EEG-Based Analysis of Motor Imagery and Multi-Speed Passive Pedaling: Implications for Brain–Computer Interfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
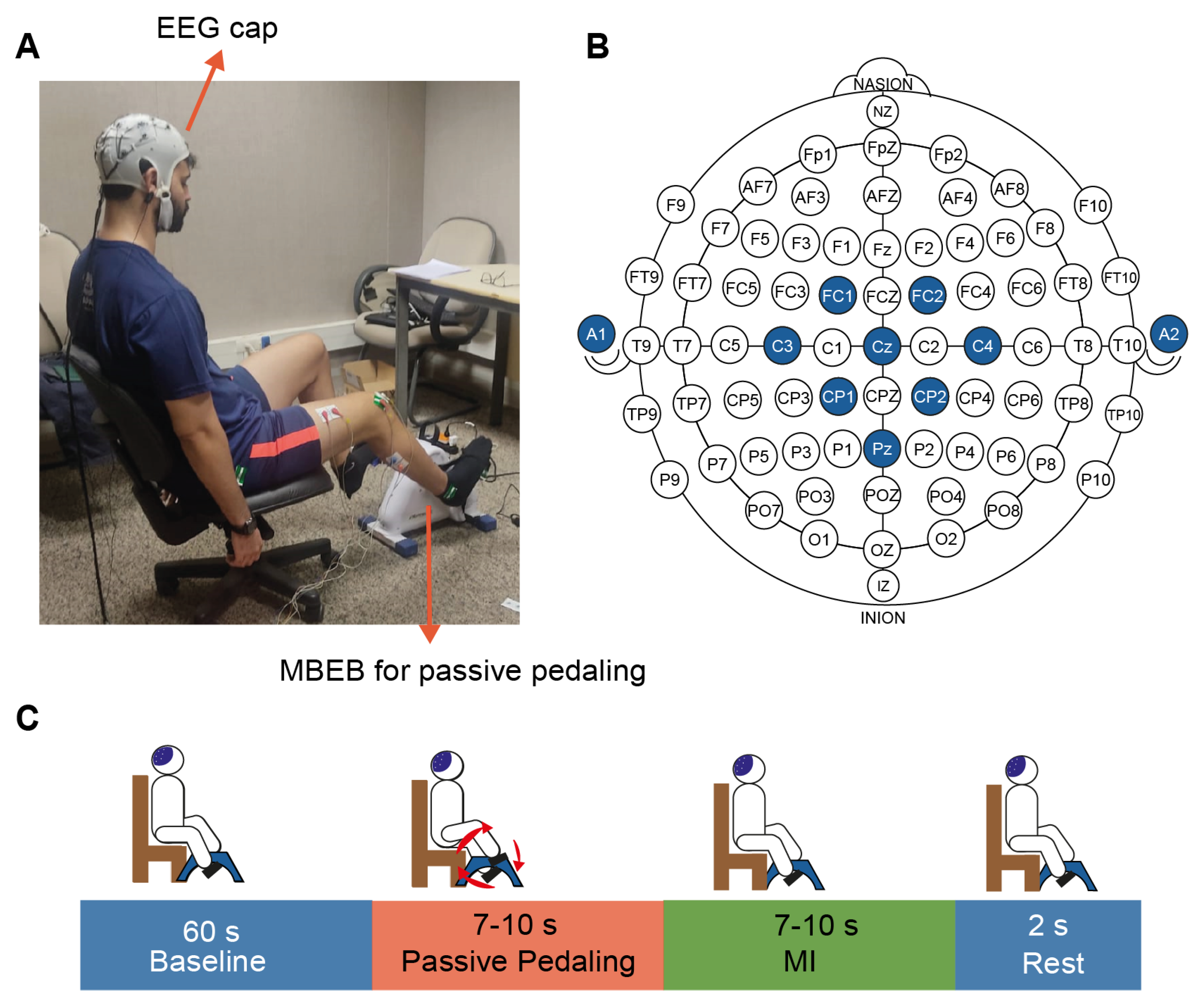
2.2. Experimental Setup
2.3. Experimental Protocol
- 1.
- The participant rested their feet on the minibike pedals and fixed their eye gazes on a black screen for 60 s without performing mental tasks (baseline).
- 2.
- Following this, the MMEB provided PP for a period of time ranging between 7 and 10 s at one of the three configured speeds: 30 (low), 45 (medium), and 60 rpm (high). Trial durations were pseudorandomized while maintaining equal representation of each length.
- 3.
- The subject was instructed to imagine pedaling movements for the same period of time as aforementioned. The individual was instructed to perform the mental task simulating the same speed that was passively received in stage 2.
- 4.
- 5.
- Stages 2 to 4 were repeated until 10 trials per class were performed, completing a total of 30 trials.
2.4. Data Analysis
2.5. Machine Learning Classifiers
- 1.
- PP Velocity Classification: EEG segments corresponding to three PP speeds (e.g., slow, medium, fast) were used as separate classes.
- 2.
- MI: EEGs from MI tasks performed after each PP condition were classified into three corresponding speed-related classes.
- 3.
- Cross-Condition: Classifiers were trained on PP EEG data and tested on MI data to evaluate generalization across conditions.
2.5.1. Pre-Processing
2.5.2. Common Spatial Patterns
2.5.3. Riemannian Geometry
2.5.4. Classification
2.6. Deep Learning Classifier
2.7. Performance Metrics
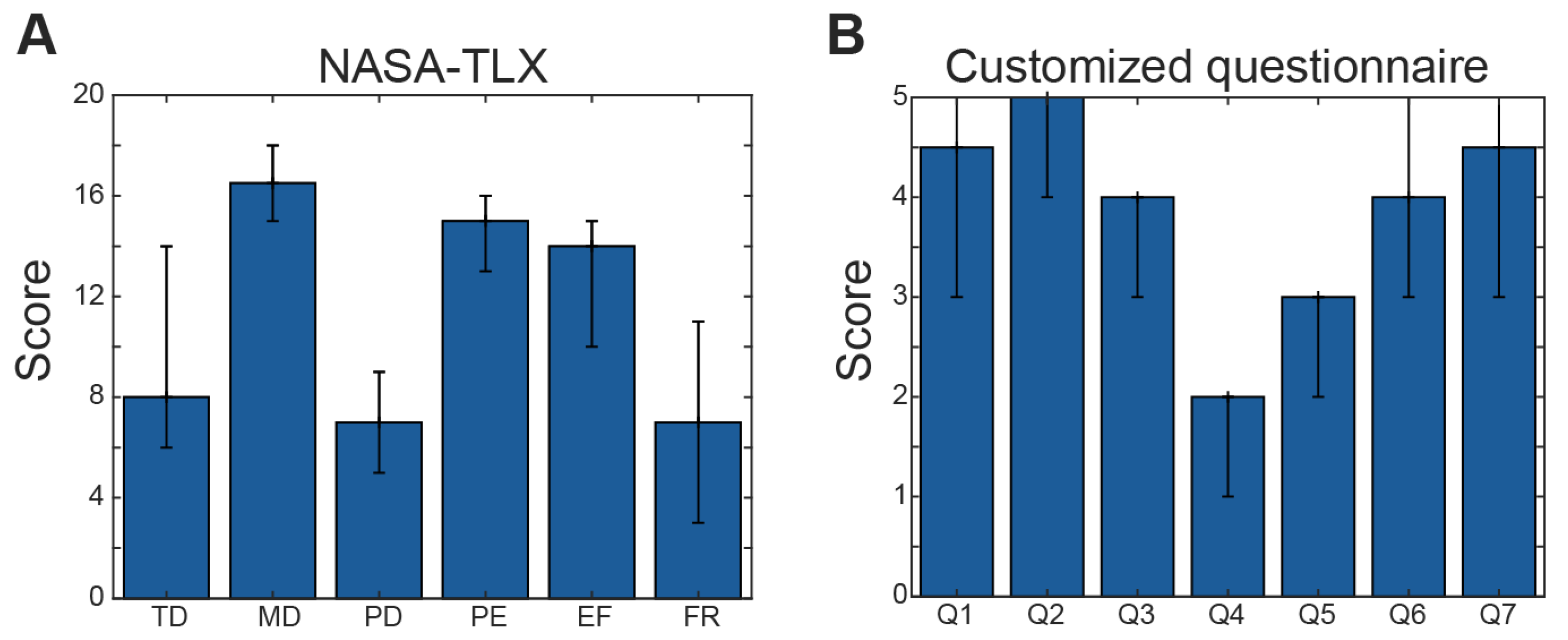
2.8. Subjective Questionnaires
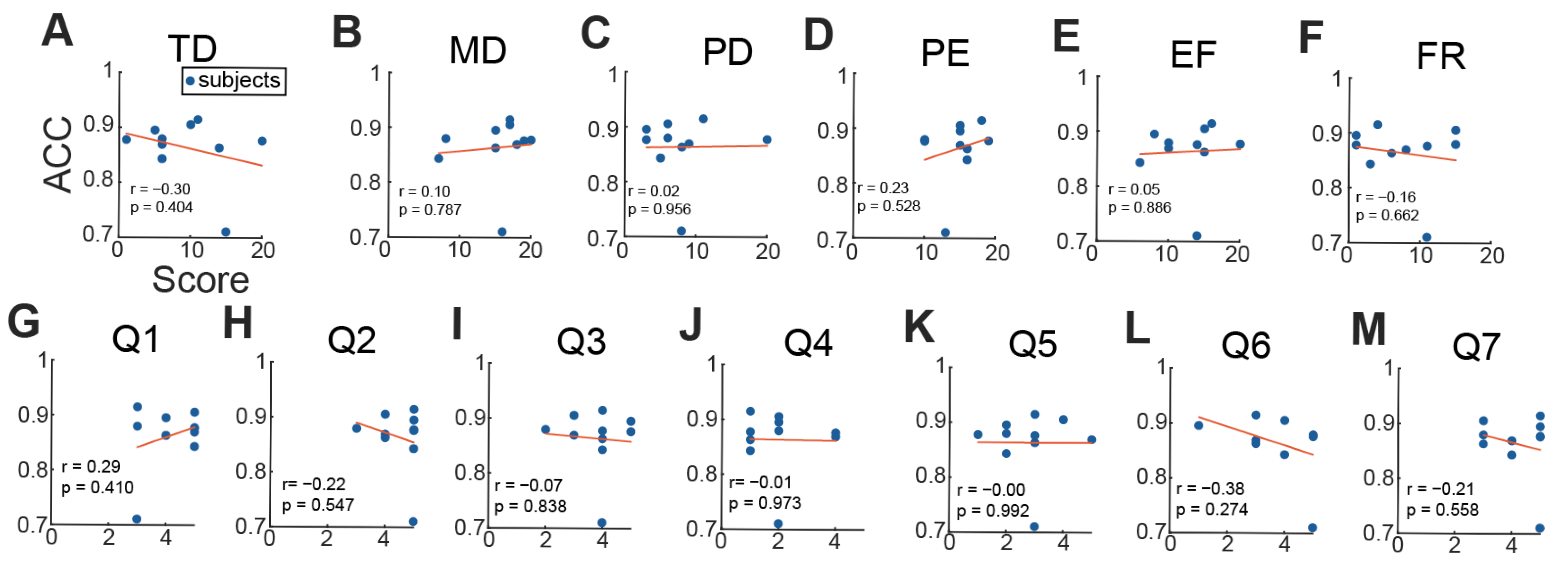
2.9. Statistical Analysis
3. Results
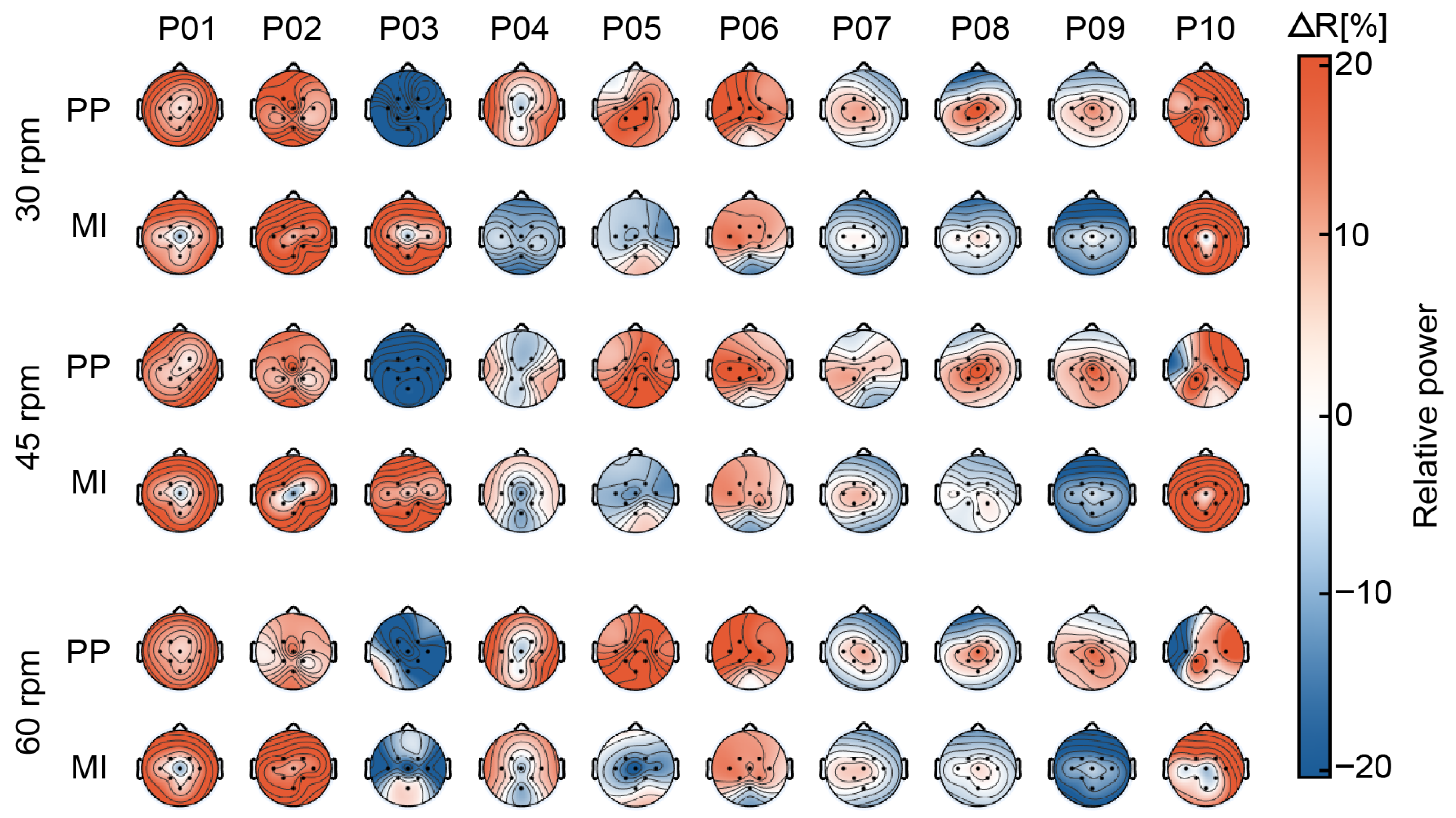
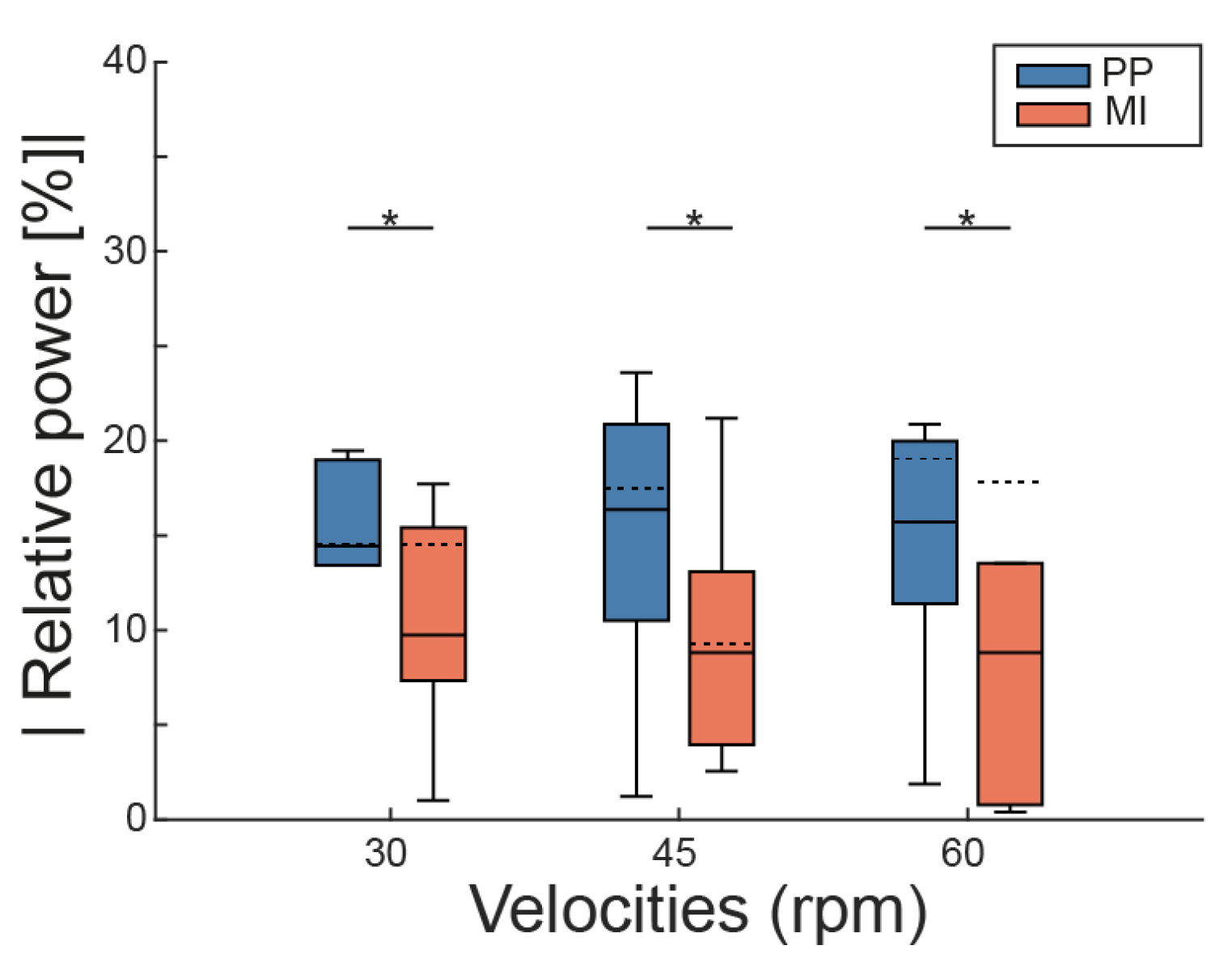
3.1. Spatial Analysis of Relative Power
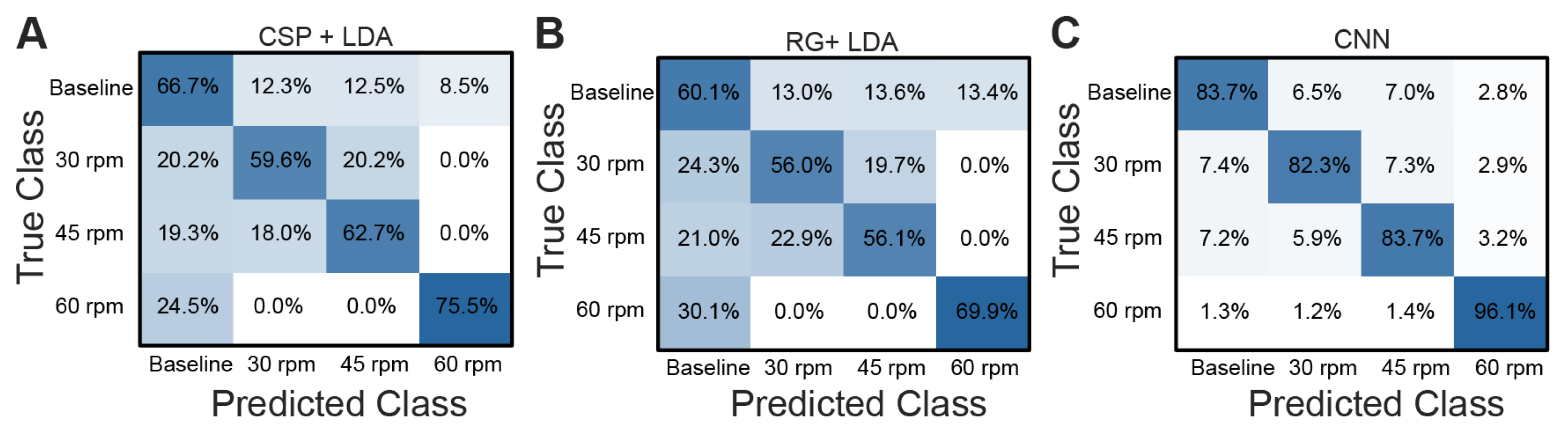
3.2. Classification Performance
3.3. Subjective Responses
4. Discussion
4.1. Strong Cortical Activation Around Cz
4.2. Topographic Patterns May Not Align with Cz
4.3. Effectiveness of CNN Multiclass Classification of Pedaling Tasks at Varying Velocities
4.4. Clinical Implications for Pedal-Based Rehabilitation BCIs
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendelow, A.D. Stroke: Pathophysiology, Diagnosis, and Management; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Romero-Laiseca, M.A.; Delisle-Rodriguez, D.; Cardoso, V.; Gurve, D.; Loterio, F.; Nascimento, J.H.P.; Krishnan, S.; Frizera-Neto, A.; Bastos-Filho, T. A low-cost lower-limb brain-machine interface triggered by pedaling motor imagery for post-stroke patients rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 988–996. [Google Scholar] [CrossRef]
- Fujita, K.; Kobayashi, Y.; Miaki, H.; Hori, H.; Tsushima, Y.; Sakai, R.; Nomura, T.; Ogawa, T.; Kinoshita, H.; Nishida, T.; et al. Pedaling improves gait ability of hemiparetic patients with stiff-knee gait: Fall prevention during gait. J. Stroke Cerebrovasc. Dis. 2020, 29, 105035. [Google Scholar] [CrossRef]
- Lima, J.P.; Silva, L.A.; Delisle-Rodriguez, D.; Cardoso, V.F.; Nakamura-Palacios, E.M.; Bastos-Filho, T.F. Unraveling Transformative Effects after tDCS and BCI Intervention in Chronic Post-Stroke Patient Rehabilitation—An Alternative Treatment Design Study. Sensors 2023, 23, 9302. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, Y.; Wang, L.; Song, S.; Chen, S.; Yang, L.; Liu, H.; Wang, H.; Shi, G.; Han, C.; et al. Effect of BCI-Controlled Pedaling Training System With Multiple Modalities of Feedback on Motor and Cognitive Function Rehabilitation of Early Subacute Stroke Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2569–2577. [Google Scholar] [CrossRef]
- Dawson-Elli, A.R.; Adamczyk, P.G. Design and Validation of a Lower-Limb Haptic Rehabilitation Robot. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1584–1594. [Google Scholar] [CrossRef]
- Guggenberger, R.; Heringhaus, M.; Gharabaghi, A. Brain-machine neurofeedback: Robotics or electrical stimulation? Front. Bioeng. Biotechnol. 2020, 8, 639. [Google Scholar] [CrossRef]
- Lebedev, M.A.; Nicolelis, M.A. Brain-machine interfaces: From basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef]
- Blanco-Diaz, C.F.; Serafini, E.R.d.S.; Bastos-Filho, T.; Dantas, A.F.O.d.A.; Santo, C.C.d.E.; Delisle-Rodriguez, D. A Gait Imagery-Based Brain–Computer Interface with Visual Feedback for Spinal Cord Injury Rehabilitation on Lokomat. IEEE Trans. Biomed. Eng. 2025, 72, 102–111. [Google Scholar] [CrossRef]
- Juan, J.V.; Martínez, R.; Iáñez, E.; Ortiz, M.; Tornero, J.; Azorín, J.M. Exploring EEG-based motor imagery decoding: A dual approach using spatial features and spectro-spatial Deep Learning model IFNet. Front. Neuroinformatics 2024, 18, 1345425. [Google Scholar] [CrossRef]
- Tanuma, A.; Fujiwara, T.; Yamaguchi, T.; Ro, T.; Arano, H.; Uehara, S.; Honaga, K.; Mukaino, M.; Kimura, A.; Liu, M. After-effects of pedaling exercise on spinal excitability and spinal reciprocal inhibition in patients with chronic stroke. Int. J. Neurosci. 2017, 127, 73–79. [Google Scholar] [CrossRef]
- Rodríguez-Ugarte, M.; Iáñez, E.; Ortíz, M.; Azorín, J.M. Personalized Offline and Pseudo-Online BCI Models to Detect Pedaling Intent. Front. Neuroinformatics 2017, 11, 45. [Google Scholar] [CrossRef]
- Delisle-Rodriguez, D.; Silva, L.; Bastos-Filho, T. EEG changes during passive movements improve the motor imagery feature extraction in BCIs-based sensory feedback calibration. J. Neural Eng. 2023, 20, 016047. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Hillis, A.E. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010, 9, 895–905. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.T.; Jeong, J.H.; Kim, L.; Lee, S.J.; Kim, H. Developing a motor imagery-based real-time asynchronous hybrid BCI controller for a lower-limb exoskeleton. Sensors 2020, 20, 7309. [Google Scholar] [CrossRef]
- Donati, A.R.; Shokur, S.; Morya, E.; Campos, D.S.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.; Xu, Z.; Wang, P.; Wu, X.; Zhang, D. Cognitive Workload Recognition Using EEG Signals and Machine Learning: A Review. IEEE Trans. Cogn. Dev. Syst. 2022, 14, 799–818. [Google Scholar] [CrossRef]
- Ferrero, L.; Quiles, V.; Ortiz, M.; Iáñez, E.; Gil-Agudo, Á.; Azorín, J.M. Brain-computer interface enhanced by virtual reality training for controlling a lower limb exoskeleton. Iscience 2023, 26, 106675. [Google Scholar] [CrossRef]
- Klepl, D.; Wu, M.; He, F. Graph neural network-based eeg classification: A survey. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 493–503. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Quiles, V.; Ferrero, L.; Iáñez, E.; Ortiz, M.; Gil-Agudo, Á.; Azorín, J.M. Brain-machine interface based on transfer-learning for detecting the appearance of obstacles during exoskeleton-assisted walking. Front. Neurosci. 2023, 17, 1154480. [Google Scholar] [CrossRef]
- Soriano-Segura, P.; Ortiz, M.; Iáñez, E.; Azorín, J.M. Design of a brain-machine interface for reducing false activations of a lower-limb exoskeleton based on error related potential. Comput. Methods Programs Biomed. 2024, 255, 108332. [Google Scholar] [CrossRef]
- Storzer, L.; Butz, M.; Hirschmann, J.; Abbasi, O.; Gratkowski, M.; Saupe, D.; Schnitzler, A.; Dalal, S.S. Bicycling and walking are associated with different cortical oscillatory dynamics. Front. Hum. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Arvaneh, M.; Guan, C.; Ang, K.K.; Quek, C. Optimizing the channel selection and classification accuracy in EEG-based BCI. IEEE Trans. Biomed. Eng. 2011, 58, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Tortora, S.; Ghidoni, S.; Chisari, C.; Micera, S.; Artoni, F. Deep learning-based BCI for gait decoding from EEG with LSTM recurrent neural network. J. Neural Eng. 2020, 17, 046011. [Google Scholar] [CrossRef] [PubMed]
- Cleland, B.T.; Schindler-Ivens, S. Brain activation during passive and volitional pedaling after stroke. Mot. Control 2019, 23, 52–80. [Google Scholar] [CrossRef] [PubMed]
- Mullens, C.H.; Brown, D.A. Visual feedback during pedaling allows individuals poststroke to alter inappropriately prolonged paretic vastus medialis activity. J. Neurophysiol. 2018, 119, 2334–2346. [Google Scholar] [CrossRef]
- Ang, K.K.; Chin, Z.Y.; Wang, C.; Guan, C.; Zhang, H. Filter bank common spatial pattern algorithm on BCI competition IV datasets 2a and 2b. Front. Neurosci. 2012, 6, 39. [Google Scholar] [CrossRef]
- Lee, M.H.; Kwon, O.Y.; Kim, Y.J.; Kim, H.K.; Lee, Y.E.; Williamson, J.; Fazli, S.; Lee, S.W. EEG dataset and OpenBMI toolbox for three BCI paradigms: An investigation into BCI illiteracy. GigaScience 2019, 8, giz002. [Google Scholar] [CrossRef]
- Kusano, K.; Hayashi, M.; Iwama, S.; Ushiba, J. Improved motor imagery skills after repetitive passive somatosensory stimulation: A parallel-group, pre-registered study. Front. Neural Circuits 2025, 18, 1510324. [Google Scholar] [CrossRef]
- Nakashima, A.; Moriuchi, T.; Matsuda, D.; Nakamura, J.; Fujiwara, K.; Ikio, Y.; Hasegawa, T.; Mitunaga, W.; Higashi, T. Continuous repetition motor imagery training and physical practice training exert the growth of fatigue and its effect on performance. Brain Sci. 2022, 12, 1087. [Google Scholar] [CrossRef]
- Zenia, N.Z.; Tarng, S.; Dizaji, L.G.; Hu, Y. EEG Features to Quantify the NASA-TLX Factors of Cognitive Workload. IEEE Trans. Hum.-Mach. Syst. 2025, 55, 372–382. [Google Scholar] [CrossRef]
- Dong, E.; Li, C.; Li, L.; Du, S.; Belkacem, A.N.; Chen, C. Classification of multi-class motor imagery with a novel hierarchical SVM algorithm for brain–computer interfaces. Med. Biol. Eng. Comput. 2017, 55, 1809–1818. [Google Scholar] [CrossRef]
- Barachant, A.; Bonnet, S.; Congedo, M.; Jutten, C. Multiclass brain–computer interface classification by Riemannian geometry. IEEE Trans. Biomed. Eng. 2011, 59, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Yger, F.; Berar, M.; Lotte, F. Riemannian approaches in brain-computer interfaces: A review. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kumar, L. Subject-independent trajectory prediction using pre-movement EEG during grasp and lift task. Biomed. Signal Process. Control 2023, 86, 105160. [Google Scholar] [CrossRef]
- Blanco-Diaz, C.F.; Guerrero-Mendez, C.D.; de Andrade, R.M.; Badue, C.; De Souza, A.F.; Delisle-Rodriguez, D.; Bastos-Filho, T. Decoding lower-limb kinematic parameters during pedaling tasks using deep learning approaches and EEG. Med. Biol. Eng. Comput. 2024, 62, 3763–3779. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Delisle-Rodriguez, D.; Romero-Laiseca, M.A.; Loterio, F.A.; Gurve, D.; Floriano, A.; Valadão, C.; Silva, L.; Krishnan, S.; Frizera-Neto, A.; et al. Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity. Sensors 2021, 21, 2020. [Google Scholar] [CrossRef]
- Höller, Y.; Bergmann, J.; Kronbichler, M.; Crone, J.S.; Schmid, E.V.; Thomschewski, A.; Butz, K.; Schütze, V.; Höller, P.; Trinka, E. Real movement vs. motor imagery in healthy subjects. Int. J. Psychophysiol. 2013, 87, 35–41. [Google Scholar] [CrossRef]
- Gu, L.; Yu, Z.; Ma, T.; Wang, H.; Li, Z.; Fan, H. EEG-based classification of lower limb motor imagery with brain network analysis. Neuroscience 2020, 436, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luo, L.; Pi, M.; Li, Z.; Li, Q.; Zhao, K.; Huang, J. EEG-Based Volitional Control of Prosthetic Legs for Walking in Different Terrains. IEEE Trans. Autom. Sci. Eng. 2021, 18, 530–540. [Google Scholar] [CrossRef]
- Tariq, M.; Trivailo, P.M.; Simic, M. Mu-Beta event-related (de) synchronization and EEG classification of left-right foot dorsiflexion kinaesthetic motor imagery for BCI. PLoS ONE 2020, 15, e0230184. [Google Scholar] [CrossRef]
- Severens, M.; Perusquia-Hernandez, M.; Nienhuis, B.; Farquhar, J.; Duysens, J. Using Actual and Imagined Walking Related Desynchronization Features in a BCI. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 877–886. [Google Scholar] [CrossRef]
- Luu, T.P.; Nakagome, S.; He, Y.; Contreras-Vidal, J.L. Real-time EEG-based brain-computer interface to a virtual avatar enhances cortical involvement in human treadmill walking. Sci. Rep. 2017, 7, 8895. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, S.; Luu, T.P.; He, Y.; Ravindran, A.S.; Contreras-Vidal, J.L. An empirical comparison of neural networks and machine learning algorithms for EEG gait decoding. Sci. Rep. 2020, 10, 4372. [Google Scholar] [CrossRef] [PubMed]
- Quiles, V.; Ferrero, L.; Iáñez, E.; Ortiz, M.; Cano, J.M.; Azorín, J.M. Detecting the speed change intention from EEG signals: From the offline and pseudo-online analysis to an online closed-loop validation. Appl. Sci. 2022, 12, 415. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, S.; Xing, J.; He, H. A CNN-based compare network for classification of SSVEPs in human walking. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2986–2990. [Google Scholar] [CrossRef]
- Sauvage, C.; Jissendi Tchofo, P.; Manto, M.; Habas, C. Brain areas involved in the control of speed during amotor sequence of the foot: Real movement versus mental imagery. J. Neuroradiol. 2013, 42, 115–125. [Google Scholar] [CrossRef]
- Sebastián-Romagosa, M.; Cho, W.; Ortner, R.; Sieghartsleitner, S.; Von Oertzen, T.J.; Kamada, K.; Laureys, S.; Allison, B.Z.; Guger, C. Brain–computer interface treatment for gait rehabilitation in stroke patients. Front. Neurosci. 2023, 17, 1256077. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Lafleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-Based Brain-Computer Interfaces Using Motor-Imagery: Techniques and Challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef]
- Gonzalez-Cely, A.; Blanco-Diaz, C.; Delisle-Rodriguez, D.; Bastos-Filho, T. EEG-Based Multi-Class Classification for Recognizing Pedaling Velocities: A Promising Approach for Brain-Computer Interface-Enhanced Lower-Limb Robotic Rehabilitation. In Proceedings of the 2024 10th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Heidelberg, Germany, 1–4 September 2024; pp. 223–228. [Google Scholar] [CrossRef]


| Approach | Features | Classifier | ACC | Ref. |
|---|---|---|---|---|
| Rest and pedaling MI | RG, CSP | LDA, ANN | 0.69, 0.80 | [2,10] |
| Attention level on virtual pedaling | Frequency | - | - | [5] |
| Rest and pedaling intention | Frequency | SVM | 0.77 | [12] |
| Rest and sitting and walking MI | Filter bank CSP | SVM | 0.80 | [15] |
| Left- and right-foot MI | Time–frequency | SVM | 0.75 | [40] |
| Leg MI during ascending stairs, descending stairs, and floor walking | CSP | SVM | 0.81 | [41] |
| Right and left dorsiflexion | Time–frequency | KNN | 0.81 | [42] |
| Walking and non-walking, both executed and imagined | ERD | - | 0.80 | [43] |
| Decoding continuous lower-limb | ANN and KF | - | - | [44,45] |
| Rest and pedaling MI at 30 rpm, 45 rpm and 60 rpm | CSP,- | LDA, CNN | 0.76, 0.87 | Ours |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Diaz, C.F.; Gonzalez-Cely, A.X.; Delisle-Rodriguez, D.; Bastos-Filho, T.F. EEG-Based Analysis of Motor Imagery and Multi-Speed Passive Pedaling: Implications for Brain–Computer Interfaces. Signals 2025, 6, 52. https://doi.org/10.3390/signals6040052
Blanco-Diaz CF, Gonzalez-Cely AX, Delisle-Rodriguez D, Bastos-Filho TF. EEG-Based Analysis of Motor Imagery and Multi-Speed Passive Pedaling: Implications for Brain–Computer Interfaces. Signals. 2025; 6(4):52. https://doi.org/10.3390/signals6040052
Chicago/Turabian StyleBlanco-Diaz, Cristian Felipe, Aura Ximena Gonzalez-Cely, Denis Delisle-Rodriguez, and Teodiano Freire Bastos-Filho. 2025. "EEG-Based Analysis of Motor Imagery and Multi-Speed Passive Pedaling: Implications for Brain–Computer Interfaces" Signals 6, no. 4: 52. https://doi.org/10.3390/signals6040052
APA StyleBlanco-Diaz, C. F., Gonzalez-Cely, A. X., Delisle-Rodriguez, D., & Bastos-Filho, T. F. (2025). EEG-Based Analysis of Motor Imagery and Multi-Speed Passive Pedaling: Implications for Brain–Computer Interfaces. Signals, 6(4), 52. https://doi.org/10.3390/signals6040052







