Studies on the Effects of Process Conditions on Separation of B1, B2 and B3 Vitamin Mixture Using HILIC and RPLC Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Columns
2.3. Chromatography
3. Results and Discussion
3.1. Effect of Mobile Phase Composition on Retention of Vitamins
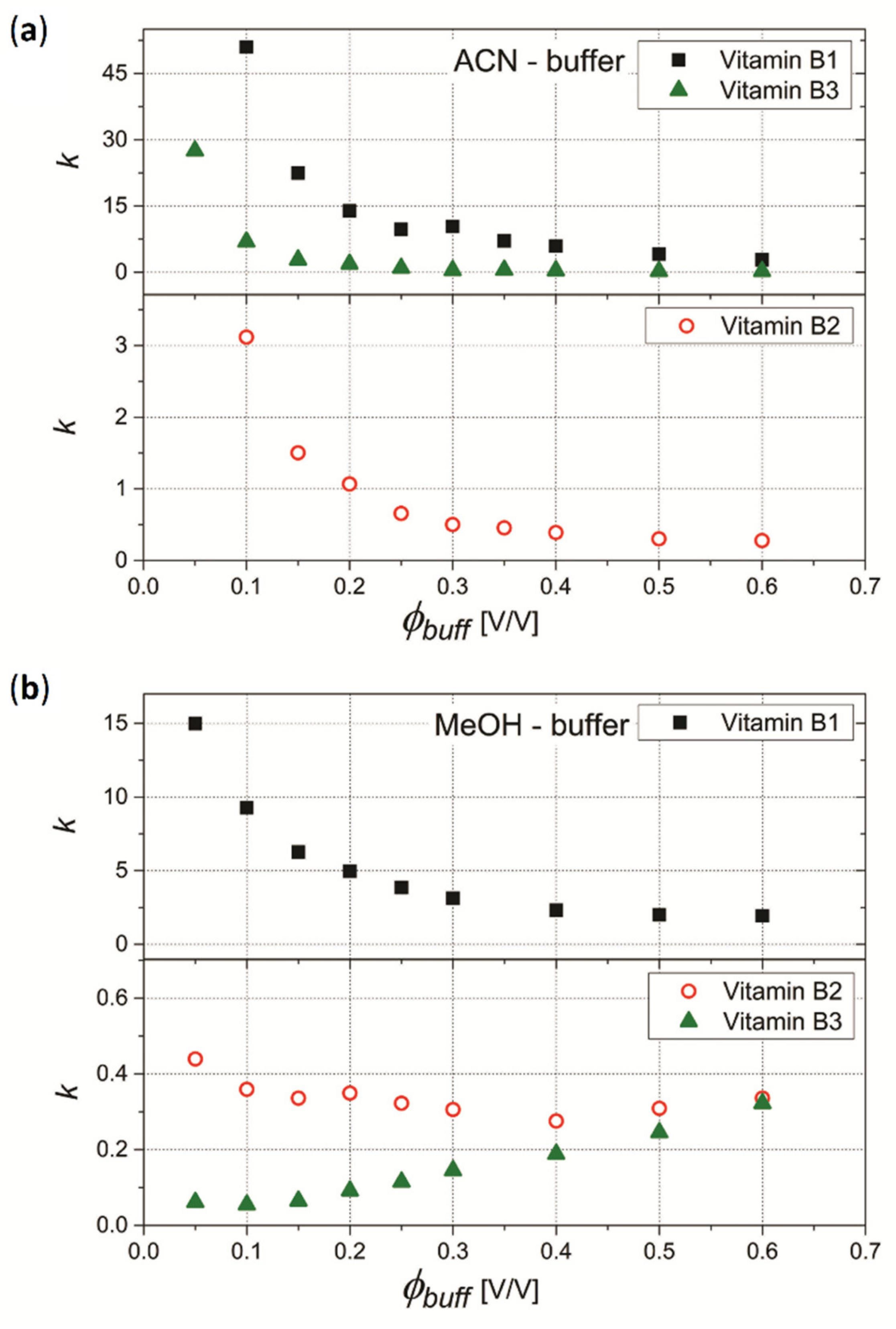
3.1.1. Column A (Acclaim™ Mixed-Mode HILIC-1)
3.1.2. Column E (Eurospher II 100–5 HILIC)
3.1.3. Column N (Nucleodur C18 Gravity-SB)
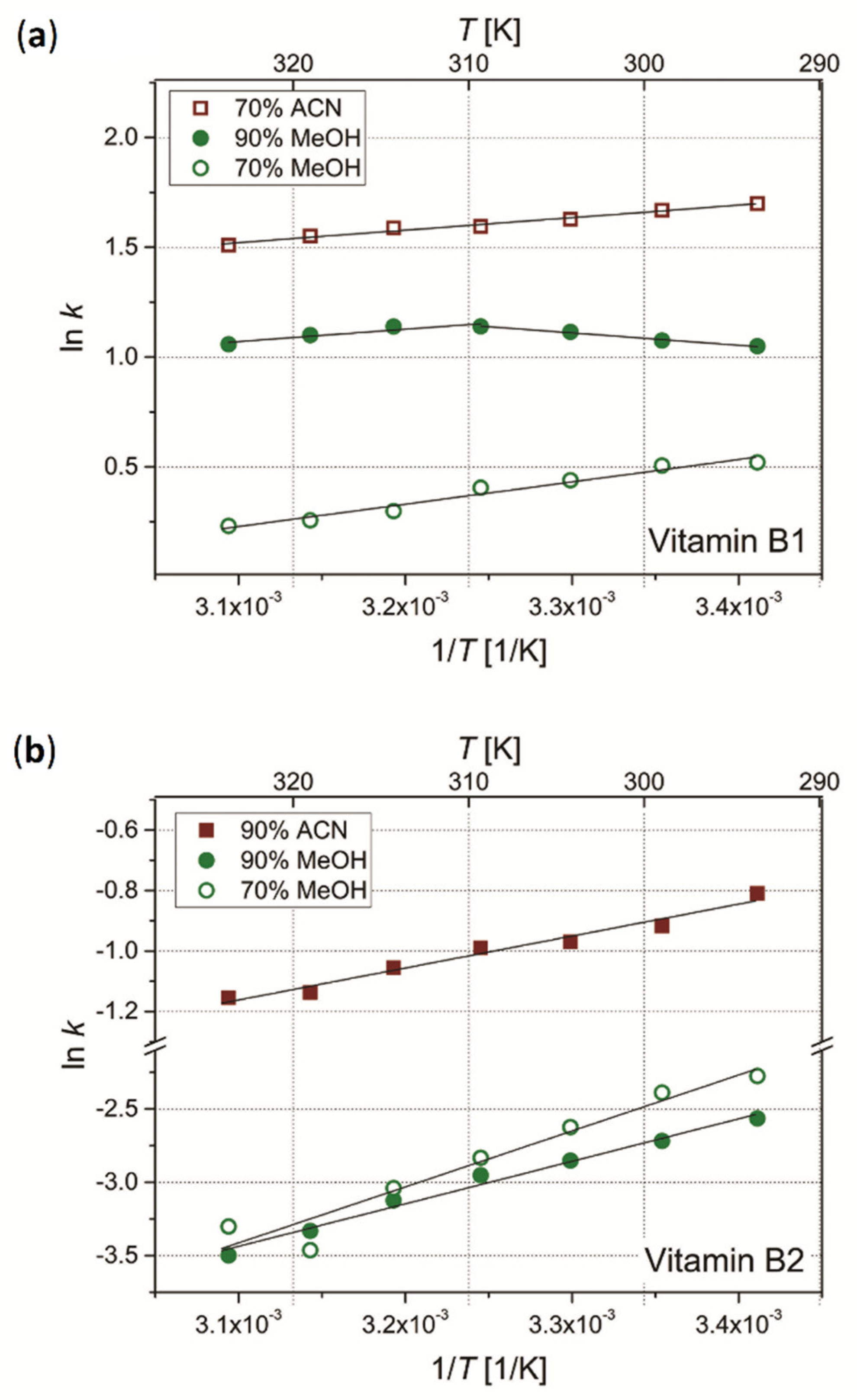
3.2. Effect of Temperature on Retention of Vitamins
3.2.1. Column A (Acclaim™ Mixed- Mode HILIC-1)
3.2.2. Column E (Eurospher II 100–5 HILIC)
3.2.3. Column N (Nucleodur C18 Gravity-SB)
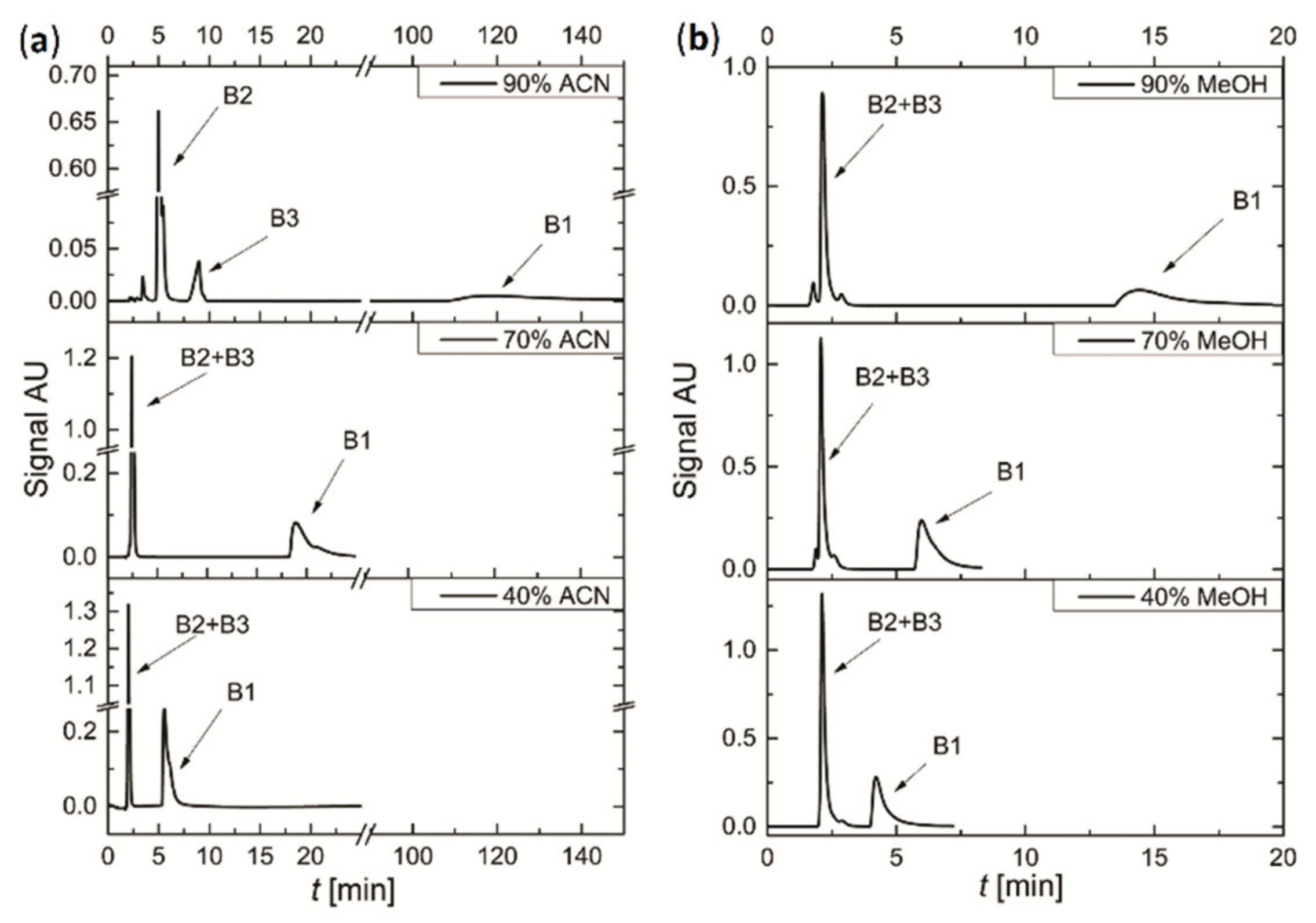
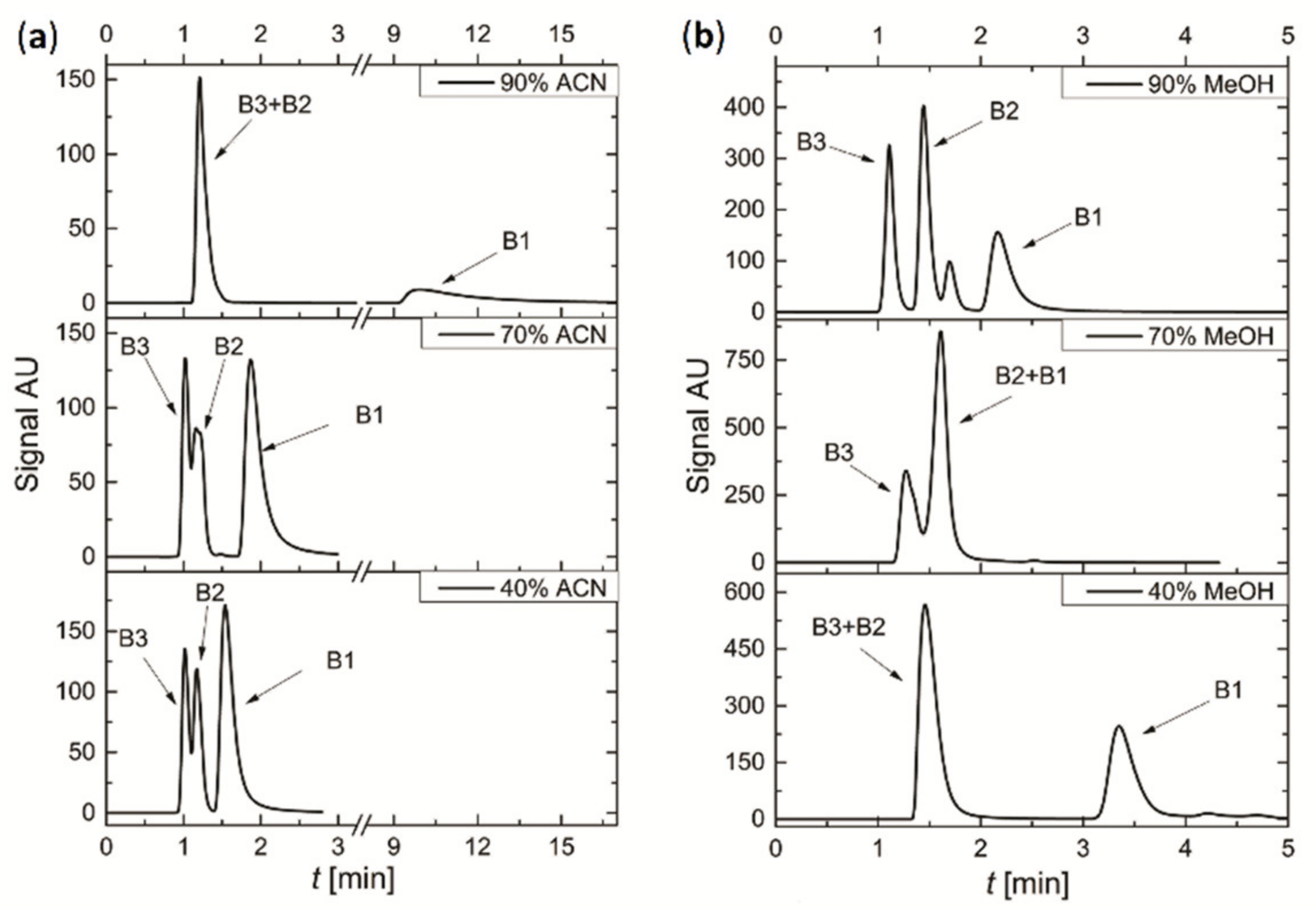
3.3. Preliminary Separation of the Mixture of Vitamins
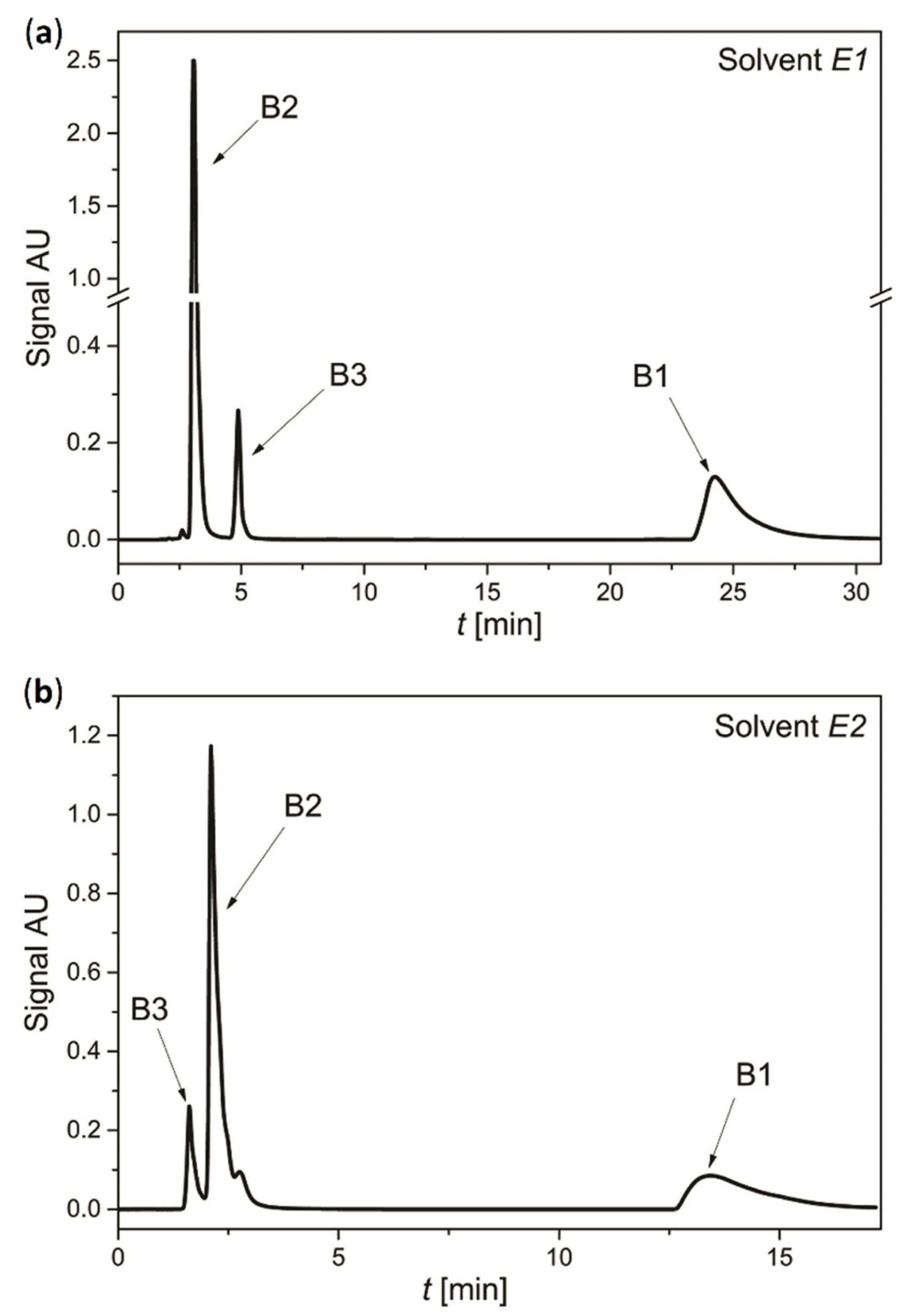
3.4. Separation of Mixture of Vitamins
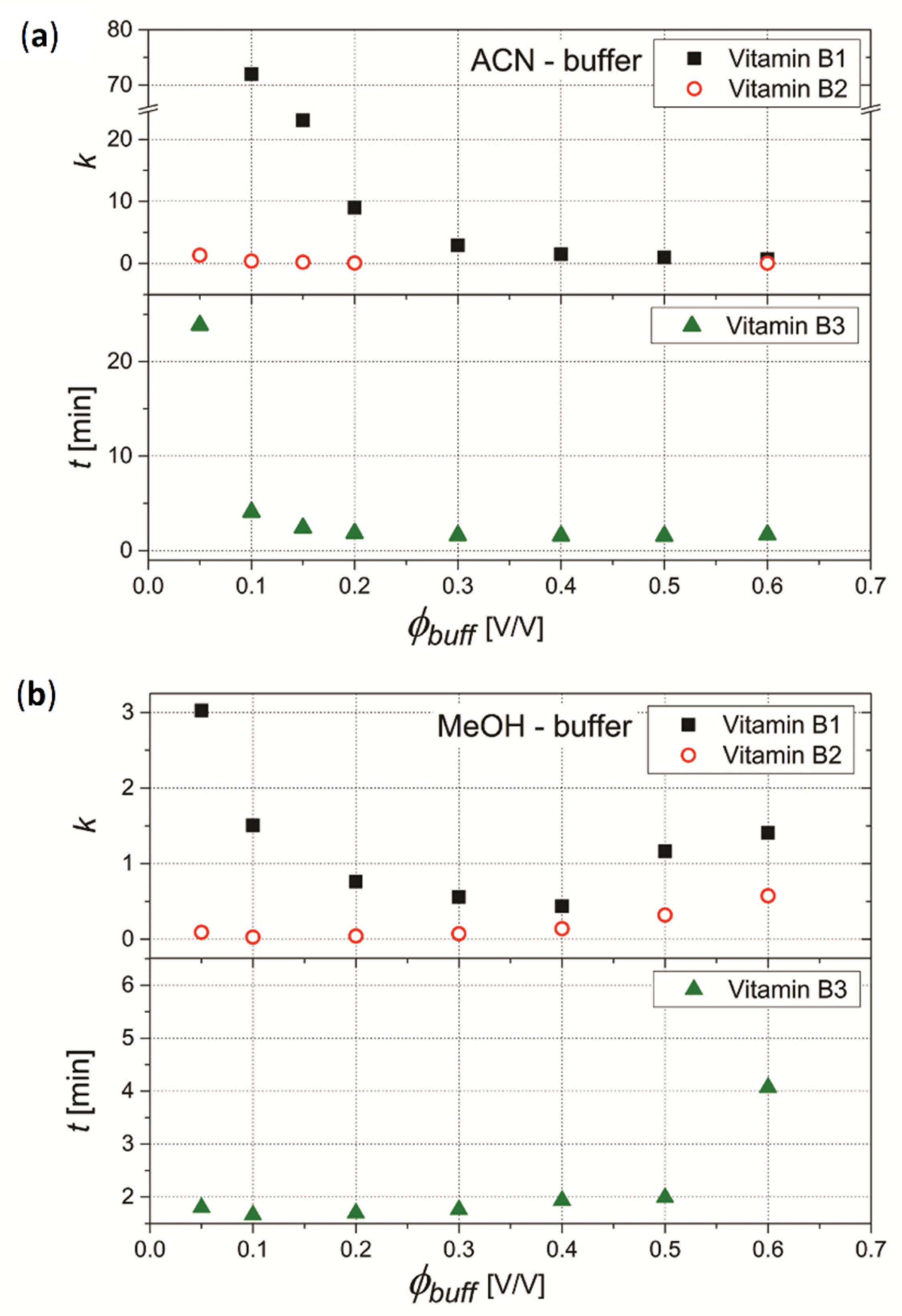
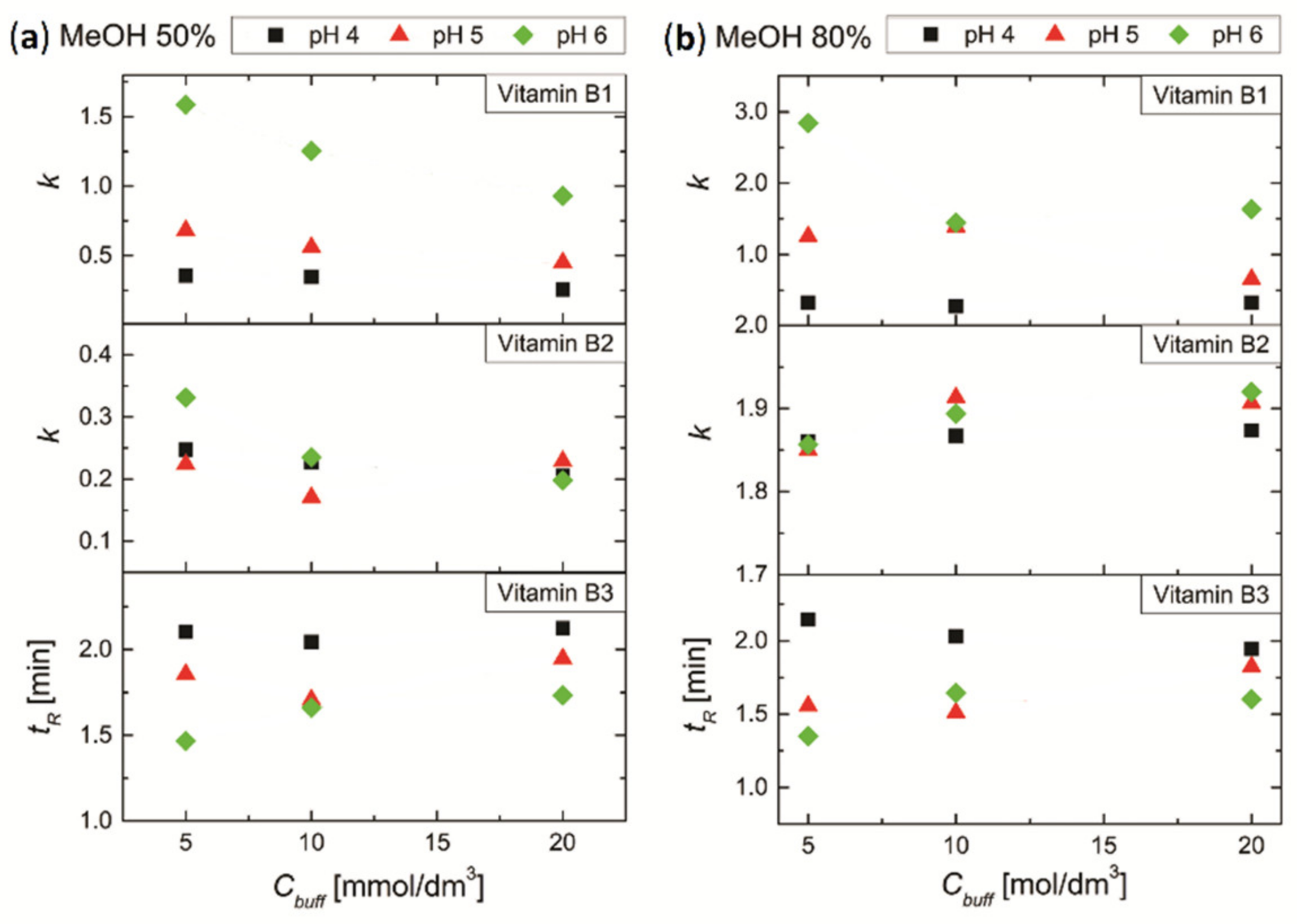
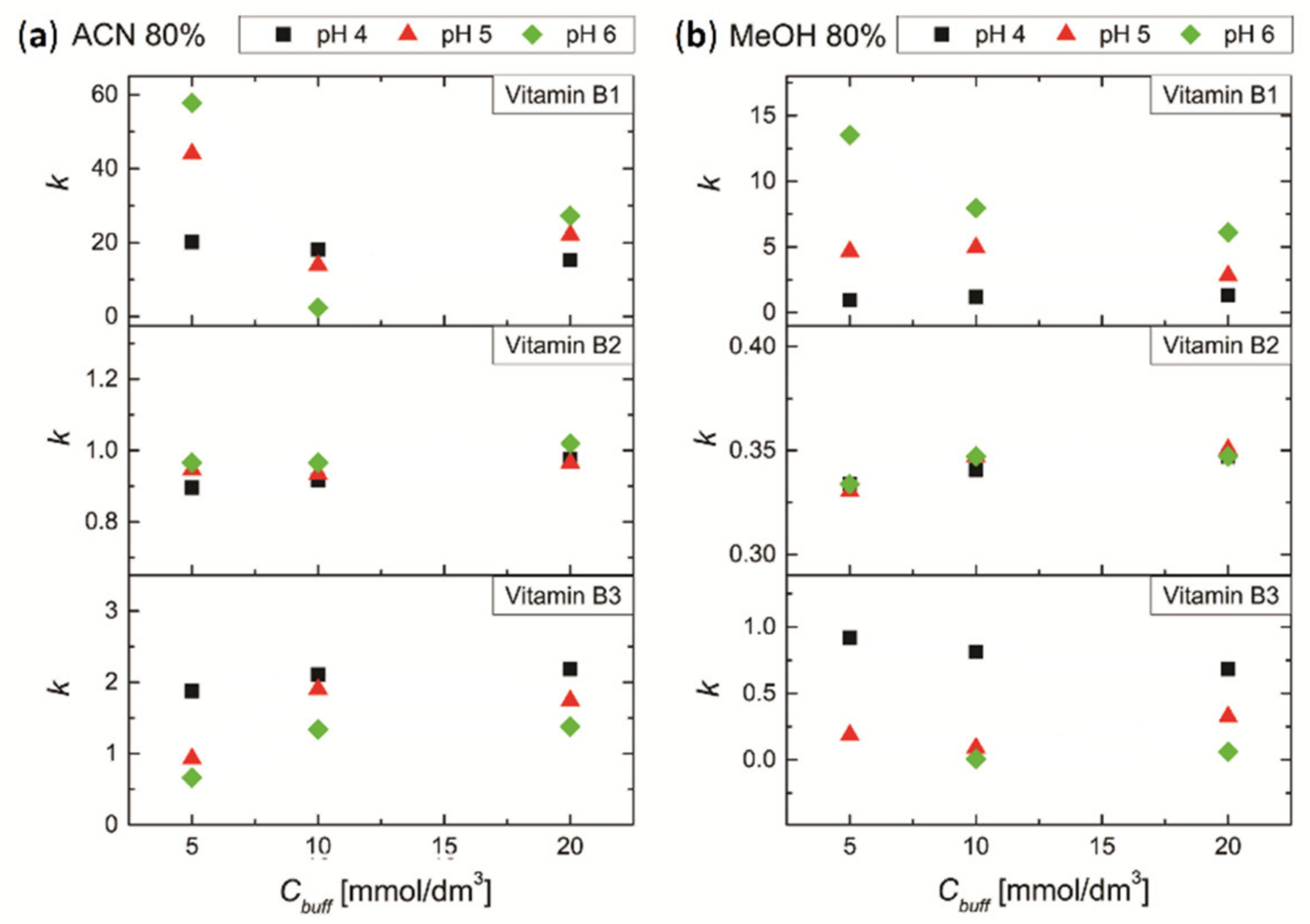
3.4.1. Effect of pH and Concentration of Buffer Salt on the Retention of Vitamins B1, B2 and B3
3.4.2. Effect of pH and Concentration of Buffer Salt on the Separation of Mixture of Vitamins B1, B2 and B3
4. Conclusions
- Since B1 and B3 vitamins are present in ionic form in the test conditions, the dominant trend along with the growth of the mobile phase pH was the increase in vitamin B1 retention and the decrease in vitamin B3 retention due to the opposite electrostatic interactions of the analyzed substances with modifying ligands and free silanol groups of adsorbents. The effects of pH for neutral vitamin B2 retention were insignificant and, for most systems, it was associated with a moderate increase in retention;
- The unequivocal effect of buffering salt concentration on the retention of analyzed vitamins was dependent on the properties of the given analyte, the stationary phase type and the organic solvent used for eluent. Depending on the particular chromatographic system, an increase or decrease in retention with an increase in the concentration of ammonium acetate in the eluent was observed;
- The obtained results of temperature effects on retention of the vitamins in selected systems indicate the dominant exothermic nature of the sorption processes with increasing temperature. There were also few observed systems for which the energy processes were endothermic, resulting from the contribution of adsorption interactions in the global retention mechanism. However, the general conclusion from the temperature studies is that application of higher process temperatures is not justified since the energy expenditure would not be balanced by possible improvement in the separation quality;
- As a result of two-step optimization of the process conditions, a HILIC chromatographic system has been proposed involving Nucleodur® C18 Gravity-SB column—although it is not an HILIC-dedicated one—and the eluent consists of 90% methanol, pH 6 and buffering salt (ammonium acetate) concentration Cbuff = 20 mmol/dm3. The system enables the separation of a mixture of vitamins B1, B2 and B3 in a shorter time than in the systems described in literature, in the isocratic conditions and using methanol, which is a more environmentally friendly organic solvent compared to commonly used acetonitrile.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Zaid, E.A.A.; Eissa, M.A. Thompson Seedless Grapevines Growth and Quality as Affected by Glutamic Acid, Vitamin B, and Algae. J. Soil Sci. Plant Nutr. 2019, 19, 725–733. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Chapman, L.M. The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 2020, 295, 12002–12013. [Google Scholar] [CrossRef]
- Seck, M.; Linton, J.A.V.; Allen, M.S.; Castagnino, D.S.; Chouinard, P.Y.; Girard, C.L. Apparent ruminal synthesis of B vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J. Dairy Sci. 2017, 100, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Castagnino, D.S.; Kammes, K.L.; Allen, M.S.; Gervais, R.; Chouinard, P.Y.; Girard, C.L. Particle length of silages affects apparent ruminal synthesis of B vitamins in lactating dairy cows. J. Dairy Sci. 2016, 99, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Castagnino, D.S.; Harvatine, K.J.; Allen, M.S.; Gervais, R.; Chouinard, P.Y.; Girard, C.L. Short communication: Effect of fatty acid supplements on apparent ruminal synthesis of B vitamins in lactating dairy cows. J. Dairy Sci. 2017, 100, 8165–8169. [Google Scholar] [CrossRef] [Green Version]
- De Leenheer, A.P.; Lambert, W. Modern Chromatographic Analysis Of Vitamins; Marcel Dekker Inc.: New York, NY, USA, 2000; p. 564. [Google Scholar]
- Fotsing, L.; Fillet, M.; Bechet, I.; Hubert, P.; Crommen, J. Determination of six water-soluble vitamins in a pharmaceutical formulation by capillary electrophoresis. J. Pharm. Biomed. Anal. 1997, 15, 1113–1123. [Google Scholar] [CrossRef]
- Dziomba, S.; Kowalski, P.; Baogonekczek, T. Field-amplified sample stacking-sweeping of vitamins B determination in capillary electrophoresis. J. Chromatogr. A 2012, 1267, 224–230. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Kowalska, S.; Szłyk, E. New procedure for column-switching isotachophoretic determination of vitamins B1 and B6 in beer samples. J. Food Compos. Anal. 2017, 57, 80–86. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Hosu, A.; Puscas, A. Thin-layer chromatography with stationary phase gradient as a method for separation of water-soluble vitamins. J. Chromatogr. A 2012, 1223, 142–146. [Google Scholar] [CrossRef]
- Dinelli, G.; Bonetti, A. Micellar electrokinetic capillary chromatography analysis of water-soluble vitamins and multi-vitamin integrators. Electrophoresis 1994, 15, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, T.; Zhang, L.; Li, H.; Fang, Y. Separation and determination of three water-soluble vitamins in pharmaceutical preparations and food by micellar electrokinetic chromatography with amperometric electrochemical detection. Anal. Chim. Acta 2001, 437, 123–129. [Google Scholar] [CrossRef]
- Navarro-Pascual-Ahuir, M.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Determination of water-soluble vitamins in energy and sport drinks by micellar electrokinetic capillary chromatography. Food Control 2016, 63, 110–116. [Google Scholar] [CrossRef]
- Ghorbani, A.R.; Momenbeik, F.; Khorasani, J.H.; Amini, M.K. Simultaneous micellar liquid chromatographic analysis of seven water-soluble vitamins: Optimization using super-modified simplex. Anal. Bioanal. Chem. 2004, 379, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, Y.; Du, Y.; Xing, D. Pressurized capillary electrochromatographic analysis of water-soluble vitamins by combining with on-line concentration technique. J. Chromatogr. A 2007, 1154, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lebiedzińska, A.; Marszałł, M.L.; Kuta, J.; Szefer, P. Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B1 (thiamine), B6 (pyridoxamine, pyridoxal and pyridoxine) and B12 in animal and plant foods. J. Chromatogr. A 2007, 1173, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Leporati, A.; Catellani, D.; Suman, M.; Andreoli, R.; Manini, P.; Niessen, W.M.A. Application of a liquid chromatography tandem mass spectrometry method to the analysis of water-soluble vitamins in Italian pasta. Anal. Chim. Acta 2005, 531, 87–95. [Google Scholar] [CrossRef]
- Viñas, P.; López-Erroz, C.; Balsalobre, N.; Hernández-Córdoba, M. Reversed-phase liquid chromatography on an amide stationary phase for the determination of the B group vitamins in baby foods. J. Chromatogr. A 2003, 1007, 77–84. [Google Scholar] [CrossRef]
- Klejdus, B.; Petrlová, J.; Potěšil, D.; Adam, V.; Mikelová, R.; Vacek, J.; Kizek, R.; Kubáň, V. Simultaneous determination of water- and fat-soluble vitamins in pharmaceutical preparations by high-performance liquid chromatography coupled with diode array detection. Anal. Chim. Acta 2004, 520, 57–67. [Google Scholar] [CrossRef]
- Heudi, O.; Kilinç, T.; Fontannaz, P. Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultra-violet detection: Application to polyvitaminated premixes. J. Chromatogr. A 2005, 1070, 49–56. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; Yao, S. High-performance liquid chromatography/electrospray ionization-mass spectrometry for simultaneous determination of taurine and 10 water-soluble vitamins in multivitamin tablets. Anal. Chim. Acta 2006, 569, 169–175. [Google Scholar] [CrossRef]
- Vidović, S.; Stojanović, B.; Veljković, J.; Pražić-Arsić, L.; Roglić, G.; Manojlović, D. Simultaneous determination of some water-soluble vitamins and preservatives in multivitamin syrup by validated stability-indicating high-performance liquid chromatography method. J. Chromatogr. A 2008, 1202, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Mendiola, J.A.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef]
- Meisser Redeuil, K.; Longet, K.; Bénet, S.; Munari, C.; Campos-Giménez, E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2015, 1422, 89–98. [Google Scholar] [CrossRef]
- Cellar, N.A.; McClure, S.C.; Salvati, L.M.; Reddy, T.M. A new sample preparation and separation combination for precise, accurate, rapid, and simultaneous determination of vitamins B1, B2, B3, B5, B6, B7, and B9 in infant formula and related nutritionals by LC-MS/MS. Anal. Chim. Acta 2016, 934, 180–185. [Google Scholar] [CrossRef]
- Márquez-Sillero, I.; Cárdenas, S.; Valcárcel, M. Determination of water-soluble vitamins in infant milk and dietary supplement using a liquid chromatography on-line coupled to a corona-charged aerosol detector. J. Chromatogr. A 2013, 1313, 253–258. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Ziobrowski, P.; Zapała, L.; Zapała, W. Studies on the retention behavior of quercetin, phenol and caffeine as test substances on selected neutral and charged Hydrophilic Interaction Liquid Chromatography stationary phases. Sep. Sci. Plus 2022, 1–8. [Google Scholar] [CrossRef]
- McCalley, D.V. Is hydrophilic interaction chromatography with silica columns a viable alternative to reversed-phase liquid chromatography for the analysis of ionisable compounds? J. Chromatogr. A 2007, 1171, 46–55. [Google Scholar] [CrossRef]
- Dos Santos Pereira, A.; David, F.; Vanhoenacker, G.; Sandra, P. The acetonitrile shortage: Is reversed HILIC with water an alternative for the analysis of highly polar ionizable solutes? J. Sep. Sci. 2009, 32, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.H.; Alden, B.A.; Berthelette, K.; Field, J.A.; Lawrence, N.L.; McLaughlin, J.; Patel, A.V. Characterization of a highly stable zwitterionic hydrophilic interaction chromatography stationary phase based on hybrid organic/inorganic particles. J. Sep. Sci. 2022, 45, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Subirat, X.; Abraham, M.H.; Roses, M. Characterization of hydrophilic interaction liquid chromatography retention by a linear free energy relationship. Comparison to reversed- and normal-phase retentions. Anal. Chim. Acta 2019, 1092, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Den Uijl, M.J.; Schoenmakers, P.J.; Pirok, B.W.; van Bommel, M.R. Recent applications of retention modelling in liquid chromatography. J Sep. Sci. 2021, 44, 88–114. [Google Scholar] [CrossRef]
- Ziobrowski, P.; Chutkowski, M.; Przywara, M.; Zapała, L.; Kosińska-Pezda, M.; Zapała, W. Analysis of adsorption energy distribution in selected hydrophilic-interaction chromatography systems with amide, amine, and zwitterionic stationary phases. J. Sep. Sci. 2021, 44, 2577–2586. [Google Scholar] [CrossRef]
- Kaliszan, R. QSRR: Quantitative structure-(chromatographic) retention relationships. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Fiamegos, Y.C.; Stalikas, C.D. HILIC separation and quantitation of water-soluble vitamins using diol column. J. Sep. Sci. 2009, 32, 909–917. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Fiamegos, Y.C.; Stalikas, C.D. Study of the behavior of water-soluble vitamins in HILIC on a diol column. Chromatographia 2010, 71, 751–759. [Google Scholar] [CrossRef]
- Karatapanis, A.E.; Fiamegos, Y.C.; Stalikas, C.D. A revisit to the retention mechanism of hydrophilic interaction liquid chromatography using model organic compounds. J. Chromatogr. A 2011, 1218, 2871–2879. [Google Scholar] [CrossRef]
- Yang, Y.; Boysen, R.I.; Hearn, M.T.W. Selectivity differences of water-soluble vitamins separated on hydrophilic interaction stationary phases. J. Sep. Sci. 2013, 36, 1897–1903. [Google Scholar] [CrossRef]
- Langer, S.; Lodge, J.K. Determination of selected water-soluble vitamins using hydrophilic chromatography: A comparison of photodiode array, fluorescence, and coulometric detection, and validation in a breakfast cereal matrix. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 960, 73–81. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.M.; Karger, B.L. Distribution Phenomena Of Mobile-Phase Components And Determination Of Dead Volume In Reversed-Phase Liquid Chromatography. Anal. Chem. 1980, 52, 2249–2257. [Google Scholar] [CrossRef]
- Jandera, P. Stationary and mobile phases in hydrophilic interaction chromatography: A review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Letzel, T. Main interactions and influences of the chromatographic parameters in HILIC separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef]








| Column | Acclaim™ Mixed-Mode HILIC-1 (Dionex, Sunnyvale, CA USA) | Eurospher II 100–5 HILIC (Knauer, Berlin, Germany) | Nucleodur ® C18 Gravity-SB (Macherey-Nagel, Oensingen, Switzerland) |
|---|---|---|---|
| Symbolic name | Column A | Column E | Column N |
| Stationary phase type | Alkyl diol on silica gel substrate | Zwitterionic (ammonium–sulphonic acid) on silica gel | Monomeric octadecyl on silica gel substrate |
| Dimensions [mm] | 4.6 × 150 | 4.6 × 150 | 4 × 125 |
| Particle diameter [μm] | 5 | 5 | 5 |
| Specific area [m2/g] | 300 | 320 | 338 |
| Pore size [Å] | 120 | 100 | 110 |
| Hold-up time [cm3] | 1.820 ± 0.008 | 1.495 ± 0.007 | 0.897 ± 0.004 |
| Total porosity εt, | 0.73 | 0.60 | 0.43 |
| Phase ratio Φ | 0.3697 | 0.6670 | 1.1320 |
| ACN–Buffer 90:10 | ACN–Buffer 70:30 | |||||
|---|---|---|---|---|---|---|
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | - | - | - | −4.73 | 0.9808 | 1.1 × 10−5 |
| Vitamin B2 | −8.78 | 0.9663 | 4.52 × 10−5 | - | - | - |
| MeOH–buffer 90:10 | MeOH–buffer 70:30 | |||||
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | −4.65/4.66 | 0.8255/0.9867 | 5.99 × 10−2/4.44 × 10−2 | −8.46 | 0.9606 | 6.73 × 10−5 |
| Vitamin B2 | −24.10 | 0.9827 | 8.32 × 10−5 | −31.74 | 0.9293 | 2.92 × 10−5 |
| ACN–Buffer 90:10 | ACN–Buffer 70:30 | |||||
|---|---|---|---|---|---|---|
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | −0.27 | −0.195 | 0.8903 | −10.01 | 0.8790 | 1.14 × 10−3 |
| Vitamin B2 | −9.35 | 0.9112 | 5.19 × 10−4 | −5.11 | 0.9478 | 1.36 × 10−4 |
| Vitamin B3 | −3.23 | 0.2099 | 0.1682 | −4.55 | 0.3058 | 0.1146 |
| MeOH–buffer 90:10 | MeOH–buffer 70:30 | |||||
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | −7.80 | 0.9923 | 1.11 × 10−6 | −3.70 | 0.9864 | 4.71 × 10−6 |
| Vitamin B2 | −5.22 | 0.9157 | 4.56 × 10−4 | −5.88 | 0.9214 | 3.82 × 10−4 |
| Vitamin B3 | −8.36 | 0.3506 | 0.0946 | −23.92 | 0.9833 | 7.79 × 10−6 |
| ACN–Buffer 90:10 | ACN–Buffer 70:30 | |||||
|---|---|---|---|---|---|---|
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | 8.21 | 0.8672 | 1.44 × 10−3 | 5.28 | 0.5164 | 0.0417 |
| Vitamin B2 | 0.23 | 0.4094 | 0.0723 | 1.66/−0.98 | 0.9494/0.9217 | 3.18 × 10−3/0.126 |
| Vitamin B3 | −0.74 | 0.7150 | 0.0102 | −0.05 | 0.2260 | 0.158 |
| MeOH–buffer 90:10 | MeOH–buffer 70:30 | |||||
| ΔH [kJ/mol] | Adj.R2 | Prob > F | ΔH [kJ/mol] | Adj.R2 | Prob > F | |
| Vitamin B1 | −3.98 | 0.9001 | 6.85 × 10−4 | −0.93 | 0.7688 | 5.97 × 10−3 |
| Vitamin B2 | −1.31 | 0.7528 | 7.09 × 10−3 | −2.48 | 0.5578 | 0.033 |
| Vitamin B3 | −0.43 | 0.5957 | 0.0258 | −1.61 | 0.4938 | 0.0472 |
| ACN–Ammonium Acetate Buffer (pH 5) | ||||||||
|---|---|---|---|---|---|---|---|---|
| φbuff [v/v] | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | |
| Column A | α1–2 | 9.13 | 3.07 | - | - | - | - | - |
| Rs1–2 | 9.24 | 2.14 | 0.65 | 0.89 | 0.89 | 0.96 | 1.18 | |
| α2–3 | - | 184 | 102 | - | - | - | 10.44 | |
| Rs2–3 | - | 8.79 | 7.60 | 4.94 | 8.87 | 3.27 | 2.10 | |
| Column E | α1–2 | - | 2.23 | 1.79 | 1.10 | 1.03 | 1.22 | 1.22 |
| Rs1–2 | - | 12.7 | 4.10 | 0.21 | 0.05 | 0.28 | 0.27 | |
| α2–3 | - | 1.54 | 13.0 | 20.6 | 15.1 | 13.5 | 10.1 | |
| Rs2–3 | - | 2.52 | 4.10 | 7.44 | 10.2 | 6.54 | 7.15 | |
| Column N | α1–2 | 0.11 | 1.35 | 4.19 | 5.72 | 6.98 | 4.89 | 5.18 |
| Rs1–2 | 0.18 | 0.36 | 0.93 | 0.89 | 1.12 | 0.79 | 0.77 | |
| α2–3 | 287 | 32.9 | 8.56 | 4.34 | 3.18 | 3.69 | 2.98 | |
| Rs2–3 | 7.30 | 3.53 | 2.68 | 2.08 | 1.58 | 1.70 | 1.25 | |
| MeOH–ammonium acetate buffer (pH 5) | ||||||||
| φbuff [v/v] | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | |
| Column A | α1–2 | - | - | - | - | 7.77 | - | 2.07 |
| Rs1–2 | 0.79 | 0.75 | 0.82 | 0.80 | 0.62 | 2.07 | 2.09 | |
| α2–3 | 32.37 | 51.70 | 17.29 | 7.60 | 3.11 | 3.63 | 2.45 | |
| Rs2–3 | 7.73 | 1.11 | 0.89 | 0.93 | 0.67 | 1.78 | 2.08 | |
| Column E | α1–2 | 7.14 | 6.55 | 3.81 | 2.11 | 1.46 | 1.35 | 1.47 |
| Rs1–2 | 1.77 | 1.38 | 1.45 | 0.84 | 0.44 | 0.27 | 0.06 | |
| α2–3 | 34.07 | 25.75 | 14.18 | 10.22 | 8.38 | 6.44 | 5.69 | |
| Rs2–3 | 5.99 | 5.98 | 5.91 | 4.87 | 4.28 | 3.67 | 3.18 | |
| Column N | α1–2 | 4.79 | 3.53 | 2.52 | 2.05 | 1.37 | 1.16 | 1.15 |
| Rs1–2 | 1.71 | 1.53 | 1.36 | 0.98 | 0.58 | 0.21 | 0.21 | |
| α2–3 | 4.20 | 2.54 | 1.29 | 1.06 | 1.52 | 2.36 | 4.37 | |
| Rs2–3 | 2.96 | 2.07 | 0.44 | 0.13 | 1.03 | 2.01 | 4.26 | |
| Column Type | Column A | Column E | Column N | ||||
|---|---|---|---|---|---|---|---|
| Mobile phase properties | Eluent indication | A1 | A2 | E1 | E2 | N1 | N2 |
| Organic modifier | ACN | MeOH | ACN | MeOH | ACN | MeOH | |
| Buffer content fbuff [-] | 0.6 | 0.5 | 0.2 | 0.2 | 0.4 | 0.1 | |
| Buffer salt concentration Cbuff [mmol/dm3] | 10 | 20 | 20 | 10 | 10 | 20 | |
| Buffer pH [-] | 5 | 6 | 4 | 6 | 6 | 6 | |
| Selectivity coefficient | α1–2 | - | - | 2.23 | 57.86 | 6.27 | 4.24 |
| α2–3 | 22.36 | 4.89 | 6.95 | 22.93 | 7.97 | 3.45 | |
| Column resolution coefficient | Rs1–2 | 3.02 | 1.49 | 4.44 | 1.29 | 1.37 | 1.27 |
| Rs2–3 | 12.48 | 1.76 | 18.21 | 8.65 | 3.48 | 1.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutkowski, M.; Ziobrowski, P.; Przywara, M.; Kamińska, J.; Zapała, W. Studies on the Effects of Process Conditions on Separation of B1, B2 and B3 Vitamin Mixture Using HILIC and RPLC Chromatography. AgriEngineering 2022, 4, 566-591. https://doi.org/10.3390/agriengineering4030038
Chutkowski M, Ziobrowski P, Przywara M, Kamińska J, Zapała W. Studies on the Effects of Process Conditions on Separation of B1, B2 and B3 Vitamin Mixture Using HILIC and RPLC Chromatography. AgriEngineering. 2022; 4(3):566-591. https://doi.org/10.3390/agriengineering4030038
Chicago/Turabian StyleChutkowski, Marcin, Piotr Ziobrowski, Mateusz Przywara, Justyna Kamińska, and Wojciech Zapała. 2022. "Studies on the Effects of Process Conditions on Separation of B1, B2 and B3 Vitamin Mixture Using HILIC and RPLC Chromatography" AgriEngineering 4, no. 3: 566-591. https://doi.org/10.3390/agriengineering4030038
APA StyleChutkowski, M., Ziobrowski, P., Przywara, M., Kamińska, J., & Zapała, W. (2022). Studies on the Effects of Process Conditions on Separation of B1, B2 and B3 Vitamin Mixture Using HILIC and RPLC Chromatography. AgriEngineering, 4(3), 566-591. https://doi.org/10.3390/agriengineering4030038







