Abstract
Background: The number of women veterans has been rising steadily since the Gulf War and many assume the functions of their male counterparts. Women face unique obstacles in their service, and it is imperative that differences in physiology not be overlooked so as to provide better and appropriate care to our women in uniform. Despite this influx and incorporation of female talent, dedicated reports contrasting female and male veterans are rare, outside of specific psychological studies. We therefore attempt to contrast gut constituents, absorption, innate immune system, and nutritional differences to provide a comprehensive account of similarities and differences between female and male veterans, from our single-center perspective, as this has not been carried out previously. Herein, we obtained a detailed roster of commonly used biomedical tests and some novel entities to detect differences between female and male veterans. The objective of this study was to detect differences in the innate immune system and other ancillary test results to seek differences that may impact the health of female and male veterans differently. Methods: To contrast biochemical and sociomedical parameters in female and male veterans, we studied the data collected on 450 female veterans and contrasted them to a group of approximately 1642 males, sequentially from 1995 to 2022, all selected because of above-average risk for CRC. As part of this colorectal cancer (CRC) screening cross-sectional and longitudinal study, we also collected stool, urine, saliva, and serum specimens. We used ELISA testing to detect stool p87 shedding by the Adnab-9 monoclonal and urinary organ-specific antigen using the BAC18.1 monoclonal. We used the FERAD ratio (blood ferritin/fecal p87), a measure of the innate immune system to gauge the activity of the innate immune system (InImS) by dividing the denominator p87 (10% N-linked glycoprotein detected by ELISA) into the ferritin level (the enumerator, a common lab test to assess anemia). FERAD ratios have not been performed elsewhere despite past Adnab-9 commercial availability so we have had to auto-cite our published data where appropriate. Results: Many differences between female and males were detected. The most impressive differences were those of the InImS where males clearly had the higher numbers (54,957 ± 120,095) in contrast to a much lower level in females (28,621 ± 66,869), which was highly significantly different (p < 0.004). Mortality was higher in males than females (49.4% vs. 24.1%; OR 3.08 [2.40–3.94]; p < 0.0001). Stool p87, which is secreted by Paneth cells and may have a protective function, was lower in males (0.044 ± 0.083) but higher in females (0.063 ± 0.116; p < 0.031). Immunohistochemistry of the Paneth cell-fixed p87 antigen was also higher in females (in the descending colon and rectum). In contrast, male ferritin levels were significantly higher (206.3 ± 255.9 vs. 141.1 ± 211.00 ng/mL; p < 0.0006). Females were less likely to be diabetic (29.4 vs. 37.3%; OR 0.7 [0.55–0.90]; p < 0.006). Females were also more likely to use NSAIDs (14.7 vs. 10.7%, OR 1.08 [1.08–2.00]; p < 0.015). Females also had borderline less GI bleeding by fecal immune tests (FITs), with 13.2% as opposed to 18.2% in males (OR 0.68 [0.46–1.01]; p = 0.057), but were less inclined to have available flexible sigmoidoscopy (OR 0.68 [0.53–0.89]; p < 0.004). Females also had more GI symptomatology, a higher rate of smoking, and were significantly younger than their male counterparts. Conclusions: This study shows significant differences with multiple parameters in female and male veterans.
1. Introduction
Given the above abstract summary that we present, there is clearly a burning need to make these data available to the readership and acquaint them with the current state of the art. We have tried to include state-of-the-art topics but did not limit ourselves to allow for as many as possible subspecialists and primary care providers to have access to a panoply of biochemical testing and have provided references for our readers.
To further elaborate, from 1975 to 2025, the National Library of Medicine counted 9141 articles that purport to show differences between male and female veterans [1]. However, if psychiatric papers are excluded, the total is reduced by 2091 to 7050. If articles reporting biochemical differences are sought, the total is less than 50 contrasting males with female veterans [2]. As a case in point, a VA study published in 2018 looking at almost 5 million veterans, drew attention to the highest weight gain in women veterans who were not diabetic from 2000 to 2014 (0.36 kg/yr) and drew attention to this disturbing trend [3]. In the same year, investigators looked at the effects of the Motivating Overweight/Obese Veterans Everywhere (MOVE!) weight management program in a much smaller cohort and concluded that while women lost less weight than men, the authors concluded that the barrier to weight loss in women should be addressed [4]. Later, a more intensive trial using the Veterans Affairs Diabetes Prevention Program (VA-DPP) and contrasting the outcomes with the MOVE! Program showed greater weight loss and participation at 6 months, but at 12 months, the results were equivalent (<5% body weight loss in both women and men) in the MOVE! Program, with similar health expenditures [5]. Clearly, the focus may be too narrow and testing should encompass a broad range of biochemical and immune parameters as we have recently proposed [6,7,8]. Given the above changing realities of a greater representation of female veterans in the US military, we set out to carefully obtain examples in the current literature as to the equity of treatment for both sexes and, as a primary objective, we aimed to obtain a detailed account of potential differences between female and male veterans evaluated sequentially from 1995 to 2022 using a prospectively acquired database, which followed up with veterans regarding the incidence of colorectal neoplasia. The demographics represent a cross-sectional view of thstudy the follow-up aspects, and the longitudinal aspect of this study.
2. Results
The overall number of patients in the trial is shown in Table 1.

Table 1.
Demographics and survival outcomes of the 2294 veterans enrolled.
The number of males was greater than that of females, which is typical in contemporary veteran studies of this type. Table 1 provides the data typically presented from most available publications, with female veterans being generally much younger than male veterans, and consequently, there was less mortality in females, as shown in Table 1. The p87 stool determination was performed in ~1000 patients, about 42% of the entire cohort. The VAMC computerized patient record (CPRS) was the repository of most common blood and urine testing accumulated over the lifetime of this study by the primary care provider (PCP).
Underlying co-morbidities are also very important to consider. These include substance abuse and smoking and alcohol consumption, here noted to be more dominant in females. In the Discussion section, the co-morbidities mentioned in multiple publications will be consulted. Females are less susceptible to diabetes, but almost twice as many are affected by GI symptomatology. However, Barrett’s esophagus is significantly greater in males. These collective observations are presented in Table 2.

Table 2.
Existing disorders. These are explored in 12 common scenarios, as summarized below.
Health-seeking behavior can be difficult to contrast between the sexes, but we feel that gender-specific tests can be compared in terms of frequency of testing for males, prostate-specific antigen (PSA), and females, mammography and Papanicolaou Smear [PS]. The latter is congruous (no change in use frequency), but the frequency of mammography in females is less than that of PSA in males, as seen in Table 3, and certainly constitutes a barrier to this important intervention.

Table 3.
Health-seeking behavior. There are widely divergent results, suggesting specific needs and/or disparities between males and females.
Although health-seeking behavior may extend to preventative measures, they are not monolithic, as there are many means to achieving these protections, as shown in Table 4. Most modalities are at parity, aside from flexible sigmoidoscopy. Cancer is far more prevalent in males, perhaps due to their increased age and lifestyle changes.

Table 4.
Screening for colorectal neoplasia.
Markers of the InImS are important and can also predict neoplasia [6], resistance to diseases such as COVID-19 [7], and even regression of preneoplastic conditions [8]. Figure 1 shows significantly lower FERAD ratios in females than males.
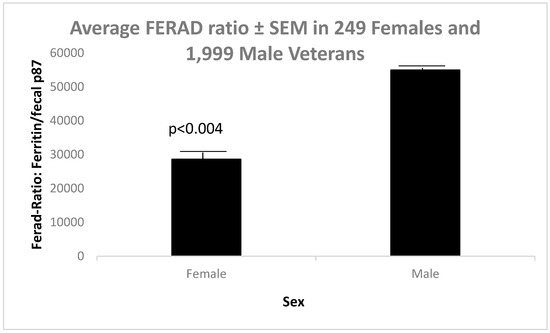
Figure 1.
Significant differences in FERAD ratios in female and male veterans.
In the interest of investigating the FERAD ratios in the African American (AA) cohort, Figure 2 below shows that the above broad demographic is also replicated in AA veterans.
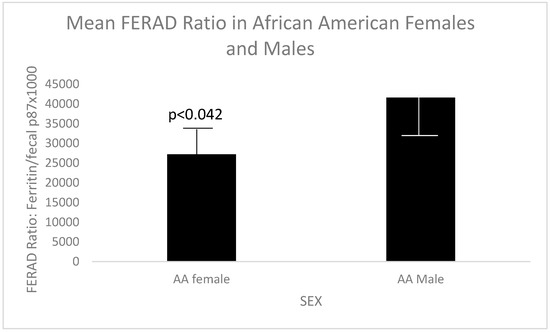
Figure 2.
Significantly different FERAD ratios in AA female and male veterans.
In Table 5 below, we contrast our database results in females and males with those of equivalent studies in the extant literature.

Table 5.
Comparison of the male and female database with the literature.
The data for Figure 3 were taken from Table 1, Table 2 and Table 3, and only age was excluded since an age disparity is seen quite often between female and male veterans.
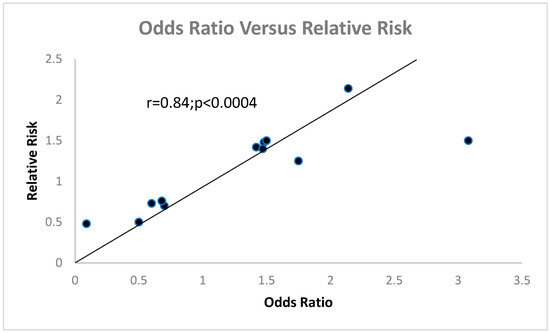
Figure 3.
This scattergram shows a highly significant correlation between OR and RR.
Since there are no similar comparison studies of the InImS contrasting female to male veterans, we offer our two published studies on this topic, the first describing the InImS in veterans with and without COVID-19 [6] and the second on gastrointestinal expression of p87 in females and males in colorectal and esophageal neoplasia [7,8,9]. For completeness’s sake, a depiction of the Western blot of the stool bands is presented below in Figure 4.
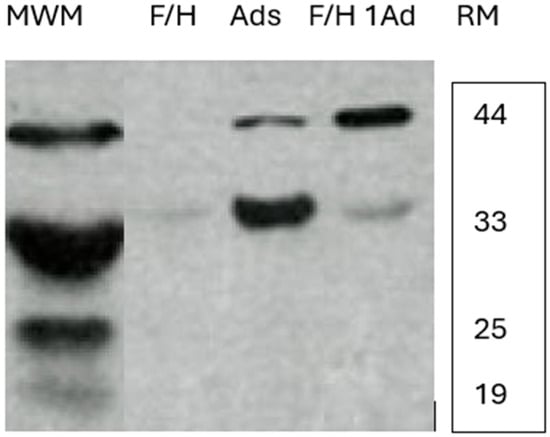
Figure 4.
Western blots of female and male stools showing relative mobility of the bands. Western blot showing p87 stool bands at approximately 44 and 30 kDa. RM—relative mobility.
The first lane shows molecular weight markers (MWM): F/H—a 51-year old Caucasian male patient with a family history of CRC but no neoplasia found at colonoscopy; Ads—a 61-year-old Caucasian female with two small left-sided adenomas; and a 64-year-old African American male with a family history of CRC and a right-sided adenoma.
3. Discussion
In summary, female veterans were statistically younger than male veterans, with no difference in ethnicity and, importantly, in survival days, despite a higher percentage of deaths in males, which we shall expand upon by drawing on previous studies.
Of the limited references available, the most informative is that of Frayne et al. [10]. The authors, writing in 2007, used a cross-sectional study to explore how utilization and cost of healthcare differ. They find that females generate higher costs than men when aged over 65 years, but the converse is true when considering veterans younger than age 65 in the inpatient setting. Outpatient use is significantly greater in female veterans over all age groups but is exponentially higher when considering the 55–74-year-olds. Most importantly, in the preceding year, Frayne et al. [11] showed that all age-groups women veterans from all age groups carry the same burden of disease as male veterans. They also point out that women veterans only utilize available VHA healthcare, and such healthcare is not accommodating to women veterans. Frayne et al. [11] also point out that the utilization of Medicare in all veterans may be responsible for the decline in outpatient utilization in both sexes of veterans above age 65. Although the VHA has made improvements to provide a great array of services and accessibility for women veterans, the effectiveness of these efforts is not available and only 10% of women veterans actually access healthcare despite an increase in women in the military, reaching 20% at the time of publishing. Despite the statistics provided, the article only lists demographics of age and ethnicity, social and military status (service connection), marital status, and any medical condition. For this reason, we have endeavored to make the remarkable differences between male and female veterans available in the context of colorectal neoplasia. Other publications, such as those that describe unmet needs in veterans (93.9% males and 6.1% females), from the VA HERO CARE Survey [12] of 7955 veterans 55 years of age or older are especially valuable in determining timely interventions. A paper from the same era looked at veteran satisfaction at 25 VHA centers and showed a generally good assessment of healthcare amongst the veterans interviewed [13] and provided some demographics for each center. The center from which our data were collected was not represented. Women veterans were more likely to seek out VHA healthcare than men on returning from the Gulf and Afghanistan wars [14], but demographics were similar to those cited above (Table 2). Demographic data were provided in an interesting article on veteran infertility [15], where the overall incidence of male infertility was 10.8 cases/10,000 patient years compared to 93.6/10,000 patient years, and ethnicity data were provided. The age spread was different with males 18 years or older versus women aged 20–49, which may account for some of the discrepancy. In a landmark article published in 2022 [16], there was a described association with infertility following self-reported toxic exposures in veterans 20–45 years of age, with <10 years of separation from active duty. The study criteria were met in 727 men (51.7%) and 592 women (49.6%). In those who failed to conceive, there were 7.61 ± 3.87 exposures versus 7.13 ± 3.67 in those who conceived, p = 0.03. In males, there were 13.14 ± 4.19 versus 12.54 ± 4.10, respectively, at a p of <0.006. It would have been more useful if the paper had reported categorical data. In a study of male breast cancer [17], interestingly, 56% of males had cancer in their left breast, while laterality in females was similar (i.e., 50% vs. 49%). The principal author had noted a high frequency of left parotid hypertrophy in male veterans, with changes potentially related to left breast hypertrophy/gynecomastia and even metastasis from the left breast to the parotid, which have been explored but have yet to be proven [18].
There was a greater breadth of data incorporating BMI, and smoking and alcohol data. To bring in a different perspective, we quote a retrospective cohort study of Canadian veterans, ref. [19], detailing that Canadian female veterans represent 14% of Canadian veterans as opposed to 10% of US (from an online source with no posting or access date) and 11% of either UK or Australian female veterans. They do provide data on COPD (NSS) and diabetes (1.5% in males and 1.3% female (p < 0.0001), with a higher prevalence in males (4.8 vs. 2.5%)). Asthma and hypertension were more prevalent in male Canadian veterans (p = 0.004 and p < 0.0001 respectively). Since cardiovascular (CVD) deaths outpace other contenders, we feel compelled to offer a paper that focuses on women as compared to men in terms of CVD risk, in a cross-sectional Diabetes Epidemiologic Cohorts (DEpiC) study. In general, men outpace women in all risk categories except for obesity [20]. Again, the data are limited to CVD risk factors but does include ethnicity, smoking, regionality, mental disorders, and alcohol.
In terms of limitations, the major problem is the limited literature that has accrued, and well as the paucity of female veterans available compared to males. Not all veterans complied with specimen collection and follow-up, which thus decreased the statistical power of this study. On the other hand, there were strengths in the general numbers of males, and the unique biomarkers we were able to use to tease out the differences between the sexes. The sample size calculations were geared to CRC initially in males and hence fell afoul of the slightly lower female prevalence, but some effort was directed to gathering a true proportion of female veterans representative of their numbers.
In a recent article by Lee at al. [21], dealing with improvements in health equity by race and sex from the veteran experience, the patient numbers were small (n = 49) but the freelisting strategy was interesting. This is based on eliciting responses in the form of lists. They included people with co-morbidities and created a “salience score” for comparison. The spectrum ran the gamut of topics from virtual care to racism. The responses provided insights for improving care extending into sex and race, and “no impact” was the most common answer to the questions posed. Unfortunately, all groups reported “discrimination”, but despite this, most groups reported satisfaction with their VHA healthcare.
To bring some other facets of veteran experience where differences between the sexes may abound, we introduce the interaction of females into the Jail Diversion Programs [22,23]. Here, diversion programs mainly center on drug abuse, mental health issues, and trauma. There were 1025 total participants. Few statistically significant differences were noted between the sexes, but females did report more sexual trauma and post-traumatic stress disorders. In contrast to jailed veterans, Campbell et al. studied dimensions of social support in a cross-sectional study [24]. There were 34,331 respondents, including civilians and female veterans, a who had a significantly smaller social network size wwhen contrasted to civilians.
4. Materials and Methods
This study was a prospective, comparative cohort study with both a cross-sectional and longitudinal design. We constructed a patient database of veterans at increased risk of CRC and noted those who had contracted neoplasia prospectively. We compared the historic biomarker baseline response using the FERAD ratio. This ratio was obtained by dividing ferritin blood levels by the estimated fecal p87, a Paneth cell marker. The p87 molecule is bound by the Adnab-9 murine monoclonal, raised against colorectal adenoma antigens [3]. At initiation of this study, the antibody was commercially available (Dako, Carpinteria, CA 93013, USA). We refined the interrelationship of the FERAD ratio by correlating it to published PDL-1 responses for various cancers [9]. Briefly, fecal and other body fluids were collected after obtaining written informed consent. The inclusion criteria included willingness to participate in a long-term study and having a colonoscopy typically ordered by the initiative of patient’s physician. The exclusion criteria were a state of health that excluded colonoscopy, the inability to annotate dates on the stool cards, and non-compliance. There was also a phase-2 section to this study, where approximately 10% of enrollees allowed forcep biopsies by colonoscopy of regions of the colon to be taken [6]. The biopsies were placed in 10% buffered formalin and fixed in a paraffin block which was sectioned into 5 micron slices for immunohistochemistry. Non-fixed tissue was extracted as described previously and enabled a comparison between fixed and native antigens. The follow-up repeat colonoscopy was ideally 4–5 years. This study ran from 1995 to 2022. This study was approved by the Wayne State University Institutional Review Board, and all patient gave informed, written consent.
Colonic washings (effluent) were also collected during coloscopy from aggregated pools in the rectum. Unstimulated saliva and urine samples were likewise collected and stored at −70 degrees Celsius. In the preliminary reports, the stored samples yielded identical results after storage for 10 years. At room temperature, p87 protein was unaffected after exposure to ambient temperatures for 6 days. This was of practical importance as most participants mailed in their stool cards, and the local mail delivery took approximately 5 days. ELISA testing was performed in accordance with the manufacturers’ directions and was preceded by protein determination of the fecal samples by the Lowry method. Stool was diluted in phosphate-buffered saline, and 5 µg of protein was plated in wells of a 96-well microtiter plate, in triplicate. We incubated the plates overnight at 4 °C, with the primary Adnab-9 antibody that binds the p87 antigen on half the plate and the other half contained a monoclonal antibody of indifferent binding of the same IgG2a epitope (UPC10), which provided the background binding. Preliminary blocking of the plate by a 5% bovine serum albumin solution prevented non-specific binding. All washes between the primary and secondary antibody applications were performed with PBS and 5% Tween-20. The reagents using the ABC technique as instructed were supplied by Vector Labs (Newark, New Jersey, CA 94560, USA) or by DakoCytomation Inc. (Carpinteria, California, CA 93013, USA), for immunohistochemistry.
The final sandwich ELISA step required adding 40 µL of p-nitro-phenyl phosphate to develop a yellow color that was read on a Thermo-Fisher cyto-spectrophotometer at 405 nm (Carlsbad, CA 92008, USA). The results were expressed as optical density (OD) minus background. The same p87 reagents were used to run estern blots on BioRad electrophoresis equipment and transfer blotters (Hercules, CA 94547, USA) using 10% nitrocellulose gels incorporating molecular weight markers and PVDF membranes. The InImS marker FERAD ratio was also combined occasionally by division with the effluent p87 (FEREFF) value to explore additional biomarker advances.
The Wayne State University Institutional Review Board (Ethics Committee) and those other participation medical centers reviewed and approved the above protocols (H09–62-94). The results were analyzed using an Instat Inc. statistical package (now Veristat Inc. Southborough, MA 01772, USA). The ordinal data conformed to normality (using the Kolmogorov–Smirnov test); the ordinal data was analyzed by two-tailed Students t-test, and non-ordinal data by Chi-square. Linear correlation was analyzed by the least-squares method. The r coefficient values were depicted on graphs where applicable. Probability values were regarded as significant at the <0.05 level, and non-ordinal confidence intervals were presented at the 95% level with Chi-square, Pearson coefficient, or Fisher’s Exact tests where applicable. Where samples were limited, we used a 1-tailed t-test if the results were congruous with similar measured trends. The criteria for high-risk individuals for colonoscopy were standard but are in the purview of the primary care provider. A 5-year follow-up interval is standard, and fecal blood testing is usually performed before the colonoscopy. The sample size for power was achieved using the Veristat statistical package. A flow diagram has been published [6].
5. Conclusions
In summary, with most conclusions sorted by results and guided by sound statistical guidance from the literature [25], we feel that, drilling down for maximal data points and attention to non-ordinal data [26] wherever possible, will augment the prevalent race and sex differences, which are important in their own right. However, this approachmay ignore additional data [27,28,29,30] that are broad-based and provide in-depth understanding using new biomarkers, thus providing a new data “homunculus”.
Author Contributions
Conceptualization, M.T.; methodology, M.R. and J.H.; validation, N.F.R., S.F. and D.B.; formal analysis, M.T.; investigation, M.T.; data curation, M.T.; writing—original draft preparation, M.T.; writing—review and editing, F.A. and B.M.; supervision, F.A. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
There was no external funding for this paper.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Wayne State University (#H 09-62-94 2000/8/17).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data will be made available from the corresponding authors under the conditions of the Veterans Health Administration.
Acknowledgments
The authors acknowledge the capable assistance of our librarians Susanna Sheltrow, Andrea Snyr, Ereny Demian, and Yosef Tobi for performing the urine and saliva assays; Yi Xu for performing the ELISA; and Mary Pat Moyer for the expert proofreading. This paper is dedicated to the memory of Dennis Ahnen, an erudite practitioner of the art of gastrointestinal medicine who is universally missed for his good advice and humanity, and to our mentor, Eva Klein, the birthmother of cancer immunology. The opinions expressed in this paper are not necessarily those of the US federal government. This work was, however, conducted under the auspices of the US Government and, hence, in the public domain.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=Differences+between+male+and+female+veterans+in+the+USA (accessed on 23 March 2025).
- Ahnen, D.J.; Guerciolini, R.; Hauptman, J.; Blotner, S.; Woods, C.J.; Wargovich, M.J. Effect of orlistat on fecal fat, fecal biliary acids, and colonic cell proliferation in obese subjects. Clin. Gastroenterol Hepatol. 2007, 5, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Tamas, M.J.; Khakharia, A.; Rothenberg, R.B.; Phillips, L.S. Weight Trends in Veterans with and Without Diabetes, 2000 to 2014. Obesity (Silver Spring) 2018, 26, 1949–1957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batch, B.C.; Goldstein, K.; Yancy, W.S., Jr.; Sanders, L.L.; Danus, S.; Grambow, S.C.; Bosworth, H.B. Outcome by Gender in the Veterans Health Administration Motivating Overweight/Obese Veterans Everywhere Weight Management Program. J. Womens Health 2018, 27, 32–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moin, T.; Damschroder, L.J.; AuYoung, M.; Maciejewski, M.L.; Datta, S.K.; Weinreb, J.E.; Steinle, N.I.; Billington, C.; Hughes, M.; Makki, F.; et al. Diabetes Prevention Program Translation in the Veterans Health Administration. Am. J. Prev. Med. 2017, 53, 70–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Antaki, F.; Rambus, M.A.; Yang, Y.-X.; Kaplan, D.; Rodriguez, R.; Maliakkal, B.; Majumdar, A.; Demian, E.; Tobi, Y.Y.; et al. The Non-Invasive Prediction of Colorectal Neoplasia (NIPCON) Study 1995-2022: A Comparison of Guaiac-Based Fecal Occult Blood Test (FOBT) and an Anti-Adenoma Antibody, Adnab-9. Int. J. Mol. Sci. 2023, 24, 17257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Bluth, M.H.; Rossi, N.F.; Demian, E.; Talwar, H.; Tobi, Y.Y.; Sochacki, P.; Levi, E.; Lawson, M.; McVicker, B. In the SARS-CoV-2 Pandora Pandemic: Can the Stance of Premorbid Intestinal Innate Immune System as Measured by Fecal Adnab-9 Binding of p87:Blood Ferritin, Yielding the FERAD Ratio, Predict COVID-19 Susceptibility and Survival in a Prospective Population Database? Int. J. Mol. Sci. 2023, 19, 7536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Khoury, N.; Al-Subee, O.; Sethi, S.; Talwar, H.; Kam, M.; Hatfield, J.; Levi, E.; Hallman, J.; Moyer, M.P.; et al. Predicting Regression of Barrett’s Esophagus-Can All the King’s Men Put It Together Again? Biomolecules 2024, 14, 1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Talwar, H.; Rossi, N.F.; Lockette, W.; McVicker, B. A Practical Format to Organize Cancer Constellations Using Innate Immune System Biomarkers: Implications for Early Diagnosis and Prognostication. Int. J. Transl. Med. 2024, 4, 726–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frayne, S.M.; Yu, W.; Yano, E.M.; Ananth, L.; Iqbal, S.; Thrailkill, A.; Phibbs, C.S. Gender and use of care: Planning for tomorrow’s Veterans Health Administration. J. Womens Health 2007, 16, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Frayne, S.M.; Parker, V.A.; Christiansen, C.L.; Loveland, S.; Seaver, M.R.; Kazis, L.E.; Skinner, K.M. Health status among 28,000 women veterans. The VA Women’s Health Program Evaluation Project. J. Gen. Intern. Med. 2006, 21 (Suppl. 3), S40–S46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Davis, S.; Tyagi, P.; Bouldin, E.D.; Hansen, J.; Brintz, B.; Noel, P.; Rupper, R.; Trivedi, R.; Kinosian, B.; Intrator, O.; et al. Sex differences in unmet needs between male and female older Veterans. J. Women Aging 2024, 36, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Zickmund, S.L.; Burkitt, K.H.; Gao, S.; Stone, R.A.; Jones, A.L.; Hausmann, L.R.M.; Switzer, G.E.; Borrero, S.; Rodriguez, K.L.; Fine, M.J. Racial, Ethnic, and Gender Equity in Veteran Satisfaction with Health Care in the Veterans Affairs Health Care System. J. Gen. Intern. Med. 2018, 33, 305–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duggal, M.; Goulet, J.L.; Womack, J.; Gordon, K.; Mattocks, K.; Haskell, S.G.; Justice, A.C.; A Brandt, C. Comparison of outpatient health care utilization among returning women and men veterans from Afghanistan and Iraq. BMC Health Serv. Res. 2010, 10, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kroll-Desrosiers, A.; Copeland, L.A.; Mengeling, M.A.; Mattocks, K.M. Infertility Services for Veterans Enrolled in Veterans Health Administration Care. J. Gen. Intern. Med. 2023, 38, 2347–2353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancuso, A.C.; Mengeling, M.A.; Holcombe, A.; Ryan, G.L. Lifetime infertility and environmental, chemical, and hazardous exposures among female and male US veterans. Am. J. Obstet. Gynecol. 2022, 22, 744.e1–744.e12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Adepoju, B.; Yacur, M.; Maron, D.; Sharma, M.C. Gender Disparity in Breast Cancer: A Veteran Population-Based Comparison. Clin. Breast Cancer 2021, 21, e471–e478. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.K.; Lim, Y.J.; Kim, W. Breast Cancer Metastasis to the Parotid: A Case Report with Imaging Findings. Am. J. Case Rep. 2021, 22, e934311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- St Cyr, K.; Aiken, A.B.; Cramm, H.; Whitehead, M.; Kurdyak, P.; Mahar, A.L. Sex-specific differences in physical health and health services use among Canadian Veterans: A retrospective cohort study using healthcare administrative data. BMJ Mil Health 2023, 169, 430–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vimalananda, V.G.; Miller, D.R.; Christiansen, C.; Wang, W.; Tremblay, P.; Fincke, B.G. Cardiovascular disease risk factors among women veterans at VA medical facilities. J. Gen. Intern. Med. 2013, 28 (Suppl. S2), S517–S523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, N.S.; Keddem, S.; Sorrentino, A.E.; Jenkins, K.A.; Long, J.A. Health Equity in the Veterans Health Administration from Veterans’ Perspectives by Race and Sex. JAMA Netw. Open 2024, 5, e2356600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasin, D.S.; Grant, B.F. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: Review and summary of findings. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1609–1640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.W. Smoking prevalence among US veterans. J. Gen. Intern. Med. 2010, 25, 147–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verne, Z.T.; Fields, J.Z.; Verne, G.N.; Zhang, B.B.; Thacker, A.L.; Zhou, Q. Onset of Irritable Bowel Syndrome, Dyspepsia, Diarrhea, Bloating, and Constipation in Deployed Gulf War Veterans. Int. J. Gasteroenterol. 2024, 8, 5–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weeda, E.R.; Bishu, K.G.; Ward, R.; Axon, R.N.; Taber, D.J.; Gebregziabher, M. Joint effect of race/ethnicity or location of residence and sex on low density lipoprotein-cholesterol among veterans with type 2 diabetes: A 10-year retrospective cohort study. BMC Cardiovasc. Disord. 2020, 20, 449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upchurch, D.M.; Wong, M.S.; Yuan, A.H.; Haderlein, T.P.; McClendon, J.; Christy, A.; Washington, D.L. COVID-19 Infection in the Veterans Health Administration: Gender-specific Racial and Ethnic Differences. Womens Health Issues 2022, 32, 41–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stainbrook, K.; Hartwell, S.; James, A. Female Veterans in Jail Diversion Programs: Differences from and Similarities to Their Male Peers. Psychiatr. Serv. 2016, 67, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Stainbrook, K.; Penney, D.; Elwyn, L. The opportunities and challenges of multi-site evaluations: Lessons from the jail diversion and trauma recovery national cross-site evaluation. Eval. Program Plan. 2015, 50, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.B.; Gray, K.E.; Hoerster, K.D.; Fortney, J.C.; Simpson, T.L. Differences in functional and structural social support among female and male veterans and civilians. Soc. Psychiatry Psychiatr. Epidemiol. 2021, 56, 375–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, C.O.; Kohlmann, T. When to use the odds ratio or the relative risk? Int. J. Public Health 2008, 53, 165–167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).