Abstract
Background: Colorectal cancer (CRC) represents one of the leading causes of cancer-related morbidity and mortality globally. Although national screening programs in Europe and the United States have demonstrated success in reducing incidence and death rates among populations aged 50 and above, a concerning increase in early-onset colorectal cancer (EOCRC), defined as diagnosis before age 50, has emerged. Methods: This paper is a narrative literature review comparing American and European CRC screening guidelines. A comprehensive search was conducted using the PubMed database with emphasis on publications from the past ten years. Results: The United States has adapted more swiftly to EOCRC trends by lowering the recommended screening age to 45, supported by modeling studies showing life-years gained and improved cost-effectiveness. In contrast, European programs remain largely organized and cost-efficient but predominantly initiate screening at age 50, potentially missing high-risk younger adults. EOCRC appears to demonstrate unique molecular and pathological features compared to late-onset CRC. Participation and adherence to screening also vary significantly between regions and modalities, with colonoscopy remaining the gold standard but less scalable than fecal immunochemical tests. Conclusions: The rising incidence of EOCRC calls for a reassessment of CRC screening policies. While the European model emphasizes equity and structure, its slower responsiveness to epidemiological changes may lead to late detection in younger cohorts. The American model’s earlier screening age addresses emerging trends but faces challenges in implementation equity. A hybrid approach may provide the optimal management, balancing public health benefit with system sustainability.
1. Introduction
Colorectal cancer (CRC) remains a significant global public health concern. It is the third most commonly diagnosed malignancy in males, following prostate and lung cancer, and ranks also third in females, after breast and lung cancers [1]. In Europe, approximately 290,000 males and 240,000 females were diagnosed in 2022, and the statistics estimate that the incidence will rise by 34% and 24.5% respectively [1]. Furthermore, despite notable advancements in therapeutic strategies, CRC continues to account for approximately 10% of all cancer-related mortality worldwide [1]. In North America, the incidence of CRC in 2022 was considerably lower, with approximately 97,000 cases in males and 86,000 in females. The implementation of national screening programs, alongside increased colonoscopy rates and improvements in diet and lifestyle, has contributed to a stabilization or even decline in incidence in many developed nations [2]. However, studies conducted in recent years have reported an increasing incidence of CRC among individuals under the age of 50—defined as early-onset CRC (EOCRC)—a population that, until recently, had not been included in screening programs for this malignancy [3,4]. Moreover, the number of EOCRC cases is increasing in both low- and high-income countries [5]. While the underlying causes of this trend remain unclear, several factors are being considered, including genetic predisposition, lifestyle behaviors, obesity, and environmental exposures [5].
Numerous risk factors were found to be associated with CRC and they can be broadly categorized as modifiable and non-modifiable [6]. Tobacco use, alcohol consumption, obesity, a diet rich in saturated fatty acids and deficient in fruits and vegetables, sedentary behavior, diabetes and gut microbiota constitute important modifiable risk factors [6,7]. Among the non-modifiable risk factors are ethnicity, male sex, advanced age, a range of hereditary mutations, inflammatory bowel diseases, cystic fibrosis, acromegaly, history of cholecystectomy, and exposure to androgenic therapies [6].
By 2002, a financial analysis of the US Preventive Service Task Force demonstrated that CRC screening was considered cost-effective compared to no screening; however, no single primary strategy was identified as superior [8]. The standardized effort to implement CRC screening in Europe began in 2003, when the Council of the European Union issued its first recommendation for population-based screening, alongside programs for breast and cervical cancer [9]. Although countries like Germany and the Czech Republic had already initiated their own screening efforts prior to this, the 2003 recommendation marked a pivotal moment in promoting CRC screening across the entire European region [10]. By 2016, 22 of 28 EU member states had implemented organized, population-based CRC screening programs, signaling the effort to combat the high mortality of CRC complications [11]. Efforts to launch CRC screening programs continue into 2025, as demonstrated by Romania’s ROCCAS 3 pilot study, which is funded by the European Union [12].
As of 2025, the European Commission recommends initiating CRC screening at age 50 and continuing until age 75, based on epidemiological evidence, risk–benefit evaluations, and cost-effectiveness analyses [13]. However, increasing attention has been directed toward EOCRC, following numerous studies reporting a rising incidence in individuals under the age of 50, largely attributed to lifestyle and dietary changes [4,5,14,15]. In response to this trend, the American Cancer Society revised its guidelines in 2018 to lower the recommended screening initiation age to 45 years, aiming to mitigate the rising burden of EOCRC [14].
The primary objective of our study is to evaluate the effectiveness of the American CRC screening programs in comparison to the European guidelines and to provide recommendations in light of the alarming increase in EOCRC incidence.
2. Results
2.1. Early-Onset Colorectal Cancer (EOCRC)
In recent decades, there has been ongoing debate among experts regarding whether EOCRC constitutes a distinct biological subtype of CRC or merely represents the same malignancy manifesting at a younger age [15,16,17,18,19,20]. Approximately 20% of EOCRC cases exhibit microsatellite instability (MSI), a molecular hallmark commonly associated with hereditary nonpolyposis colorectal cancer (HNPCC), comparable to the 10–15% prevalence reported in sporadic CRC overall [17,18,19,20,21]. However, the underlying mechanisms of MSI appear to differ by age group: in late-onset CRC (LOCRC), MSI typically results from MLH1 gene silencing through promoter hypermethylation—an acquired and non-inheritable event observed in up to 75% of cases [19]. Conversely, in younger patients, MSI is more frequently attributable to germline mutations linked to Lynch syndrome [19].
While MSI-positive tumors are generally characterized by indolent behavior and proximal colonic location, this classical pattern is not consistently observed in EOCRC [21]. Instead, EOCRC is more frequently associated with unfavorable histopathological features, including poor differentiation, mucinous architecture, and the presence of signet ring cells—traits commonly found in hereditary CRC syndromes [22]. Additionally, individuals with EOCRC show an increased propensity for developing synchronous or metachronous tumors and a higher incidence of polyp formation during surveillance colonoscopy [23]. As previously noted, EOCRC tends to predominantly affect the distal colon and rectum [24].
The risk factors associated with EOCRC are largely similar to those identified in CRC overall and include familial cancer syndromes, dietary patterns, chronic inflammatory conditions, tobacco use, alcohol consumption, obesity, ethnicity, and physical inactivity [25]. Among these, obesity plays a particularly important role in CRC pathogenesis, as it promotes chronic inflammation within visceral adipose tissue and contributes to insulin resistance—both of which are known to enhance oxidative stress and pro-tumorigenic pathways [26]. Consequently, regular physical activity is regarded as a protective factor in the prevention of colon cancer, though its protective effect appears less pronounced in rectal cancer [27]. Tobacco-related carcinogens may reach the colorectal epithelium either through ingestion or via systemic circulation, potentially contributing to carcinogenesis by inducing deoxyribonucleic acid (DNA) damage or disrupting the expression of key oncogenes and tumor suppressor genes [28]. Epidemiological studies exploring the association between cigarette smoking and CRC incidence have identified correlations with specific molecular subtypes, including microsatellite instability-high (MSI-high), CpG island methylator phenotype-positive (CIMP-positive), and BRAF-mutated CRC [28]. These observations suggest that epigenetic mechanisms may play a significant role in the pathogenesis of smoking-related CRC [28].
EOCRC is frequently diagnosed at more advanced stages, a phenomenon largely attributed to the absence of standardized screening programs for individuals under the age of 50—except in cases with a known familial history of the disease [29]. Moreover, the presence of a distinct genetic profile, coupled with aggressive histopathological characteristics, may contribute to the accelerated progression of the neoplasm [30]. The prognostic assessment of CRC in patients under 50 years of age remains controversial. While some studies suggest a poorer overall prognosis in this population, others report comparable five-year survival rates to those observed in patients diagnosed after the age of 50, highlighting the need for further research to clarify these discrepancies [31].
2.2. Advantages and Disadvantages of the Screening Programme
The burden of CRC among young adults is increasing at an alarming rate, with a significant rise in diagnoses made at advanced or metastatic stages [32]. This trend cannot be solely attributed to detection bias, as multiple studies have demonstrated a disproportionate increase in advanced-stage EOCRC relative to localized disease [33]. The determinants of disease susceptibility appear to have evolved over time, as evidenced by the rising incidence of CRC among white adults aged 50 to 54 years since 2005—an age group traditionally eligible for screening—thereby reversing a long-standing decline. This shift is largely attributed to a pronounced birth cohort effect, beginning with individuals born in the 1950s, who, along with subsequent generations, are anticipated to experience elevated CRC risk as they age [34].
Furthermore, mortality rates among young adults with CRC are on the rise, contrary to expectations based on improved screening and early detection initiatives [35]. Notably, the median overall survival for patients with EOCRC remains lower than that of their older counterparts [35]. Importantly, findings by Chen et al. indicate that late-stage presentation in EOCRC is not associated with delays in symptom onset or diagnosis, underscoring the potential benefit of initiating screening protocols at earlier ages to improve prognosis in younger populations [36].
Historically, individuals aged 50–55 years were considered to be at a higher risk of CRC compared to those aged 45–49 [37]. However, the screening uptake in the older age group is nearly double that observed in the younger cohort, prompting the hypothesis that the actual risk between these two age groups may be more similar than previously believed [37]. Additionally, the rate of increase in CRC incidence among younger generations is nearly twice that observed in older populations, reinforcing growing concerns regarding the rising prevalence of EOCRC [38].
Clinical trials have demonstrated that a single negative sigmoidoscopy or colonoscopy provides prolonged protection against CRC, with protective effects lasting up to 17 and 20 years, respectively—currently the longest follow-up durations reported in the literature [39]. An analysis of the American Cancer Society’s CRC screening guidelines reveals a marked shift in strategic focus [40]. The 2018 revision departed from a narrow emphasis on mortality reduction and instead adopted a broader objective: to reduce the overall incidence of CRC and improve long-term quality of life through earlier detection and preventive interventions [40].
Despite the potential advantages of initiating CRC screening at the age of 45, patient compliance remains a critical determinant of program effectiveness. Data from both European countries and the United States indicate screening adherence rates between 40% and 60%, while compliance with follow-up recommendations following diagnosis reaches approximately 80% [41,42,43]. Predictive modeling conducted by Chen et al. demonstrated that suboptimal patient adherence may substantially diminish the anticipated reductions in CRC-related mortality and the survival benefits associated with earlier detection [44].
It is important to note that these predictive models are based exclusively on patient age, without incorporating additional variables such as sex, family history, dietary patterns, obesity, or smoking status [45]. This limitation highlights the need for further research to evaluate the true benefit of earlier screening when accounting for a broader range of risk factors. The overall efficacy of a screening program is heavily dependent on participation rates, which should ideally exceed 80%—a threshold that is often difficult to achieve in real-world settings [46].
Expanding screening to individuals under 50 years of age raises additional concerns, including the potential diversion of resources and clinical attention from older, higher-risk populations to a demographic with comparatively lower absolute risk. Furthermore, the economic burden associated with CRC screening and treatment is considerable. In the United States, the cost per CRC case can exceed $100,000, while across Europe, the total economic impact—including direct medical costs and indirect societal costs—is estimated to surpass €13 billion annually [47,48].
A critical issue in the formulation of CRC screening guidelines is the quality and type of evidence upon which recommendations are based. Ideally, guidelines should be informed by robust data derived from well-designed, reproducible randomized controlled trials (RCTs), which represent the highest standard of clinical evidence [49]. In practice, however, the absence of such data often necessitates reliance on simulation models. While these models are valuable tools, their predictive accuracy is inherently limited by underlying assumptions and projections.
Nevertheless, in the context of limited long-term outcome data for various screening modalities, simulation-based studies remain essential for evaluating the comparative effectiveness of different strategies [50]. Notably, earlier versions of the U.S. Preventive Services Task Force (USPSTF) guidelines have also incorporated modeling analyses, particularly those developed by the Cancer Intervention and Surveillance Modeling Network (CISNET).
2.3. Techniques Used for Screening
A broad array of methods is available for the early detection of CRC, each balancing diagnostic accuracy, invasiveness, cost-efficiency, and patient compliance. International guidelines tailor their recommendations based on population risk, test performance, and healthcare infrastructure.
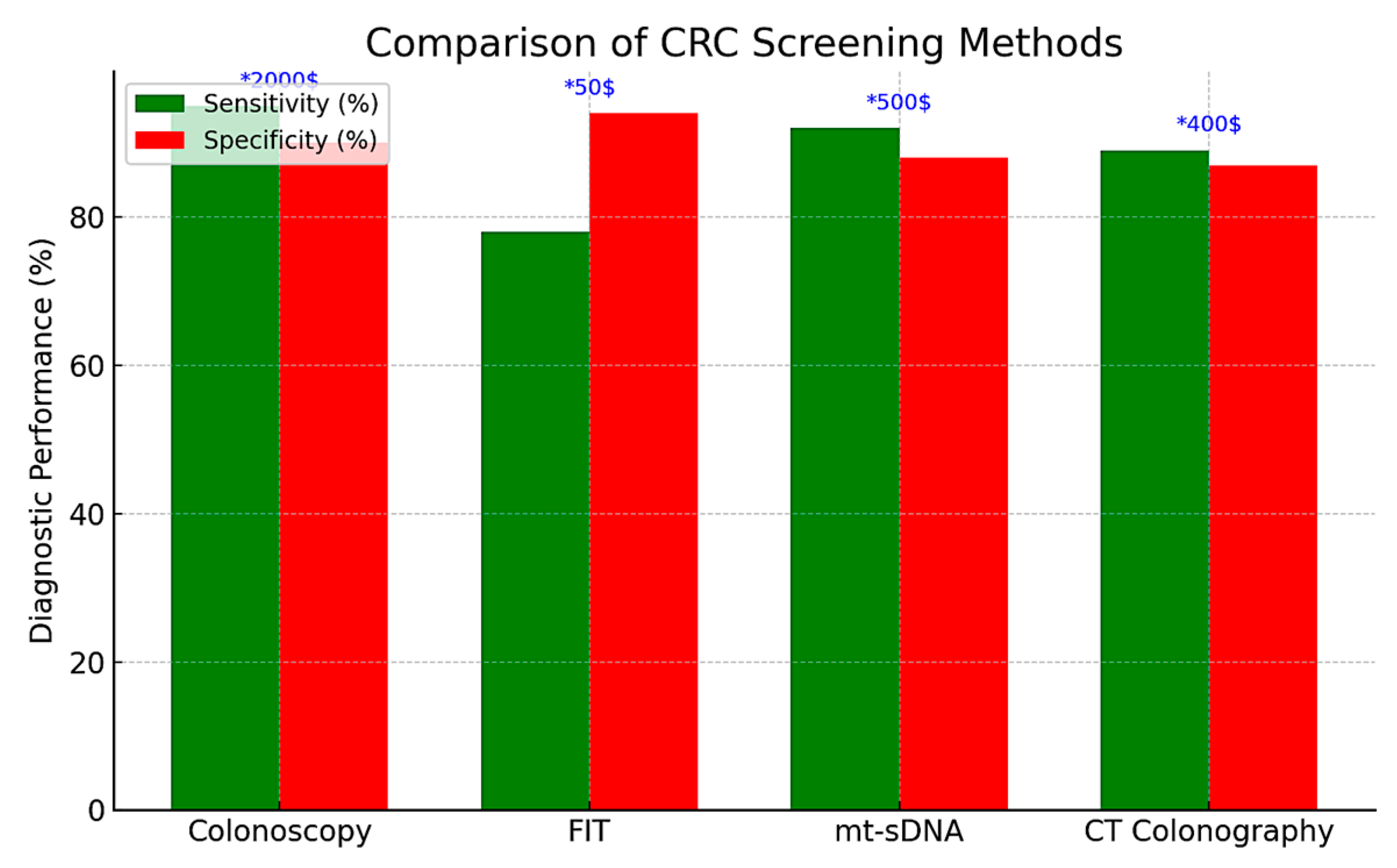
Colonoscopy remains the gold standard for CRC screening in both the United States and Europe, providing the highest sensitivity (approximately 95%) and the distinct advantage of enabling immediate biopsy or polypectomy during the same procedure. It is typically recommended every 10 years for average-risk individuals, starting at age 45 in the United States and at age 50 in most European countries. However, its invasive nature, the requirement for bowel preparation and sedation, as well as its cost (exceeding $2000 per procedure in the U.S.), pose significant limitations to its scalability in population-wide screening programs [51,52].
As a non-invasive alternative, the fecal immunochemical test (FIT) has emerged as the primary screening modality in most European countries. FIT detects occult blood in stool and has largely replaced the older guaiac-based fecal occult blood test (gFOBT) due to its superior sensitivity (approximately 79% for CRC and 30–35% for advanced adenomas), high specificity (94%), and the absence of dietary restrictions [53,54,55]. Its low cost (approximately $50) and ease of use make it particularly suitable for annual or biennial population-based screening programs, enhancing participation rates and offering greater cost-effectiveness—as measured by quality-adjusted life years (QALYs) gained—compared to colonoscopy [53,54,55].
Multitarget stool DNA (mt-sDNA) tests combine the FIT with molecular biomarkers—such as KRAS mutations and methylated BMP3/NDRG4—to improve detection rates. These assays demonstrate high sensitivity for CRC (approximately 92%) and moderate sensitivity for advanced adenomas (42–46%), albeit with slightly reduced specificity (87–89%) and substantially higher costs (approximately $600), factors that hinder their widespread implementation in large-scale screening programs [56,57,58]. Recommended at three-year intervals, mt-sDNA testing is included in the U.S. Preventive Services Task Force (USPSTF) guidelines; however, its adoption remains limited due to inconsistent insurance coverage and concerns regarding affordability.
Computed tomography (CT) colonography, also known as virtual colonoscopy, provides non-invasive imaging of the entire colon and is recommended at five-year intervals according to U.S. screening guidelines. This method demonstrates high sensitivity (over 90%) for detecting larger lesions (≥6 mm), including both CRC and advanced polyps. However, it does not allow for biopsy or polypectomy, often requiring a subsequent conventional colonoscopy when abnormalities are identified. In Europe, the adoption of CT colonography remains limited, largely due to resource constraints and reduced diagnostic accuracy for smaller or flat lesions, for which sensitivity is estimated at approximately 70% [59].
Flexible sigmoidoscopy represents a less invasive and more resource-efficient alternative for examining the distal colon and is recommended at intervals of 5 to 10 years. Although still employed in certain European regions, its major limitation lies in the inability to visualize and detect lesions located in the proximal (right-sided) colon, thereby reducing its overall sensitivity for comprehensive CRC screening [60].
Emerging screening modalities—such as capsule endoscopy and blood-based biomarker assays (e.g., Septin 9)—offer increased convenience but have yet to demonstrate the sensitivity and specificity required for widespread clinical implementation. Capsule endoscopy exhibits sensitivity rates of up to 88% for polyps ≥ 6 mm; however, it demonstrates limited ability to detect serrated lesions and remains costly and largely inaccessible for routine population-based screening [61,62]. Similarly, the Septin 9 blood test has shown modest sensitivity for CRC (approximately 69%) and poor diagnostic performance for detecting adenomas, thereby limiting its utility as a primary screening tool [52].
In summary, effective CRC screening requires the selection of appropriate modalities based on healthcare infrastructure, resource availability, and patient-specific factors (Table 1). Although colonoscopy remains the most comprehensive tool for both diagnosis and intervention, the fecal immunochemical test (FIT) continues to be the preferred method in many European population-based programs due to its cost-effectiveness and ease of implementation. In contrast, the U.S. screening framework includes a wider array of options—such as multitarget stool DNA (mt-sDNA) testing and CT colonography—but faces ongoing challenges related to adherence, uniformity, and equitable access, particularly among younger individuals. Ultimately, selecting the optimal screening strategy necessitates a careful balance between diagnostic performance, economic sustainability, and accessibility to ensure timely detection and reduce CRC-related morbidity and mortality. The comparative evaluation of CRC screening methods is presented in Table 1 and Figure 1.

Table 1.
Comparison between potential screening methods for CRC * [63,64,65,66,67]. (* All prices listed in the table reflect estimates based on costs in the United States. Actual costs may vary in other countries depending on healthcare systems, insurance coverage, and local pricing policies).
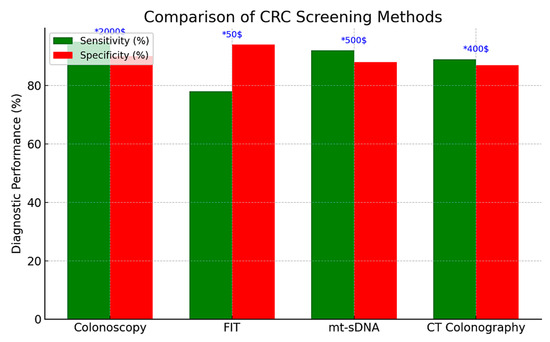
Figure 1.
Comparison of CRC screening methods.
2.4. Screening in Europe
CRC screening in Europe is primarily guided by the European Commission’s recommendation to initiate screening at age 50 and continue until ages 74–75, employing the fecal immunochemical test (FIT) as the principal method or colonoscopy as the most comprehensive diagnostic tool. In countries such as the Netherlands, Slovenia, and the Basque Country (Spain), organized national screening programs have achieved participation rates exceeding 60%, which have been associated with significant reductions in both CRC incidence and mortality [68].
Nonetheless, the implementation of CRC screening remains heterogeneous across the continent. While many Western European countries have established robust, population-based programs, several Eastern European nations either lack organized screening altogether or remain in pilot phases, with participation rates as low as 14% in some regions [69]. These disparities contribute to marked differences in CRC-related outcomes and mortality between regions.
An emerging concern is the increasing incidence of EOCRC. Recent epidemiological data suggest that this trend is not confined to North America; countries such as Germany and France have also reported annual increases in EOCRC among younger adults [70]. A 2019 study by Cardoso et al. highlighted that current age-based screening thresholds may overlook a substantial proportion of younger individuals who could benefit from early detection, particularly in the context of evolving lifestyle and dietary risk factors [71].
Moreover, simulation-based health economic evaluations indicate that lowering the screening initiation age to 45 may be both clinically advantageous and cost-effective. A 2024 modeling study conducted in Germany assessed the impact of including individuals aged 45–49 in the national CRC screening program and reported a notable decrease in incidence rates, alongside improvements in cost-efficiency metrics compared to existing standards [72].
Given the growing burden of EOCRC and the shifting epidemiological landscape, a reevaluation of the current European guidelines to consider earlier screening initiation may represent a critical step toward reducing CRC-related morbidity and mortality.
2.5. Screening in America
In the United States, CRC screening is primarily opportunistic but notably diversified, offering multiple screening modalities to individuals beginning at age 45 for those at average risk. Since 2018, the American Cancer Society (ACS) has recommended initiating screening at age 45—a position subsequently endorsed by the U.S. Preventive Services Task Force (USPSTF) in 2021 through a Grade B recommendation for average-risk adults [73].
Despite these updated guidelines and mandated insurance coverage, screening uptake among individuals aged 45–49 remains suboptimal. As of 2021, only 20% of individuals in this age group were up to date with CRC screening; stool-based tests were markedly underutilized (2.4%), while colonoscopy accounted for the majority of screenings (17.8%) [74]. Furthermore, disparities in screening uptake persist across socioeconomic and racial/ethnic groups, with particularly low participation rates observed among uninsured individuals and certain minority populations [75]. Modeling studies suggest that lowering the screening initiation age increases the demand for colonoscopies—by approximately 810 additional procedures per 1000 individuals screened—but may yield moderate to substantial net gains in life-years saved and long-term cost reductions by facilitating earlier cancer detection and reducing the burden of late-stage treatment [75].
Overall, the U.S. screening model emphasizes early detection through a wide range of available modalities. However, its effectiveness remains limited by persistent inequities in implementation and inconsistent engagement, particularly among newly eligible younger adults.
2.6. Comparative Analysis: Europe vs. America
The key distinction between Europe and the United States in CRC screening lies in the coordination of strategies and responsiveness to emerging epidemiological trends. European countries prioritize structured, population-based programs designed to ensure standardized access and cost-effectiveness, but these systems have been comparatively slower to adapt to the increasing incidence of EOCRC. In contrast, the U.S. has adopted a more flexible and adaptive approach, lowering the recommended screening age to 45 in response to shifting epidemiological data. However, this model continues to face challenges in maintaining consistent participation, particularly among newly eligible younger adults.
This divergence highlights deeper systemic differences: while the European model promotes equity, scalability, and organized outreach, it may lack the agility to promptly adjust to evolving trends. Conversely, the U.S. system allows for rapid policy changes but remains vulnerable to disparities in healthcare access. Notably, the earlier screening threshold implemented in the U.S. is increasingly supported by growing evidence of rising EOCRC incidence, suggesting that a similar approach could be considered in Europe, especially in countries with well-developed healthcare infrastructure capable of accommodating broader age-based screening criteria without undermining program integrity. Key comparative aspects are summarized in Table 2.

Table 2.
Comparison between Europe and United States regarding CRC screening.
3. Discussions
Effective screening must satisfy classical public health criteria, such as those defined by Wilson and Jungner [76]. These criteria state that the disease should be common, detectable in a preclinical phase, and that screening methods must be accurate, cost-effective, and capable of improving clinical outcomes [76].
Screening is a well-established and extensively studied public health strategy that originated in the 20th century in the United States, with the introduction of X-ray testing for tuberculosis and serological testing for syphilis. These initiatives were soon followed by screening programs for diabetes, cardiovascular diseases, and various cancers after World War II [77,78].
Four foundational elements were identified as essential for the development of effective screening programs:
- (1)
- the availability of reliable and valid diagnostic tests
- (2)
- the introduction of effective treatments for screen-detectable diseases
- (3)
- the development of key conceptual tools (e.g., sensitivity, specificity, predictive values)
- (4)
- improved and expanded access to healthcare.
CRC meets these criteria through its high incidence, long asymptomatic phase, the existence of detectable and treatable precursor lesions (e.g., adenomatous polyps), and the strong association between earlier stage at diagnosis and improved survival. Consequently, CRC screening has been described as a “gold standard” model for screening evaluation [79,80].
Furthermore, CRC screening has been shown to be cost-effective, although the optimal strategy continues to be debated [8]. Organized screening programs should incorporate public education, quality assurance measures, and equitable access to diagnostic and therapeutic services. Participation rates above 70% are considered essential to achieve a meaningful population-level impact.
In Europe, current guidelines generally recommend initiating CRC screening at age 50 and continuing until around age 74–75 [81]. However, only a few countries have reconsidered their age thresholds in light of the increasing incidence of EOCRC. Implementation remains heterogeneous across the continent: countries such as the Netherlands, Slovenia, and the United Kingdom have established national FIT-based programs with participation rates exceeding 60%. In contrast, many Eastern European countries still rely on opportunistic or regional programs, with participation rates as low as 14% [5]. These disparities are largely attributable to differences in healthcare infrastructure, funding, and the prioritization of preventive services.
The United States adopts a markedly different approach—screening is primarily opportunistic, offers a broader range of screening modalities, and recommends initiation at age 45 for average-risk individuals. Although more agile and responsive to shifting epidemiological trends, the U.S. system faces persistent challenges in ensuring equitable access across socioeconomic and racial/ethnic groups. European countries may benefit from adopting similar flexibility regarding initiation age—particularly lowering it to 45—while preserving the strengths of their organized, equity-focused programs.
It is also important to highlight the role of hereditary CRC syndromes, such as Lynch syndrome, which require individualized screening strategies. European guidelines recommend colonoscopic surveillance every 1–2 years starting at age 25 for individuals with MutL homolog 1 (MLH1) or MutS homolog 2 (MSH2) mutations, and at around age 35 for those with MutS homolog 6 (MSH6) or Postmeiotic Segregation Increased 2 (PMS2) mutations [82]. The use of high-definition endoscopy or chromoendoscopy is advised to enhance lesion detection [83,84]. These personalized approaches align with the broader trend toward earlier and risk-adapted screening, serving as a model for precision prevention in high-risk populations.
The latest findings from the literature provide additional depth to the ongoing debate on optimal colorectal cancer screening strategies. Le et al. conducted a comprehensive analysis of EOCRC in patients under 40 years and demonstrated that while most cases are sporadic, almost 18% harbored germline mutations, over half of which were linked to Lynch syndrome–associated genes. Moreover, somatic mutations were detected in the vast majority of cases (94%), with TP53, APC, and KRAS being the most frequently altered genes [85]. These results underline the role of genetic predisposition in a subgroup of EOCRC patients and reinforce the scientific rationale for lowering the age of screening initiation. In parallel, Malibary highlighted the Euro-Asian experience with colorectal cancer screening, pointing to both the heterogeneity of regional guidelines and the rapid incorporation of novel diagnostic tools, such as blood-based biomarkers (e.g., methylated SEPT9), multitarget stool DNA/RNA assays, and artificial intelligence-enhanced colonoscopy [86]. These innovations, combined with regional epidemiological data, stress the importance of tailoring screening approaches to population risk profiles and available resources. Taken together, these recent contributions complement our comparative analysis of European and U.S. guidelines, strengthening the rationale for a hybrid screening model that integrates genetic insights, emerging technologies, and context-specific strategies to maximize early detection, public health benefit, and healthcare sustainability.
4. Materials and Methods
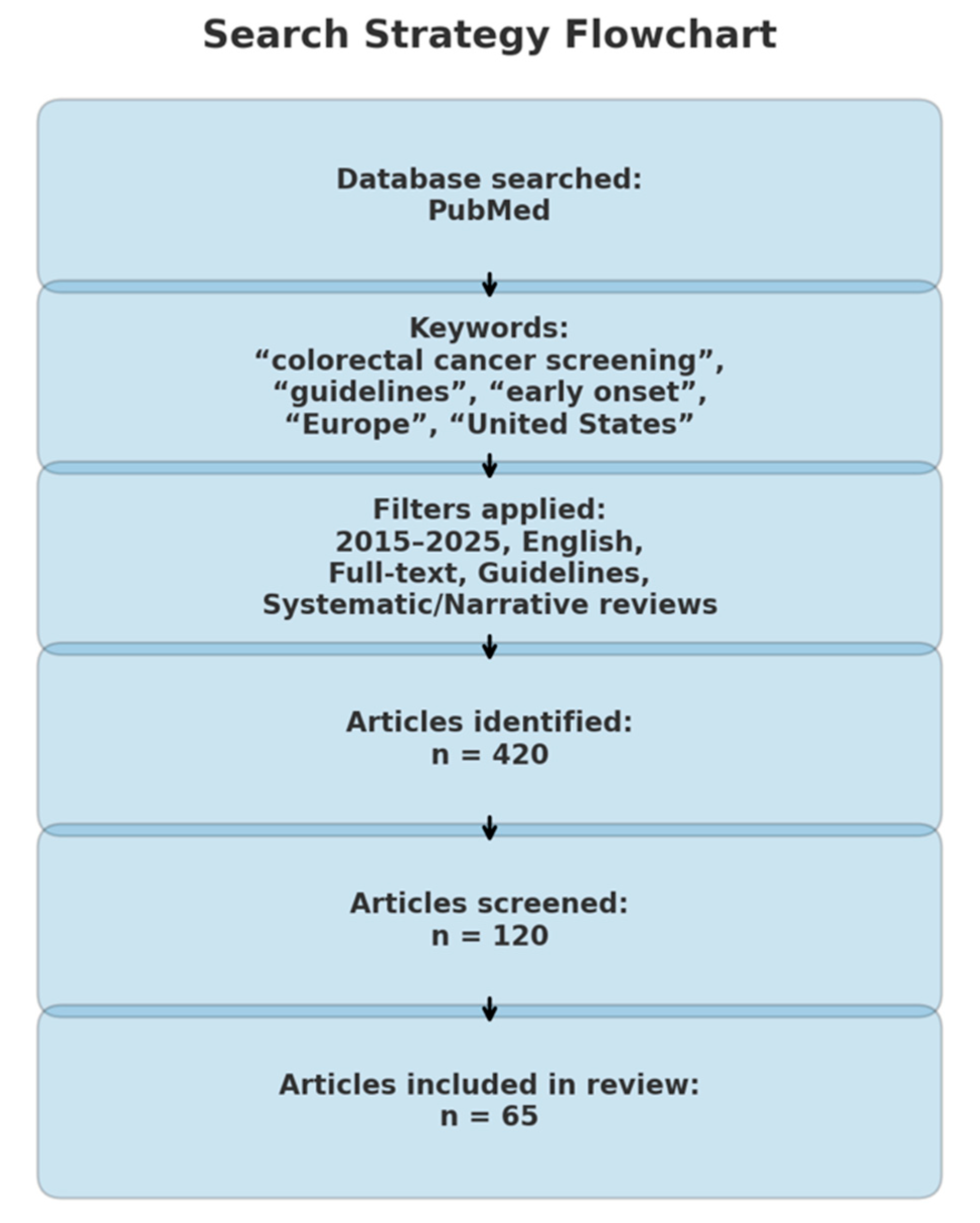
This paper is a descriptive, narrative review that explores CRC screening strategies in both Europe and the United States, synthesizing evidence that may highlight the need for adaptation in current screening practices. A comprehensive search of the medical literature was conducted using the PubMed database for the period 2015–2025, targeting articles published within the past decade. The search strategy employed a combination of relevant keywords, including: “CRC screening,” “guidelines,” “early onset,” “late onset,” “Europe,” “United States,” “recommendations,” “age 45,” and “age 50” (Figure 2).
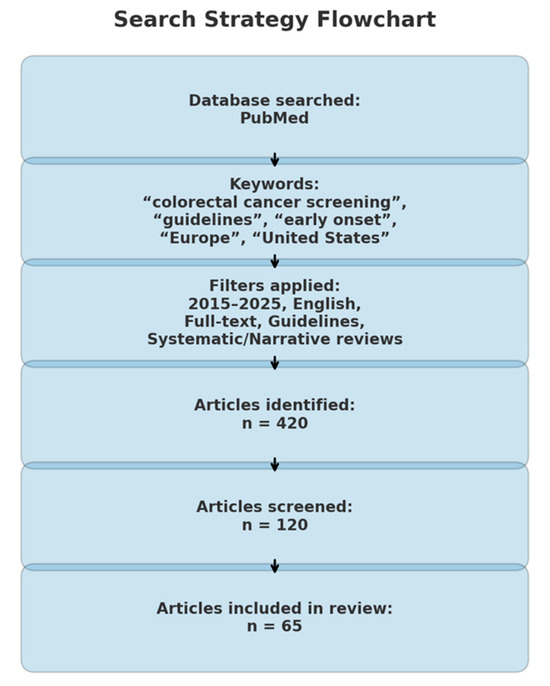
Figure 2.
Search strategy flowchart.
All retrieved articles were initially screened based on titles and abstracts to determine relevance. Studies were eligible for full-text review if they met the following inclusion criteria: availability in full text, publication in English, and classification as clinical practice guidelines, meta-analyses, or systematic or narrative reviews addressing EOCRC, screening modalities, or implementation strategies in Europe or the United States (Figure 2). Articles not aligned with these criteria were excluded (Figure 2). The selection and review processes were carried out independently by the authors at all stages.
5. Conclusions
The accelerating incidence of EOCRC across Western populations calls for a forward-looking recalibration of screening paradigms. While recent U.S. policy shifts signal a growing alignment between epidemiological evidence and clinical practice, European systems face a pivotal opportunity: to evolve from static models to more dynamic, risk-adapted frameworks. Proactive strategies, such as integrating genetic risk profiling, lowering screening thresholds, and leveraging digital health tools for targeted outreach—could redefine early detection. Particularly in nations with robust healthcare infrastructures, phased expansion of screening programs to younger cohorts may yield substantial long-term benefits. Looking ahead, the challenge lies not in reaffirming current limitations but in designing agile systems capable of anticipating and adapting to the burden of CRC.
Author Contributions
Conceptualization, V.B. and A.N.; methodology, V.A.I., T.F.G. and G.G.; software, V.B. and A.N.; validation, V.A.I., G.G. and C.C.D.; formal analysis, V.B., A.N., V.A.I. and G.G.; investigation, V.B., A.N. and T.F.G.; resources, V.B., A.N. and T.F.G.; data curation, V.B., A.N., V.A.I. and G.G.; writing—original draft preparation, V.B. and A.N.; writing—review and editing, V.A.I., G.G. and C.C.D.; supervision, V.A.I., G.G. and C.C.D.; project administration, V.A.I.; funding acquisition, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Global Cancer Observatory. Cancer Today. Lyon, France: International Agency for Research on Cancer. 2025. Available online: https://gco.iarc.who.int/today/en (accessed on 16 August 2025).
- Ait Ouakrim, D.; Pizot, C.; Boniol, M.; Malvezzi, M.; Negri, E.; Bota, M.; Jenkins, M.A.; Bleiberg, H.; Autier, P. Trends in colorectal cancer mortality in Europe: Retrospective analysis of the WHO mortality database. BMJ 2015, 351, h4970. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Webber, E.M.; Goddard, K.A.; Scrol, A.; Piper, M.; Williams, M.S.; Zallen, D.T.; Calonge, N.; Ganiats, T.G.; Janssens, A.C.J.W.; et al. Family history and the natural history of colorectal cancer: Systematic review. Genet. Med. 2015, 17, 702–712. [Google Scholar] [CrossRef]
- Kasi, P.M.; Shahjehan, F.; Cochuyt, J.J.; Li, Z.; Colibaseanu, D.T.; Merchea, A. Rising proportion of young individuals with rectal and colon cancer. Clin. Colorectal Cancer 2019, 18, e87–e95. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Baban, I.A.; Gheorghe, G.; Barbu, A.; Antonie, N.I.; Georgescu, T.F.; Bratu, M.R.; Diaconu, C.C.; Mambet, C.; Bleotu, C.; et al. Clinical, Immunohistochemical, and Inflammatory Profiles in Colorectal Cancer: The Impact of MMR Deficiency. Diagnostics 2025, 15, 2141. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef]
- Pignone, M.; Saha, S.; Hoerger, T.; Mandelblatt, J. Cost-effectiveness analyses of colorectal cancer screening: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002, 137, 96–104. [Google Scholar] [CrossRef]
- European Parliament/EU Monitor. Recommendation 2003/878—Cancer Screening. EU Monitor. 2003. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vitgbgirpwzy (accessed on 16 August 2025).
- Zavoral, M.; Suchanek, S.; Zavada, F.; Dusek, L.; Muzik, J.; Seifert, B.; Fric, P. Colorectal cancer screening in Europe. World J. Gastroenterol. 2009, 15, 5907–5915. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Cancer-Preventive Interventions. Availability and use of colorectal cancer screening. In Colorectal Cancer Screening; International Agency for Research on Cancer: Lyon, France, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553201/ (accessed on 16 August 2025).
- Institutul Clinic Fundeni; Institutul Național de Sănătate Publică. ROCCAS 3: Consolidarea Capacității Sistemului de Sănătate Pentru Implementarea Sustenabilă a Programului Național Organizat de Screening în Cancerul Colorectal; Cod SMIS 319263. Institutul Clinic Fundeni. Available online: https://icfundeni.ro/roccas-3/ (accessed on 16 August 2025).
- Segnan, N.; Patnick, J.; von Karsa, L. Screening for colorectal cancer: European guidelines. Ann. Oncol. 2012, 23, ix53–ix67. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline genetic features of young individuals with colorectal cancer. Gastroenterology 2018, 154, 897–905.e1. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Baban, I.-A.; Barbu, A.; Georgescu, T.F.; Tiuca, L.-C.; Iacobus, N.A.; Diaconu, C.C. Prognostic differences between early-onset and late-onset colorectal cancer. Medicina 2025, 61, 390. [Google Scholar] [CrossRef]
- de la Chapelle, A.; Hampel, H. Clinical relevance of microsatellite instability in colorectal cancer. J. Clin. Oncol. 2010, 28, 3380–3387. [Google Scholar] [CrossRef]
- Losi, L.; Di Gregorio, C.; Pedroni, M.; Ponti, G.; Roncucci, L.; Scarselli, A.; Genuardi, M.; Baglioni, S.; Marino, M.; Rossi, G.; et al. Molecular genetic alterations and clinical features in early-onset colorectal carcinomas and their role for the recognition of hereditary cancer syndromes. Am. J. Gastroenterol. 2005, 100, 2280–2287. [Google Scholar] [CrossRef]
- Liang, J.T.; Huang, K.C.; Cheng, A.L.; Jeng, Y.M.; Wu, M.S.; Wang, S.M. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br. J. Surg. 2003, 90, 205–214. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. 2012, 25, 1128–1139. [Google Scholar] [CrossRef]
- Carr, P.R.; Weigl, K.; Jansen, L.; Walter, V.; Erben, V.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Healthy lifestyle factors associated with lower risk of colorectal cancer irrespective of genetic risk. Gastroenterology 2018, 155, 1805–1815.e5. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; DesMeules, M. Energy intake, physical activity, energy balance, and cancer: Epidemiologic evidence. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 472, pp. 191–215. [Google Scholar] [CrossRef]
- Limsui, D.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Weisenberger, D.J.; Laird, P.W.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J. Natl. Cancer Inst. 2010, 102, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Castells, A.; Marzo-Castillejo, M.; Mascort, J.J.; Amador, F.J.; Andreu, M.; Bellas, B.; Ferrández, A.; Ferrándiz, J.; Giráldez, M.; Gonzalo, V.; et al. Clinical practice guideline. Prevention of colorectal cancer. 2009 update. Asociación Española de Gastroenterología. Gastroenterol. Hepatol. 2009, 32, 717.e1–717.e58. [Google Scholar] [PubMed]
- Lin, J.-T.; Wang, W.-S.; Yen, C.-C.; Liu, J.-H.; Yang, M.-H.; Chao, T.-C.; Chen, P.-M.; Chiou, T.-J. Outcome of colorectal carcinoma in patients under 40 years of age. J. Gastroenterol. Hepatol. 2005, 20, 900–905. [Google Scholar] [CrossRef]
- Yeo, S.A.; Chew, M.H.; Koh, P.K.; Tang, C.L. Young colorectal carcinoma patients do not have a poorer prognosis: A comparative review of 2,426 cases. Tech. Coloproctol. 2013, 17, 653–661. [Google Scholar] [CrossRef]
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.; Vierkant, R.A.; Aakre, J.A.; Larson, D.W. Young-onset colorectal cancer in patients with no known genetic predisposition: Can we increase early recognition and improve outcome? Medicine 2008, 87, 259–263. [Google Scholar] [CrossRef]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 2017, 318, 572–574. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. [Google Scholar] [CrossRef]
- Chen, F.W.; Sundaram, V.; Chew, T.A.; Ladabaum, U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin. Gastroenterol. Hepatol. 2017, 15, 728–737.e3. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M. SEER Cancer Statistics Review, 1975–2014. Available online: https://scholar.google.com/scholar_lookup?title=SEER%20Cancer%20Statistics%20Review,%201975-2014 (accessed on 16 August 2025).
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J. Long-term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: The UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.M.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016, 315, 2576–2594. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Altenhofen, L.; Stock, C.; Hoffmeister, M. Prevention, early detection, and overdiagnosis of colorectal cancer within 10 years of screening colonoscopy in Germany. Clin. Gastroenterol. Hepatol. 2015, 13, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, P.; Chen, H.-C.; Kim, J.I.; Efron, J.; Weiss, E.G.; Nogueras, J.J.; Vernava, A.M.; Wexner, S.D. High compliance rates observed for follow-up colonoscopy post polypectomy are achievable outside of clinical trials: Efficacy of polypectomy is not reduced by low compliance for follow up. Colorectal Dis. 2004, 6, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef]
- Chen, C.; Stock, C.; Hoffmeister, M.; Brenner, H. Optimal age for screening colonoscopy: A modeling study. Gastrointest. Endosc. 2019, 89, 1017–1025.e12. [Google Scholar] [CrossRef]
- Wells, B.J.; Kattan, M.W.; Cooper, G.S.; Jackson, L.; Koroukian, S. Colorectal Cancer Predicted Risk Online (CRC-PRO) calculator using data from the Multi-Ethnic Cohort Study. J. Am. Board Fam. Med. 2014, 27, 42–55. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey; CDC: Atlanta, GA, USA, 2025. Available online: https://www.cdc.gov/nchs/nhis/index.html (accessed on 16 August 2025).
- Anderson, J.C.; Samadder, J.N. To screen or not to screen adults 45–49 years of age: That is the question. Am. J. Gastroenterol. 2018, 113, 1750–1753. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Naber, S.K.; Doria-Rose, V.P.; Pabiniak, C.; Johanson, C.; Fischer, S.E.; Lansdorp-Vogelaar, I.; Kuntz, K.M. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: Modeling study for the US Preventive Services Task Force. JAMA 2016, 315, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Princic, N.; Miller-Wilson, L.-A.; Wilson, K.; Limburg, P. Healthcare costs of colorectal cancer screening and events following colonoscopy among commercially insured average-risk adults in the United States. Curr. Med. Res. Opin. 2022, 38, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-García, A.Z.; Quintero, E. Role of colonoscopy in colorectal cancer screening: Available evidence. Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101838. [Google Scholar] [CrossRef] [PubMed]
- Pignone, M.; Lanier, B.; Kluz, N.; Valencia, V.; Chang, P.; Olmstead, T. Effectiveness and cost-effectiveness of mailed FIT in a safety net clinic population. J. Gen. Intern. Med. 2021, 36, 3441–3447. [Google Scholar] [CrossRef]
- Tobias, N.; Yesilda, B.; Hermann, B. Stage-specific sensitivity of fecal immunochemical tests for detecting colorectal cancer: Systematic review and meta-analysis. Am. J. Gastroenterol. 2020, 115, 56–69. [Google Scholar] [CrossRef]
- Bosch, L.J.W.; Melotte, V.; Mongera, S.; Daenen, K.L.J.; Coupé, V.M.H.; Van Turenhout, S.T.; Stoop, E.M.; de Wijkerslooth, T.R.; Mulder, C.J.J.; Rausch, C.; et al. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am. J. Gastroenterol. 2019, 114, 1909–1918. [Google Scholar] [CrossRef]
- Redwood, D.G.; Dinh, T.A.; Kisiel, J.B.; Borah, B.J.; Moriarty, J.P.; Provost, E.M.; Sacco, F.D.; Tiesinga, J.J.; Ahlquist, D.A. Cost-effectiveness of multitarget stool DNA testing vs colonoscopy or fecal immunochemical testing for colorectal cancer screening in Alaska Native people. Mayo Clin. Proc. 2021, 96, 1203–1217. [Google Scholar] [CrossRef]
- Stürzlinger, H.; Conrads-Frank, A.; Eisenmann, A.; Ivansits, S.; Jahn, B.; Janzic, A.; Jelenc, M.; Kostnapfel, T.; Bedrač, S.M.; Mühlberger, N.; et al. Stool DNA testing for early detection of colorectal cancer: Systematic review using the HTA Core Model® for rapid relative effectiveness assessment. GMS Ger. Med. Sci. 2023, 21, Doc06. [Google Scholar] [CrossRef]
- Ebner, D.W.; Johnson, H.A.; Estes, C.; Johnson, W.K.; Khan, R.S.; Thompson, G.; Kong, J.; Camardo, M.; Dore, M.; Vahdat, V.; et al. Multi-target stool DNA and the fecal immunochemical test: A systematic review and meta-analysis on test performances. Am. J. Prev. Med. 2025, 69, 107654. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Yee, J.; Johnson, C.D. CT colonography: Over two decades from discovery to practice. Abdom. Radiol. 2018, 43, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Song, X.; Zhu, G.; Ni, B.; Ma, X.; Li, J. Is flexible sigmoidoscopy screening associated with reducing colorectal cancer incidence and mortality? A meta-analysis and systematic review. Front. Oncol. 2023, 13, 1288086. [Google Scholar] [CrossRef] [PubMed]
- Loomans-Kropp, H.A.; Song, Y.; Gala, M.; Parikh, A.R.; van Seventer, E.E.; Alvarez, R.; Hitchins, M.P.; Shoemaker, R.H.; Umar, A. Methylated Septin9 (mSEPT9): A promising blood-based biomarker for the detection and screening of early-onset colorectal cancer. Cancer Res. Commun. 2022, 2, 90–100. [Google Scholar] [CrossRef]
- Ho, A.H.Y.; Lui, R.N. The current and future clinical applications of capsule endoscopy. J. Gastroenterol. Hepatol. 2024, 39, 28–33. [Google Scholar] [CrossRef]
- Murphy, J.; Halloran, S.; Gray, A. Cost-effectiveness of the faecal immunochemical test at a range of positivity thresholds compared with the guaiac faecal occult blood test in the NHS Bowel Cancer Screening Programme in England. BMJ Open 2017, 7, e017186. [Google Scholar] [CrossRef]
- Naber, S.K.; Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Fischer, S.E.; Pabiniak, C.J.; Soto, B.; Kuntz, K.M.; Lansdorp-Vogelaar, I. Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PLoS ONE 2019, 14, e0220234. [Google Scholar] [CrossRef]
- Pyenson, B.; Pickhardt, P.J.; Sawhney, T.G.; Berrios, M. Medicare cost of colorectal cancer screening: CT colonography vs. optical colonoscopy. Abdom. Imaging 2015, 40, 2966–2976. [Google Scholar] [CrossRef]
- Makhzoum, A.; Louw, J.; Paterson, W.G. Comparison of flexible sigmoidoscopy screening in average risk patients performed by nurses versus gastroenterologists. J. Can. Assoc. Gastroenterol. 2018, 1, 82–86. [Google Scholar] [CrossRef][Green Version]
- Lin, K.W. mSEPT9 blood test (Epi proColon) for colorectal cancer screening. Am. Fam. Physician 2019, 100, 10–11. [Google Scholar]
- Digestive Cancers Europe. DiCE Releases the CRC Screening White Paper. Digestive Cancers Europe. 2019. Available online: https://digestivecancers.eu/dice-releases-the-crc-screening-white-paper/ (accessed on 16 August 2025).
- Feller, A.; Schmidlin, K.; Bordoni, A.; Bouchardy, C.; Bulliard, J.; Camey, B.; Konzelmann, I.; Maspoli, M.; Wanner, M.; Zwahlen, M.; et al. Socioeconomic and demographic inequalities in stage at diagnosis and survival among colorectal cancer patients: Evidence from a Swiss population-based study. Cancer Med. 2018, 7, 1498–1510. [Google Scholar] [CrossRef]
- Tanaka, L.F.; Figueroa, S.H.; Popova, V.; Klug, S.J.; Buttmann-Schweiger, N. The rising incidence of early-onset colorectal cancer. Dtsch. Ärztebl. Int. 2023, 120, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Lwin, M.W.; Cheng, C.-Y.; Calderazzo, S.; Schramm, C.; Schlander, M. Would initiating colorectal cancer screening from age of 45 be cost-effective in Germany? An individual-level simulation analysis. Front. Public Health 2024, 12, 1307427. [Google Scholar] [CrossRef]
- Patel, S.G.; May, F.P.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.A.; Jacobson, B.C.; Shaukat, A.; Robertson, D.J. Updates on age to start and stop colorectal cancer screening: Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 285–299. [Google Scholar] [CrossRef]
- American Cancer Society. Colorectal Cancer Facts & Figures 2023–2025; American Cancer Society: Atlanta, GA, USA, 2023; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2023-2025.pdf (accessed on 16 August 2025).
- Siddique, S.; Wang, R.; Yasin, F.; Gaddy, J.J.; Zhang, L.; Gross, C.P.; Ma, X. USPSTF colorectal cancer screening recommendation and uptake for individuals aged 45 to 49 years. JAMA Netw. Open 2024, 7, e2436358. [Google Scholar] [CrossRef]
- Chandarana, M.; Shrestha, K.; Parmeshwar, R. Principles of cancer screening. Surgery 2021, 39, 221–227. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968; Available online: https://scholar.google.com/scholar_lookup?title=Principles%20and%20practice%20of%20screening%20for%20disease (accessed on 20 April 2025).
- Morabia, A.; Zhang, F. History of medical screening: From concepts to action. Postgrad. Med. J. 2004, 80, 463–469. [Google Scholar] [CrossRef]
- Stryker, S.J.; Wolff, B.G.; Culp, C.E.; Libbe, S.D.; Ilstrup, D.M.; MacCarty, R.L. Natural history of untreated colonic polyps. Gastroenterology 1987, 93, 1009–1014. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Soares, A.S.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021, 108, 484–493. [Google Scholar] [CrossRef]
- van Leerdam, M.E. Endoscopic Management of Lynch Syndrome and of Familial Risk of Colorectal Cancer. ESGE Guideline. Available online: https://www.esge.com/endoscopic-management-of-lynch-syndrome-and-of-familial-risk-of-colorectal-cancer-esge-guideline (accessed on 16 August 2025).
- Ionescu, V.A.; Gheorghe, G.; Oprita, R.; Ilie, M.; Dascalu, R.I.; Zaharia, O.; Jinga, V.; Diaconu, C.; Constantinescu, G. The Outcomes of Nutritional Support Techniques in Patients with Gastrointestinal Cancers. Gastroenterol. Insights 2022, 13, 245–257. [Google Scholar] [CrossRef]
- Le, K.T.; Pham, V.N.T.; Do, M.D.; Nguyen, T.H.; Tran, T.T. Long-Term Outcomes and Genetic Mutation Patterns in Early-Onset Colorectal Cancer. Asian J. Surg. 2025, 48, 6018–6024. [Google Scholar] [CrossRef]
- Malibary, N.H. Advancements in the Colorectal Cancer Screening, a Euro-Asian Perspective. Asian J. Surg. 2025, 48, 3487–3494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).