Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review
Abstract
1. Introduction
2. Review Methods: Identification, Selection, and Quantitative Synthesis
2.1. Search Strategy and Selection Criteria
- ("integrated sensing and communication"OR ISAC OR "joint sensing and communication")AND (health* OR clinic* OR hospital)
- ("digital twin" OR "physiological twin")AND (patient OR device OR ward OR hospital)
- (mmWave OR "millimeter wave" OR THz OR "sub-6")AND (vital OR respiration OR localization OR radar)AND (health* OR clinic*)
- FHIR OR "HL7 FHIR" AND (interoperability OR EHR)
- (security OR safety OR regulatory OR HIPAA OR FDA)AND (ISAC OR "digital twin") AND health*
2.2. Quantitative Synthesis
- ISAC only (66) is broken down into healthcare (9) vs. non-healthcare (57); DT-only (45) breaks down as healthcare (24) vs. non-healthcare (21).
- The scarcity of healthcare ISAC–DT work confirms the early stage of convergence and motivates and guides evaluation elsewhere in this paper.
3. Background and Literature Review
3.1. ISAC Technology Overview
3.1.1. Definition and Evolution of ISAC
- 1.
- 2.
- Cognitive radio and spectrum sharing (2000s): The development of cognitive radio technologies laid the groundwork for dynamic spectrum access and sharing between sensing and communication functions [11].
- 3.
- Joint radar–communication systems (2010s): Research began exploring truly integrated approaches where waveform design, signal processing, and resource allocation were optimized for both functions simultaneously [7].
- 4.
- Modern ISAC systems (2020s): Current ISAC technologies leverage advanced signal processing; multiple-input, multiple-output (MIMO) techniques; and artificial intelligence to achieve unprecedented levels of integration and performance [7].
3.1.2. Integration Principles of Sensing and Communication
3.1.3. Role of ISAC in 6G Networks
- (i)
- Ubiquitous sensing, whereby dense, distributed transceivers perceive the radio environment and provide rich context awareness [15];
- (ii)
- Centimeter-level localization for users and smart objects by exploiting wide-bandwidth and joint radar–communication signal processing [16];
- (iii)
- Gesture and activity recognition through contact-free Wi-Fi or mmWave sensing that classifies human motion patterns in real time [17];
- (iv)
- High-resolution imaging and mapping that turns the network into a distributed radar/camera array [7];
- (v)
- Non-invasive health monitoring via metrics such as heart beat, respiration, or blood-pressure tracking via RF reflections [18].
3.2. Digital-Twin Technology Overview
3.2.1. Definition and Concept
3.2.2. Current Applications in Healthcare
3.2.3. Key Enabling Technologies
- (i)
- Sensors/IoMT (ECG, radar, UWB tags, wearables, and infusion pumps) that feed raw and derived observations;
- (ii)
- The ISAC link (PHY/MAC) spanning sub-6 GHz, mmWave, UWB, and THz bands with URLLC scheduling;
- (iii)
- A data pipeline for ingestion, QA/QC, logging, and semantic mapping to FHIR, HL7, and IEEE 11073;
- (iv)
- Twin engines providing physiology-based modeling, inference/analytics, and simulation;
- (v)
- Clinical applications that realize monitoring, CDS, and personalization.
3.3. Healthcare System Challenges
3.3.1. Aging Population and Chronic Disease Management
3.3.2. Data Integration and Analysis Difficulties
3.3.3. Remote Monitoring and Telehealth Limitations
3.4. Convergence Opportunities
3.4.1. Enhanced Data Collection and Integration
3.4.2. Real-Time Feedback and Intervention
3.4.3. System-Level Optimization
4. Technical Analysis of ISAC Technology and Frequency Bands
4.1. Millimeter-Wave (mmWave) Band
4.1.1. Characteristics and Properties
4.1.2. Applications in Healthcare ISAC (mmWave)
4.1.3. Limitations and Challenges
4.2. Terahertz (THz) Band
4.2.1. Characteristics and Properties
4.2.2. Applications in Healthcare ISAC (THz)
4.2.3. Limitations and Challenges
4.3. Sub-6 GHz Band
4.3.1. Characteristics and Properties
4.3.2. Applications in Healthcare ISAC (Sub-6 GHz)
4.3.3. Limitations and Challenges
4.4. Multi-Band Integration
4.4.1. Complementary Characteristics
4.4.2. Implementation Approaches
- In-hospital hotspot sensing/telepresence: A mixed-reality telesupervised ultrasound platform on a private 5G network installed with 4.7 GHz (coverage) and 28 GHz (hotspots) base stations with a local 5G core; measured throughput reached ≈ 1.45 Gbps down/147 Mbps up, and end-to-end latencies for concurrent streams were ∼49 ms (ultrasound image), ∼196 ms (HMD view), and ∼797 ms (360° camera), enabling real-time guidance [131].
- Air–ground continuity in emergency care: A helicopter EMS program used 2.6 + 4.9 GHz dual-frequency collaborative networking to maintain access from vertical take-off/landing to cruise, with network switching delays ≤ 30 ms, supporting continuous telemetry and consultation during flight [132].
- Regional emergency response: A mixed-frequency private 5G emergency system expanded the rescue radius from ∼5 km to ∼60 km and reduced cross-district response time from ∼60 min to min, illustrating multi-band orchestration translating into operational KPIs [133].
- Private 5G SA in hospitals: A standalone private 5G build supported mobile ward rounds, remote ultrasound, and inter-hospital links with measured inter-site latency ∼14 ms and downlink/uplink rates of ∼790/91 Mbps, providing the substrate on which sub-6 coverage and high-band bursts can be scheduled per application phase [134].
4.4.3. Challenges in Multi-Band Integration
4.5. Healthcare-Specific Considerations
4.5.1. Safety and Regulatory Aspects
4.5.2. Clinical Validation Requirements
4.5.3. Integration with Healthcare Information Systems
4.5.4. Equity Perspective
4.6. Future Trends and Research Directions
4.6.1. Technological Advancements
4.6.2. Emerging Applications
4.6.3. Standardization Efforts
4.6.4. Standardization Hurdles for ISAC-Enabled Healthcare Devices
4.6.5. Security Issues and Mitigation Strategies in 6G ISAC Systems
5. Digital-Twin Technology in Healthcare
5.1. Patient-Centric Digital Twins
5.1.1. Conceptual Framework
5.1.2. Implementation Approaches
5.1.3. Current Applications and Case Studies
5.2. Healthcare Facility and System Digital Twins
5.2.1. Conceptual Framework
5.2.2. Implementation Approaches
5.2.3. Current Applications and Case Studies
5.3. Medical Device and Equipment Digital Twins
5.3.1. Conceptual Framework
5.3.2. Implementation Approaches
5.3.3. Current Applications and Case Studies
6. ISAC–DT Integration Challenges and Opportunities
6.1. Clinical Validation Status of DT–ISAC
6.2. Recommended Evaluation Protocol for DT–ISAC in Hospitals
- ISAC fidelity: MAE/LoA vs. reference; valid coverage (% time usable); alert yield (PPV, alarms/bed-day).
- Closed loop: time to detection/decision/action; execution %; override rates.
- KPIs: ED/ward LOS (median/IQR and upper tails), boarding hours, rescue/ICU transfer per 1000 patient-days, code events, staff walking distance/time share.
6.3. Technical Integration Challenges
- Data integration and freshness (ISAC → DT ingestion).
- Healthcare digital twins must harmonize heterogeneous sources—electronic health records (EHRs), medical devices, imaging, and wearables—and contact-free ISAC streams (e.g., respiration/motion and indoor localization). Variations in formats, sampling cadences, semantics, and quality require ingestion, semantic mapping, and QA pipelines that preserve data freshness for deterioration forecasting [25,26,184,185,234,235]. In practice, this means aligning ISAC sensing/link cadences with DT update policies and recording provenance so that twin states reflect current bedside context (see interoperability mechanisms in Section 4.5.3 and band-driven sensing constraints in Section 4.1.3, Section 4.2.3 and Section 4.3.3).
- Compute and placement under end-to-end latency budgets.
- Detailed physiological modeling (multi-scale, fluid-dynamics-based, or neuro-cardiac) is computationally intensive. When the twin closes the loop on ISAC (e.g., adapting beam direction, bandwidth, or sampling), end-to-end delay spans sensing, the wireless link, edge/cloud compute, and actuation. Balancing model fidelity with latency/throughput is therefore an integration constraint, not only a modeling choice [208,210,214,236]. Section 4.1.3, Section 4.2.3 and Section 4.3.3 detail band-level trade-offs that drive feasible sampling/update rates, while this section specifies how placement across edge–cloud systems affects closed-loop timeliness.
- Uncertainty and credibility with link-/sensor-induced variability.
- Twin predictions inherit uncertainty from physiologic variability, measurement noise, packet loss/reordering, and model approximations [187,237]. With ISAC in the loop, changes in link quality or sensing geometry can shift observation models and calibration, so uncertainty must be quantified and propagated with awareness of sensing/link conditions and communicated to clinicians to avoid overconfidence. This motivates VVUQ routines that condition on ISAC telemetry quality and cadence (see calibration and validation considerations in Section 6.6 and Section 4.5.2).
- Interoperability and provenance for synchronized updates.
- Despite the adoption of Fast Healthcare Interoperability Resources (FHIR), achieving consistent semantics and lineage across EHR/device data and ISAC streams remains difficult [184,235]. ISAC features (e.g., contact-free vital signs and localization) must be mapped to FHIR-compatible resources with timestamps/identifiers that the twin can trust for synchronization and replay. Practical pathways and QA tool chains are discussed in Section 4.5.3.
- Privacy and security with sensing–communication co-design.
- Digital twins carry sensitive patient data and, thus, require cryptographic protections, fine-grained access control, and secure architectures compliant with HIPAA/GDPR [26,188]. ISAC-specific considerations (e.g., exposure/power limits, side-channel/leakage surfaces, and on-link protections) must be addressed alongside application-layer measure (see safety/regulatory and security discussions in Section 4.5.1 and Section 4.6.5). Integrating these constraints with the twin’s update cadence helps ensure that privacy and safety are maintained without sacrificing timeliness.
6.4. Clinical Integration Opportunities
- Clinical decision support with ISAC-informed context.
- Patient-specific twins that simulate interventions and predict outcomes become more actionable when continuously fed by ISAC telemetry (e.g., contact-free respiration/motion and indoor localization), which keeps model states current; recommended actions can, in turn, adapt beam direction, bandwidth, or sampling in a closed loop [214,239]. Latency/throughput considerations for such loops are discussed in Section 6.3, with band-driven sensing constraints presented in Section 4.1.3, Section 4.2.3 and Section 4.3.3.
- Continuous monitoring and early warning.
- ISAC-enabled contact-free streams can reveal subtle physiologic changes before overt signs, enabling proactive interventions [25,187]. Operating points depend on sensing band and link budget (mmWave/THz for resolution; sub-6 GHz for coverage/penetration) and on update policies that preserve data freshness for twin ingestion (see Section 4.1.3, Section 4.2.3 and Section 4.3.3 and Section 6.3).
- Personalized treatment planning with richer observability.
- ISAC-derived context (mobility, respiration patterns, and environmental dynamics) improves parameterization and boundary conditions for individualized models, supporting tailored therapy while controlling risk [185,208]. Credibility hinges on calibration and validation procedures that account for sensing/link variability (see Section 4.5.2 and Section 6.6).
- In silico testing and closed-loop execution.
- Virtual testing of interventions prior to clinical execution enhances safety and optimizes strategies, particularly in high-risk settings [210,240]. When deployed with ISAC, recommended actions can be executed with closed-loop adaptations of the sensing/communication stack, subject to safety/regulatory and security constraints (see Section 4.5.1 and Section 4.6.5).
- Remote and distributed care.
- For home and rural scenarios, sub-6 GHz links improve range and reliability so that twins remain synchronized under patchy connectivity, while secure pipelines protect patient data [26,188]. Interoperability and provenance for mixed EHR/device/ISAC streams are covered in Section 4.5.3, and end-to-end timeliness considerations are discussed in Section 6.3.
6.5. Organizational and Workflow Integration
- Workflow integration and alert routing.
- Twin outputs must be embedded into existing pathways (rounds, escalation, and discharge) without undue burden and with clear ownership for closed-loop actions that may reconfigure ISAC sensing/communication (e.g., beam direction, bandwidth, and sampling) [187,242]. Practical workflow fit depends on sensing-band and placement choices for contact-free monitoring and localization (see band trade-offs in Section 4.1.3, Section 4.2.3 and Section 4.3.3 and latency- and throughput-aware pipelines in Section 6.3).
- Training and competency.
- Clinicians and biomedical engineers require training to interpret ISAC-derived telemetry (contact-free respiration/motion and indoor localization), understand twin assumptions and uncertainty, and incorporate outputs into decision-making [25,26]. Competency also includes provenance-aware data handling and interoperability (FHIR/EHR) so that updates ingested by the twin are trusted and timely (see Section 4.5.2, Section 4.5.3 and Section 6.6).
- Governance, safety, and security.
- Effective governance balances clinical leadership and technical expertise to manage data use, system integrity, and ethics [208,243,244]. For ISAC–DT, this explicitly includes RF exposure/power limits and spectrum coexistence in clinical areas, along with privacy and security controls aligned to HIPAA/GDPR. Related considerations are detailed in Section 4.5.1 and Section 4.6.5.
- Change management and human in the loop.
- Introducing ISAC–DT loops requires strategies that address resistance, build trust, and clarify the boundary between automation and clinician oversight [185,210]. Alarm policies, escalation paths, and fallback behaviors should be co-designed so that latency budgets and alert routing remain compatible with staffing patterns (see integration aspects in Section 6.3).
- Outcome measurement and continuous improvement.
- Organizations should track clinical, operational, and economic endpoints to assess the impact of ISAC–DT deployments, including patient outcomes (length of stay, complications, and satisfaction), operational flow (throughput, and time to intervention), and technical indicators that reflect ISAC coupling (update freshness, end-to-end latency, and alert precision/recall) [26,63]. Evaluation frameworks and validation considerations are discussed in Section 4.5.2.
6.6. Model Calibration Challenges for Physiological Digital Twins
- Credibility and VVUQ anchored in clinical use.
- Calibration must produce models whose predictive claims align with clinical endpoints and uncertainty bounds that clinicians can act upon. This requires VVUQ routines tailored to the target pathway (e.g., early warning and therapy planning) and reported in a reproducible manner [245,246]. Guidance on validation endpoints appears in Section 4.5.2.
- Online calibration under ISAC timing and freshness.
- With ISAC-derived telemetry (e.g., radar-based respiration/motion and indoor localization) feeding the twin, calibration and re-calibration must respect end-to-end latency budgets and update policies so that twin states remain current for decision support. The sensing band and link budget govern feasible sampling and assimilation rates (Section 4.1.3, Section 4.2.3 and Section 4.3.3); integration pipelines and placement choices for timely updates are discussed in Section 6.3.
- Data assimilation and synchronization across scales.
- Continuous multimodal streams—radar-derived vital signs, electrocardiography, and imaging—must be assimilated with consistent timestamps and provenance. Temporal misalignment, jitter, and dropouts can destabilize filters used in calibration and degrade predictive accuracy [7,23,136,190,248]. Provenance capture and semantic mapping for synchronized updates are covered in Section 4.5.3.
- Personalization under uncertainty.
- Patient-specific parameters should reflect genetic variation, lifestyle factors, and heterogeneous disease trajectories, yet limited longitudinal data increase estimation uncertainty. Methods that propagate uncertainty from sensors and links through calibrated parameters to outputs help avoid overconfidence [23,245,247,249]. Reporting practices for uncertainty and calibration quality are summarized in Section 4.5.2.
- Resource-aware model fidelity and placement.
- Physiological models (multi-scale or fluid dynamics-based) are computationally demanding; fidelity must be balanced against latency/throughput constraints of the ISAC link and the edge–cloud compute path [208,210,214,236]. Practical operating points that keep closed-loop operation feasible are linked to band-dependent constraints in Section 4.1.3, Section 4.2.3 and Section 4.3.3 and integration considerations in Section 6.3.
- Privacy, safety, and governance implications for calibration data.
- Calibration often requires access to sensitive raw signals and intermediate states. Pipelines must comply with HIPAA/GDPR while respecting RF exposure/power limits and spectrum coexistence in clinical areas [26,188]. Related safety/regulatory and security considerations appear in Section 4.5.1 and Section 4.6.5.
- From prototypes to reproducible evidence.
- Collectively, these hurdles motivate physics-informed machine learning, Bayesian data-assimilation pipelines, open benchmark datasets, and harmonized VVUQ protocols tailored to physiological twins so that prototypes can mature into reliable clinical decision-support systems [245,248]. Alignment with the ISAC sensing/communication cadence and provenance standards (Section 4.5.3) helps ensure that calibration results are both credible and operationally timely.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3GPP | 3rd Generation Partnership Project (mobile standards body) |
| 6G | Sixth-generation mobile-network paradigm |
| ACK | Acknowledgment latency (“ack”) |
| AUROC | Area under the receiver operating characteristic curve |
| AUPRC | Area under the precision–recall curve |
| CI | Confidence interval |
| DICOM | Digital Imaging and Communications in Medicine (medical image format) |
| DT | Digital Twin—continuously updated virtual replica |
| ED | Emergency Department |
| EHR | Electronic Health Record |
| EMA | European Medicines Agency (EU regulator) |
| ETSI GR ISC 001 | ETSI Report “Integrated Sensing and Communication—Use Cases for 6G” |
| FHIR | Fast Healthcare Interoperability Resources (HL7 standard) |
| GDPR | General Data Protection Regulation (EU data-privacy law) |
| HbA1c | Glycated hemoglobin; 3-month average of blood-glucose control |
| HIPAA | Health Insurance Portability and Accountability Act (US data-privacy law) |
| HL7 | Health Level Seven International (health-data standards organization) |
| ICNIRP | International Commission on Non-Ionizing Radiation Protection |
| ICU | Intensive Care Unit |
| IoMT | Internet of Medical Things |
| IoT | Internet of Things |
| ISAC | Integrated Sensing and Communication |
| ITS | Interrupted time-series |
| KPI | Key performance indicator |
| LoA | Limits of agreement |
| LOS | Length of stay |
| MAE | Mean absolute error |
| MAPE | Mean absolute percentage error |
| MIMO | Multiple-input multiple-output |
| mmWave | Millimeter-wave band (30–300 GHz) |
| PD | Power density |
| PPV | Positive predictive value |
| PPV@alert | Positive predictive value at alert threshold |
| RF | Radio frequency |
| RFIC | Radio-frequency integrated circuit |
| ROI | Return on investment |
| SAR | Specific absorption rate (RF-exposure metric) |
| THz | Terahertz band (0.1–10 THz) |
| TIPPSS | Trust, Identity, Privacy, Protection, Safety, and Security |
| TRL | Technology readiness level |
| VVUQ | Verification, Validation, and Uncertainty Quantification |
References
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241565042 (accessed on 9 September 2025).
- Assistant Secretary for Planning and Evaluation. Health Care Workforce: Key Issues, Challenges, and the Path Forward. 2024. Available online: https://aspe.hhs.gov/sites/default/files/documents/82c3ee75ef9c2a49fa6304b3812a4855/aspe-workforce.pdf (accessed on 9 September 2025).
- World Health Organization. Pulse Survey on Continuity of Essential Health Services During the COVID-19 Pandemic; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1 (accessed on 9 September 2025).
- HIMSS. Interoperability in Healthcare. 2019. Available online: https://legacy.himss.org/resources/interoperability-healthcare (accessed on 9 September 2025).
- Gazzarata, R.; Almeida, J.; Lindsköld, L.; Cangioli, G.; Gaeta, E.; Fico, G.; Chronaki, C.E. HL7 Fast Healthcare Interoperability Resources (HL7 FHIR) in Digital Healthcare Ecosystems for Chronic Disease Management: A Scoping Review. Int. J. Med. Inform. 2024, 189, 105507. [Google Scholar] [CrossRef] [PubMed]
- Tabari, P.; Costagliola, G.; De Rosa, M.; Boeker, M. State-of-the-art fast healthcare interoperability resources (FHIR)–based data model and structure implementations: Systematic scoping review. JMIR Med. Inform. 2024, 12, e58445. [Google Scholar] [CrossRef]
- Liu, F.; Cui, Y.; Masouros, C.; Grant, P.M.; Petropulu, A.P.; Hanzo, L. Integrated Sensing and Communications: Toward Dual-Functional Wireless Networks for 6G and Beyond. IEEE J. Sel. Areas Commun. 2022, 40, 1728–1767. [Google Scholar] [CrossRef]
- Elayan, H.; Aloqaily, M.; Guizani, M. Digital twin for intelligent context-aware IoT healthcare systems. IEEE Internet Things J. 2021, 8, 16749–16757. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, Z.; Hawbani, A.; Yu, K.; Zhang, Y.; Guizani, M. ELITE: An Intelligent Digital Twin-Based Hierarchical Routing Scheme for Softwarized Vehicular Networks. IEEE Trans. Mob. Comput. 2023, 22, 5231–5247. [Google Scholar] [CrossRef]
- Liu, F.; Masouros, C.; Petropulu, A.P.; Griffiths, H.; Hanzo, L. Joint Radar and Communication Design: Applications, State-of-the-Art, and the Road Ahead. IEEE Trans. Commun. 2020, 68, 3834–3862. [Google Scholar] [CrossRef]
- Akyildiz, I.F.; Lee, W.; Vuran, M.C.; Mohanty, S. NeXt Generation/Dynamic Spectrum Access/Cognitive Radio Wireless Networks: A Survey. Comput. Netw. 2006, 50, 2127–2159. [Google Scholar] [CrossRef]
- Wang, S.; Dai, W.; Wang, H.; Li, G.Y. Robust Waveform Design for Integrated Sensing and Communication. IEEE Trans. Signal Process. 2024, 72, 3122–3138. [Google Scholar] [CrossRef]
- Subramaniyan, M.; Venkatasamy, T.K.; Hossen, M.J. Adaptive Resource Allocation and Routing for Integrated Sensing and Communications for Wireless Technologies. EURASIP J. Wirel. Commun. Netw. 2025, 2025, 33. [Google Scholar] [CrossRef]
- Zhang, J.A.; Liu, F.; Masouros, C.; Heath, R.W.; Feng, Z.; Zheng, L.; Petropulu, A.P. An Overview of Signal Processing Techniques for Joint Communication and Radar Sensing. IEEE J. Sel. Top. Signal Process. 2021, 15, 1295–1315. [Google Scholar] [CrossRef]
- Saad, W.; Bennis, M.; Chen, M. A Vision of 6G Wireless Systems: Applications, Trends, Technologies, and Open Research Problems. IEEE Netw. 2020, 34, 134–142. [Google Scholar] [CrossRef]
- Behravan, A.; Yajnanarayana, V.; Keskin, M.F.; Chen, H.; Shrestha, D.; Abrudan, T.E.; Svensson, T.; Schindhelm, K.; Wolfgang, A.; Lindberg, S.; et al. Positioning and Sensing in 6G: Gaps, Challenges, and Opportunities. IEEE Veh. Technol. Mag. 2023, 18, 30–39. [Google Scholar] [CrossRef]
- Miao, F.; Huang, Y.; Lu, Z.; Ohtsuki, T.; Gui, G.; Sari, H. Wi-Fi Sensing Techniques for Human Activity Recognition: Brief Survey, Potential Challenges, and Research Directions; ACM: New York, NY, USA, 2025; Volume 57, pp. 1–30. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, H.; Mao, C.; Han, J.; Xu, W. Non-intrusive Human Vital Sign Detection Using mmWave Sensing Technologies: A Review. ACM Trans. Sens. Netw. 2023, 20, 1–36. [Google Scholar] [CrossRef]
- Vallée, A. Digital Twin for Healthcare Systems. Front. Digit. Health 2023, 5, 1253050. [Google Scholar] [CrossRef]
- Trauer, J.; Schweigert-Recksiek, S.; Engel, C.; Spreitzer, K.; Zimmermann, M. What is a digital twin?—Definitions and insights from an industrial case study in technical product development. In Proceedings of the Design Society: Design Conference, Online (originally scheduled for Cavtat, Croatia), 26–29 October 2020; Cambridge University Press: Cambridge, MA, USA, 2020; Volume 1, pp. 757–766. [Google Scholar] [CrossRef]
- Grieves, M.; Vickers, J. Digital Twin: Mitigating Unpredictable, Undesirable Emergent Behaviour in Complex Systems. In Transdisciplinary Perspectives on Complex Systems; Springer: Cham, Switzerland, 2017; pp. 85–113. [Google Scholar] [CrossRef]
- Kritzinger, W.; Karner, M.; Traar, G.; Henjes, J.; Sihn, W. Digital Twin in Manufacturing: A Categorical Literature Review and Classification. IFAC-PapersOnLine 2018, 51, 1016–1022. [Google Scholar] [CrossRef]
- Rasheed, A.; San, O.; Kvamsdal, T. Digital Twin: Values, Challenges and Enablers From a Modeling Perspective. IEEE Access 2020, 8, 21980–22012. [Google Scholar] [CrossRef]
- Soman, R.K.; Farghaly, K.; Mills, G.; Whyte, J. Digital twin construction with a focus on human twin interfaces. Autom. Constr. 2025, 170, 105924. [Google Scholar] [CrossRef]
- Fuller, A.; Fan, Z.; Day, C.; Barlow, C. Digital Twin: Enabling Technologies, Challenges and Open Research. IEEE Access 2020, 8, 108952–108971. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S.; et al. Digital Twins to Personalize Medicine. Genome Med. 2019, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Viola, F.; Del Corso, G.; De Paulis, R.; Verzicco, R. GPU accelerated digital twins of the human heart open new routes for cardiovascular research. Sci. Rep. 2023, 13, 8230. [Google Scholar] [CrossRef]
- Gonsard, A.; Genet, M.; Drummond, D. Digital Twins for Chronic Lung Diseases. Eur. Respir. Rev. 2024, 33, 240159. [Google Scholar] [CrossRef]
- Penverne, Y.; Martinez, C.; Cellier, N.E.A. A Simulation-Based Digital Twin Approach to Assessing the Organisation of Response to Emergency Calls. NPJ Digit. Med. 2024, 7, 385. [Google Scholar] [CrossRef]
- Karakra, A.; Fontanili, F.; Lamine, E.H.; Lamothe, J.; Taweel, A. Pervasive Computing Integrated Discrete Event Simulation for a Hospital Digital Twin. In Proceedings of the 2018 IEEE/ACS 15th International Conference on Computer Systems and Applications (AICCSA), Aqaba, Jordan, 28 October–1 November 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Zhong, D.; Xia, Z.; Zhu, Y.; Duan, J. Overview of Predictive Maintenance Based on Digital Twin Technology. Heliyon 2023, 9, e14534. [Google Scholar] [CrossRef] [PubMed]
- Samei, E. The Future of In Silico Trials and Digital Twins in Medicine. PNAS Nexus 2025, 4, pgaf123. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, R.; Seo, P.; Zhao, L.; Tegenge, M.; Zhu, H. Role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm. Res. 2022, 39, 1669. [Google Scholar] [CrossRef]
- Wang, H.; Arulraj, T.; Ippolito, A.; Popel, A.S. From virtual patients to digital twins in immuno-oncology: Lessons learned from mechanistic quantitative systems pharmacology modeling. NPJ Digit. Med. 2024, 7, 189. [Google Scholar] [CrossRef]
- Ekström, A.M.; Ottersen, O.P. Digital twin for pandemic monitoring and prevention: Urgent need for agreements for global data sharing. Proc. Natl. Acad. Sci. USA 2023, 120, e2311969120. [Google Scholar] [CrossRef]
- Mulder, S.T.; Omidvari, A.H.; Rueten-Budde, A.J.; Huang, P.H.; Kim, K.H.; Bais, B.; Rousian, M.; Hai, R.; Akgun, C.; van Lennep, J.R.; et al. Dynamic digital twin: Diagnosis, treatment, prediction, and prevention of disease during the life course. J. Med. Internet Res. 2022, 24, e35675. [Google Scholar] [CrossRef] [PubMed]
- Jameil, A.K.; Al-Raweshidy, H.S. Enhancing Offloading with Cybersecurity in Edge Computing for Digital Twin-Driven Patient Monitoring. IET Wirel. Sens. Syst. 2024, 14, 363–380. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Song, X.; Shu, L.; Li, Z. The Digital Twin in Medicine: A Key to the Future of Healthcare? Front. Med. 2022, 9, 9330225. [Google Scholar] [CrossRef]
- Mekki, Y.M.; Luijten, G.; Hagert, E.; Belkhair, S.; Varghese, C.; Qadir, J.; Solaiman, B.; Bilal, M.; Dhanda, J.; Egger, J.; et al. Digital Twins for the Era of Personalized Surgery. NPJ Digit. Med. 2025, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 9 September 2025).
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing Populations: The Challenges Ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Older Adults | Chronic Disease Indicators. 2023. Available online: https://www.cdc.gov/cdi/indicator-definitions/older-adults.html (accessed on 9 September 2025).
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of Multimorbidity and Implications for Health Care, Research, and Medical Education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with Multimorbidity: A Systematic Review of the Literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging. What Is Long-Term Care? 2023. Available online: https://www.nia.nih.gov/health/long-term-care/what-long-term-care (accessed on 9 September 2025).
- Torab-Miandoab, A.; Satyal, M.; Wang, Y.E.A. Interoperability of Heterogeneous Health Information Systems: A Systematic Literature Review. BMC Med. Inform. Decis. Mak. 2023, 23, 18. [Google Scholar] [CrossRef]
- Peng, C.; Goswami, P. Meaningful integration of data from heterogeneous health services and home environment based on ontology. Sensors 2019, 19, 1747. [Google Scholar] [CrossRef]
- Syed, R.; Eden, R.; Makasi, T.; Chukwudi, I.; Mamudu, A.; Kamalpour, M.; Kapugama Geeganage, D.; Sadeghianasl, S.; Leemans, S.J.; Goel, K.; et al. Digital health data quality issues: Systematic review. J. Med. Internet Res. 2023, 25, e42615. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.I.; Mondéjar, A.G.; da Silva, A.C.; Silva-Calpa, G.; Teixeira, M.F.; Carvalho, F.; Raposo, A.; Endler, M. Real-time data analysis in health monitoring systems: A comprehensive systematic literature review. J. Biomed. Inform. 2022, 127, 104009. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Roundtable on Value & Science-Driven Health Care. Healthcare Data as a Public Good: Privacy and Security. In Clinical Data as the Basic Staple of Health Learning: Creating and Protecting a Public Good: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2010; p. 5. Available online: https://www.ncbi.nlm.nih.gov/books/NBK54293/ (accessed on 9 September 2025).
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth benefits and barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef]
- Drake, C.; Zhang, Y.; Chaiyachati, K.H.; Polsky, D. The limitations of poor broadband internet access for telemedicine use in rural America: An observational study. Ann. Intern. Med. 2019, 171, 382–384. [Google Scholar] [CrossRef]
- Gandrup, J.; Ali, S.M.; McBeth, J.; Van Der Veer, S.N.; Dixon, W.G. Remote Symptom Monitoring Integrated into Electronic Health Records: A Systematic Review. J. Am. Med. Inform. Assoc. 2020, 27, 1752–1763. [Google Scholar] [CrossRef]
- Zon, M.; Ganesh, G.; Deen, M.J.; Fang, Q. Context-aware medical systems within healthcare environments: A systematic scoping review to identify subdomains and significant medical contexts. Int. J. Environ. Res. Public Health 2023, 20, 6399. [Google Scholar] [CrossRef]
- Serrano, L.P.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Eldaly, A.S.; Haider, C.R.; Felton, C.L.; Paulson, M.R.; Maniaci, M.J.; et al. Benefits and challenges of remote patient monitoring as perceived by health care practitioners: A systematic review. Perm. J. 2023, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Du, Y.; Zhang, Q.; Jiang, W.; Cui, Y.; Meng, Z.; Wu, H.; Feng, Z. Integrated Sensing and Communication Driven Digital Twin for Intelligent Machine Network. IEEE Internet Things Mag. 2024, 7, 60–67. [Google Scholar] [CrossRef]
- Huang, N.; Wang, T.; Wu, Y.; Wu, Q.; Quek, T.Q.S. Integrated Sensing and Communication Assisted Mobile Edge Computing: An Energy-Efficient Design via Intelligent Reflecting Surface. IEEE Wirel. Commun. Lett. 2022, 11, 2085–2089. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, K.; Han, J.; Sui, Q.; Li, Z. Photonic Integrated Sensing and Communication System Harnessing Submarine Fiber-Optic Cables for Coastal Event Monitoring. IEEE Commun. Mag. 2022, 60, 110–116. [Google Scholar] [CrossRef]
- De Lima, C.F.; Belot, D.; Berkvens, R.; Bourdoux, A.; Dardari, D.; Guillaud, M.; Isomursu, M.; Lohan, E.; Miao, Y.; Barreto, A.N.; et al. Convergent Communication, Sensing and Localization in 6G Systems: An Overview of Technologies, Opportunities and Challenges. IEEE Access 2021, 9, 26902–26925. [Google Scholar] [CrossRef]
- Jameil, A.K.; Al-Raweshidy, H. A Digital Twin Framework for Real-Time Healthcare Monitoring: Leveraging AI and Secure Systems for Enhanced Patient Outcomes. Discov. Internet Things 2025, 5, 37. [Google Scholar] [CrossRef]
- Spitzer, M.; Dattner, I.; Zilcha-Mano, S. Digital Twins and the Future of Precision Mental Health. Front. Psychiatry 2023, 14, 1082598. [Google Scholar] [CrossRef]
- Elkefi, S.; Asan, O. Digital twins for managing health care systems: Rapid literature review. J. Med. Internet Res. 2022, 24, e37641. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Mustofa, R.; Hossain, N.U.I.; Islam, M.S. Smart Health Practices: Strategies to Improve Healthcare Efficiency Through Digital Twin Technology. Smart Health 2025, 36, 100541. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zhang, N.; Zhu, B. Advancing Emergency Care With Digital Twins. JMIR Aging 2025, 8, e71777. [Google Scholar] [CrossRef]
- Zheng, R.; Ng, S.T.; Shao, Y.; Li, Z.; Xing, J. Leveraging Digital Twin for Healthcare Emergency Management System: Recent Advances, Critical Challenges, and Future Directions. Reliab. Eng. Syst. Saf. 2025, 261, 111079. [Google Scholar] [CrossRef]
- Koul, S.; Mishra, V.; Taylor, I. Enhancing Hospital Operations Efficiency Through Digital Twin Technology. In Blockchain and Digital Twin for Smart Hospitals; Elsevier: Amsterdam, The Netherlands, 2025; pp. 511–528. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz–300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Strazza, C.; Olivieri, N.; De Rose, A.; Stevens, T.; Leen, P.; Daniel, T.; Marina, B. Technology Readiness Level—Guidance Principles for Renewable Energy Technologies—Final Report; Technical report; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Redondi, A.E.C.; Innamorati, C.; Gallucci, S.; Fiocchi, S.; Matera, F. A Survey on Future Millimeter-Wave Communication Applications. IEEE Access 2024, 12, 133165–133182. [Google Scholar] [CrossRef]
- Bressler, M.; Zhu, J.; Olick-Gibson, J.; Haefner, J.; Zhou, S.; Chen, Q.; Mazur, T.; Hao, Y.; Carter, P.; Zhang, T. Millimeter wave-based patient setup verification and motion tracking during radiotherapy. Med. Phys. 2024, 51, 2967–2974. [Google Scholar] [CrossRef]
- Soumya, A.; Krishna Mohan, C.; Cenkeramaddi, L.R. Recent Advances in mmWave-Radar-Based Sensing, Its Applications, and Machine Learning Techniques: A Review. Sensors 2023, 23, 8901. [Google Scholar] [CrossRef] [PubMed]
- Shahjehan, W.; Rathore, R.S.; Shah, S.W.; Aljaidi, M.; Sadiq, A.S.; Kaiwartya, O. A Review on Millimeter-Wave Hybrid Beamforming for Wireless Intelligent Transport Systems. Future Internet 2024, 16, 337. [Google Scholar] [CrossRef]
- Al-Samman, A.M.; Azmi, M.H.; Al-Gumaei, Y.A.; Al-Hadhrami, T.; Abd. Rahman, T.; Fazea, Y.; Al-Mqdashi, A. Millimeter Wave Propagation Measurements and Characteristics for 5G System. Appl. Sci. 2020, 10, 335. [Google Scholar] [CrossRef]
- Mirbeik, A.; Najafizadeh, L.; Ebadi, N. A Synthetic Ultra-Wideband Transceiver for Millimeter-Wave Imaging Applications. Micromachines 2023, 14, 2031. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, J.A.; Zhang, H.; Xu, M. Vital Sign Monitoring in Dynamic Environment via mmWave Radar and Camera Fusion. IEEE Trans. Mob. Comput. 2024, 23, 4163–4180. [Google Scholar] [CrossRef]
- Ahn, S.; Choi, M.; Lee, J.; Kim, J.; Chung, S. Non-Contact Fall Detection System Using 4D Imaging Radar for Elderly Safety Based on a CNN Model. Sensors 2025, 25, 3452. [Google Scholar] [CrossRef]
- Zeng, X.; Báruson, H.S.L.; Sundvall, A. Walking step monitoring with a millimeter-wave radar in real-life environment for disease and fall prevention for the elderly. Sensors 2022, 22, 9901. [Google Scholar] [CrossRef]
- Liu, X.; Cao, J.; Tang, S.; Wen, J. Wi-sleep: Contactless sleep monitoring via wifi signals. In Proceedings of the 2014 IEEE Real-Time Systems Symposium, Rome, Italy, 2–5 December 2014; pp. 346–355. [Google Scholar] [CrossRef]
- Huang, X.; Cheena, H.; Thomas, A.; Tsoi, J.K. Indoor detection and tracking of people using mmwave sensor. J. Sens. 2021, 2021, 6657709. [Google Scholar] [CrossRef]
- Hao, Z.; Yan, H.; Dang, X.; Ma, Z.; Jin, P.; Ke, W. Millimeter-wave radar localization using indoor multipath effect. Sensors 2022, 22, 5671. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Joshi, K.R.; Rajavi, Y.; Taghivand, M.; Pauly, J.M.; Poon, A.S.Y.; Scott, G.C. A Millimeter-Wave Digital Link for Wireless MRI. IEEE Trans. Med. Imaging 2017, 36, 574–583. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Y.; Jin, D.; Su, L.; Vasilakos, A. A survey of millimeter wave communications (mmWave) for 5G: Opportunities and challenges. Wirel. Netw. 2015, 21, 2657–2676. [Google Scholar] [CrossRef]
- Akdeniz, M.R.; Liu, Y.; Samimi, M.K.; Sun, S.; Rangan, S.; Rappaport, T.S.; Erkip, E. Millimeter wave channel modeling and cellular capacity evaluation. IEEE J. Sel. Areas Commun. 2014, 32, 1164–1179. [Google Scholar] [CrossRef]
- Rappaport, T.S.; Xing, Y.; MacCartney, G.R.; Molisch, A.F.; Mellios, E.; Zhang, J. Overview of Millimeter Wave Communications for Fifth-Generation (5G) Wireless Networks—With a Focus on Propagation Models. IEEE Trans. Antennas Propag. 2017, 65, 6213–6230. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, G.; Wang, J.; Zhou, Z. Reconfigurable Intelligent Surface–Assisted Millimeter Wave Networks: Cell Association and Coverage Analysis. Electronics 2023, 12, 4270. [Google Scholar] [CrossRef]
- Rangan, S.; Rappaport, T.S.; Erkip, E. Millimeter-wave cellular wireless networks: Potentials and challenges. Proc. IEEE 2014, 102, 366–385. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, X.; Sun, S.; Huang, Y.; Al-Tahmeesschi, A.; Jiang, Y. Energy-Efficiency of Millimeter-Wave Full-Duplex Relaying Systems: Challenges and Solutions. IEEE Access 2016, 4, 4848–4860. [Google Scholar] [CrossRef]
- Nagatsuma, T.; Ducournau, G.; Renaud, C.C. Advances in Terahertz Communications Accelerated by Photonics. Nat. Photonics 2016, 10, 371–379. [Google Scholar] [CrossRef]
- Gallot, G. Terahertz Sensing in Biology and Medicine. Photoniques 2020, 101, 53–56. [Google Scholar] [CrossRef]
- Ajito, K. Terahertz Spectroscopy for Pharmaceutical and Biomedical Applications. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 1140–1145. Available online: https://ieeexplore.ieee.org/abstract/document/7334624 (accessed on 9 September 2025).
- Siegel, P.H. Terahertz technology. IEEE Trans. Microw. Theory Tech. 2002, 50, 910–928. [Google Scholar] [CrossRef]
- Rappaport, T.S.; Xing, Y.; MacCartney, G.R.; Molisch, A.F.; Mellios, E.; Zhang, J. Wireless Communications and Applications above 100 GHz: Opportunities and Challenges for 6G and Beyond. IEEE Access 2019, 7, 78729–78757. [Google Scholar] [CrossRef]
- Tonouchi, M. Cutting-Edge Terahertz Technology. Nat. Photonics 2007, 1, 97–105. [Google Scholar] [CrossRef]
- Dutta, M.; Bhalla, A.S.; Guo, R. THz imaging of skin burn: Seeing the unseen—An overview. Adv. Wound Care 2016, 5, 338–348. [Google Scholar] [CrossRef]
- Khani, M.E.; Osman, O.B.; Harris, Z.B.; Chen, A.; Zhou, J.W.; Singer, A.J.; Arbab, M.H. Accurate and early prediction of the wound healing outcome of burn injuries using the wavelet Shannon entropy of terahertz time-domain waveforms. J. Biomed. Opt. 2022, 27, 116001. [Google Scholar] [CrossRef]
- Penkov, N.V. Terahertz spectroscopy as a method for investigation of hydration shells of biomolecules. Biophys. Rev. 2023, 15, 833–849. [Google Scholar] [CrossRef]
- Weisenstein, C.; Wigger, A.K.; Richter, M.; Sczech, R.; Bosserhoff, A.K.; Bolívar, P.H. THz detection of biomolecules in aqueous environments—Status and perspectives for analysis under physiological conditions and clinical use. J. Infrared Millimeter Terahertz Waves 2021, 42, 607–646. [Google Scholar] [CrossRef]
- Rong, Y.; Theofanopoulos, P.C.; Trichopoulos, G.C.; Bliss, D.W. A new principle of pulse detection based on terahertz wave plethysmography. Sci. Rep. 2022, 12, 6347. [Google Scholar] [CrossRef] [PubMed]
- Hoog Antink, C.; Schulz, R.; Rohr, M.; Wenzel, K.; Liebermeister, L.; Kohlhaas, R.; Preu, S. Estimating thoracic movement with high-sampling rate THz technology. Sensors 2023, 23, 5233. [Google Scholar] [CrossRef]
- Ma, J.; Shrestha, R.; Adelberg, J.; Yeh, C.Y.; Hossain, Z.; Knightly, E.; Jornet, J.M.; Mittleman, D.M. Security and Eavesdropping in Terahertz Wireless Links. Nature 2018, 563, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Singh Virdi, J.; Babakhani, A.; Roberts, I.P. A Survey on Advancements in THz Technology for 6G: Systems, Circuits, Antennas, and Experiments. IEEE Open J. Commun. Soc. 2025, 6, 1998–2016. [Google Scholar] [CrossRef]
- Liu, K.; Feng, Y.; Han, C.; Chang, B.; Chen, Z.; Xu, Z.; Li, L.; Zhang, B.; Wang, Y.; Xu, Q. High-Speed 0.22 THz Communication System with 84 Gbps for Real-Time Uncompressed 8K Video Transmission of Live Events. Nat. Commun. 2024, 15, 52370. [Google Scholar] [CrossRef]
- Alsaedi, W.K.; Ahmadi, H.; Khan, Z.; Grace, D. Spectrum Options and Allocations for 6G: A Regulatory and Standardization Review. IEEE Open J. Commun. Soc. 2023, 4, 1787–1812. [Google Scholar] [CrossRef]
- Hashemi, H. The indoor radio propagation channel. Proc. IEEE 1993, 81, 943–968. [Google Scholar] [CrossRef]
- Seidel, S.; Rappaport, T.; Jain, S.; Lord, M.; Singh, R. Path loss, scattering and multipath delay statistics in four European cities for digital cellular and microcellular radiotelephone. IEEE Trans. Veh. Technol. 1991, 40, 721–730. [Google Scholar] [CrossRef]
- Andrews, J.G.; Buzzi, S.; Choi, W.; Hanly, S.V.; Lozano, A.; Soong, A.C.K.; Zhang, J.C. What Will 5G Be? IEEE J. Sel. Areas Commun. 2014, 32, 1065–1082. [Google Scholar] [CrossRef]
- Boccardi, F.; Heath, R.W.; Lozano, A.; Marzetta, T.L.; Popovski, P. Five disruptive technology directions for 5G. IEEE Commun. Mag. 2014, 52, 74–80. [Google Scholar] [CrossRef]
- Wang, C.-X.; Haider, F.; Gao, X.; You, X.-H.; Yang, Y.; Yuan, D.; Aggoune, H.M.; Haas, H.; Fletcher, S.; Hepsaydir, E. Cellular architecture and key technologies for 5G wireless communication networks. IEEE Commun. Mag. 2014, 52, 122–130. [Google Scholar] [CrossRef]
- Abu-Ali, N.; Taha, A.E.M.; Salah, M.; Hassanein, H. Uplink Scheduling in LTE and LTE-Advanced: Tutorial, Survey and Evaluation Framework. IEEE Commun. Surv. Tutor. 2014, 16, 1239–1265. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Baldemair, R.; Cheng, T.; Parkvall, S.; Larsson, D.; Koorapaty, H.; Frenne, M.; Falahati, S.; Grövlen, A.; et al. 5G New Radio: Unveiling the Essentials of the Next Generation Wireless Access Technology. IEEE Commun. Stand. Mag. 2019, 3, 30–37. [Google Scholar] [CrossRef]
- Parkvall, S.; Blankenship, Y.W.; Blasco, R.; Dahlman, E.; Fodor, G.; Grant, S.J.; Stare, E.; Stattin, M. 5G NR Release 16: Start of the 5G Evolution. IEEE Commun. Stand. Mag. 2020, 4, 56–63. [Google Scholar] [CrossRef]
- Gupta, A.; Jha, R.K. A Survey of 5G Network: Architecture and Emerging Technologies. IEEE Access 2015, 3, 1206–1232. [Google Scholar] [CrossRef]
- European Telecommunications Standards Institute. Integrated Sensing And Communications (ISAC): Use Cases and Deployment Scenarios (ETSI GR ISC 001 V1.1.1); Technical report; European Telecommunications Standards Institute: Valbonne, France, 2025; Available online: https://www.etsi.org/deliver/etsi_gr/ISC/001_099/001/01.01.01_60/gr_ISC001v010101p.pdf (accessed on 9 September 2025).
- Toker, O.; Adla, R. A sub-6 GHz vital signs sensor using software defined radios. Eng. Proc. 2020, 2, 38. [Google Scholar] [CrossRef]
- Liu, Y.; Al Kalaa, M.O. Testbed as a Regulatory Science Tool (TRUST): A Design Model for Evaluating 5G-Enabled Medical Devices. IEEE Access 2023, 11, 81563–81576. [Google Scholar] [CrossRef]
- Chinnaperumal, S.; Periyasamy, M.; Alhussan, A.A.; Kannan, S.; Khafaga, D.S.; Raju, S.K.; Eid, M.M.; El-Kenawy, E.S.M. Secure and intelligent 5G-enabled remote patient monitoring using ANN and Choquet integral fuzzy VIKOR. Sci. Rep. 2025, 15, 9913. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, K.; Xiong, J.; Liu, L.; Ma, H. Pushing the Limits of WiFi Sensing with Low Transmission Rates. IEEE Trans. Mob. Comput. 2024, 23, 10265–10279. [Google Scholar] [CrossRef]
- Moltchanov, D.; Samuylov, A.; Lisovskaya, E.; Kovalchukov, R.; Begishev, V.; Sopin, E.; Gaidamaka, Y.; Koucheryavy, Y. Performance Characterization and Traffic Protection in Street Multi-Band Millimeter-Wave and Microwave Deployments. IEEE Trans. Wirel. Commun. 2021, 21, 163–178. [Google Scholar] [CrossRef]
- Swami, P.; Mishra, M.K.; Bhatia, V.; Ratnarajah, T.; Trivedi, A. Performance Analysis of sub-6 GHzmmWave NOMA Hybrid-HetNets Using Partial CSI. IEEE Trans. Veh. Technol. 2022, 71, 12958–12971. [Google Scholar] [CrossRef]
- Liu, X.; Meng, X.; Duan, H.; Hu, Z.; Wang, M. A Survey on Secure WiFi Sensing Technology: Attacks and Defenses. Sensors 2025, 25, 1913. [Google Scholar] [CrossRef]
- Xu, D.; Khalili, A.; Yu, X.; Kwan Ng, D.W.; Schober, R. Integrated Sensing and Communication in Distributed Antenna Networks. In Proceedings of the 2023 IEEE International Conference on Communications Workshops (ICC Workshops), Rome, Italy, 28 May–1 June 2023; pp. 1457–1462. [Google Scholar] [CrossRef]
- Gao, Z.; Wan, Z.; Zheng, D.; Tan, S.; Masouros, C.; Ng, D.W.K.; Chen, S. Integrated Sensing and Communication with mmWave Massive MIMO: A Compressed Sampling Perspective. IEEE Trans. Wirel. Commun. 2023, 22, 1745–1762. [Google Scholar] [CrossRef]
- Wang, X.; Fei, Z.; Zhang, J.A.; Xu, J. Partially-Connected Hybrid Beamforming Design for Integrated Sensing and Communication Systems. IEEE Trans. Commun. 2022, 70, 6648–6660. [Google Scholar] [CrossRef]
- Ma, M.L.; Zhao, D.; Hu, Z.J.; Wang, Y.; Liang, F.; Wang, B.Z. Increasing Microwave Penetration Depth in the Human Body by a Complex Impedance Match of Skin Interface with a Two-Layered Medium. Electronics 2024, 13, 3915. [Google Scholar] [CrossRef]
- Bayesteh, A.; He, J.; Chen, Y.; Zhu, P.; Ma, J.; Shaban, A.W.; Yu, Z.; Zhang, Y.; Zhou, Z.; Wang, G. Integrated Sensing and Communication (ISAC)—From Concept to Practice; Technical report; Huawei Technologies: Shenzhen, China, 2022; Available online: https://www.huawei.com/en/huaweitech/future-technologies/integrated-sensing-communication-concept-practice (accessed on 9 September 2025).
- Hu, J.; Niyato, D.; Luo, J. Cross-Domain Learning Framework for Tracking Users in RIS-Aided Multi-Band ISAC Systems With Sparse Labeled Data. IEEE J. Sel. Areas Commun. 2024, 42, 2754–2768. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, Q.Y. Joint Resource Allocation for User-Centric Cell-Free Integrated Sensing and Communication Systems. IEEE Commun. Lett. 2023, 27, 2338–2342. [Google Scholar] [CrossRef]
- Zhang, J.A.; Rahman, M.L.; Huang, X.; Guo, Y.J.; Chen, S.; Heath, R.W. Perceptive Mobile Networks: Cellular Networks With Radio Vision via Joint Communication and Radar Sensing. IEEE Veh. Technol. Mag. 2020, 16, 20–30. [Google Scholar] [CrossRef]
- Deng, R.; Di, B.; Zhang, H.; Niyato, D.; Han, Z.; Poor, H.V.; Song, L. Reconfigurable Holographic Surfaces for Future Wireless Communications. IEEE Wirel. Commun. 2022, 28, 126–131. [Google Scholar] [CrossRef]
- Kim, M.; Son, M.H.; Moon, S.; Cha, W.C.; Jo, I.J.; Yoon, H. Correction: A Mixed Reality-Based Telesupervised Ultrasound Education Platform on 5G Network Compared to Direct Supervision: Prospective Randomized Pilot Trial. JMIR Serious Games 2025, 13, e77586. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, Y.; Chen, C.; Huang, D.; Wang, J.; Li, X.; Ji, Z.; Li, Q.; Li, Z. 5G Key Technologies for Helicopter Aviation Medical Rescue. J. Med. Internet Res. 2024, 26, e50355. [Google Scholar] [CrossRef]
- Lu, J.; Ling, K.; Zhong, W.; He, H.; Ruan, Z.; Han, W. Construction of a 5G-based, three-dimensional, and efficiently connected emergency medical management system. Heliyon 2023, 9, e13826. [Google Scholar] [CrossRef]
- Chen, B.; Shi, X.; Feng, T.; Jiang, S.; Zhai, Y.; Ren, M.; Liu, D.; Wang, C.; Gao, J. Construction and Application of a Private 5G Standalone Medical Network in a Smart Health Environment: Exploratory Practice From China. J. Med. Internet Res. 2024, 26, e52404. [Google Scholar] [CrossRef]
- Hakimi, A.; Galappaththige, D.; Tellambura, C. A roadmap for NF-ISAC in 6G: A comprehensive overview and tutorial. Entropy 2024, 26, 773. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, F.; Li, Y.; Zhang, K.; Huang, H.; Zou, J.; Li, X.; Dong, Y.; Dong, F.; Zhu, J.; et al. Integrated Sensing and Communications: Recent Advances and Ten Open Challenges. IEEE Internet Things J. 2024, 11, 19094–19120. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Z.; Luo, J. Multi-Band Reconfigurable Holographic Surface Based ISAC Systems: Design and Optimization. In Proceedings of the ICC 2023—IEEE International Conference on Communications, Rome, Italy, 28 May–1 June 2023; pp. 2927–2932. [Google Scholar] [CrossRef]
- Wang, Q.; Kakkavas, A.; Gong, X.; Stirling-Gallacher, R.A. Towards Integrated Sensing and Communications for 6G. In Proceedings of the 2022 2nd IEEE International Symposium on Joint Communications & Sensing (JC&S), Seefeld, Austria, 9–10 March 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Europe, M. Interoperability Standards in Digital Health. 2021. Available online: https://www.medtecheurope.org/wp-content/uploads/2021/10/mte_interoperability_digital_health_white-paper_06oct21.pdf (accessed on 9 September 2025).
- IEEE C95.1-2019; IEEE Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz(Revision of IEEE Std C95.1-2005/ Incorporates IEEE Std C95.1-2019/Cor 1-2019). IEEE: New York, NY, USA, 4 October 2019. [CrossRef]
- Electromagnetic Compatibility (EMC) of Medical Devices. Technical report, U.S. Food and Drug Administration. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/electromagnetic-compatibility-emc-medical-devices (accessed on 9 September 2025).
- General Principles of Software Validation; Guidance for Industry and FDA Staff. Technical Report, U.S. Food and Drug Administration. 2002. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-principles-software-validation (accessed on 9 September 2025).
- Health Insurance Portability and Accountability Act of 1996. Public Law 104–191; 1996. Available online: https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf (accessed on 9 September 2025).
- General Data Protection Regulation (GDPR). 2018. Available online: https://gdpr-info.eu/ (accessed on 9 September 2025).
- Haveman, M.E.; van Rossum, M.C.; Vaseur, R.M.E.; van der Riet, C.; Schuurmann, R.C.L.; Hermens, H.J.; de Vries, J.-P.P.M.; Tabak, M. Continuous Monitoring of Vital Signs with Wearable Sensors During Daily Life Activities: Validation Study. JMIR Form. Res. 2022, 6, e30863. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhang, D.; Li, Y.; Lu, Z.; Chen, J.; Wang, H.; Zhou, F.; Pu, Y.; Hu, Y.; Ma, L.; et al. Monitoring Long-Term Cardiac Activity with Contactless Radio Frequency Signals. Nat. Commun. 2024, 15, 10598. [Google Scholar] [CrossRef]
- Bujan, B.; Fischer, T.; Dietz-Terjung, S.; Bauerfeind, A.; Jedrysiak, P.; Sundrup, M.G.; Hamann, J.; Schöbel, C. Clinical Validation of a Contactless Respiration Rate Monitor. Sci. Rep. 2023, 13, 3480. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, K.; Breteler, M.; Van Wolfwinkel, L.; Rheineck Leyssius, A.; Kossen, S.; Kalkman, C.; Van Zaane, B.; Peelen, L. Wireless non-invasive continuous respiratory monitoring with FMCW radar: A clinical validation study. J. Clin. Monit. Comput. 2016, 30, 797–805. [Google Scholar] [CrossRef]
- Toften, S.; Kjellstadli, J.T.; Thu, O.K.F.; Ellingsen, O.J. Noncontact Longitudinal Respiratory Rate Measurements in Healthy Adults Using a Radar-Based Sleep Monitor (Somnofy): Validation Study. JMIR Biomed. Eng. 2022, 7, e36618. [Google Scholar] [CrossRef]
- FHIR Release 5: HL7 Fast Healthcare Interoperability Resources. 2023. Available online: https://www.hl7.org/fhir/ (accessed on 9 September 2025).
- IEEE ISO 11073-10101-2020; ISO IEEE International Standard—Health Informatics-Device Interoperability-Part 10101: Point-of-Care Medical Device Communication-Nomenclature. IEEE: New York, NY, USA, 28 August 2020. [CrossRef]
- Lindsay, M.R.; Lytle, K. Implementing Best Practices to Redesign Workflow and Optimize Nursing Documentation in the Electronic Health Record. Appl. Clin. Inform. 2022, 13, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Manco, C.; Dolci, T.; Azzalini, F.; Barbierato, E.; Gribaudo, M.; Tanca, L. HEALER: A Data Lake Architecture for Healthcare. In Proceedings of the 2023 Workshops of the EDBT/ICDT Joint Conference, EDBT/ICDT-WS 2023, CEUR-WS, Ioannina, Greece, 28 March 2023; Volume 3379. Available online: https://ceur-ws.org/Vol-3379/DataPlat_2023_602.pdf (accessed on 9 September 2025).
- Monlezun, D.J.; Omutoko, L.; Oduor, P.; Kokonya, D.; Rayel, J.; Sotomayor, C.; Girault, M.I.; Uriarte, M.D.l.; Sinyavskiy, O.; Aksamit, I.; et al. Digitalization of Health Care in Low- and Middle-Income Countries. Bull. World Health Organ. 2024, 102, 1–15. Available online: https://cdn.who.int/media/docs/default-source/bulletin/online-first/blt.24.291643.pdf (accessed on 9 September 2025).
- Forum, W.E. How Digital Tools Can Reduce Health Inequity in Low- and Middle-Income Countries. 2025. Available online: https://www.weforum.org/stories/2025/03/digital-tools-reduce-health-inequity-low-middle-income-countries/ (accessed on 9 September 2025).
- Rofougaran, R. The Role of mmWave in Eliminating Challenges of Real-World 5G. EE Times. 21 March 2022. Available online: https://www.eetimes.com/the-role-of-mmwave-in-eliminating-challenges-of-real-world-5g/ (accessed on 9 September 2025).
- Chen, R.; Yan, B.; Chang, M.C.F. A Review of Circuits and Systems for Advanced Sub-THz Transceivers in Wireless Communication. Electronics 2025, 14, 861. [Google Scholar] [CrossRef]
- González-Prelcic, N.; Keskin, M.F.; Kaltiokallio, O.; Valkama, M.; Dardari, D.; Shen, X.; Shen, Y.S.; Bayraktar, M.; Wymeersch, H. The Integrated Sensing and Communication Revolution for 6G: Vision, Techniques, and Applications. Proc. IEEE 2024, 112, 676–723. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, W.; Xing, C.; Zhao, N.; Al-Dhahir, N.; Karagiannidis, G.K.; Yang, X. Intelligent integrated sensing and communication: A survey. Sci. China Inf. Sci. 2025, 68, 131301. [Google Scholar] [CrossRef]
- Sherazi, H.H.R.; Zorbas, D.; O’Flynn, B. A Comprehensive Survey on RF Energy Harvesting: Applications and Performance Determinants. Sensors 2022, 22, 2990. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhang, L.; Zheng, Y.N.; Liu, S.; Huang, Z.R.; Liang, J.C.; Renzo, M.D.; Galdi, V.; Cui, T.J. Integrated Sensing and Communication Based on Space-Time-Coding Metasurfaces. Nat. Commun. 2025, 16, 1836. [Google Scholar] [CrossRef] [PubMed]
- De la Paz, E.; Maganti, N.H.; Trifonov, A.; Jeerapan, I.; Mercier, P.P.; Wang, J. A Self-Powered Ingestible Wireless Biosensing System for Real-Time in situ Monitoring of Gastrointestinal Tract Metabolites. Nat. Commun. 2022, 13, 7405. [Google Scholar] [CrossRef]
- Selvaraj, M.; Sreeja, B.S.; Aly, M.A.S. Terahertz-Based Biosensors for Biomedical Applications: A Review. Methods 2025, 234, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lerman, I.; Bu, Y.; Singh, R.; Silverman, H.A.E.A. Next Generation Bioelectronic Medicine: Making the Case for Non-Invasive Closed-Loop Autonomic Neuromodulation. Bioelectron. Med. 2025, 11, 1. [Google Scholar] [CrossRef]
- Haque, A.; Milstein, A.; Fei-Fei, L. Illuminating the Dark Spaces of Healthcare with Ambient Intelligence. Nature 2020, 585, 193–202. [Google Scholar] [CrossRef] [PubMed]
- IEEE Draft Standard for Information Technology—Telecommunications and Information Exchange Between Systems Local and Metropolitan Area Networks—Specific Requirements—Part 11: Wireless LAN Medium Access Control (MAC) and Physical Layer (PHY) Specifications Amendment 4: Enhancements for Wireless LAN Sensing. In IEEE P802.11bf/D3.0, December 2023, pp. 1–227, 21 January 2024. Available online: https://ieeexplore.ieee.org/servlet/opac?punumber=10412009 (accessed on 9 September 2025).
- Lemus-Zúñiga, L.G.; Félix, J.M.; Fides-Valero, A.; Benlloch-Dualde, J.V.; Martinez-Millana, A. A Proof-of-Concept IoT System for Remote Healthcare Based on Interoperability Standards. Sensors 2022, 22, 1646. [Google Scholar] [CrossRef]
- 2933-2024; IEEE/UL Standard for Clinical Internet of Things (IoT) Data and Device Interoperability with TIPPSS—Trust, Identity, Privacy, Protection, Safety, and Security. IEEE: New York, NY, USA, 2024. [CrossRef]
- Alkhateeb, A.; Charan, G.; Osman, T.; Hredzak, A.; Morais, J.; Demirhan, U.; Srinivas, N. DeepSense 6G: A Large-Scale Real-World Multi-Modal Sensing and Communication Dataset. IEEE Commun. Mag. 2023. [Google Scholar] [CrossRef]
- Standard for Vocabulary for Integrated Sensing and Communication Systems. Draft PAR Approved June 2023. Available online: https://standards.ieee.org/ieee/3384/11323/ (accessed on 9 September 2025).
- Study on Integrated Sensing and Communication (Release 19). 2024. Available online: https://www.3gpp.org/dynareport/22837.htm (accessed on 9 September 2025).
- U.S. Food and Drug Administration. Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions; U.S. Food and Drug Administration: Silver Spring, MD, USA, June 2025. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cybersecurity-medical-devices-quality-system-considerations-and-content-premarket-submissions (accessed on 9 September 2025).
- Islam, S.M.M. Radar-based remote physiological sensing: Progress, challenges, and opportunities. Front. Physiol. 2022, 13, 955208. [Google Scholar] [CrossRef]
- Ayaz, M.; Pasha, M.F.; Alzahrani, M.Y.; Budiarto, R.; Stiawan, D. The Fast Health Interoperability Resources (FHIR) standard: Systematic literature review of implementations, applications, challenges and opportunities. JMIR Med. Inform. 2021, 9, e21929. [Google Scholar] [CrossRef]
- Su, N.; Liu, F.; Masouros, C. Sensing-Assisted Eavesdropper Estimation: An ISAC Breakthrough in Physical Layer Security. IEEE Trans. Wirel. Commun. 2024, 23, 3162–3174. [Google Scholar] [CrossRef]
- Zou, J.; Masouros, C.; Liu, F.; Sun, S. Securing the Sensing Functionality in ISAC Networks: An Artificial Noise Design. IEEE Trans. Veh. Technol. 2024, 73, 17800–17805. [Google Scholar] [CrossRef]
- Ye, X.; Mao, Y.; Yu, X.; Fu, L. Intelligent Omni-Surface-Aided Integrated Sensing and Communications Based on Deep Reinforcement Learning with Knowledge Transfer. IEEE Trans. Wirel. Commun. 2025, 24, 4344–4360. [Google Scholar] [CrossRef]
- Günlü, O.; Bloch, M.R.; Schaefer, R.F.; Yener, A. Secure Integrated Sensing and Communication. IEEE J. Sel. Areas Inf. Theory 2023, 4, 40–53. [Google Scholar] [CrossRef]
- Tian, S.; Ju, Y.; Liu, L.; Pei, Q.; Zhang, N.; Wu, C.; Mumtaz, S. Secure Terahertz Indoor Communications Using Blockage Feature-Based Artificial Noise in 6G. In Proceedings of the GLOBECOM 2023—2023 IEEE Global Communications Conference, Kuala Lumpur, Malaysia, 4–8 December 2023; pp. 7261–7266. [Google Scholar] [CrossRef]
- Chen, J.; Wu, K.; Niu, J.; Li, Y. Joint active and passive beamforming in RIS-Assisted secure ISAC systems. Sensors 2024, 24, 289. [Google Scholar] [CrossRef]
- Jang, S.; Kim, N.; Kim, G.; Lee, B. Recent Trend of Rate-Splitting Multiple Access-Assisted Integrated Sensing and Communication Systems. Electronics 2024, 13, 4579. [Google Scholar] [CrossRef]
- Clerckx, B.; Mao, Y.; Jorswieck, E.A.; Yuan, J.; Love, D.J.; Erkip, E.; Niyato, D. A Primer on Rate-Splitting Multiple Access: Tutorial, Myths, and Frequently Asked Questions. IEEE J. Sel. Areas Commun. 2023, 41, 1265–1308. [Google Scholar] [CrossRef]
- Barricelli, B.R.; Casiraghi, E.; Fogli, D. A Survey on Digital Twin: Definitions, Characteristics, Applications, and Design Implications. IEEE Access 2019, 7, 167653–167671. [Google Scholar] [CrossRef]
- Minerva, R.; Lee, G.M.; Crespi, N. Digital Twin in the IoT Context: A Survey on Technical Features, Scenarios, and Architectural Models. Proc. IEEE 2020, 108, 1785–1824. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, H.; Liu, A.; Nee, A.Y.C. Digital Twin in Industry: State-of-the-Art. IEEE Trans. Ind. Inform. 2019, 15, 2405–2415. [Google Scholar] [CrossRef]
- Papachristou, K.; Katsakiori, P.F.; Papadimitroulas, P.; Strigari, L.; Kagadis, G.C. Digital Twins’ Advancements and Applications in Healthcare, Towards Precision Medicine. J. Pers. Med. 2024, 14, 1101. [Google Scholar] [CrossRef]
- De Benedictis, A.; Mazzocca, N.; Somma, A.; Strigaro, C. Digital Twins in Healthcare: An Architectural Proposal and Its Application in a Social Distancing Case Study. IEEE J. Biomed. Health Inform. 2022, 27, 5143–5154. [Google Scholar] [CrossRef]
- Amofa, S.; Xia, Q.; Xia, H.; Obiri, I.A.; Adjei-Arthur, B.; Yang, J.; Gao, J. Blockchain-secure patient Digital Twin in healthcare using smart contracts. PLoS ONE 2024, 19, e0286120. [Google Scholar] [CrossRef]
- Nagaraj, D.; Khandelwal, P.; Steyaert, S.; Gevaert, O. Augmenting Digital Twins with Federated Learning in Medicine. Lancet Digit. Health 2023, 5, e251–e253. [Google Scholar] [CrossRef] [PubMed]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The Digital Twin to Enable the Vision of Precision Cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef] [PubMed]
- Niederer, S.A.; Lumens, J.; Trayanova, N.A. Computational models in cardiology. Nat. Rev. Cardiol. 2019, 16, 100–111. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Zare, F.; Masoumi Shahrbabak, S.; Brattain, L.; Hahn, J.O.; Inan, O.T.; Mukkamala, R.; Palmer, J.; Paydarfar, D.; et al. Building digital twins for cardiovascular health: From principles to clinical impact. J. Am. Heart Assoc. 2024, 13, e031981. [Google Scholar] [CrossRef]
- Qian, S.; Ugurlu, D.; Fairweather, E.; Toso, L.D.; Deng, Y.; Strocchi, M.; Cicci, L.; Jones, R.E.; Zaidi, H.; Prasad, S.; et al. Developing cardiac digital twin populations powered by machine learning provides electrophysiological insights in conduction and repolarization. Nat. Cardiovasc. Res. 2025, 4, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Shamanna, P.; Erukulapati, R.S.; Shukla, A.; Shah, L.; Willis, B.; Thajudeen, M.; Kovil, R.; Baxi, R.; Wali, M.; Damodharan, S.; et al. One-year outcomes of a digital twin intervention for type 2 diabetes: A retrospective real-world study. Sci. Rep. 2024, 14, 25478. [Google Scholar] [CrossRef]
- Digital Twin Precision Therapy for Type 2 Diabetes Mellitus. 2025. Available online: https://clinicaltrials.gov/study/NCT05181449 (accessed on 9 September 2025).
- Somers, R.; Walkinshaw, N.; Mark Hierons, R.; Elliott, J.; Iqbal, A.; Walkinshaw, E. Configuration testing of an artificial pancreas system using a digital twin: An evaluative case study. Softw. Test. Verif. Reliab. 2025, 35, e70000. [Google Scholar] [CrossRef]
- Griffiths, M.; Kubeyev, A.; Laurie, J.; Giorni, A.; Zillmann da Silva, L.A.; Sivasubramaniam, P.; Beer, P.; Foster, M.; Biankin, A.V.; Asghar, U. Transforming cancer care and therapeutic development by predicting individual patient responses to treatment. In Proceedings of the 36th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Abstract Book, Barcelona, Spain, 23–25 October 2024; Available online: https://event.eortc.org/ena2024/wp-content/uploads/sites/33/2024/10/ENA-2024-Abstracts.pdf (accessed on 9 September 2025).
- Sandrone, S. Digital twins in neuroscience. J. Neurosci. 2024, 44. [Google Scholar] [CrossRef]
- Fekonja, L.S.; Schenk, R.; Schröder, E.; Tomasello, R.; Tomšič, S.; Picht, T. The digital twin in neuroscience: From theory to tailored therapy. Front. Neurosci. 2024, 18, 1454856. [Google Scholar] [CrossRef]
- Andres, A.; Roland, M.; Wickert, K.; Diebels, S.; Stöckl, J.; Herrmann, S.; Reinauer, F.; Leibinger, R.; Pavlov, A.; Schuppener, L.; et al. Advantages of digital twin technology in orthopedic trauma Surgery–Exploring different clinical use cases. Sci. Rep. 2025, 15, 19987. [Google Scholar] [CrossRef]
- Diniz, P.; Grimm, B.; Garcia, F.; Fayad, J.; Ley, C.; Mouton, C.; Oeding, J.F.; Hirschmann, M.T.; Samuelsson, K.; Seil, R. Digital twin systems for musculoskeletal applications: A current concepts review. Knee Surgery Sport. Traumatol. Arthrosc. 2025, 33, 1892–1910. [Google Scholar] [CrossRef] [PubMed]
- Moyaux, T.; Liu, Y.; Bouleux, G.; Cheutet, V. An Agent-Based Architecture of the Digital Twin for an Emergency Department. Sustainability 2023, 15, 3412. [Google Scholar] [CrossRef]
- Davahli, M.R.; Karwowski, W.; Taiar, R. A System Dynamics Simulation Applied to Healthcare: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5741. [Google Scholar] [CrossRef]
- Bouleux, G.; Bril El Haouzi, H.; Cheutet, V.; Demesure, G.; Derigent, W.; Moyaux, T.; Trilling, L. Requirements for a Digital Twin for an Emergency Department. In Service Oriented, Holonic and Multi-Agent Manufacturing Systems for Industry of the Future; Studies in Computational Intelligence; Springer: Cham, Switzerland, 2023; pp. 130–141. [Google Scholar] [CrossRef]
- Mutashar, H.Q.; Mahmoud, S.M.; Abu-Alsaad, H.A. Cloud-based Digital Twin Framework and IoT for Smart Emergency Departments in Hospitals. Eng. Technol. Appl. Sci. Res. 2025, 15, 22269–22277. [Google Scholar] [CrossRef]
- Xue, J.; Li, Z.; Zhang, S. Multi-resource Constrained Elective Surgical Scheduling with Nash Equilibrium toward Smart Hospitals. Sci. Rep. 2025, 15, 3946. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, M.H.; Song, Y.S.; Lee, J.G.; Park, J.Y.; Li, K.J. Building an Indoor Digital Twin—A Use-Case for a Hospital Digital Twin to Analyze COVID-19 Transmission. ISPRS Int. J. Geo-Inf. 2024, 13, 460. [Google Scholar] [CrossRef]
- Boschert, S.; Rosen, R. Digital Twin—The Simulation Aspect. In Mechatronic Futures: Challenges and Solutions for Mechatronic Systems and Their Designers; Hehenberger, P., Bradley, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–74. [Google Scholar] [CrossRef]
- Xames, M.D.; Topcu, T.G. A Systematic Literature Review of Digital Twin Research for Healthcare Systems: Research Trends, Gaps, and Realization Challenges. IEEE Access 2024, 12, 4099–4126. [Google Scholar] [CrossRef]
- Durão, L.F.C.; Haag, S.; Anderl, R.; Schützer, K.; Zancul, E. Digital twin requirements in the context of industry 4.0. In Proceedings of the 15th IFIP WG 5.1 International Conference on Product Lifecycle Management to Support Industry 4.0, Turin, Italy, 2–4 July 2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 204–214. [Google Scholar] [CrossRef]
- Shamayleh, A.; Awad, M.; Farhat, J. IoT Based Predictive Maintenance Management of Medical Equipment. J. Med. Syst. 2020, 44, 72. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; He, X.; Li, Z. Digital Twin in Healthcare: Recent Updates and Challenges. Digit. Health 2023, 9, 20552076221149651. [Google Scholar] [CrossRef]
- Tekinerdogan, B. On the Notion of Digital Twins: A Modeling Perspective. Systems 2023, 11, 15. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, J.; Qi, Q.; Zhang, M.; Zhang, H.; Sui, F. Digital twin-driven product design, manufacturing and service with big data. Int. J. Adv. Manuf. Technol. 2018, 94, 3563–3576. [Google Scholar] [CrossRef]
- Wang, E.; Tayebi, P.; Song, Y.T. Cloud-Based Digital Twins’ Storage in Emergency Healthcare. Int. J. Networked Distrib. Comput. 2023, 11, 75–87. [Google Scholar] [CrossRef]
- Rücker, N.; Pflüger, L.; Maier, A. Hardware Failure Prediction on Imbalanced Times Series Data. J. Digit. Imaging 2021, 34, 182–189. [Google Scholar] [CrossRef]
- AbdElSalam, M.; Bensalem, S.; Delacourt, A.; He, W.; Katsaros, P.; Kekatos, N.; Ruiz, R.N.; Peled, D.; Ponchant, M.; Ryad, I.; et al. Digital Twin for the Formal Analysis of a Depth of Anaesthesia Controller. Simulation 2025, 101, 3. [Google Scholar] [CrossRef]
- Ortiz-Barrios, M.; Petrillo, A.; Arias-Fonseca, S.; McClean, S.; de Felice, F.; Nugent, C.; Uribe-López, S.A. An AI-Based Multiphase Framework for Improving Mechanical Ventilator Availability in Emergency Departments During Respiratory Disease Seasons: A Case Study. Int. J. Emerg. Med. 2024, 17, 45. [Google Scholar] [CrossRef]
- Ducrée, J. On-board reagent storage and release by solvent-selective, rotationally opened membranes: A digital twin approach. Microfluid. Nanofluidics 2022, 26, 39. [Google Scholar] [CrossRef]
- Koopsen, T.; Gerrits, W.; van Osta, N.; van Loon, T.; Wouters, P.; Prinzen, F.W.; Vernooy, K.; Delhaas, T.; Teske, A.J.; Meine, M.; et al. Virtual Pacing of a Patient’s Digital Twin to Predict Left Ventricular Reverse Remodelling After Cardiac Resynchronization Therapy. EP Eur. 2024, 26, euae009. [Google Scholar] [CrossRef]
- Toften, S.; Kjellstadli, J.T.; Kværness, J.; Pedersen, L.; Laugsand, L.E.; Thu, O.K.F. Contactless and continuous monitoring of respiratory rate in a hospital ward: A clinical validation study. Front. Physiol. 2024, 15, 1502413. [Google Scholar] [CrossRef] [PubMed]
- Huguet, M.; Pehlivan, C.; Ballereau, F.; Dodane-Loyenet, A.; Fontanili, F.; Garaix, T.; Yordanov, Y.; Augusto, V.; Tazarourte, K.; Redjaline, A. Indoor positioning systems provide insight into emergency department systems enabling proposal of designs to improve workflow. Commun. Med. 2025, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Stites, M.; Surprise, J.; McNiel, J.; Northrop, D.; De Ruyter, M. Continuous Capnography Reduces the Incidence of Opioid-Induced Respiratory Rescue by Hospital Rapid Resuscitation Team. J. Patient Saf. 2021, 17, e557–e561. [Google Scholar] [CrossRef]
- Taenzer, A.H.; Pyke, J.B.; McGrath, S.P.; Blike, G.T. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study. Anesthesiology 2010, 112, 282–287. [Google Scholar] [CrossRef]
- Campbell, M.; Piaggio, G.; Elbourne, D.; Altman, D. Consort 2010 statement: Extension to cluster randomised trials. BMJ 2012, 345, e5661. [Google Scholar] [CrossRef]
- Hemming, K.; Haines, T.; Chilton, P.; Girling, A.; Lilford, R. The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 2015, 350, h391. [Google Scholar] [CrossRef]
- Wagner, A.; Soumerai, S.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef]
- Bernal, J.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Liu, X.; Rivera, S.C.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Ashrafian, H.; Beam, A.L.; Chan, A.W.; Collins, G.S.; Deeks, A.D.J.; et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Lancet Digit. Health 2020, 2, e537–e548. [Google Scholar] [CrossRef]
- Rivera, S.C.; Liu, X.; Chan, A.W.; Denniston, A.K.; Calvert, M.J.; Ashrafian, H.; Beam, A.L.; Collins, G.S.; Darzi, A.; Deeks, J.J.; et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Lancet Digit. Health 2020, 2, e549–e560. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc, G.; Davies, L.; Goodman, D.; Batalden, P.; Davidoff, F.; Stevens, D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual. Saf. 2016, 25, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.F.; Moons, K.G.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Group†, P. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Carbonaro, A.; Marfoglia, A.; Nardini, F.; Mellone, S. CONNECTED: Leveraging digital twins and personal knowledge graphs in healthcare digitalization. Front. Digit. Health 2023, 5, 1322428. [Google Scholar] [CrossRef]
- Marfoglia, A.; Nardini, F.; Arcobelli, V.A.; Moscato, S.; Mellone, S.; Carbonaro, A. Towards Real-World Clinical Data Standardization: A Modular FHIR-Driven Transformation Pipeline to Enhance Semantic Interoperability in Healthcare. Comput. Biol. Med. 2025, 187, 109745. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.C.; Rose, A.; DeMarco, K.R.; Dawson, J.R.D.; Han, Y.; Jeng, M.T.; Harvey, R.D.; Santana, L.F.; Ripplinger, C.M.; Vorobyov, I.; et al. A Multiscale Predictive Digital Twin for Neurocardiac Modulation. J. Physiol. 2023, 601, 3789–3812. [Google Scholar] [CrossRef]
- Sel, K.; Hawkins-Daarud, A.; Chaudhuri, A.; Osman, D.; Bahai, A.; Paydarfar, D.; Willcox, K.; Chung, C.; Jafari, R. Survey and Perspective on Verification, Validation, and Uncertainty Quantification of Digital Twins for Precision Medicine. NPJ Digit. Med. 2025, 8, 40. [Google Scholar] [CrossRef]
- Tortora, M.; Pacchiano, F.; Ferraciolli, S.F.; Criscuolo, S.; Gagliardo, C.; Jaber, K.; Angelicchio, M.; Briganti, F.; Caranci, F.; Tortora, F.; et al. Medical Digital Twin: A Review on Technical Principles and Clinical Applications. J. Clin. Med. 2025, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Grieb, N.; Schmierer, L.; Kim, H.U.; Strobel, S.; Schulz, C.; Meschke, T.; Kubasch, A.S.; Brioli, A.; Platzbecker, U.; Neumuth, T.; et al. A Digital Twin Model for Evidence-Based Clinical Decision Support in Multiple Myeloma Treatment. Front. Digit. Health 2023, 5, 1324453. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Lim, B.; Kwon, O.S.; Kim, M.H.; Kim, D.; Park, J.W.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Joung, B.; et al. Clinical Usefulness of Digital Twin-Guided Virtual Amiodarone Test in Patients with Atrial Fibrillation Ablation. NPJ Digit. Med. 2024, 7, 297. [Google Scholar] [CrossRef]
- Madni, A.M.; Madni, C.C.; Lucero, S.D. Leveraging Digital Twin Technology in Model-Based Systems Engineering. Systems 2019, 7, 7. [Google Scholar] [CrossRef]
- Mills, G.; Wilson, D.; Symons, A.; Roufaeel, I.; Scully, P.; Shanks, D.; Bhaskar, C.; Eames, I.; Jayaram, H.; Sivaprasad, S.; et al. Iterative Digital Twin Development for High-Technology Healthcare Buildings: The case of a new ophthalmology diagnostic hub. In Routledge Handbook of Smart Built Environment; Routledge: London, UK, 2025; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003383840-11/iterative-digital-twin-development-high-technology-healthcare-buildings-grant-mills-duncan-wilson-anne-symons-irinie-roufaeel-peter-scully-david-shanks-cephas-bhaskar-ian-eames-hari-jayaram-sobha-sivaprasad-paul-foster (accessed on 9 September 2025).
- Pellegrino, G.; Gervasi, M.; Angelelli, M.; Corallo, A. A Conceptual Framework for Digital Twin in Healthcare: Evidence from a Systematic Meta-Review. Inf. Syst. Front. 2024, 27, 7–32. [Google Scholar] [CrossRef]
- Huang, P.h.; Kim, K.h.; Schermer, M. Ethical Issues of Digital Twins for Personalized Health Care Service: Preliminary Mapping Study. J. Med. Internet Res. 2022, 24, e33081. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Tshinanu, F.M. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef]
- Pathmanathan, P.; Gray, R.A. Ensuring reliability of safety-critical clinical applications of computational cardiac models. Front. Physiol. 2013, 4, 358. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, P.; Strocchi, M.; Bishop, M.J.; Boyle, P.M.; Plank, G. From bits to bedside: Entering the age of digital twins in cardiac electrophysiology. Europace 2024, 26, euae295. [Google Scholar] [CrossRef] [PubMed]
- Pathmanathan, P.; Cordeiro, J.M.; Gray, R.A. Comprehensive uncertainty quantification and sensitivity analysis for cardiac action potential models. Front. Physiol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Popescu, D.M.; Shade, J.K. Machine learning in arrhythmia and electrophysiology. Circ. Res. 2021, 128, 544–566. [Google Scholar] [CrossRef]
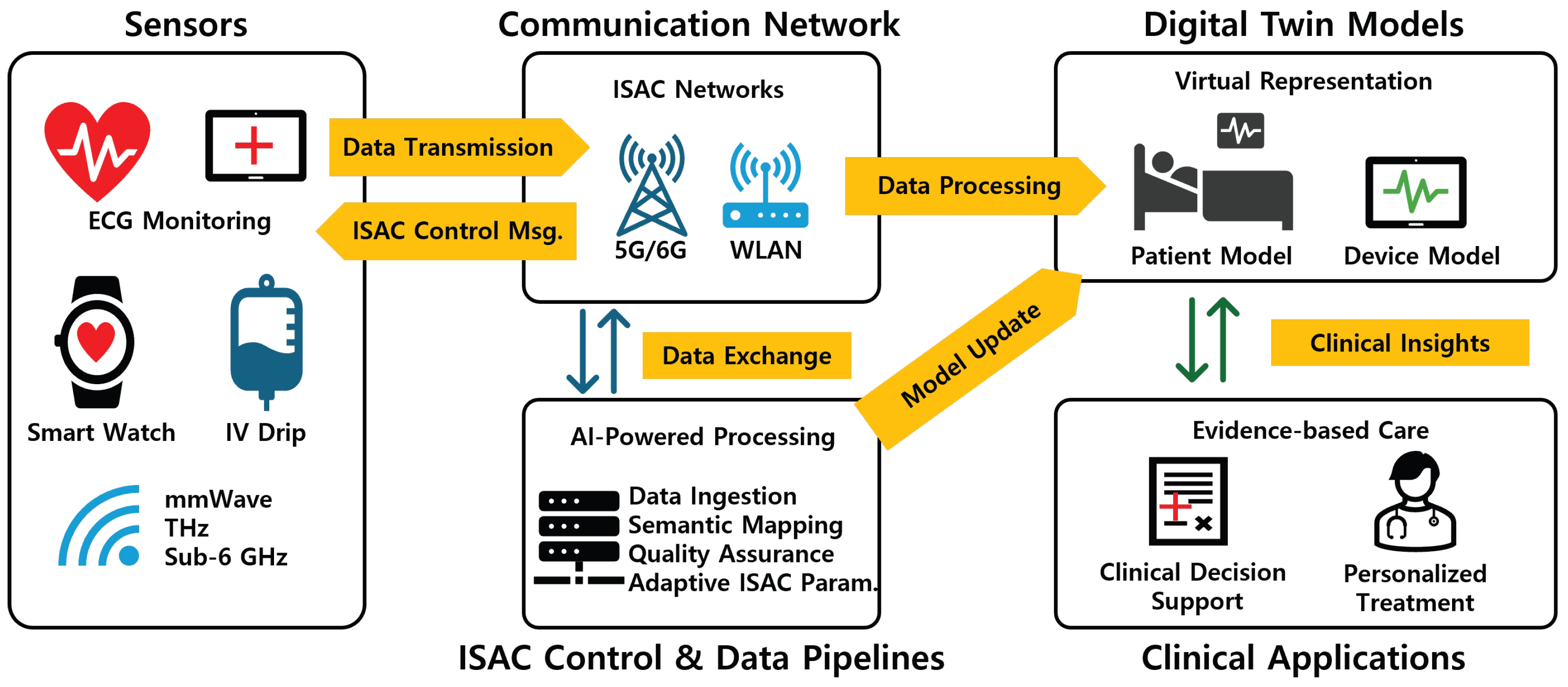
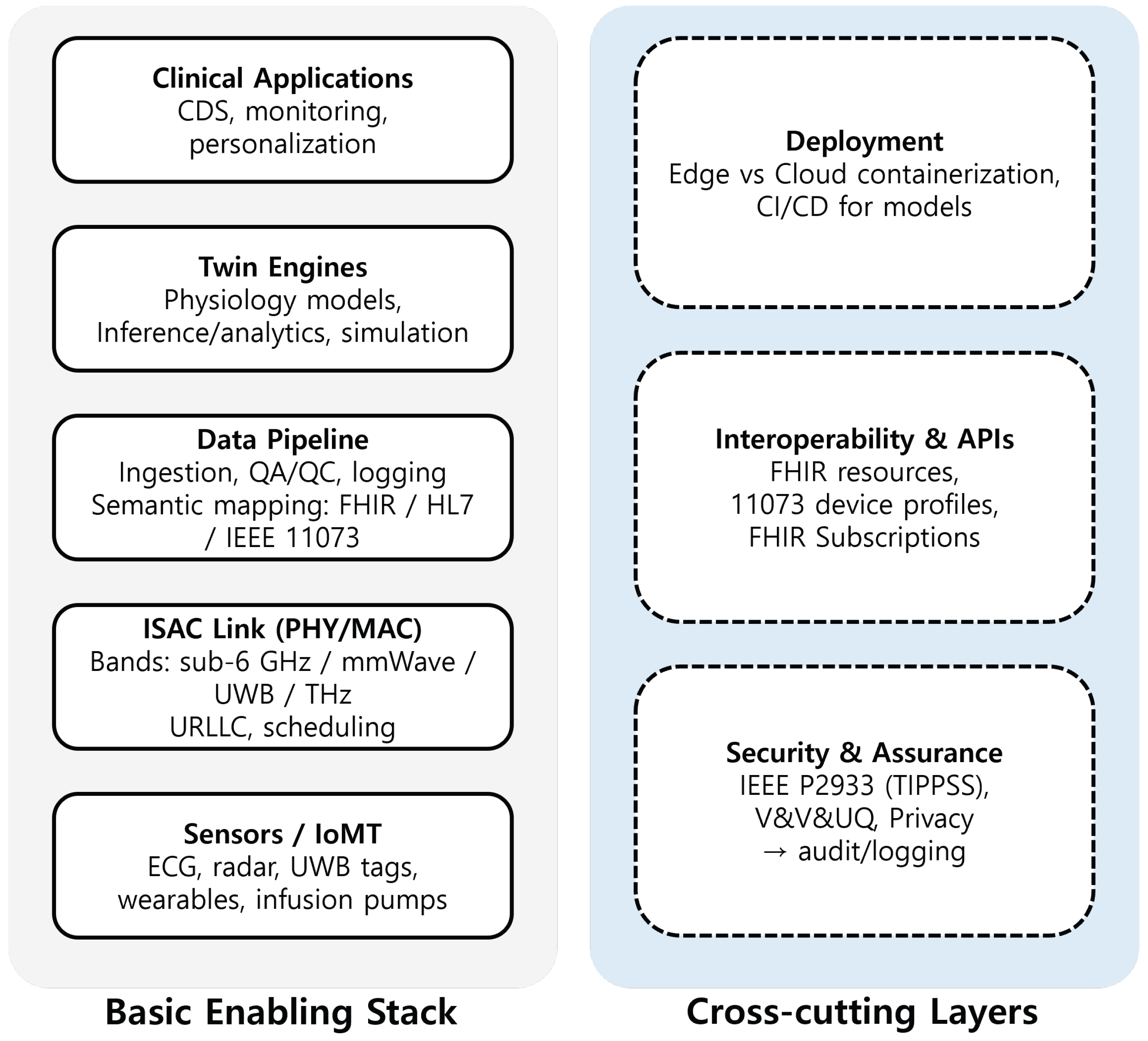
| Domain | DT | ISAC | ISAC–DT | Other | Total |
|---|---|---|---|---|---|
| Healthcare | 24 | 9 | 4 | 38 | 75 |
| Non-healthcare | 21 | 57 | 6 | 59 | 143 |
| Total | 45 | 66 | 10 | 97 | 218 |
| Domain | RF/Radar Sensing | Comm. Tech | General ISAC | Total |
|---|---|---|---|---|
| Healthcare | 8 | 0 | 1 | 9 |
| Non-healthcare | 14 | 24 | 19 | 57 |
| Total | 22 | 24 | 20 | 66 |
| Database | DT | ISAC | ISAC–DT | Other | Total |
|---|---|---|---|---|---|
| Elsevier | 16 | 3 | 1 | 12 | 32 |
| IEEE | 4 | 24 | 3 | 6 | 37 |
| IEEE Xplore | 2 | 26 | 2 | 7 | 37 |
| Springer | 11 | 2 | 0 | 8 | 21 |
| PubMed | 9 | 0 | 0 | 3 | 12 |
| ACM | 0 | 6 | 0 | 7 | 13 |
| MDPI | 3 | 5 | 1 | 8 | 17 |
| Year | DT | ISAC | ISAC–DT | Other | Total |
|---|---|---|---|---|---|
| 2020 | 3 | 1 | 0 | 2 | 6 |
| 2021 | 5 | 2 | 0 | 3 | 10 |
| 2022 | 7 | 10 | 1 | 6 | 24 |
| 2023 | 8 | 15 | 2 | 9 | 34 |
| 2024 | 9 | 20 | 3 | 12 | 44 |
| 2025 | 13 | 18 | 4 | 21 | 56 |
| Total | 45 | 66 | 10 | 53 | 218 |
| Area | Observed Limitation in Prior Work | Research Need/Direction | Where Covered |
|---|---|---|---|
| External validity |
|
| Section 4.5.2 |
| DT synchronization |
|
| Section 6 Section 4.5.3 |
| Latency-aware inference |
|
| Section 6 Section 6.3 |
| Model calibration & VVUQ |
|
| Section 6.6 |
| Semantic interoperability |
|
| Section 4.5.3 |
| Security, privacy, and safety |
|
| Section 4.5.1 Section 4.6.5 Section 4.6.4 |
| Spectrum and hardware limits |
|
| Section 4.3.3 Section 4.1.3 Section 4.2.3 Section 4.5.1 |
| Organizational adoption |
|
| Section 6.5 |
| Equity and infrastructure |
|
| Section 4.5.4 |
| Frequency Band | Typical Sensing Resolution | Penetration Through Tissue/Obstacles | Usable Bandwidth | Approx. Hardware Cost (2025) | Safety Metric and Compliance | TRL |
|---|---|---|---|---|---|---|
| mmWave (30–300 GHz) | Medium (centimeter level) | Moderate (LOS preferred) | 2–4 GHz per channel | Moderate (phased-array RFICs) | Power density ≤ 10 W m−2 | 6–7 |
| THz (0.1–10 THz) | High (sub-millimeter) | Low (surface only) < 1 mm in tissue | 10–100 GHz per channel | High (lab prototypes) | Power density ≤ 1 W m−2 | 3–4 |
| Sub-6 GHz | Low (decimeter level) | High (good wall and tissue penetration) | ≤200 MHz per channel | Low (commodity chipsets) | SAR-based, within ICNIRP limits | 8–9 |
| Band (§ Link) | Specific Examples/Techniques | Key Characteristics |
|---|---|---|
| mmWave (30–300 GHz; see Section 4.1.2) | ||
| Vital-sign monitoring | Contact-free breathing and pulse estimation at 60/77 GHz | Accuracy comparable to that of clinical contact sensors (reported prototypes/studies) |
| Gait analysis and fall detection | Fine-grained human motion tracking; gait symmetry/stride measures | Useful for older adults or patients with limited mobility |
| Sleep monitoring | Respiration, body motion, and heart-related signals during sleep | PSG-comparable metrics without electrodes/belts |
| Indoor localization | Centimeter-level localization/tracking (patients/staff/equipment) | Feeds operational digital twins for workflow/resource optimization |
| High-volume data transfer | Streaming of high-volume medical data (e.g., real-time sensor feeds and raw MRI/CT image streams) | Abundant bandwidth for timely twin synchronization |
| THz (0.1–10 THz; see Section 4.2.2) | ||
| Biomedical imaging (non-invasive) | THz imaging for skin/thermal wound/dental/lesion assessment | Non-ionizing; sensitive to water-content changes |
| Spectroscopy for biosensing | Molecular/biomarker sensing; hydration assessment via THz spectroscopy | Distinct spectral signatures enable identification |
| Ultra-high-resolution vital-sign monitoring | Vital-sign sensing with very fine spatial/temporal granularity | Ultra-fine sensing resolution for physiology-aware DT inputs |
| Physical-layer security | Directionality and limited propagation range studied for PLS | Reflection-path risks noted; security aspects investigated |
| Ultra-high-speed data links | 0.22 THz link, achieving 84 Gb/s over 1.26 km | Real-time large DT datasets/uncompressed video support |
| Sub-6 GHz (see Section 4.3.2) | ||
| Facility-wide monitoring | Tracking of movement/utilization/occupancy across wards/facilities | Facility-level digital-twin components |
| Vital-sign sensing | Respiration/heart-related sensing at several meters, even through obstacles | Room/home tolerance with obstacle penetration |
| Human activity recognition | ADL/mobility detection; fall alerts | Enriches patient digital twins with behavioral context |
| Medical device connectivity | Connectivity for wearables and environmental sensors | Standards/protocols facilitate interoperability and system integration |
| Remote patient monitoring | Reliable updates in challenging or rural environments | Greater range/reliability than higher bands |
| DT Scope | Specific Applications/Cases | Reported Evidence/Key Outcomes |
|---|---|---|
| Patient-centric digital twins—see Section 5.1.3 | ||
| Cardiovascular (patient-level DT) | Digital twins that integrate cardiac imaging, electrophysiology, and biophysical simulation | Personalization of pacemaker programming and therapy selection; population-scale heart-twin cohorts discussed |
| Diabetes management | Real-world study of the Twin Health platform | Twelve-month real-world study reporting HbA1c reduction (mean decrease reported) |
| Oncology | FarrSight®-Twin-type oncology twins that generate virtual patients for therapy optimization | Replication of phase-II/III trial outcomes and improved therapy optimization outlined |
| Neurology | Brain-twin framework fusing neuro-imaging, genetics, and neural models | Pathway from in silico cortex models to personalized neuromodulation |
| Orthopedics | Musculoskeletal/orthopedic twins for gait analysis, rehabilitation, and implant design | Case-study evidence showing applicability to surgeon planning/implant design |
| Healthcare facility/system digital twins—see Section 5.2.3 | ||
| Emergency department flow | Twins model patient arrivals, triage processes, treatment flows, and discharge pathways | Surge-scenario simulation; optimized staff utilization reported |
| Operating room management | Twins simulate surgical scheduling, procedure durations, turnover times, and resource needs | Robust scheduling and throughput under uncertainty described |
| Infection control | Twins model airflow patterns, contact networks, and facility layouts | Prediction/mitigation of nosocomial transmission discussed |
| Energy/sustainability | Twins optimize HVAC operations, lighting, and related building systems | Energy-efficiency gains and operational-cost impacts outlined |
| Medical device and equipment digital twins—see Section 5.3.3 | ||
| Imaging/ultrasound systems | Digital twins monitor critical components (e.g., transducers) | Predictive maintenance/reliability improvements described |
| Infusion/delivery devices | Twins track medication delivery accuracy and battery/pressure health | Remote performance monitoring and anomaly detection reported |
| Ventilation/gas delivery | Twins support optimization of gas delivery | Remote monitoring and performance optimization described (including pandemic-period operations) |
| Clinical lab/analyzers | Laboratory automation systems digitally twinned | Reagent-use optimization and maintenance of analytical quality discussed |
| Implantable/therapeutic devices | Pacemakers, defibrillators, and neurostimulators with DT-enabled monitoring | Shift from reactive to proactive maintenance/therapy management noted |
| Use Case and Setting | Sample/Period and Design | Key Numeric Findings |
|---|---|---|
| Contactless respiratory rate (UWB radar) Setting: University hospital emergency ward (adults at rest) | Sample/period: Duration: median of 42 min Design: Method comparison vs. reference (Nox T3s) | Bias of – breaths/min 95% limits of agreement breaths/min Trend concordance of No missed/false clinical alarms No gap min [221] |
| ED staff indoor positioning (UWB IPS) Setting: French Level-1 Emergency Department | Sample/period: 46 days Tags: 27 Design: Observational tracking + ML classifier | Doctors’ care-related time share of 26– Triage/ICU nurses Nurses’ walking distance rises with occupancy Job-category classifier accuracy of [222] |
| Continuous monitoring and outcomes (capnography/oximetry) Setting: Med-surg wards (PCA opioids) | Period: 2012–2015 Design: Before/after (capnography rollout) | Opioid-induced respiratory rescue incidence Transfers to higher level of care reduced by (7.6→1.6 per month) [223] |
| Continuous pulse-ox surveillance and rescue/ICU transfer Setting: Postoperative units | Period: 11 mo before/10 mo after Design: Before/after (surveillance with pager alerts) | Rescue events per 1000 discharges ICU transfers per 1000 patient-days (Comparison units: no change) [224] |
| DT for flow (simulation/pilots) Setting: Hospital ED/wards (various) | Design: Simulation/pilot reports | DT models validated against site data Scenarios report reduced waiting time and LOS (Simulation evidence; limited real-world endpoints) [63] |
| Item | Requirement |
|---|---|
| Design |
|
| Population |
|
| Intervention |
|
| Comparator |
|
| Outcomes |
|
| Metrics |
|
| Operational |
|
| Generalization |
|
| Safety |
|
| Governance |
|
| Economics |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Oh, S.; Kim, G. Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review. Signals 2025, 6, 51. https://doi.org/10.3390/signals6040051
Kim Y, Oh S, Kim G. Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review. Signals. 2025; 6(4):51. https://doi.org/10.3390/signals6040051
Chicago/Turabian StyleKim, Youngboo, Seungmin Oh, and Gayoung Kim. 2025. "Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review" Signals 6, no. 4: 51. https://doi.org/10.3390/signals6040051
APA StyleKim, Y., Oh, S., & Kim, G. (2025). Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review. Signals, 6(4), 51. https://doi.org/10.3390/signals6040051







