Pre- and Postoperative Risk Factors for Hirschsprung-Associated Enterocolitis in Vietnamese Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Analysis
3. Results
3.1. General Characteristics
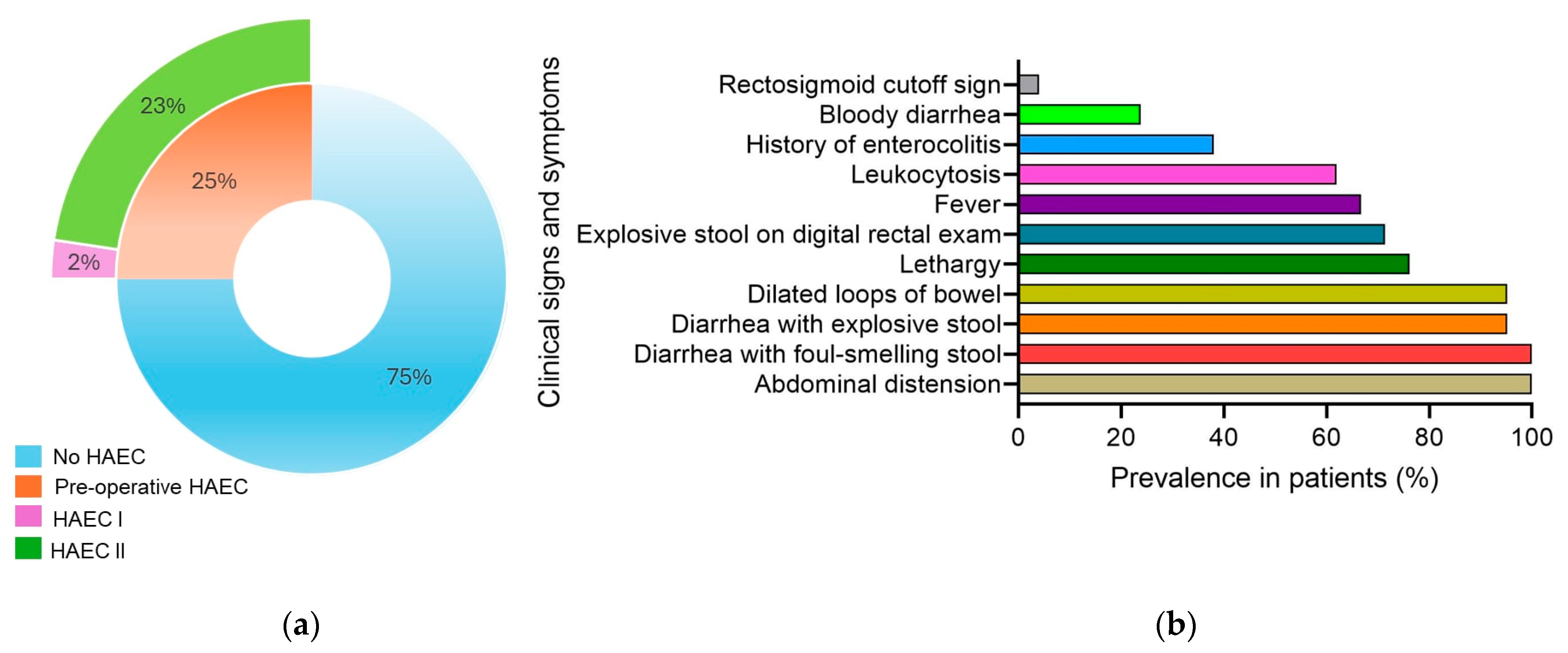
3.2. Preoperative Enterocolitis
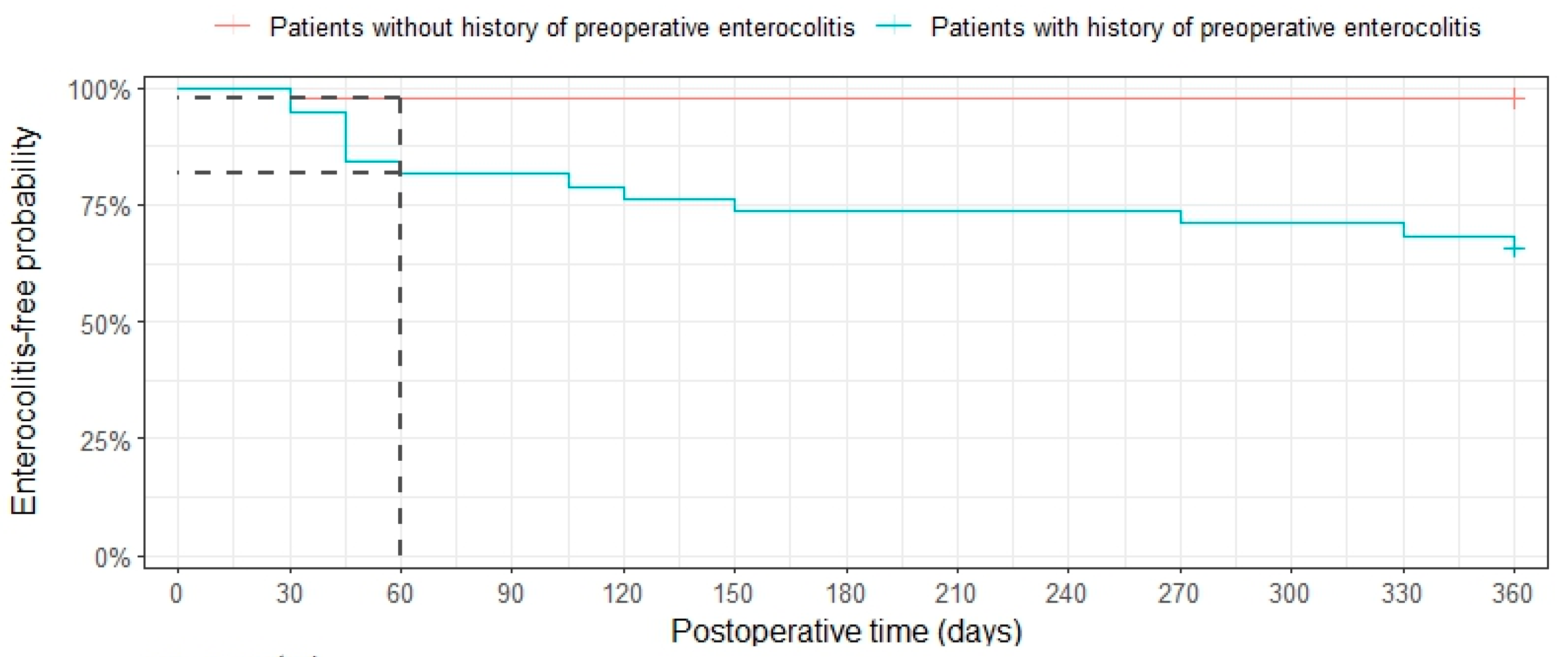
3.3. Postoperative Enterocolitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence intervals |
| HAEC | Hirschsprung-associated enterocolitis |
| Hb | Hemoglobin |
| HSCR | Hirschsprung’s disease |
| OR | Odds ratio |
| TEPT | Transanal endorectal pull-through |
| WHO | World Health Organization |
References
- Gosain, A.; Frykman, P.K.; Cowles, R.A.; Horton, J.; Levitt, M.; Rothstein, D.H.; Langer, J.C.; Goldstein, A.M. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr. Surg. Int. 2017, 33, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Holscheneider, A.M. Hirschsprung’s Disease and Allied Disorder, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Pecoraro, A.R.; Hunter, C.E.; Bennett, W.E.; Markel, T.A. Factors Affecting Higher Readmission Rates and Costs in Pediatric Patients With Hirschsprung Disease. J. Surg. Res. 2021, 268, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Lewit, R.A.; Kuruvilla, K.P.; Fu, M.; Gosain, A. Current understanding of Hirschsprung-associated enterocolitis: Pathogenesis, diaginosis and treatment. J. Pediatr. Surg. 2022, 31, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Vieten, D.; Spicer, R. Enterocolitis complicating Hirschsprung’s disease. Semin. Pediatr. Surg. 2004, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, L.C.; Skarda, D.E.; Rollins, M.D.; Bucher, B.T. Hirschsprung-associated enterocolitis in children treated at US children’s hospitals. J. Pediatr. Surg. 2020, 55, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yan, J.; Zhang, Z.; Kai, W.; Wang, Z.; Chen, Y. Risk factors for Hirschsprung-associated enterocolitis following Soave: A retrospective study over a decade. BMC Pediatr. 2022, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.J.; Nataraja, R.; Pacilli, M. Prevention and management of recurrent postoperative Hirschsprung’s disease obstructive symptoms and enterocolitis: Systematic review and meta-analysis. J. Pediatr. Surg. 2018, 53, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.C.; Osman, F.; Teitelbaum, D.H.; Caty, M.G.; Langer, J.C. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: A Delphi analysis. J. Pediatr. Surg. 2009, 44, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Onis, M.D.; Garza, C.; Onyango, A.W.; Martorell, R. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar]
- Levitt, M.A.; Hamrick, M.C.; Eradi, B.; Bischoff, A.; Hall, J.; Peña, A. Transanal, full-thickness, Swenson-like approach for Hirschsprung disease. J. Pediatr. Surg. 2013, 48, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Gunadi; Luzman, R.A.; Kencana, S.M.S.; Arthana, B.D.; Ahmad, F.; Sulaksmono, G.; Rastaputra, A.S.; Arini, G.P.; Pitaka, R.T.; Dwihantoro, A.; et al. Comparison of Two Different Cut-Off Values of Scoring System for Diagnosis of Hirschsprung-Associated Enterocolitis After Transanal Endorectal Pull-Through. Front. Pediatr. 2021, 9, 705663. [Google Scholar] [CrossRef] [PubMed]
- Cher, J.; Wu, C.; Adams, S. Hirschsprung-Associated Enterocolitis. In Gastrointestinal Diseases and Their Associated Infections; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–247. [Google Scholar]
- Frykman, P.K.; Short, S.S. Hirschsprung-associated enterocolitis: Prevention and therapy. Semin. Pediatr. Surg. 2012, 21, 328–335. [Google Scholar] [CrossRef]
- Zhang, X.M.; Sun, D.; Xu, Q.; Liu, H.; Li, Y.; Wang, D.; Wang, J.; Zhang, Q.; Hou, P.M.; Mu, W.M.; et al. Risk factors for Hirschsprung disease-associated enterocolitis: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 2509–2524. [Google Scholar] [CrossRef] [PubMed]
- Yulianda, D.; Sati, A.I.; Makhmudi, A.G. Risk factors of preoperative Hirschsprung-associated enterocolitis. BMC Proc. 2019, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Hagens, J.; Reinshagen, K.; Tomuschat, C. Prevalence of Hirschsprung-associated enterocolitis in patients with Hirschsprung disease. Pediatr. Surg. Int. 2022, 38, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Frykman, P.K.; Nordenskjöld, A.; Kawaguchi, A.; Hui, T.T.; Granström, A.L.; Cheng, Z.; Tang, J.; Underhill, D.M.; Iliev, I.; Funari, V.A.; et al. Characterization of Bacterial and Fungal Microbiome in Children with Hirschsprung Disease with and without a History of Enterocolitis: A Multicenter Study. PLoS ONE 2015, 10, e0124172. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Tanaka, H.; Endo, N. Predictive factors for the development of postoperative Hirschsprung-associated enterocolitis in children operated during infancy. Pediatr. Surg. Int. 2021, 37, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Le-Nguyen, A.; Righini-Grunder, F.; Piché, N.; Faure, C.; Aspirot, A. Factors influencing the incidence of Hirschsprung associated enterocolitis (HAEC). J. Pediatr. Surg. 2019, 54, 959–963. [Google Scholar] [CrossRef] [PubMed]

| Variables | Samples (n = 84) | |
|---|---|---|
| Age at diagnosis (months) | Mean ± S.D. | 7.2 ± 10.7 |
| Interquartile range | 1.5–8 | |
| Range | 1–68 | |
| Gender | Male | 71 (84.5%) |
| Female | 13 (15.5%) | |
| Gestation | Full term | 81 (96.4%) |
| Pre-term | 3 (3.6%) | |
| Congenital abnormalities | Down syndrome | 1 (1.2%) |
| Genitourinary defects | 4 (4.8%) | |
| Cardiac defects | 7 (8.4%) | |
| None | 82 (85.6%) | |
| Clinical presentation | Abdominal distension | 78 (92.9%) |
| Delayed meconium passage | 73 (86.9%) | |
| High-pressure rectal decompression | 38 (45.2%) | |
| Palpable fecal mass | 6 (7.1%) |
| Patients with HAEC (n = 21) | Patients Without HAEC (n = 63) | OR [95% CI] | p-Value a | |
|---|---|---|---|---|
| Percentile of weight for age b | 0.001 ** | |||
| Normal | 7 (13%) | 47 (87%) | 1 | |
| Underweight | 12 (48%) | 13 (52%) | 0.17 [0.05; 0.50] | |
| Malnutrition | 2 (50%) | 2 (50%) | 0.16 [0.01; 1.71] | |
| Overweight | 0 (0%) | 1 (100%) | - | |
| History of HAEC | 3.53 [1.26; 10.3] | 0.024 * | ||
| Yes | 12 (41.4%) | 17 (58.6%) | ||
| No | 9 (16.4%) | 46 (83.6%) | ||
| Preoperative medical care | 0.18 [0.04; 0.61] | 0.010 * | ||
| Yes | 3 (8.82%) | 31 (91.2%) | ||
| No | 18 (36.0%) | 32 (64.0%) | ||
| Enemas radiology aganglionosis | 1.23 [0.16; 35.1] | 1.000 | ||
| Short | 20 (25.3%) | 59 (74.7%) | ||
| Long | 1 (20%) | 4 (80%) | ||
| Congenital anomalies | 0.40 [0.11; 1.57] | 0.164 | ||
| Yes | 7 (58.3%) | 7 (58.3%) | ||
| No | 56 (77.8%) | 16 (22.2%) |
| Patients with HAEC (n = 14) | Patients Without HAEC (n = 69) | p-Value a | |
|---|---|---|---|
| History of preoperative HAEC | <0.001 ** | ||
| Yes | 11 (37.9%) | 18 (62.1%) | |
| No | 3 (5.56%) | 51 (94.4%) | |
| Procedure | 0.581 | ||
| Swenson-like | 10 (18.2%) | 45 (81.8%) | |
| SWlike—open | 2 (28.6%) | 5 (7.4%) | |
| SWlike—lap | 0 (0.0%) | 7 (100%) | |
| TEPT | 2 (14.3%) | 12 (85.7%) | |
| Complication | 0.002 ** | ||
| Stricture | 4 (80.0%) | 1 (20.0%) | |
| None | 10 (12.8%) | 68 (87.2%) | |
| Hb (g/dL) | 11.7 (2.94%) | 11.5 (2.23%) | 0.823 |
| Blood loss (mL) | 12.1 (9.73%) | 8.91 (7.58%) | 0.224 |
| Aganglionic length (cm) | 9.67 | 6.7 | 0.031 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viet, H.T.; Minh, T.H.; Truong, N.V.; Phuong, A.H.T.; Nguyen, B.-U.; The, H.C.; Dang, C.P.; Uy, L.T.N. Pre- and Postoperative Risk Factors for Hirschsprung-Associated Enterocolitis in Vietnamese Children. Gastrointest. Disord. 2025, 7, 17. https://doi.org/10.3390/gidisord7010017
Viet HT, Minh TH, Truong NV, Phuong AHT, Nguyen B-U, The HC, Dang CP, Uy LTN. Pre- and Postoperative Risk Factors for Hirschsprung-Associated Enterocolitis in Vietnamese Children. Gastrointestinal Disorders. 2025; 7(1):17. https://doi.org/10.3390/gidisord7010017
Chicago/Turabian StyleViet, Hoang Tran, Tuan Huynh Minh, Nhan Vu Truong, Anh Huynh Thi Phuong, Bich-Uyen Nguyen, Hao Chung The, Cong Phi Dang, and Linh Truong Nguyen Uy. 2025. "Pre- and Postoperative Risk Factors for Hirschsprung-Associated Enterocolitis in Vietnamese Children" Gastrointestinal Disorders 7, no. 1: 17. https://doi.org/10.3390/gidisord7010017
APA StyleViet, H. T., Minh, T. H., Truong, N. V., Phuong, A. H. T., Nguyen, B.-U., The, H. C., Dang, C. P., & Uy, L. T. N. (2025). Pre- and Postoperative Risk Factors for Hirschsprung-Associated Enterocolitis in Vietnamese Children. Gastrointestinal Disorders, 7(1), 17. https://doi.org/10.3390/gidisord7010017






