRef]
- Peiró, S.; Gordon, M.H.; Blanco, M.; Pérez-Llamas, F.; Segovia, F.; Almajano, M.P. Modelling Extraction of White Tea Polyphenols: The Influence of Temperature and Ethanol Concentration. Antioxidants 2014, 3, 684–699. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence So Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef]
- Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-E.; Lin, L.-H. DPPH scavenging capacity of extracts from Camellia seed dregs using polyol compounds as solvents. Heliyon 2019, 5, e02315. [Google Scholar] [CrossRef]
- Jakupović, L.; Kalvarešin, M.; Bukovina, K.; Poljak, V.; Vujić, L.; Končić, M.Z. Optimization of Two Eco-Friendly Extractions of Black Medick (Medicago lupulina L.) Phenols and Their Antioxidant, Cosmeceutical, α-Glucosidase and α-Amylase Inhibitory Properties. Molecules 2021, 26, 1610. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Calhelha, R.C.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules 2020, 25, 2497. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carménère Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Teh, S.-S.; Niven, B.E.; Bekhit, A.E.-D.; Carne, A.; Birch, E.J. The Use of Microwave and Pulsed Electric Field as a Pretreatment Step in Ultrasonic Extraction of Polyphenols from Defatted Hemp Seed Cake (Cannabis sativa) Using Response Surface Methodology. Food Bioprocess Technol. 2014, 7, 3064–3076. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.-P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.-J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef]
- Saldaña, G.; Cebrián, G.; Abenoza, M.; Sánchez-Gimeno, C.; Álvarez, I.; Raso, J. Assessing the efficacy of PEF treatments for improving polyphenol extraction during red wine vinifications. Innov. Food Sci. Emerg. Technol. 2017, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Mazza, L.; Nutrizio, M.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed electric fields- and ultrasound-assisted green extraction of valuable compounds from Origanum vulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 56, 4834–4842. [Google Scholar] [CrossRef]
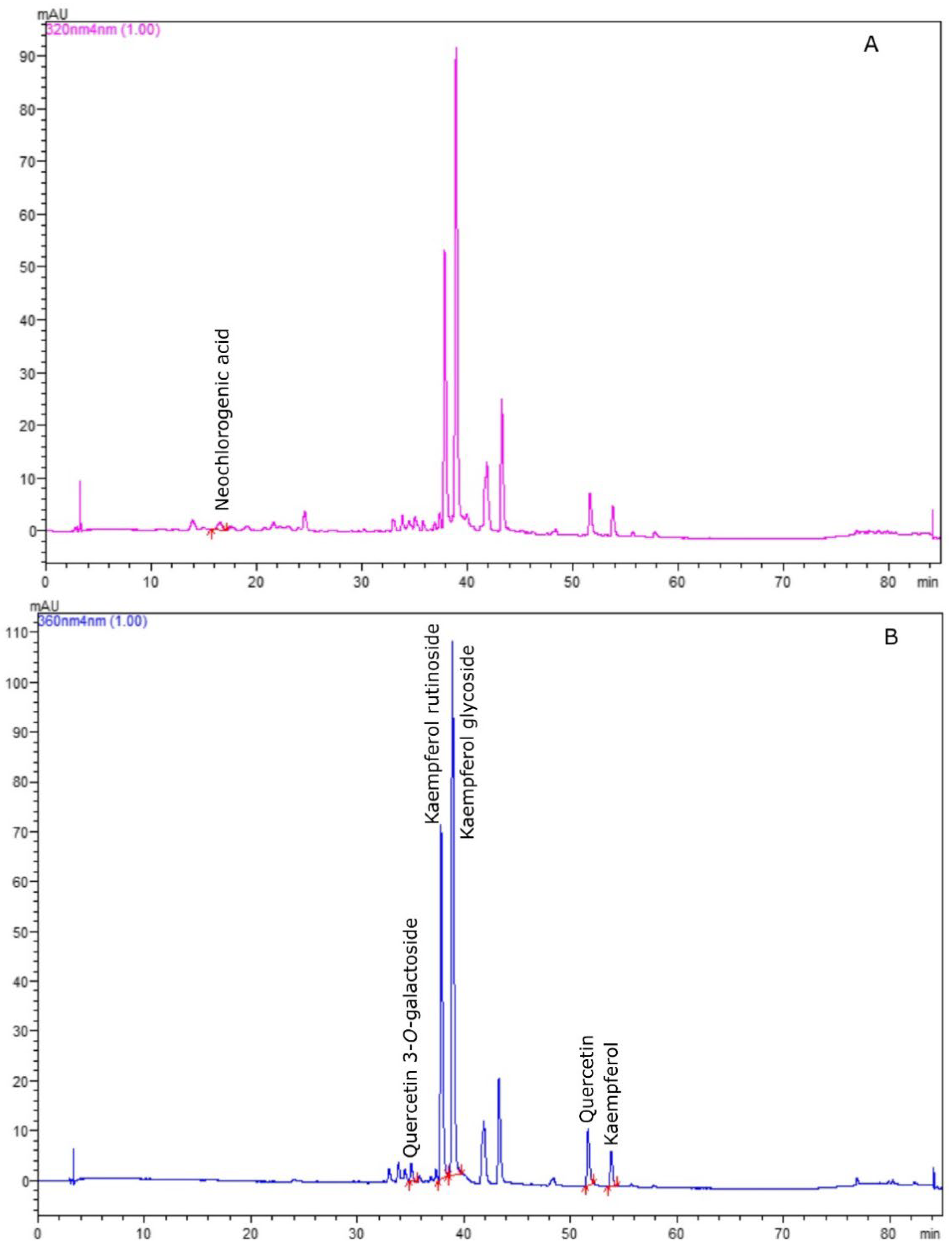
| Compound | Polyphenol Content (mg g−1) | ||
|---|---|---|---|
| Without PEF | PEF 30 min | PEF 60 min | |
| Neochlorogenic acid | 0.42 ± 0.03 A | 0.52 ± 0.05 a | 0.51 ± 0.06 a |
| Kaempferol 3-O-β-rutinoside | 18 ± 1 A | 22 ± 2 a | 24 ± 3 a |
| Kaempferol 3-glucoside | 28 ± 2 A | 35 ± 3 a | 40 ± 4 a |
| Quercetin 3-O-galactoside | 0.23 ± 0.02 A | 0.29 ± 0.03 a | 0.36 ± 0.04 a |
| Quercetin | 1.7 ± 0.2 A | 2.4 ± 0.2 a | 2.8 ± 0.3 a |
| Kaempferol | 1.8 ± 0.2 A | 2.6 ± 0.3 a | 3.1 ± 0.3 a |
| Total identified | 50.2 | 62.8 | 70.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntourtoglou, G.; Drosou, F.; Dourtoglou, V.G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering 2022, 4, 847-854. https://doi.org/10.3390/agriengineering4040054
Ntourtoglou G, Drosou F, Dourtoglou VG, Athanasiadis V, Chatzimitakos T, Bozinou E, Lalas SI. Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering. 2022; 4(4):847-854. https://doi.org/10.3390/agriengineering4040054
Chicago/Turabian StyleNtourtoglou, George, Fotini Drosou, Vassilis G. Dourtoglou, Vassilis Athanasiadis, Theodoros Chatzimitakos, Eleni Bozinou, and Stavros I. Lalas. 2022. "Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment" AgriEngineering 4, no. 4: 847-854. https://doi.org/10.3390/agriengineering4040054
APA StyleNtourtoglou, G., Drosou, F., Dourtoglou, V. G., Athanasiadis, V., Chatzimitakos, T., Bozinou, E., & Lalas, S. I. (2022). Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering, 4(4), 847-854. https://doi.org/10.3390/agriengineering4040054









