Abstract
Background/Objectives: This study aimed to identify molecular variants associated with the progression of gastric precancerous lesions in a follow-up study conducted on patients from Southwestern Colombia. Methods: Whole-exome sequencing (WES) was performed on patients enrolled in the Colombian chemoprevention trial, who were classified into two groups—progression and regression—based on changes in the severity of their gastric precancerous lesions over 16 years of follow-up. The bioinformatics pipeline included steps for quality control, mapping, variant calling, filtering, and annotation. Associations between molecular variants and lesion progression were analyzed using Fisher’s exact test and the Cochran–Armitage trend test. Additionally, functional impact and pathway enrichment analyses were performed for variants that showed significant associations. Results: Thirty-eight molecular variants from thirty-seven participants were associated with the progression of gastric precancerous lesions. These variants were found in tumor suppressor genes like CDKN2A and CDK4, which are involved in cell cycle regulation and apoptosis. Additionally, variants were identified in extracellular matrix regulators such as COL23A1, LAMA2, and TNR. Other noteworthy findings included variants in FLT1, which is linked to VEGF signaling in angiogenesis, and APOB, which is involved in modulating inflammatory responses. Furthermore, alterations in genes associated with the hemostatic system, such as FGA and F5, underscored the connection between hemostasis and carcinogenesis. Conclusions: This exploratory analysis highlighted some molecular variants that may affect the function, structure, and expression of key proteins involved in cancer development, contributing to the progression of gastric precancerous lesions.
1. Introduction
Gastric cancer (GC) was responsible for approximately 660,175 deaths worldwide in 2022, making it the sixth most prevalent cancer in terms of both mortality and incidence [1]. The most common subtype is intestinal-type gastric cancer, which typically develops through a series of pathological stages. This progression begins with non-atrophic gastritis (NAG) and can advance to multifocal atrophic gastritis (MAG), intestinal metaplasia (IM), dysplasia, and ultimately GC [2]. A primary factor in the onset of this disease is infection with Helicobacter pylori (H. pylori) [2]. Helicobacter pylori is a Gram-negative, flagellated, and microaerophilic bacterium [3] that colonizes the gastric epithelium, where it releases virulence factors such as VacA and CagA, disrupting host cell functions. Additionally, it secretes enzymes including ureases, proteases, catalases, lipases, mucinases, and dismutases, which degrade the protective mucosal layer, enabling bacterial survival in the acidic gastric environment and facilitating evasion of host immune defenses [4]. Persistent H. pylori infection induces chronic gastric inflammation, a key driver in the progression from gastritis to precancerous lesions and, ultimately, gastric cancer [2]. This highlights its critical role as a major risk factor in gastric carcinogenesis.
In Colombia, GC is the leading cause of cancer-related deaths [1]. The Andean region of Nariño in Southwestern Colombia exhibits some of the highest incidence rates in the country (150 per 100,000) [5,6]. This high-mountain region is characterized by the convergence of several factors that contribute to an increased risk of gastric malignancy. These include a high prevalence of H. pylori infection, reaching 60% in children and 90% in adults; a significant prevalence (38.6%) of precursor lesions to malignancy; high consumption of salted, smoked, and dried foods; and low intake of micronutrients and antioxidants [7]. Therefore, it is essential to study genetic alterations that may modulate the effects of environmental factors exposure by regulating multiple biological pathways involved in gastric carcinogenesis [8].
Since 1991, a cohort of patients from this region has been monitored to evaluate the effects of anti-H. pylori therapy and antioxidant supplementation on the histological progression of gastric precancerous lesions [9]. Initial findings from a six-year follow-up indicated that all therapeutic interventions led to reductions in lesions, although the effects were not additive [9]. By the twelve-year mark, the benefits of H. pylori eradication continued, while the temporary effects of antioxidant supplementation diminished [10]. At 16 years, there was a decrease in diagnoses of MAG; however, advanced lesions such as IM and dysplasia did not show any reduction, highlighting the negative impact of prolonged H. pylori infection [11]. A follow-up after 20 years confirmed the protective effect of anti-H. pylori treatment against lesion progression [12]. Despite these positive outcomes, some patients still experienced histological progression, suggesting that host genetic susceptibility may play a significant role in the advancement of gastric precancerous lesions [13].
Several host genetic factors contribute to chronic gastritis and its progression to gastric cancer, including adaptive cellular mechanisms triggered by H. pylori infection, such as inflammation, oxidative stress, endoplasmic reticulum stress, the unfolded protein response, and autophagy [14]. These processes are influenced by various genes and molecular variants that can modulate their activity. For instance, polymorphisms in genes such as IL-1β, TNF-α, IL-8, and IL-1RN have been studied as potential genetic determinants of chronic inflammation and gastric cancer risk, as they can alter the expression of proinflammatory cytokines involved in the immune response [8,15].
Several genetic polymorphisms have been linked to an increased risk of gastric cancer [16], including mutations in key oncogenes such as EGFR, HER2, and KRAS, as well as in the tumor suppressor gene TP53 [17]. On the other hand, previous studies have identified molecular variants in genes such as MUC2, XRCC1, OGG1, IL-8, NFKB1, and CD14, which have been associated with the progression of gastric precancerous lesions in European and Asian populations through candidate SNP genotyping [18,19,20]. However, many genes involved in gastric carcinogenesis remain unexplored, highlighting the need for further research in this area.
Whole-exome sequencing (WES) is a powerful and cost-effective tool in the biomarker detection and discovery phase which enables the simultaneous analysis of hundreds of mutations [17,21]. Unlike targeted approaches, WES allows for an extensive examination of a patient’s DNA without being restricted to a predefined set of genes [22]. Moreover, it provides high coverage of coding regions, ensuring the reliable detection of single nucleotide variants (SNVs) and insertions/deletions (InDels), which are among the most common alterations [23]. Additionally, studies have reported the incidental identification of variants outside the target regions, attributed to the random fragmentation of genomic DNA during exome library preparation [24]. WES has proven particularly valuable in identifying genetic alterations linked to gastric cancer, validating molecular variants in well-established cancer-related genes, and uncovering novel variants implicated in carcinogenesis. These genes primarily function in genome integrity (TP53, BRCA2), chromatin remodeling (ARID1A), and cell adhesion (CDH1, FAT4, CTNNA1) [25,26]. By leveraging WES, this study aimed to identify a broad spectrum of molecular variants across multiple genes, offering a more comprehensive understanding of the genetic landscape of carcinogenesis.
We aimed to identify genetic alterations, including SNVs and InDels, that might influence the inflammatory processes associated with H. pylori infection and carcinogenesis, thereby affecting the progression of gastric lesions. We analyzed whole-exome sequencing (WES) data from patients in southwestern Colombia who had been followed for gastric precancerous lesions.
2. Results
2.1. Molecular Variants Associated with the Progression of Gastric Precancerous Lesions
The study included two groups of patients: 21 patients with the highest progression scores (Progression group) and 16 patients with the highest regression scores (Regression group). Supplementary Table S1 describes the sex and histological diagnosis of the analyzed population. A total of 11,731 genetic variants were identified across 776 genes out of the 911 candidate genes. Among these variants, 59.5% were in intronic regions, 34.4% were in exonic regions, and 5% were found in UTR regions (Supplementary Figure S1). Additionally, 1.1% comprised variants found upstream or downstream, splicing variants, and non-coding RNA (ncRNA) variants. Of the 776 genes analyzed, 98.6% (765 genes) showed alterations in the progression group, while 97.7% (758 genes) exhibited changes in the regression group. Variants common to both groups accounted for 95.5% (745 genes).
Figure 1 presents a flowchart summarizing the methodological steps followed in the study, including the total number of variants identified at each stage, up to the final selection of those significantly associated. Table 1 presents 38 significant associations (p-value < 0.01) between the analyzed molecular variants and the progression of gastric precancerous lesions, in comparison to the regression group. Notably, genes such as CDKN2A (rs11515), TNR (rs2228359), and CAPN2 (rs17599) showed significant differences in allele frequencies between the two groups, indicating their potential involvement in lesion progression. Three variants in the FLT1 gene, which is associated with angiogenesis, displayed significant differences in minor allele frequency (MAF) between the progression and regression groups (e.g., rs17537350: MAF of 0.67 vs. 0.28, p-value < 0.002). Variants in the TNK2 gene, known for its role in signaling pathways, also exhibited significant associations, with exonic variants such as rs2278034 showing an MAF of 0.52 in the progression group compared to 0.22 in the regression group (p-value < 0.009). Several variants in the SEMA3A gene, which is related to an extracellular matrix organization, demonstrated strong associations as well (e.g., rs7789342 with MAF of 0.79 vs. 0.47, p-value < 0.007). Additionally, variants in the APOB gene (e.g., rs676210) suggested a potential role in inflammation, with a progression MAF of 0.50 compared to a regression MAF of 0.19 (p-value < 0.007). Furthermore, variants in the genes LAMA2, COL23A1, and FAT4, which are crucial in extracellular matrix regulation, were strongly associated with lesion progression (e.g., rs17057184 in LAMA2: MAF of 0.33 vs. 0.06, p-value < 0.009). Lastly, multiple exonic variants in the F5 gene showed significant associations (e.g., rs6027 with MAF of 0.29 vs. 0.03, p-value < 0.005), suggesting their roles in hemostasis and carcinogenesis.
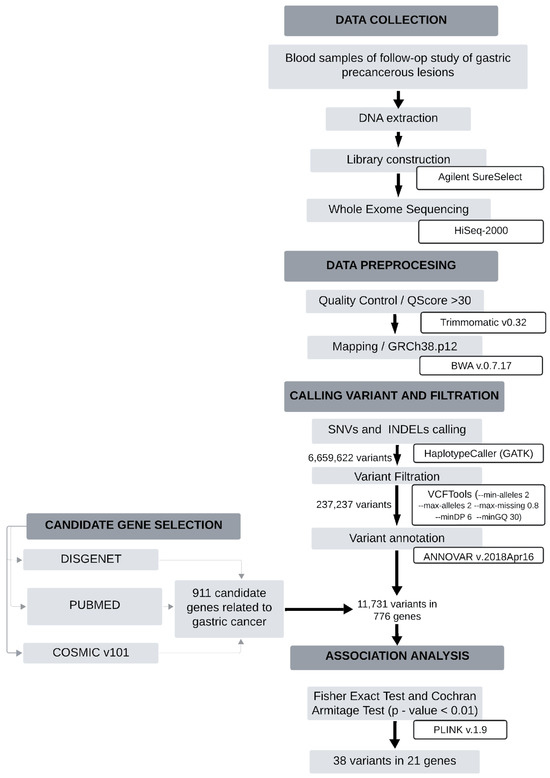
Figure 1.
Flowchart summarizing the methodological steps for identifying significantly associated variants in the study.

Table 1.
Molecular variants associated with the progression of gastric precancerous lesions.
2.2. Functional Impact Prediction of Nonsynonymous Variants
Nine variants located in the F5, FGA, TNK2, CAPN2, COL23A1, and APOB genes are nonsynonymous variants (Table 2). For the F5 gene, the variant rs6027 (D2222G) was predicted to be damaging according to SIFT and PROVEAN and was categorized as “probably damaging” by PolyPhen, indicating a significant functional impact. In contrast, the variants rs1800595 (H1327R) and rs6030 (M1764V) were predicted to be tolerated or benign by all tools, suggesting minimal impact on protein function. The variant rs6018 (N817T) showed mixed predictions; it is deemed damaging by SIFT and PROVEAN, while PolyPhen suggested a benign effect. For the FGA gene, the variant rs6050 (T331A) was predicted to be tolerated and benign by all assessment tools, indicating a low functional impact. In the TNK2 gene, the variant rs56260729 (P757L) was also predicted to be tolerated and benign, suggesting it has minimal effect on the protein. The CAPN2 gene featured the variant rs17599 (K490Q), which was predicted to be damaging by SIFT and PROVEAN, but benign according to PolyPhen, suggesting a potential moderate impact. Within the COL23A1 gene, the variant rs61739424 (R267W) was predicted to be damaging by SIFT and PROVEAN, with no prediction available from PolyPhen, suggesting a significant functional impact. Lastly, the APOB gene showed the variant rs676210 (P2739L), which was predicted to be damaging by SIFT and PROVEAN and classified as “probably damaging” by PolyPhen, strongly suggesting a deleterious effect.

Table 2.
Functional impact of the nonsynonymous variants.
2.3. Enriched Biological Pathways Linked to the Progression of Gastric Precancerous Lesions
Table 3 presents the enriched pathways significantly associated with the progression of gastric precancerous lesions based on the molecular variants identified in the study. The pathway with the highest significance was Post-translational Protein Phosphorylation (R-HSA-8957275), which included genes such as APOB, CDH2, F5, and FGA, with a p-value of 0.000025. This highlighted the importance of protein modification processes in lesion progression. Additionally, the Cell Cycle Regulation in G1 Phase pathways (R-HSA-69231, R-HSA-69236) connected variants in CDK4 and CDKN2A to cyclin D-associated events and G1 phase regulation, which are crucial for cell cycle progression, with a p-value of 0.002392. Another significant pathway was Extracellular Matrix (ECM) Organization (R-HSA-1474244), where variants in the genes CAPN2, COL23A1, FGA, LAMA2, and TNR underscored the importance of ECM remodeling, with a p-value of 0.000105. The ECM Proteoglycans pathway (R-HSA-3000178), associated with LAMA2 and TNR, emphasized the structural and functional roles of proteoglycans in maintaining tissue integrity, with a p-value of 0.006982. The Degradation of the ECM pathway (R-HSA-1474228) involved genes such as CAPN2 and COL23A1 that participate in ECM breakdown, potentially facilitating tumor invasion and progression, with a p-value of 0.022382. Moreover, the Toll-like Receptor (TLR) Regulation and Cascades pathways (R-HSA-5686938, R-HSA-168898) regulate immune responses through TLR signaling, featuring genes APOB and FGA, and showed significant enrichment with p-values of 0.000443 and 0.026409, respectively. The Non-Receptor Tyrosine Kinase Signaling pathway (R-HSA-9006927) included variants in CDK4 and RAC1, which are critical for cell proliferation and differentiation, with a p-value of 0.003583. The VEGF Signaling pathway (R-HSA-194138), involving FLT1 and RAC1, highlights angiogenesis as a key process in lesion progression, with a p-value of 0.013237. Finally, the Hemostasis and Fibrin Clot Formation pathways (R-HSA-109582, R-HSA-140877) involved genes F5 and FGA, linking blood clotting processes with gastric lesion progression, carrying p-values of 0.023201 and 0.001883.

Table 3.
Reactome pathways of genes with molecular variants that are significantly associated (p-value < 0.05).
3. Materials and Methods
3.1. Patients and Study Samples
This study utilized blood samples from a biobank managed by the Registro Poblacional de Cáncer de Cali (RPCC) in Colombia, as part of the Colombian chemoprevention trial. Histopathological data, which included global diagnoses such as normal histology, non-atrophic gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia, were obtained as previously described [9,10]. A histopathology score was developed by assigning numerical values to the subdivisions of the global diagnoses, as detailed in the cohort follow-up studies [9,10].
Patients were selected based on the variation in their histopathological scores over a 16-year follow-up period. Histopathological progression was defined as an increase in the score assigned to the histological diagnosis at the end of follow-up, compared to the baseline diagnosis. In contrast, histopathological regression was defined as a decrease in the score at follow-up compared to baseline.
3.2. Sample Preparation and Exome Sequencing
DNA was extracted using the salting-out method, and its concentration was measured with a Thermo Scientific NanoDrop 2000 spectrophotometer (Waltham, MA, USA). Exome enrichment was performed using the Agilent V6 and SureSelect V6-Post platforms (Agilent Technologies, Inc., Santa Clara, CA, USA). Libraries, which contained inserts of approximately 300 bp, were sequenced on Illumina HiSeq2000 and NextSeq2000 platforms (Illumina, Inc., San Diego, CA, USA), producing 100 bp paired-end reads at 100X coverage. Sequencing services were provided by Novogene Corporation Inc. (Sacramento, CA, USA) and the Translational Genomics Core Laboratory at Louisiana State University.
3.3. Bioinformatics Analysis
Quality control of the raw data was performed using FastQC version 0.11.7. Adapter trimming and the filtering of sequences with a Q Score below 30 were conducted using Trimmomatic version 0.32 [27]. The reads were then mapped to the GRCh38.p12 reference genome utilizing BWA version 0.7.17 with the BWA-MEM algorithm and default parameters [28]. The alignment files were processed with SAMtools version 1.6 [29]. During post-alignment processing, PCR duplicates were marked using Picard and removed from further analyses.
For variant calling, we used the HaplotypeCaller in GATK v.4.0.2.1. Single Nucleotide Variants (SNVs) and InDels were called simultaneously using gVCF mode (Genomic Variant Call Format) [30]. Variants and genotypes were filtered based on read depth and quality with VCFtools version 0.1.16, applying the following parameters: min-alleles 2, max-alleles 2, max-missing 0.8, minDP 30, and minGQ 30.
3.4. Selection of Candidate Genes
We identified human candidate genes that influence gastric carcinogenesis to focus our search for molecular variants. This list was created through a comprehensive review of various databases and the relevant literature. The criteria used for selecting candidate genes included the following: (i) the DISGENET repository (https://disgenet.com/, accessed on 15 December 2023), utilizing search terms such as “carcinoma of the stomach (diagnosis)”, “gastric adenocarcinoma”, “malignant neoplasm of the stomach”, and “neoplasm, stomach” [31]; (ii) a PUBMED search conducted in December 2023 employing the terms (stomach cancer OR gastric cancer) AND (exome sequencing OR whole genome sequencing) AND (review). Based on these criteria, we selected five NGS studies [25,26,32,33,34] and three reviews [8,35,36]; (iii) a search of the COSMIC v101 database (https://cancer.sanger.ac.uk/, accessed on 15 December 2023) for the most mutated genes associated with “intestinal adenocarcinoma”. In total, we selected 911 human candidate genes related to gastric cancer.
3.5. Annotation and Genotyping
Single nucleotide variants (SNVs) and insertions/deletions (InDels) were annotated using ANNOVAR version 2018Apr16 [37] which incorporated data from RefSeq/NCBI and dbSNP. Molecular variants in candidate genes were identified based on the annotated files.
3.6. Statistical Analysis
Patients were divided into two groups: those with progression and those with regression based on histopathological changes. We analyzed the associations between molecular variants and lesion progression using Fisher’s exact test [38] and the Cochran–Armitage trend test [39], both implemented in PLINK v1.9 software [40]. Fisher’s exact test was employed to assess global associations, ensuring reliable results in cases with small sample sizes. Meanwhile, the Cochran–Armitage trend test was applied specifically under an additive genetic model to evaluate whether allele frequency exhibited a directional trend associated with lesion progression. This model is particularly useful for detecting a dose-response relationship, where the presence of more copies of a specific allele might correlate with a higher risk of progression [41]. Their p-values may be similar or vary depending on the nature of the association, with both tests contributing to a more comprehensive understanding of the genetic factors involved.
3.7. Enrichment Analysis and Functional Impact
Functional enrichment analysis was conducted using the SNPnexus web server and Reactome version 90 database to identify pathways affected by significant variants. The key pathways identified included extracellular matrix organization, VEGF signaling, immune system regulation, and cell cycle control, all of which are important in carcinogenesis and lesion progression. Pathways with a p-value of less than 0.05 were considered significantly enriched. Additionally, the functional impact of nonsynonymous variants was predicted using PolyPhen2 [42], SIFT version 1.2 [43], and PROVEAN version 1.1.5 [44].
4. Discussion
The introduction of next-generation sequencing (NGS) technology has significantly advanced basic research in oncology by enabling the identification of genetic alterations linked to various types of cancer, including gastric cancer. This research utilized whole exome sequencing to pinpoint genetic variants associated with the progression of gastric precancerous lesions, which pose a high risk for developing the disease. These molecular variants allowed us to identify genes potentially involved in different hallmarks of gastric tumorigenesis. Specifically, we found variants in genes associated with the following: sustained proliferative signaling, driven by alterations in cell cycle regulation; chronic inflammation, which may contribute to immune dysregulation and oxidative stress, leading to cumulative tissue damage and genomic instability; angiogenesis, promoted by dysregulation of the VEGF signaling pathway; invasion and metastasis, facilitated by genes involved in extracellular matrix degradation, enabling tumor cell dissemination; and tumor progression, influenced by changes in hemostatic balance that enhance immune evasion, invasion, and metastatic potential.
This cohort study included patients from the Andean region of the Nariño department in Colombia, an area with a notably high incidence of gastric cancer and precancerous lesions [45,46]. The analysis of candidate genes identified 38 variants that may be associated with the progression of gastric precancerous lesions. These variants were found in 23 genes involved in various biological processes related to carcinogenesis (Table 3). Notably, the CDK4 and CDKN2A genes are critical for cell signaling and regulating the cell cycle, both of which contribute to the uncontrolled proliferation characteristic of cancer cells [47]. The CDK4 gene encodes a kinase that interacts with D-type cyclins, facilitating progression through the G1 phase of the cell cycle as the cell prepares to initiate DNA synthesis [48]. Previous studies have reported that the CycD-CDK4 complex is often constitutively activated in several cancers, including gastric cancer, leading to unchecked cell proliferation [49,50]. This suggests that CDK4 may act as a driver of carcinogenesis in gastric tissues [51]. Another important regulator of the cell cycle is CDKN2A, which is classified as part of the tumor suppressor family. CDKN2A encodes a protein known as p16, which inhibits CDK4/6 activity. When p16 expression is reduced, the activity of the CycD-CDK4/6 complex increases, leading to abnormal phosphorylation of the retinoblastoma gene [52]. This process accelerates cell growth. Additionally, CDKN2A encodes p14, which plays a role in the post-transcriptional regulation of TP53 expression [53]. Consequently, alterations in this gene can disrupt cell cycle regulation, resulting in the uncontrolled proliferation of abnormal cells [54]. The C540G variant (rs11515) is in an untranslated region (UTR) that is crucial for regulating gene expression [55]. In melanoma, this variant is linked to low p53 expression and a significantly shorter progression time from primary to metastatic melanoma [56]. Similarly, in breast cancer, the variant has been associated with reduced p16 protein expression and an increased proliferative capacity [53]. However, another study reported conflicting results, indicating no association between this variant and the risk of adenocarcinomas of the upper gastrointestinal tract in Caucasian populations [57]. Several factors may explain the lack of association and discrepancies in findings, including differences in cancer etiology, genetic variability across populations, disparities in age between case and control groups, absence of data on major risk factors, such as gastroesophageal reflux, H. pylori infection, alcohol consumption, and finally, the small sample size.
In our study cohort, the genetic variant was present in all individuals with progression of gastric precancerous lesions (N = 21), suggesting its potential role in the development of these conditions. However, given our sample size, further studies in larger populations are necessary to validate these findings and assess their broader applicability.
A key process driving cancer progression is the dysregulation of extracellular matrix (ECM) components, which play a crucial role in regulating cellular proliferation, migration, and apoptosis. Dysregulation of the ECM will compromise gastric tissue structure and function, ultimately contributing to gastric cancer progression. These alterations have been observed in gastric precancerous lesions, highlighting their potential role in early tumorigenesis [58]. In this study, molecular alterations were identified in genes associated with key ECM components, including COL23A1, LAMA2, and TNK2. The COL23A1 gene encodes a member of the transmembrane collagens that are involved in cell–matrix interactions and maintaining structural integrity [59]. Similarly, the LAMA2 gene encodes a subunit of laminin, an essential ECM component that influences cell adhesion, differentiation, and migration [60]. Alterations in the expression levels of both genes have been implicated in cancer development [59,61]. In the case of the COL23A1 gene, a non-synonymous alteration (R267W) was identified exclusively in the progression group, with a statistically significant association (p-value < 0.01 in two tests). Computational predictions consistently classified this variant as deleterious, suggesting it may lead to significant structural and functional disruptions in the encoded protein. Additionally, in gastric precancerous lesions, the expression of tenascin—a component of the ECM—shows a slight increase, which becomes more pronounced in invasive tumors. This suggests the involvement tenascin in both the progression of malignant growth and the processes of tissue injury repair. Alterations in the expression and function of genes encoding ECM elements lead to modifications in its composition, affecting tissue stiffness and architecture. This impacts cell communication and facilitates tumor cell migration and invasion, and this is observed from the early stages of gastric carcinogenesis [58].
In this study, several genes involved in Vascular Endothelial Growth Factor (VEGF) signaling were identified, including FLT1. This gene encodes a receptor tyrosine kinase known as Vascular Endothelial Growth Factor Receptor 1 (VEGFR1), which plays a critical role in angiogenesis and tumor progression [62]. Angiogenesis plays a major role in the multi-step carcinogenesis process since the gastric mucosa undergoes important histological changes, which may require gaining access to the vasculature to receive an adequate supply of nutrients and oxygen [63]. In gastric cancer, FLT1 expression is significantly elevated compared to normal tissues, and this increase is associated with a poorer prognosis [64]. Additionally, FLT1 is expressed in monocytes, where it regulates their activation and chemotaxis, thereby linking tumor-promoting inflammation to FLT1 signaling [65]. In gastric cancer, where chronic and persistent inflammation is the main driving force behind malignant transformation, angiogenesis and tumor-promoting inflammation are tightly linked processes that play essential roles in cancer progression [63,66]. Both tumor and stromal cells release angiogenic factors, driving endothelial cell proliferation. In turn, these endothelial cells contribute to sustained inflammation, further stimulating angiogenesis by secretion of cytokines, proteases, growth factors, and proangiogenic molecules, creating a feedback loop. Additionally, abnormal vascular structures can lead to hypoxia, acidosis, and DNA damage, fostering a tumor-supportive microenvironment [63]. Our association analysis identified three molecular variants of FLT1: two synonymous variants and one located in the 5′ untranslated region (UTR). The variant in the 5′ UTR region, c.-59C>T (rs55927955), may play a pivotal role in regulating gene expression and potentially altering FLT1. Variants in untranslated regions (UTRs) can influence key post-transcriptional processes, including pre-mRNA processing, mRNA stability, translation efficiency, and subcellular localization, all of which may impact gene expression [55].
The development of gastric cancer (GC) is closely linked to chronic inflammation, primarily triggered by H. pylori. This process initiates a cascade of cellular events that promote the malignant transformation of normal gastrointestinal epithelial cells [67]. Apolipoproteins play a significant role in modulating inflammation by neutralizing the hydrophobic signals of biological molecules, such as Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs). This action helps prevent the uncontrolled activation of the immune system [68,69]. Additionally, apolipoproteins have demonstrated an inhibitory effect on NF-κB activation, which limits the production of pro-inflammatory cytokines and prevents excessive and persistent immune responses that can lead to tissue damage [68]. APOB has been proposed as a biomarker in various types of cancer, including hepatocellular, kidney, and breast cancer [69]. Recently, low levels of APOB have been associated with an increased risk of gastric cancer [70]. In this study, two molecular variants of the APOB gene were identified. The non-synonymous variant P2739L (rs676210) was predicted to have a deleterious effect on protein function due to conformational changes resulting from a rigid proline substitution, potentially disrupting the structure and splicing of the protein, according to the HOPE database. This variant is in linkage disequilibrium with the intronic variant c.3697-79C>T (rs673548), both of which have been previously associated with lipid metabolism and coronary heart disease, but not with cancer [71]. A study conducted in an Asian population found a significant association between this variant and serum APOB levels. It was observed that individuals with two mutant alleles (AA) had lower serum levels of APOB compared to those with a heterozygous genotype [72]. These findings suggest that alterations in APOB expression and function may contribute to persistent inflammation, thereby promoting the carcinogenic cascade in gastric cancer.
Functional enrichment analysis indicates that changes in the hemostasis process may be associated with the progression of gastric lesions. Studies have identified a connection between the processes involved in carcinogenesis, such as primary tumor growth, cancer cell invasion, immune evasion, angiogenesis, and metastatic processes, and components of the hemostatic system [73,74]. One such component is coagulation factor V (F5), which plays a crucial role in the blood clotting cascade. The activation of the coagulation system, influenced by F5, can be advantageous for tumor development [75]. Research has shown that F5 is expressed at higher levels in breast cancer and is strongly associated with poor survival outcomes in gastric cancer [75,76]. Four non-synonymous genetic variants were identified in the F5 gene. The first variant, D2222G (rs6027), is predicted to have a deleterious effect. A study in breast cancer has shown a significant correlation between this variant and tumor size, with an odds ratio of 3.97 (95% CI 1.16–13.52, p-value 0.028) [76,77]. The second variant, H1327R (rs1800595), is predicted to be benign or tolerant; however, it is in linkage disequilibrium (LD) with the third variant, N817T (rs6018), which is a deleterious variant associated with response to the drug warfarin [78]. Additionally, the FGA gene, which encodes the fibrinogen alpha subunit, a component of blood clots [79,80], contains the non-synonymous variant T331A (rs6050). While this variant has not been directly linked to cancer risk, FGA is significant in gastric cancer due to its elevated serum expression levels in patients compared to healthy individuals, as well as its diagnostic accuracy in detecting both early and advanced disease stages. As a result, FGA is proposed as a potential tumor-associated biomarker [80].
Despite its contributions, this study has limitations, including a small sample size, the unknown contribution of the genetic ancestral fraction of the study population, and lack of p-value adjustment for multiple comparisons. The small sample size may limit the statistical power to detect significant genetic associations, and the findings might not fully generalize to larger populations. While we applied False Discovery Rate (FDR) correction to account for multiple testing, no variants remained significant after adjustment, which is expected in studies with limited sample sizes. For this reason, we report unadjusted p-values while acknowledging the potential for false positives. Future studies should consider increasing the cohort size and employing statistical corrections for multiple testing to strengthen the robustness of the findings and enhance their generalizability. Validation in larger cohorts is necessary to confirm these associations. Finally, our study used computational predictions to evaluate the potential impact of genetic variants on protein structure and function. However, future functional studies are necessary to verify the biological implications of the identified variants.
5. Conclusions
This exploratory study identified 38 molecular variants potentially contributing to the progression of gastric precancerous lesions, emphasizing their potential as biomarkers for early detection and therapeutic targets. These findings open new avenues for exploring their clinical applications, including personalized treatment strategies, while also enhancing our understanding of the molecular mechanisms underlying gastric carcinogenesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gidisord7020030/s1, Figure S1: Types of variants identified in 773 genes implicated in gastric cancer development. “Other”: category that includes upstream/downstream variants, intron splice site variants, exon splicing variants, and variants in non-coding RNA transcripts; Table S1: Sex and histological diagnosis of the analyzed population.
Author Contributions
Conceptualization, L.M.-O. and A.C.; Data curation, L.M.-O. and A.C.; Formal analysis, L.M.-O. and A.C.; Funding acquisition, L.M.-O. and A.C.; Investigation, L.M.-O., L.E.B. and A.C.; Methodology, L.M.-O., J.Z., J.G. and A.C.; Project administration, A.C.; Resources, J.Z., J.G. and L.E.B.; Supervision, A.C.; Validation, A.C.; Visualization, L.M.-O. and A.C.; Writing—original draft, L.M.-O.; Writing—review and editing, L.M.-O., J.Z., J.G., L.E.B. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad del Valle, grant number CI 71356, and Vanderbilt University—NIH—USA, Prime Award No. 2 P01 CA028842, Subaward No. VUMC3239. L.M.-O. was funded by Gobernacion de Nariño—Fundación CEIBA, doctoral grant number 1091, 2018. J.Z. was partially funded by NIH/NIGMS grant number P20 GM121288-06. The APC was funded by Vice-Rectory for Research, Universidad del Valle, grant number CI 71356.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Health, Universidad del Valle (code E 034-023, approved date 4 August 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Nelson Rivera, Diana Lopez, Francisco Aguayo, and Heiber Cardenas for their contribution to this work.
Conflicts of Interest
The authors declare no conflicts of interest. The founders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MAG | Multifocal Atrophic Gastritis |
| IM | Intestinal Metaplasia |
| GC | Gastric Cancer |
| WES | Whole Exome Sequencing |
References
- IARC GLOBOCAN 2022: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2022. Available online: https://gco.iarc.who.int (accessed on 21 February 2024).
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.M.; Zambrano, R.; Uribe, P.T.; Arturo, B.L.; Jaramillo, M.d.S.; Lopez, P.A.; Perez, J.M. Asociación Clínica, Patológica y Microbiológica de Helicobacter Pylori En Biopsias Gástricas En El Departamento de Caldas-Colombia. Rev. Gastroenterol. México 2018, 38, 144–150. [Google Scholar]
- Cervantes, E. Helicobacter Pylori: Mecanismos de Patogenicidad. Rev. Latinoam. Patol. Clín. 2016, 63, 100–109. [Google Scholar]
- Correa, P.; Cuello, C.; Duque, E.; Burbano, L.; García, F.; Bolaños, O.; Brown, C.; Haenszel, W. Gastric Cancer in Colombia. III. Natural History of Precursor Lesions. J. Natl. Cancer Inst. 1976, 57, 1027–1035. [Google Scholar] [CrossRef]
- Pardo, C.; Cendales, R. Incidencia, Mortalidad y Prevalencia de Cáncer en Colombia 2007–2011, 1st ed.; Instituto Nacional de Cancerologia: Bogotá, Colombia, 2015; ISBN 9789585883253. [Google Scholar]
- Bedoya-Urresta, Á.; Yépez, Y.; Calvache, D.; Cifuentes, Y.; Lucero, N.; González, P.; Bedoya, G.Á.; Manosalva, E.; Martínez, T.; Peñalosa, A.; et al. Proyecto Urkunina 5000-Investigación de La Prevalencia de Lesiones Precursoras y Del Efecto de La Erradicación de Helicobacter Pylori Como Prevención Primaria Del Cáncer Gástrico En El Departamento de Nariño. Rev. Colomb. Cirugía 2018, 33, 345–352. [Google Scholar] [CrossRef]
- Chiurillo, M.A. Role of Gene Polymorphisms in Gastric Cancer and Its Precursor Lesions: Current Knowledge and Perspectives in Latin American Countries. World J. Gastroenterol. 2014, 20, 4503–4515. [Google Scholar] [CrossRef]
- Correa, P.; Fontham, E.; Bravo, J.; Bravo, L.; Ruiz, B.; Zarama, G.; Realpe, J.; Malcom, G.; Mera, R. Chemoprevention of Gastric Dysplasia: Randomized Trial of Antioxidant Supplements and Anti-Helicobacter Pylori Therapy. J. Natl. Cancer Inst. 2000, 92, 1881–1888. [Google Scholar] [CrossRef]
- Mera, R.; Fontham, E.T.H.; Bravo, L.E.; Bravo, J.C.; Piazuelo, M.B.; Camargo, M.C.; Correa, P. Long Term Follow up of Patients Treated for Helicobacter Pylori Infection. Gut 2005, 54, 1536–1540. [Google Scholar] [CrossRef]
- Mera, R.; Bravo, L.E.; Camargo, C.; Bravo, J.; Delgado, A.; Romero, J.; Yepez, M.; Realpe, J.; Schneider, B.; Morgan, D.; et al. Dynamics of Helicobacter Pylori Infection as a Determinant of Progression of Gastric Precancerous Lesions: 16-Year Follow-up of an Eradication Trial. Gut 2018, 67, 1239–1246. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Bravo, L.E.; Mera, R.M.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Washington, M.K.; Rosero, A.; Garcia, L.S.; Realpe, J.L.; et al. The Colombian Chemoprevention Trial. Twenty-Year Follow-up of a Cohort of Patients with Gastric Precancerous Lesions. Gastroenterology 2020, 160, 1106–1117. [Google Scholar] [CrossRef]
- Companioni, O.; Bonet, C.; García, N.; Ramírez-Lázaro, M.J.; Lario, S.; Mendoza, J.; Adrados, M.M.; Poves, E.; Espinosa, L.; Pozo-Kreilinger, J.J.; et al. Genetic Variation Analysis in a Follow-up Study of Gastric Cancer Precursor Lesions Confirms the Association of MUC2 Variants with the Evolution of the Lesions and Identifies a Significant Association with NFKB1 and CD14. Int. J. Cancer 2018, 143, 2777–2786. [Google Scholar] [CrossRef]
- Díaz, P.; Valderrama, M.V.; Bravo, J.; Quest, A.F.G. Helicobacter Pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front. Microbiol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Rosero-Rojas, S.C.; Chaleal-Cultid, J.A.; Pazos-Moncayo, Á.J.; Rosero-Galindo, C.Y. Polimorfismos IL1B-511 y TNF-A-308 En Una Población Infectada Con Helicobacter Pylori de Una Zona de Bajo Riesgo de Cáncer Gástrico En Nariño-Colombia. Infectio 2020, 24, 81. [Google Scholar] [CrossRef]
- Wu, L.; Schaid, D.; Sicotte, H.; Wieben, E.; Li, H.; Petersen, G. Case-Only Exome Sequencing and Complex Disease Susceptibility Gene Discovery: Study Design Considerations Lang. J. Med. Genet. 2015, 52, 10–16. [Google Scholar] [CrossRef]
- Desai, A.N.; Jere, A. Next-Generation Sequencing for Cancer Biomarker Discovery. In Next Generation Sequencing in Cancer Research; Wu, W., Choudhry, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–125. [Google Scholar]
- Li, W.Q.; Zhang, L.; Ma, J.L.; Zhang, Y.; Li, J.Y.; Pan, K.F.; You, W.C. Association between Genetic Polymorphisms of DNA Base Excision Repair Genes and Evolution of Precancerous Gastric Lesions in a Chinese Population. Carcinogenesis 2009, 30, 500–505. [Google Scholar] [CrossRef]
- Li, Z.W.; Wu, Y.; Sun, Y.; Liu, L.Y.; Tian, M.M.; Feng, G.S.; You, W.C.; Li, J.Y. Inflammatory Cytokine Gene Polymorphisms Increase the Risk of Atrophic Gastritis and Intestinal Metaplasia. World J. Gastroenterol. 2010, 16, 1788–1794. [Google Scholar] [CrossRef]
- Marín, F.; Bonet, C.; Muñoz, X.; García, N.; Pardo, M.L.; Ruiz-Liso, J.M.; Alonso, P.; Capellà, G.; Sanz-Anquela, J.M.; González, C.A.; et al. Genetic Variation in MUC1, MUC2 and MUC6 Genes and Evolution of Gastric Cancer Precursor Lesions in a Long-Term Follow-up in a High-Risk Area in Spain. Carcinogenesis 2012, 33, 1072–1080. [Google Scholar] [CrossRef]
- Meyerson, M.; Gabriel, S.; Getz, G. Advances in Understanding Cancer Genomes through Second-Generation Sequencing. Nat. Rev. Genet. 2010, 11, 685–696. [Google Scholar] [CrossRef]
- Chang, V. Whole Exome Sequencing of Pediatric Gastric Adenocarcinoma: A Germline and Somatic Mutation Analysis. Master’s Thesis, University of California, Los Angeles, CA, USA, 2012. [Google Scholar]
- Valdés-Mas, R.; Bea, S.; Puente, D.A.; López-Otín, C.; Puente, X.S. Estimation of Copy Number Alterations from Exome Sequencing Data. PLoS ONE 2012, 7, e51422. [Google Scholar] [CrossRef]
- Kim, Y.C.; Cui, J.; Luo, J.; Xiao, F.; Downs, B.; Wang, S.M. Exome-Based Variant Detection in Core Promoters. Sci. Rep. 2016, 6, 30716. [Google Scholar] [CrossRef]
- Wang, K.; Kan, J.; Yuen, S.T.; Shi, S.T.; Chu, K.M.; Law, S.; Chan, T.L.; Kan, Z.; Chan, A.S.Y.; Tsui, W.Y.; et al. Exome Sequencing Identifies Frequent Mutation of ARID1A in Molecular Subtypes of Gastric Cancer. Nat. Genet. 2011, 43, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.J.; Cutcutache, I.; Poon, S.L.; Zhang, S.L.; Mcpherson, J.R.; Tao, J.; Rajasegaran, V.; Heng, H.L.; Deng, N.; Gan, A.; et al. Exome Sequencing of Gastric Adenocarcinoma Identifies Recurrent Somatic Mutations in Cell Adhesion and Chromatin Remodeling Genes. Nat. Genet. 2012, 44, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map (SAM) Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Sivachenko, A.Y.; Cibulskis, K.; Gabriel, S.B.; et al. A Framework for Variation Discovery and Genotyping Using Next- Generation DNA Sequencing Data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Park, J.; Yoo, H.M.; Jang, W.; Shin, S.; Kim, M.; Kim, Y.; Lee, S.W.; Kim, J.G.; Gurzu, S. Distribution of Somatic Mutations of Cancerrelated Genes According to Microsatellite Instability Status in Korean Gastric Cancer. Medicine 2017, 96, e7224. [Google Scholar] [CrossRef]
- Slavin, T.; Neuhausen, S.; Rybak, C.; Solomon, I.; Nehoray, B.; Blazer, K. Genetic Gastric Cancer Susceptibility in the International Clinical Cancer Genomics Community Research Network. Cancer Genet. 2017, 216–217, 111–119. [Google Scholar] [CrossRef]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.N.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-Genome Sequencing and Comprehensive Molecular Profiling Identify New Driver Mutations in Gastric Cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef]
- Abbas, M.; Faggian, A.; Sintali, D.N.; Khan, G.J.; Naeem, S.; Shi, M.; Dingding, C. Current and Future Biomarkers in Gastric Cancer. Biomed. Pharmacother. 2018, 103, 1688–1700. [Google Scholar] [CrossRef]
- Rosero, G.C.Y.; Mejía, O.L.; Corredor, M. A Review of Polymorphisms in Genes Involved in the Development of Gastric Cancer. Rev. Colomb. Gastroenterol. 2016, 31, 391–402. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Jang, J.-Y.; Park, T. Exact Association Test for Small Size Sequencing Data. BMC Med. Genom. 2018, 11, 30. [Google Scholar] [CrossRef]
- Ghodsi, M.; Amiri, S.; Hassani, H.; Ghodsi, Z. An Enhanced Version of Cochran-Armitage Trend Test for Genome-Wide Association Studies. Meta Gene 2016, 9, 225–229. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Manning, S.E.; Ku, H.-C.; Dluzen, D.F.; Xing, C.; Zhou, Z. A Nonparametric Alternative to the Cochran-Armitage Trend Test in Genetic Case-Control Association Studies: The Jonckheere-Terpstra Trend Test. PLoS ONE 2023, 18, e0280809. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the Effects of Coding Non-Synonymous Variants on Protein Function Using the SIFT Algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN Web Server: A Tool to Predict the Functional Effect of Amino Acid Substitutions and Indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Bravo, L.E.; Van Doorn, L.J.; Realpe, J.L.; Correa, P. Virulence-Associated Genotypes of Helicobacter Pylori: Do They Explain the African Enigma? Am. J. Gastroenterol. 2002, 97, 2839–2842. [Google Scholar] [CrossRef]
- Cuello, C.; Correa, P.; Haenszel, W.; Gordillo, G.; Brown, C.; Archer, M. Gastric Cancer in Colombia. I. Cancer Risk and Suspect Enviromental Agents. J. Natl. Cancer Inst. 1976, 37, 1015–1020. [Google Scholar]
- Chatrath, A.; Ratan, A.; Dutta, A. Germline Variants That Affect Tumor Progression. Trends Genet. 2021, 37, 433–443. [Google Scholar] [CrossRef]
- Baker, S.J.; Poulikakos, P.I.; Irie, H.Y.; Parekh, S.; Reddy, E.P. CDK4: A Master Regulator of the Cell Cycle and Its Role in Cancer. Genes Cancer 2022, 13, 21–45. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 Kinases: From Basic Science to Cancer Therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef]
- Javed, A.; Yarmohammadi, M.; Korkmaz, K.S.; Rubio-Tomás, T. The Regulation of Cyclins and Cyclin-Dependent Kinases in the Development of Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 2848. [Google Scholar] [CrossRef]
- Ullah Shah, A.; Mahjabeen, I.; Kayani, M.A. Genetic Polymorphisms in Cell Cycle Regulatory Genes CCND1 and CDK4 Are Associated with Susceptibility to Breast Cancer. J. BUON 2015, 20, 985–993. [Google Scholar]
- Roa, S.J.C.; Vo, Q.; Araya, O.J.C.; Villaseca, H.M.; Guzmán, G.P.; Ibacache, S.G.; de Aretxabala, U.X.; Roa, E.I. Inactivación Del Gen CDKN2A (P16) En Cáncer de La Vesícula Biliar. Rev. Médica Chile 2004, 132, 11. [Google Scholar] [CrossRef]
- Royds, J.A.; Pilbrow, A.P.; Ahn, A.; Morrin, H.R.; Frampton, C.; Russell, I.A.; Moravec, C.S.; Sweet, W.E.; Tang, W.H.W.; Currie, M.J.; et al. The Rs11515 Polymorphism Is More Frequent and Associated With Aggressive Breast Tumors with Increased ANRIL and Decreased P16INK4a Expression. Front. Oncol. 2016, 5, 306. [Google Scholar] [CrossRef]
- Danishevich, A.; Bilyalov, A.; Nikolaev, S.; Khalikov, N.; Isaeva, D.; Levina, Y.; Makarova, M.; Nemtsova, M.; Chernevskiy, D.; Sagaydak, O.; et al. CDKN2A Gene Mutations: Implications for Hereditary Cancer Syndromes. Biomedicines 2023, 11, 3343. [Google Scholar] [CrossRef]
- Steri, M.; Idda, M.L.; Whalen, M.B.; Orrù, V. Genetic Variants in MRNA Untranslated Regions. WIREs RNA 2018, 9, e1474. [Google Scholar] [CrossRef]
- Sauroja, I.; Smeds, J.; Vlaykova, T.; Kumar, R.; Talve, L.; Hahka-Kemppinen, M.; Punnonen, K.; Jansèn, C.T.; Hemminki, K.; Pyrhönen, S. Analysis of G1/S Checkpoint Regulators in Metastatic Melanoma. Genes Chromosomes Cancer 2000, 28, 404–414. [Google Scholar] [CrossRef]
- Geddert, H.; Kiel, S.; Zotz, R.B.; Zhang, J.; Willers, R.; Gabbert, H.E.; Sarbia, M. Polymorphism of P16 INK4A and Cyclin D1 in Adenocarcinomas of the Upper Gastrointestinal Tract. J. Cancer Res. Clin. Oncol. 2005, 131, 803–808. [Google Scholar] [CrossRef]
- Moreira, A.M.; Pereira, J.; Melo, S.; Fernandes, M.S.; Carneiro, P.; Seruca, R.; Figueiredo, J. The Extracellular Matrix: An Accomplice in Gastric Cancer Development and Progression. Cells 2020, 9, 394. [Google Scholar] [CrossRef]
- Xu, F.; Chang, K.; Ma, J.; Qu, Y.; Xie, H.; Dai, B.; Gan, H.; Zhang, H.; Shi, G.; Zhu, Y.; et al. The Oncogenic Role of COL23A1 in Clear Cell Renal Cell Carcinoma. Sci. Rep. 2017, 7, 9846. [Google Scholar] [CrossRef]
- Packer, D.; Martin, P.T. Micro-Laminin Gene Therapy Can Function as an Inhibitor of Muscle Disease in the DyW Mouse Model of MDC1A. Mol. Ther. Methods Clin. Dev. 2021, 21, 274–287. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Lan, Y.-L.; Lou, J.-C.; Lyu, Y.-Z.; Hao, Y.-C.; Su, Q.-F.; Ma, B.-B.; Yuan, Z.-B.; Yu, Z.-K.; Zhang, H.-Q.; et al. Expression and Methylation Status of LAMA2 Are Associated with the Invasiveness of Nonfunctioning PitNET. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018818821296. [Google Scholar] [CrossRef]
- Shibuya, M. Involvement of Flt-1 (VEGF Receptor-1) in Cancer and Preeclampsia. Proc. Jpn. Acad. Ser. B 2011, 87, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Malespín-Bendaña, W.; Alpízar-Alpízar, W.; Figueroa-Protti, L.; Reyes, L.; Molina-Castro, S.; Une, C.; Ramírez-Mayorga, V. Helicobacter Pylori Infection Induces Gastric Precancerous Lesions and Persistent Expression of Angpt2, Vegf-A and Tnf-A in a Mouse Model. Front. Oncol. 2023, 13, 1072802. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Liu, L.; Liu, Y.; Bi, W. Co-Expression of VEGF-B and FLT-1 Correlates with Malignancy and Prognosis of Gastric Cancer. Biomark. Med. 2021, 15, 481–488. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Zhang, H.; Li, J.; He, T.; Yeo, E.-J.; Soong, D.Y.H.; Carragher, N.O.; Munro, A.; Chang, A.; Bresnick, A.R.; et al. FLT1 Signaling in Metastasis-Associated Macrophages Activates an Inflammatory Signature That Promotes Breast Cancer Metastasis. J. Exp. Med. 2015, 212, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Watanabe, M.; Baba, H. Chronic Inflammation and Gastrointestinal Cancer. J. Cancer Metastasis Treat. 2015, 1, 138. [Google Scholar] [CrossRef]
- Cho, N.; Seong, S. Apolipoproteins Inhibit the Innate Immunity Activated by Necrotic Cells or Bacterial Endotoxin. Immunology 2009, 128, e479–e486. [Google Scholar] [CrossRef]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and Cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef]
- Pih, G.Y.; Gong, E.J.; Choi, J.Y.; Kim, M.-J.; Ahn, J.Y.; Choe, J.; Bae, S.E.; Chang, H.-S.; Na, H.K.; Lee, J.H.; et al. Associations of Serum Lipid Level with Gastric Cancer Risk, Pathology, and Prognosis. Cancer Res. Treat. 2021, 53, 445–456. [Google Scholar] [CrossRef]
- Aceves-Ramírez, M.; Valle, Y.; Casillas-Muñoz, F.; Martínez-Fernández, D.E.; Parra-Reyna, B.; López-Moreno, V.A.; Flores-Salinas, H.E.; Valdés-Alvarado, E.; Muñoz-Valle, J.F.; García-Garduño, T.; et al. Analysis of the APOB Gene and Apolipoprotein B Serum Levels in a Mexican Population with Acute Coronary Syndrome: Association with the Single Nucleotide Variants Rs1469513, Rs673548, Rs676210, and Rs1042034. Genet. Res. 2022, 2022, 4901090. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Han, W.; Zhang, Q.; Shang, X.; Li, X.; Lu, F.; Liu, X. Polymorphisms in ApoB Gene Are Associated with Risk of Myocardial Infarction and Serum ApoB Levels in a Chinese Population. Int. J. Clin. Exp. Med. 2015, 8, 16571–16577. [Google Scholar]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and Cancer: Biological and Clinical Aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Moik, F.; Ay, C. Hemostasis and Cancer: Impact of Haemostatic Biomarkers for the Prediction of Clinical Outcomes in Patients with Cancer. J. Thromb. Haemost. 2022, 20, 2733–2745. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, X.-W.; Qin, Y.-Z.; Mo, X.-W.; Luo, S.-S. Identification of F5 as a Prognostic Biomarker in Patients with Gastric Cancer. BioMed Res. Int. 2020, 2020, 9280841. [Google Scholar] [CrossRef] [PubMed]
- Tinholt, M.; Sandset, P.M.; Iversen, N. Polymorphisms of the Coagulation System and Risk of Cancer. Thromb. Res. 2016, 140, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Tinholt, M.; Garred, Ø.; Borgen, E.; Beraki, E.; Schlichting, E.; Kristensen, V.; Sahlberg, K.K.; Iversen, N. Subtype-specific Clinical and Prognostic Relevance of Tumor-expressed F5 and Regulatory F5 Variants in Breast Cancer: The CoCaV Study. J. Thromb. Haemost. 2018, 16, 1347–1356. [Google Scholar] [CrossRef]
- Jorgensen, A.L.; Al-Zubiedi, S.; Zhang, J.E.; Keniry, A.; Hanson, A.; Hughes, D.A.; Eker, D.V.; Stevens, L.; Hawkins, K.; Toh, C.H.; et al. Genetic and Environmental Factors Determining Clinical Outcomes and Cost of Warfarin Therapy: A Prospective Study. Pharmacogenet. Genom. 2009, 19, 800–812. [Google Scholar] [CrossRef]
- GeneCards Gene-Fibrinogen Alpha Chain. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=FGA (accessed on 3 November 2024).
- Shi, F.; Wu, H.; Qu, K.; Sun, Q.; Li, F.; Shi, C.; Li, Y.; Xiong, X.; Qin, Q.; Yu, T.; et al. Identification of Serum Proteins AHSG, FGA and APOA-I as Diagnostic Biomarkers for Gastric Cancer. Clin. Proteom. 2018, 15, 18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).