1. Introduction
Achalasia is a rare primary esophageal motility disorder, with an estimated annual incidence of 1 to 2 cases per 100,000 individuals [
1]. It is characterized by impaired relaxation of the lower esophageal sphincter (LES) and the progressive loss of esophageal peristalsis, leading to dysphagia, regurgitation, chest pain, and weight loss [
1,
2]. According to the Chicago Classification, achalasia is further divided into three manometric subtypes: type I (classic achalasia with absent peristalsis), type II (achalasia with panesophageal pressurization), and type III (spastic achalasia with premature contractions) [
1,
2]. These subtypes differ in prevalence and therapeutic response, and advanced cases may progress to a markedly dilated “megaesophagus” [
1,
2].
Although its clinical features and treatment strategies, such as pneumatic dilation (PD), laparoscopic Heller myotomy (LHM), or peroral endoscopic myotomy (POEM), are well established, the etiology of achalasia has not been established [
3,
4,
5,
6]. Several lines of evidence suggest that genetic predisposition, viral triggers, and autoimmune mechanisms may contribute to the selective degeneration of inhibitory myenteric neurons [
7].
Among the comorbidities described in patients with achalasia, autoimmune diseases have attracted particular interest [
8,
9]. Disorders such as type 1 diabetes mellitus, systemic lupus erythematosus, and autoimmune thyroid disease have been reported at higher prevalence compared to the general population [
9,
10]. This clustering suggests that achalasia may be part of a broader autoimmune diathesis rather than an isolated neuromuscular disorder [
8,
10]. Thyroid disorders, in particular, are of interest given their high frequency in the general population and their well-established autoimmune pathogenesis [
8,
9,
10].
Isolated case reports, small observational cohorts, and recent cross-sectional studies have consistently described an association between achalasia and thyroid dysfunction, most commonly hypothyroidism due to Hashimoto’s thyroiditis and, less frequently, hyperthyroidism related to Graves’ disease [
11,
12,
13]. The reported prevalence varies widely, but several studies suggest that the risk of thyroid disease is up to three times higher in patients with achalasia than in matched controls [
14]. Nonetheless, the evidence remains limited and heterogeneous, leaving the true magnitude and clinical significance of this association uncertain.
The aim of this narrative review is to synthesize the available evidence on the relationship between achalasia and thyroid disorders. By examining epidemiological data, proposed immunopathogenic links, and potential diagnostic and therapeutic implications, we seek to provide clinicians with a clearer understanding of this emerging association and to highlight areas where further research is urgently needed.
2. Methodology
This review was conducted as a narrative synthesis of the available literature regarding the association between achalasia and thyroid disorders. Although not designed as a systematic review, an explicit and structured methodology was applied to ensure rigor and transparency. The literature search was carried out between March and August 2025, covering studies published from January 2005 to August 2025. The time frame was chosen to capture both the earliest systematic reports on the coexistence of achalasia and thyroid disease as well as the most recent clinical and immunological studies.
The electronic databases PubMed, Scopus, and Web of Science were interrogated using combinations of the following keywords: “achalasia”, “esophageal motility disorders”, “thyroid disease”, “hypothyroidism”, “hyperthyroidism”, “autoimmune thyroiditis”, and “autoimmunity”. Only full-text articles were included in order to ensure accurate interpretation of data. Reference lists of included papers were also hand-searched to identify additional relevant publications.
Studies were eligible for inclusion if they specifically reported original data on patients with achalasia and concomitant thyroid disease, or if they examined autoimmune comorbidities in achalasia cohorts and provided thyroid-specific outcomes. Both observational studies and case reports were considered eligible, given the scarcity of available data. Reviews and immunopathological analyses were included when they offered mechanistic insights relevant to thyroid autoimmunity in the context of achalasia. Studies were excluded if they did not provide thyroid-related data, if achalasia was not diagnosed by clinical, radiographic, or manometric criteria, or if the report was limited to conference abstracts without peer-reviewed full text.
Although designed as a narrative review, we sought to enhance transparency by explicitly reporting our search strategy. The electronic databases PubMed, Scopus, and Web of Science were interrogated using the following combinations of keywords: “achalasia” AND (“thyroid disease” OR “hypothyroidism” OR “hyperthyroidism” OR “autoimmune thyroiditis” OR “autoimmunity”). Reference lists of included articles were hand-searched for additional studies.
Inclusion criteria were: (I) observational cohorts, case–control studies, or case reports that described thyroid disease in patients with achalasia; (II) mechanistic or immunological studies relevant to thyroid autoimmunity in the context of achalasia; and (III) full-text, peer-reviewed articles published between January 2005 and August 2025.
Exclusion criteria included: (I) studies not providing thyroid-specific outcomes; (II) reports in which achalasia was not confirmed by clinical, radiographic, or manometric criteria; and (III) abstracts without peer-reviewed full text.
Relevant studies dealing with achalasia and thyroid disorders were selected and analyzed by the authors. Both quantitative studies and case reports were included. No formal quality assessment was performed, in line with the narrative nature of this review. Data extracted from each study included population characteristics, diagnostic criteria for achalasia and thyroid disease, prevalence rates, methodological limitations, and key clinical findings. These data were synthesized narratively and summarized in tabular and graphical form to highlight both the consistency and the limitations of the evidence base.
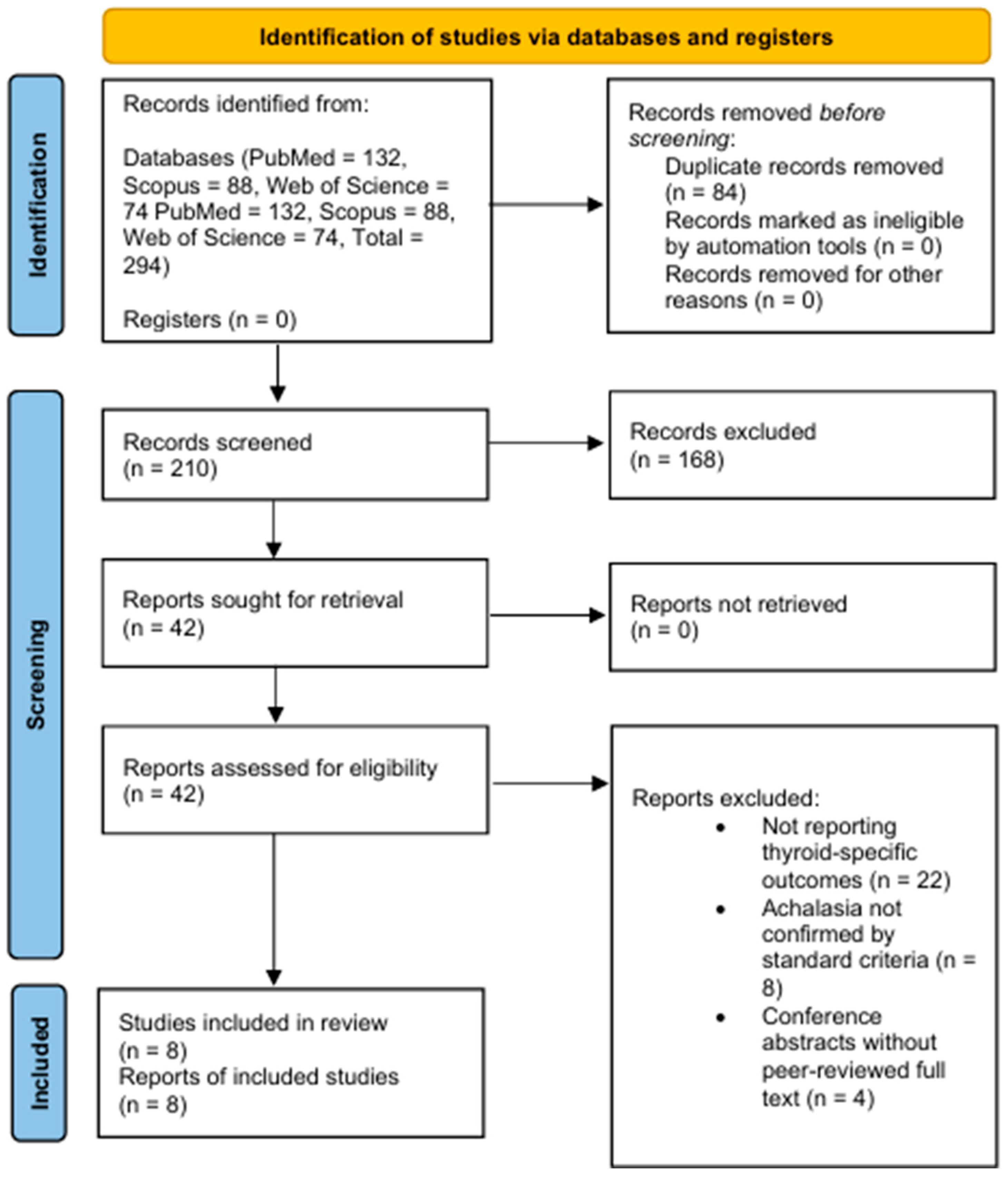
To enhance transparency, we summarized the selection process using a PRISMA-style flowchart (
Figure 1). Although this is a narrative review, we applied explicit inclusion and exclusion criteria and documented the screening process according to PRISMA recommendations. In total, 294 records were retrieved, of which 210 were screened and 42 full-texts were assessed for eligibility. Following application of the inclusion and exclusion criteria, 8 studies were included in the qualitative narrative synthesis.
3. Epidemiology of Achalasia and Thyroid Disorders
Achalasia is a relatively uncommon condition, with an incidence ranging between 1 and 2 per 100,000 population annually and a prevalence of approximately 10 per 100,000 [
1]. It typically presents in early to middle adulthood, although cases can occur at any age, and shows no clear sex predilection [
7]. According to the Chicago Classification, achalasia is currently divided into three manometric subtypes: type I (classic achalasia, with minimal pressurization), type II (achalasia with panesophageal pressurization), and type III (spastic achalasia with premature contractions) [
3]. These subtypes differ in prevalence, with type II being the most frequent, and show variable responses to treatment. In advanced stages, long-standing disease may evolve into a dilated “megaesophagus,” which carries additional diagnostic and therapeutic challenges [
3].
By contrast, thyroid disorders are among the most common endocrine conditions worldwide, affecting up to 5–10% of the general population depending on age, sex, and iodine status [
15]. Autoimmune thyroid disease, particularly Hashimoto’s thyroiditis and Graves’ disease, represents the predominant cause of both hypothyroidism and hyperthyroidism in iodine-sufficient regions [
16].
When considered together, the apparent coexistence of these two conditions becomes clinically relevant. Early reports suggested that up to one quarter of patients with achalasia may present with thyroid dysfunction. Emami et al. described thyroid abnormalities in approximately 23% of their achalasia cohort, including both hypo- and hyperthyroidism [
13].
Romero-Hernández et al. reported a threefold higher prevalence of autoimmune thyroid disease in patients with achalasia compared to controls with gastroesophageal reflux disease [
14].
More recently, a large cross-sectional study from Iran, including 393 patients diagnosed between 2006 and 2023, reported an overall prevalence of thyroid disorders of approximately 24–25% among individuals with achalasia [
17]. Autoimmune thyroiditis, confirmed by anti-thyroid peroxidase antibodies, was detected in 20.5% of tested patients [
17]. Notably, treatment outcomes for achalasia did not differ significantly between euthyroid patients and those with concomitant thyroid disease. Although limited by its cross-sectional design, this study suggests that thyroid autoimmunity may be frequent but does not necessarily influence the short-term therapeutic response [
17].
Although prevalence estimates vary across studies, the recurring signal of thyroid disease in patients with achalasia exceeds background population rates (
Table 1). This consistent finding reinforces the hypothesis of a non-random association, potentially linked to shared immunogenetic predispositions. Nevertheless, heterogeneity in study design, sample size, and diagnostic criteria limits definitive conclusions, underscoring the need for more robust epidemiological data. It is important to note that none of the included studies stratified their findings by achalasia subtype (types I–III or advanced megaesophagus). Therefore, no conclusions can be drawn regarding potential subtype-specific differences in the prevalence of thyroid disorders.
Notably, most available studies did not stratify outcomes by achalasia subtype. It therefore remains unclear whether the prevalence of thyroid disease differs between type I, II, or III achalasia, or in patients with advanced megaesophagus. This gap in the evidence highlights the need for future studies with subtype-specific analysis.
4. Immunological and Pathophysiological Mechanisms
The pathogenesis of achalasia has long been suspected to involve autoimmune mechanisms, supported by histological, immunological, and genetic findings [
7,
10,
14]. Inflammatory infiltrates composed predominantly of cytotoxic CD8+ T lymphocytes have been observed in the myenteric plexus of patients with achalasia, accompanied by neuronal degeneration and loss of inhibitory ganglion cells [
18,
19]. Several studies have demonstrated circulating autoantibodies directed against enteric neurons, although their pathogenic significance remains uncertain [
10,
14]. In parallel, an increased prevalence of HLA class II alleles associated with autoimmunity, such as HLA-DQB1*06:02, has been reported in patients with achalasia [
10].
Beyond descriptive histology, converging data suggest a cytotoxic, T-cell-mediated process targeting inhibitory nitrergic neurons of the myenteric plexus [
10,
11,
12]. A Th1/Th17-skewed milieu (e.g., interferon-γ, TNF-α, IL-17) and activated macrophages/microglia-like cells can amplify neuronal injury and glial dysfunction, culminating in loss of nitric-oxide–synthase–positive neurons and impaired LES relaxation [
10,
11,
12]. Circulating anti-enteric-neuron antibodies have been variably detected, but their pathogenicity remains uncertain; they may reflect bystander activation or epitope spreading after initial neuronal damage [
10,
11,
12]. HLA class II associations (e.g., DQ haplotypes) plausibly facilitate antigen presentation of esophageal or cross-reactive peptides, sustaining a chronic autoimmune loop within the esophageal wall [
10,
11,
12].
These observations align with the frequent detection of autoimmune thyroid disease among achalasia cohorts. Hashimoto’s thyroiditis and Graves’ disease share common immunological hallmarks, including autoreactive T cells, anti-thyroid peroxidase (TPO) and anti-thyroglobulin (Tg) antibodies, and, in the case of Graves’ disease, stimulating TSH receptor antibodies [
20]. Autoimmune thyroid disease (AITD) similarly features breakdown of central/peripheral tolerance, HLA-restricted presentation of thyroid antigens (TPO, Tg, TSHR), and a Th1/Th17-dominant cytokine signature [
20]. Shared non-HLA susceptibility loci implicated across organ-specific autoimmunity (e.g., CTLA4, PTPN22) could lower the threshold for autoreactivity in both tissues [
20]. At the interface, epitope spreading and bystander activation may link local tissue injury (esophageal or thyroid) to systemic autoimmunity, explaining clustering of AITD with achalasia in some patients [
20].
The coexistence of these two conditions may therefore reflect a broader predisposition to autoimmune reactivity, possibly mediated by shared HLA haplotypes or by molecular mimicry between viral antigens and self-antigens within both the thyroid and the esophageal myenteric plexus [
21].
Moreover, the occurrence of achalasia in the context of polyglandular autoimmune syndromes (PAS) further supports this hypothesis [
22]. Reports have documented achalasia coexisting with type 1 diabetes mellitus, Addison’s disease, and autoimmune thyroiditis, suggesting that achalasia may represent part of a systemic autoimmune spectrum rather than an isolated motility disorder [
11,
12,
18]. Several triggers may initiate or unmask autoimmunity in genetically predisposed hosts [
11,
12,
18]. Post-infectious mechanisms (molecular mimicry to viral antigens), tissue stress and microischemia, or local dysbiosis-driven immune activation have all been proposed in achalasia; in AITD, environmental factors such as iodine excess/deficit, postpartum immune rebound, infection, and psychological stress can precipitate disease onset [
11,
12,
18]. These triggers could act as the “first hit,” with cross-reactive immune responses subsequently targeting esophageal neurons or thyroid antigens in susceptible HLA backgrounds [
11,
12,
18].
Although the mechanistic links remain speculative, the convergence of epidemiological, immunogenetic, and immunohistochemical evidence strengthens the concept of a common autoimmune substrate [
11,
12,
18]. While these findings support an autoimmune contribution, they remain associative rather than demonstrative.
A plausible model is that environmental triggers in a genetically primed host ignite a Th1/Th17-dominant response that injures inhibitory myenteric neurons; ensuing antigen release and epitope spreading sustain chronic inflammation and may co-occur with thyroid autoimmunity in part via shared HLA and checkpoint pathways [
11,
12,
18]. Still, direct causality is unproven: disease-specific autoantibodies to esophageal targets are inconsistently detected, longitudinal data are scarce, and mechanistic links remain inferential [
11,
12,
18]. Prospective studies integrating immunophenotyping (single-cell profiling of esophageal/thyroid infiltrates), autoantibody repertoires, HLA/immune-gene genotyping, and viral-exposure signatures are needed to validate this model [
11,
12,
18]. Most studies are limited to small series or case reports, and direct evidence of pathogenic autoantibodies or disease-specific immune pathways is lacking.
Building upon these insights, a recent cross-sectional study further highlighted the systemic autoimmune context of achalasia, explicitly recognizing thyroid disorders among its clinical associations [
8]. This finding strengthens the concept of a shared immunological substrate underlying both esophageal motor dysfunction and thyroid autoimmunity [
8,
9].
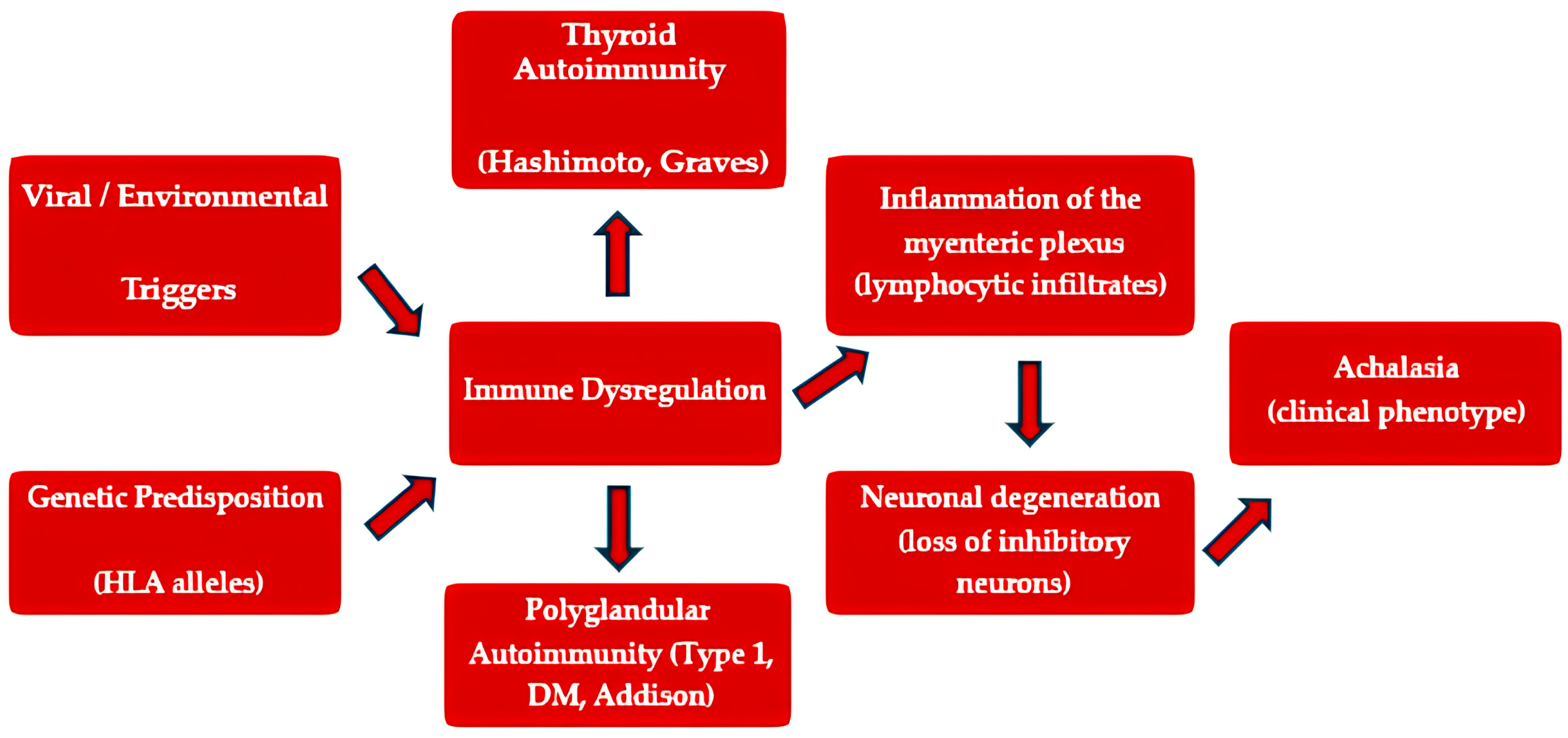
Future research integrating advanced immunophenotyping, autoantibody profiling, and genetic studies may help to elucidate the precise relationship between esophageal neuronal injury and systemic autoimmunity, particularly in relation to thyroid disease (
Figure 2).
5. Clinical Evidence and Case Reports
The clinical evidence supporting an association between achalasia and thyroid disease derived mainly from small observational studies and isolated case reports, which, despite their limitations, provide recurring signals of a non-random coexistence.
One of the earliest systematic observations was reported by Emami et al., who examined 30 patients with achalasia and found thyroid abnormalities in nearly one quarter of cases [
13]. Hypothyroidism was the most frequent condition, followed by hyperthyroidism and solitary thyroid nodules [
13]. Although the study was limited by its small cohort, it was the first to suggest a clinically significant overlap between achalasia and thyroid disorders [
13].
Further support came from case-based evidence [
12]. Quidute et al. described a patient presenting with achalasia in association with autoimmune thyroid disease, hypothesizing that both conditions might share an autoimmune background [
12].
More recently, Simon et al. reported a case of achalasia associated with hyperthyroidism, in which clinical improvement was observed after combined treatment of both disorders [
11]. These reports, while anecdotal, highlight the clinical relevance of considering thyroid function in patients with esophageal motility disorders [
11].
Larger comparative studies have reinforced these observations. In a case–control study, Romero-Hernández et al. evaluated 114 patients with achalasia against matched controls with gastroesophageal reflux disease (GERD) [
14]. Autoimmune thyroid disease was identified in 16.7% of the achalasia cohort compared with 4.2% of controls, corresponding to an approximate threefold increased risk [
14]. This remains one of the most compelling pieces of epidemiological evidence to date.
The most extensive data were recently provided by Mehrban et al. in a multicenter study of 393 Iranian patients with achalasia [
17]. They reported thyroid peroxidase (TPO) antibody positivity in 20.5% of participants, confirming a substantial burden of thyroid autoimmunity [
17]. Interestingly, the presence of thyroid disease did not appear to influence therapeutic response to PD or surgical interventions, suggesting that while the association may be etiologically significant, it does not necessarily alter short-term treatment outcomes [
17]. This lack of impact may, however, reflect the fact that thyroid dysfunctions were appropriately treated, highlighting the importance of their timely recognition and management.
These findings demonstrate a consistent enrichment of thyroid disease among patients with achalasia, particularly autoimmune hypothyroidism. Although the studies are heterogeneous and limited by sample size, they collectively strengthen the hypothesis of a shared autoimmune diathesis.
When contextualized against other autoimmune comorbidities, the strength of the achalasia–thyroid association appears particularly robust. Type 1 diabetes mellitus and systemic autoimmune disorders such as lupus or rheumatoid arthritis have also been described in achalasia cohorts, but usually at lower frequencies or in isolated case reports. By contrast, thyroid autoimmunity has been consistently documented across multiple studies, often affecting a notable proportion of patients. This suggests that thyroid disease may represent one of the most frequent and clinically relevant autoimmune comorbidities of achalasia, supporting the rationale for targeted screening.
6. Methodological Limitations of Current Evidence
Although the available literature consistently suggests an increased prevalence of thyroid disease in patients with achalasia, several methodological limitations must be acknowledged. Most published studies are limited by small sample sizes, often fewer than one hundred participants, which reduces statistical power and increases the risk of random associations. In addition, most data are derived from single-center experiences or geographically restricted cohorts, limiting the generalizability of the findings across different populations.
Another critical issue relates to the heterogeneity of diagnostic criteria used for both achalasia and thyroid disease. While the diagnosis of achalasia is generally based on manometry and imaging, variations exist in classification systems and inclusion criteria. For thyroid disorders, some studies relied solely on biochemical assessments, whereas others included antibody testing or clinical diagnosis, introducing variability that complicates direct comparison.
Most studies are cross-sectional or retrospective, which prevents conclusions about temporal or causal relationships. Consequently, it remains unclear whether thyroid autoimmunity contributes to the pathogenesis of achalasia, or whether the association reflects a broader predisposition to autoimmune comorbidity. The absence of longitudinal studies is a notable gap that prevents evaluation of whether thyroid disease precedes, coincides with, or follows the onset of achalasia.
Another important consideration is the possibility of ascertainment or surveillance bias. Patients with achalasia who are referred to tertiary centers or included in research cohorts are often subject to more intensive medical evaluation compared to the general population. Given the known tendency for autoimmune conditions to cluster, such enhanced monitoring may increase the likelihood of detecting thyroid abnormalities that might otherwise remain subclinical or undiagnosed. This increased surveillance could therefore inflate the reported prevalence of thyroid disease in achalasia cohorts, partially accounting for the consistently higher rates observed. Future studies should attempt to mitigate this bias by including population-based cohorts with standardized screening protocols.
Finally, publication bias cannot be excluded. Case reports and small series that describe positive associations are more likely to be published than negative studies, which may exaggerate the perceived strength of the relationship.
Another limitation relates to missing data. Most of the available evidence on the coexistence of achalasia and thyroid disorders is derived from retrospective studies, where incomplete datasets are common. Information on thyroid status, antibody profiles, or long-term follow-up was often unavailable or inconsistently reported. As no standardized strategies for handling missing values were applied across studies, these gaps may have introduced bias and limit the strength of pooled observations. Future prospective research with systematic data collection and predefined handling of missing variables is needed to reduce this source of uncertainty.
These limitations underscore the need for large, multicenter, prospective studies incorporating standardized diagnostic protocols and mechanistic immunological assessments to establish the true nature of the association between achalasia and thyroid disorders. These methodological shortcomings not only limit causal inference but also risk underestimating the true burden of thyroid dysfunction in achalasia.
7. Clinical Implications: Diagnosis and Management
The recognition of a potential association between achalasia and thyroid disorders carries several important implications for clinical practice. Although causality has not been definitively established, the consistent observation of autoimmune thyroid disease in patients with achalasia suggests that a more comprehensive diagnostic approach may be warranted. Routine assessment of thyroid function, particularly through serum thyroid-stimulating hormone (TSH) and thyroid peroxidase (TPO) antibodies, may be useful in newly diagnosed patients with achalasia, especially if they have symptoms such as fatigue, weight change, or temperature intolerance.
While routine thyroid function testing in all patients with achalasia may appear reasonable, current evidence is insufficient to demonstrate a clear cost–benefit advantage of universal screening. A more prudent approach could be to prioritize thyroid evaluation in patients with suggestive clinical symptoms, a personal or family history of autoimmune disease, or unexplained postoperative complaints. This strategy may optimize diagnostic yield while avoiding unnecessary investigations.
From a therapeutic standpoint, current evidence indicates that thyroid dysfunction does not significantly alter the short-term outcomes of achalasia treatment. The short- and long-term effectiveness of both POEM and LHM in the treatment of achalasia is well established [
4,
6]. In this context, the presence of concomitant thyroid disease does not appear to modify procedural efficacy, although unrecognized dysfunction may still confound postoperative symptom assessment.
In the multicenter study by Mehrban et al., the presence of thyroid autoimmunity did not affect the efficacy of PD, LHM, or POEM [
17]. Nevertheless, unrecognized thyroid disease may contribute to persistent or overlapping symptoms, complicating clinical management and obscuring accurate assessment of postoperative outcomes. For example, dysphagia, weight loss, or chest discomfort may be misattributed to achalasia progression when in fact they reflect untreated hypothyroidism or hyperthyroidism.
Beyond individual patient care, the identification of thyroid disease in achalasia raises broader considerations regarding autoimmune clustering. Clinicians should remain alert to the possibility of additional autoimmune comorbidities, such as type 1 diabetes mellitus or Addison’s disease, particularly in patients with suggestive clinical features. In this regard, achalasia might occasionally represent part of a broader polyglandular autoimmune syndrome rather than an isolated disorder. From a broader perspective, achalasia could also be viewed within the spectrum of PAS, where shared genetic and immunological substrates predispose to multi-organ involvement.
From a clinical perspective, it is conceivable that the diagnostic yield of thyroid screening could vary depending on achalasia subtype or disease stage. For example, type II achalasia, being the most common and generally more responsive to therapy, may represent the optimal setting for systematic screening. By contrast, patients with long-standing disease or megaesophagus often present with overlapping systemic symptoms such as weight loss or dysphagia, which could obscure the recognition of concomitant thyroid dysfunction. To date, no study has systematically explored these differences, underscoring the need for future research with subtype-specific analyses. However, the absence of subtype-stratified data in the available literature remains a major limitation, which prevents any firm conclusions about differential risk of thyroid disease across achalasia subtypes.
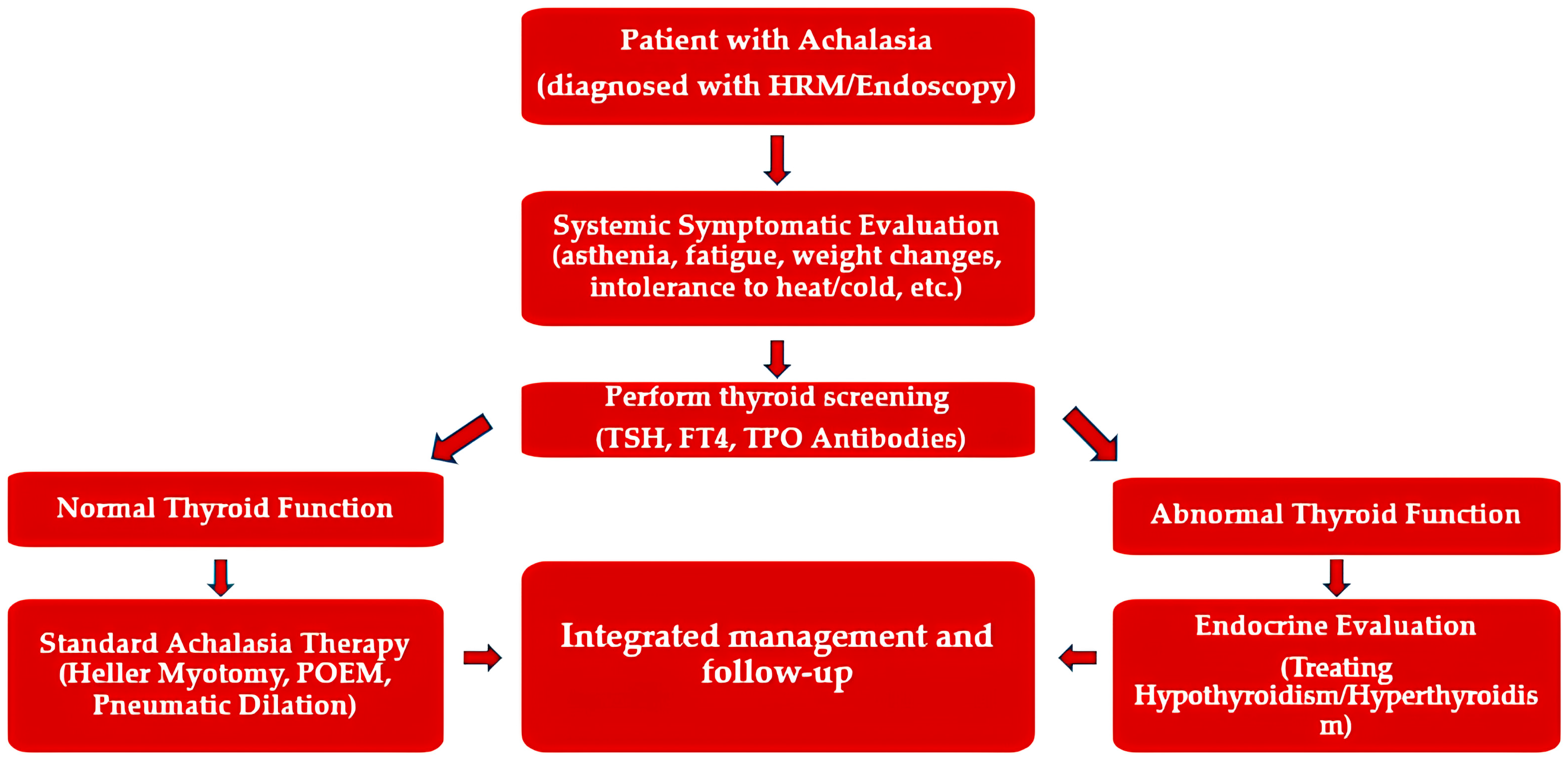
Integrating thyroid evaluation into the work-up of patients with achalasia may therefore improve diagnostic accuracy, enhance symptom management, and support early detection of broader autoimmune syndromes (
Figure 3).
In summary, while thyroid disease does not appear to modify procedural outcomes directly, its recognition is clinically relevant for optimizing patient care. Incorporating thyroid screening into routine evaluation of patients with achalasia may be a pragmatic step until more definitive evidence becomes available.
8. Future Directions and Research Perspectives
The current body of evidence suggests a non-random association between achalasia and thyroid disease, yet critical gaps remain that warrant systematic investigation. First, the absence of large, multicenter, prospective studies represents the most significant limitation in establishing the temporal relationship between the two conditions. Longitudinal follow-up would be essential to determine whether thyroid autoimmunity precedes, coincides with, or develops subsequent to the onset of achalasia. Such data would help clarify whether thyroid disease functions as a comorbidity or as a potential contributing factor to pathogenesis.
Second, standardization of diagnostic protocols is necessary. Future studies should uniformly define achalasia based on high-resolution manometry and validated classification systems, while thyroid dysfunction should be assessed using a combination of biochemical parameters and autoantibody profiles. This approach would minimize heterogeneity and improve comparability across studies.
Third, the role of genetic and immunological predisposition deserves more rigorous exploration. Genome-wide association studies, combined with advanced immunophenotyping, may identify common susceptibility loci and clarify whether shared HLA haplotypes underlie the co-occurrence of achalasia and thyroid autoimmunity. Similarly, functional studies investigating autoreactive T cells, cross-reactive epitopes, and neuronal autoantibodies could provide mechanistic insights into the autoimmune hypothesis.
Finally, the broader context of autoimmune clustering in achalasia should not be overlooked. Prospective registries that systematically screen for multiple autoimmune conditions, including thyroiditis, type 1 diabetes mellitus, Addison’s disease, and systemic autoimmune disorders, would provide valuable epidemiological data and help define whether achalasia should be considered part of a polyglandular autoimmune spectrum.
In conclusion, future research should prioritize prospective, mechanistic, and multicenter designs that integrate clinical, immunological, and genetic data. Such efforts will be critical to clarify the nature of the relationship between achalasia and thyroid disease and may ultimately reshape the clinical management of patients with esophageal motility disorders (
Table 2).
Recognizing this association in clinical practice is crucial, as it may prompt a more comprehensive patient evaluation and facilitate the earlier detection of endocrine dysfunctions. Nevertheless, the scarcity of high-quality studies, together with the predominance of small case series and individual reports, underscores the need for prospective investigations to establish causality and elucidate the underlying immunopathogenic mechanisms.
9. Conclusions
Achalasia remains a rare esophageal motility disorder with an uncertain pathogenesis, yet growing evidence indicates that autoimmune mechanisms may play a central role. Thyroid disorders, particularly autoimmune thyroiditis and hyperthyroidism, have emerged as recurrent comorbidities, suggesting a possible systemic autoimmune overlap. Recent large-scale studies have confirmed a higher prevalence of thyroid autoimmunity among patients with achalasia, although therapeutic outcomes for the esophageal disorder appear unaffected. Nonetheless, unrecognized thyroid dysfunction may contribute to persistent symptoms and adversely influence postoperative outcomes. These findings support the hypothesis that achalasia may not only represent an esophageal motility disorder but could also be part of a broader systemic autoimmune spectrum