Abstract
Background/Objectives: Colorectal cancer incidence in Bahrain occurs at a ratio of 13.4–18.8 per 100,000 persons after age standardization. This study aims to explore the relationship between colorectal cancer/abnormalities and different lifestyle factors. Secondly, it aims to explore the association between f-Hb levels, colonoscopy findings, and lifestyle factors in a FIT-positive population in Bahrain. Method: A retrospective cross-sectional study was performed for patients positive for FIT and who had a colonoscopy. Different dietary and demographic factors as well as f-Hb levels were assessed. Results: A total of 559 (M: 330; F: 229) subjects were enrolled in this study. Subjects with CRC had significantly higher f-Hb concentrations (median: 1269 μg/mg) when compared with subjects of other groups. Higher percentages of CRC as well as large and small polyps were recorded in males. However, there was no significant difference in f-Hb concentration between males and females (p = 0.90). Higher median levels were found for f-Hb in patients with Q3 (higher red meat consumption) compared to Q1 and Q2 in the category with CRC, despite there being no statistically significant differences among the groups (p = 0.742). Similar results for coffee consumption and f-Hb concentrations in the different groups have been recorded (p = 0.697). A higher quartile of red meat consumption was associated with an increase in CRC risk of 79.9%. Coffee consumption reflected a lower risk of CRC by −47% moving from Q1 to Q2, while BMI was found to be a risk factor (+44%) for CRC. Conclusion: This study highlighted that high f-Hb concentration can be used as a predictive biomarker of CRC.
1. Introduction
Colorectal cancer (CRC) is a malignancy that ranks as the third-most diagnosed cancer in the world. Bray F et al. published a study in 2024 that provided global cancer statistics for 2022. They estimated the incidence and mortality rates for 36 different cancers across 185 countries worldwide. Their study found that colon cancer is the third most commonly diagnosed cancer globally, with an estimated 1.9 million new cases and over 900,000 deaths [1]. The gradual accumulation of genetic and epigenetic alterations leads to the transformation of mucosal epithelial cells into benign adenomas. These adenomas can later evolve into the malignant form of adenocarcinoma and CRCs [2]. The progression from benign lesions to cancer can take years, allowing the detection of precancerous adenoma or early cancer through screening tests [3].
High consumption of fruits and vegetables is possibly associated with a decreased risk of colorectal cancer. The evidence suggests that a higher intake of certain fruits, such as citrus, apples, watermelon, and kiwi, is associated with a lower risk of colorectal cancer, particularly colon cancer (Terry P et al.).
The most used screening method is a fecal immunochemical test (FIT) [4], a one-sample test that uses an antibody assay to detect and quantify the human hemoglobin in the stool [5]. Previous studies have shown the association between the fecal hemoglobin (f-Hb) concentration and colorectal lesions. CRC subjects present with a higher concentration of f-Hb than subjects with adenoma or non-neoplastic findings [6,7,8].
The incidence and mortality of CRC patients around the world is varied [4]. Screening tests are an imperative tool to lessen the incidence and mortality rates of CRC [4]. Although CRC is most associated with developed countries, many of the regions that have a high number of CRC cases are those undergoing economic development and do not implement screening programs [4], or implement them insufficiently [2]. These regions tend to be less developed and therefore hold the largest number of CRC mortalities around the world, such as regions in Africa [4]. On the other hand, developed countries employ recurrent CRC screenings which lessen the rates of incidence and mortality [2].
During the last two decades, the number of new CRC cases has increased very rapidly in Bahrain [9]. It has also become the most diagnosed cancer among men and the second most diagnosed among women [1]. Similarly to Saudi Arabia, the age-standardized ratio of CRC in Bahrain is between 13.4 and 18.8 per 100,000 persons. When compared to the USA’s rate of 25.2–32.1 per 100,000 persons, Bahrain is lower [10]. As shown by Al Shaik in Bahrain, between the period of 2016 to 2019, the age of CRC patients at the time of presentation ranged from 27 to 86 years, and the median age was around 60 years for men and 58 years for women [11].
The heritability of CRC is 30–40%, while the bulk of CRC cases arise spontaneously with no prior family history (60–65%) [2] due to modifiable risk factors [2]. CRC is considered a marker of socioeconomic development and the rising incidence in transitioning countries likely reflects the Westernization of diet and lifestyle habits [1]. Several dietary and lifestyle factors have been associated with CRC development. In particular, obesity, high red and processed meat consumption, alcohol, low physical activity, and smoking have been established to increase the risk of CRC [12]. These risk factors largely apply to low-middle income countries which are observing a gradual increase in CRC as they become more Westernized [2].
The population in Bahrain is exposed to multiple modifiable risk factors for CRC as follows: 76% of Bahrainis are overweight or obese, 17.8% smoke tobacco daily [13], and similarly to other Arab Gulf Countries, dietary habits have shifted to follow an unhealthier pattern [14].
The study aims to explore the associations between CRC or colorectal abnormalities in relation to different dietary, lifestyle, and demographic factors. Furthermore, this study aims to explore the association between f-Hb concentration in relation to colonoscopy findings and lifestyle factors in a FIT-positive population in Bahrain.
2. Materials and Methods
2.1. Study Design
A retrospective cross-sectional study was performed using data extracted from the database generated from the national CRC screening campaign that was launched in 2019 in the Kingdom of Bahrain by King Hamad University Hospital (KHUH). The campaign invited the population aged between 45 and 75 to perform the FIT test. Subjects who had a positive test result were offered a colonoscopy. Some subjects underwent colonoscopies outside KHUH. Among the 11,000 participants enrolled in the campaign, 716 patients tested positive for FIT and underwent colonoscopy at the King Hamad University Hospital.
2.2. Subjects Declaration
Following approval from the Scientific Research and Development Directorate in KHUH (Ethic code reference number: 23-572) on 2nd February 2023, the study was performed in alignment with the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice. The study was conducted from 1 June 2019 to 2023. Information regarding BMI, smoking, red meat, coffee, and alcohol consumption was collected during the first medical appointment at King Hamad University Hospital.
2.3. Clinical Evaluation
Study visits were scheduled at baseline and measurements of subjects were collected during the interview at the medical checkup by the physician. The age at diagnosis was recorded at the time of colonoscopy. Body mass index (BMI) was obtained by calculations (dividing measured weight (kg) by height squared (m2)).
2.4. Smoking and Alcohol Consumption
Smokers were considered those who either claimed to have a daily smoking habit or to have discontinued smoking for less than 10 years before undergoing screening.
Consumers of alcohol were those with a usual alcohol consumption without defining the quantity.
2.5. Red Meat and Coffee Consumption
Information regarding red meat and coffee consumption was collected during the interview. The frequency of red meat consumption was defined by asking patients to report the number of portions of red meat consumed monthly. Coffee consumption was calculated as the number of cups per day. Red meat and coffee consumption have been stratified based on tertiles as follows: Q1 under 33.3%, Q2 from 33.3% to 66.6%, and Q3 above 66.6%.
2.6. Fecal Immunochemical Test (FIT)
To obtain each fecal sample from the participants, specifications and instructions were given at the screening center. There was no requirement for medication or dietary restrictions. In order to reduce the decomposition of fecal hemoglobin (f-Hb), fecal samples were placed in a sampling tube (Elken Chemical Company, Tokyo, Japan) filled with 2.0 mL of buffer solution in a sealed plastic bag and sent to the laboratory for analysis.
OC-SENSOR DIANA (Elken Chemical Company, Tokyo, Japan) was used to conduct the quantitation of f-Hb. In this immunoassay-based assessment of f-Hb, detection is through rabbit polyclonal antibodies. This assay is classified as a turbidimetric latex agglutination test. The presence and attachment of f-Hb in the sample to the latex-coated antibodies leads to a change in the light intensity and hence the absorbance, which is then measured. An automated analyzer conducts the testing and qualitative results are generated. Measurements obtained from the FIT are stated in nanograms (ng) of Hb per milliliter (mL) of buffer. The OC-SENSOR commonly applies a cut-off of 100 ng of Hb per mL buffer (equivalent to 20 micrograms of Hb per gram of feces). Regular calibration analysis was conducted once a month, and regular quality control assessments with two levels of control materials using materials from the manufacturer were conducted every working day.
2.7. Histologic Examination and Colonoscopy
Experienced gastroenterologists performed the colonoscopies (>1000 cases) using an EVIS LUCERA CV-260 colonoscope (Olympus). For the colonoscopy bowel preparation, examinees ingested 4 L of polyethylene glycol solution. Experienced pathologists examined, assessed, biopsied, or removed all of the found polypoid lesions.
Classification of polyps was performed based on their number, size, and histologic characteristics (tubular, tubulovillous, or villous adenoma; hyperplastic polyp; sessile serrated adenoma; or traditional serrated adenoma). Hyperplastic polyps and inflammatory polyps were considered normal findings. Dysplasia grade was classified as low or high. Advanced adenoma was defined as the presence of one of the following features: >10 mm in diameter, tubulovillous or villous structure, and high-grade dysplasia (HGD). High-risk adenoma (HRA) was defined as an advanced adenoma or three or more adenomas. Advanced CRN was assessed as a cancer or advanced adenoma, and overall CRN was defined as any adenoma or cancer.
2.8. Statistical Analysis
All the assays were conducted using Statistical Package for the Social Sciences, version 28.0 (SPSS Inc., Chicago, IL, USA). The normal distribution of the variables was checked using the Kolmogorov–Smirnov test and using Q-Q graphs. Descriptive statistics analyzing the raw data for each of the 5 categories and the full sample were provided, including median, interquartile range, and frequencies where appropriate. The continuous variables were verified as having a normal distribution of data and then analyzed and statistically compared between groups using one-way analysis of variance (ANOVA). Variances were considered to be statistically significant for p value < 0.05. Logistic regression was used to predict the risks (effects) of lifestyle factors and demographic characteristics on colorectal cancer or colorectal anomalies.
3. Results
3.1. Characteristics of Study Population
As shown in Figure 1, of the total 11,000 subjects who underwent FIT, 716 resulted positive and were referred to the Endoscopy Unit to undergo colonoscopy. Of the 569 FIT-positive individuals who underwent colonoscopy, 4 patients with a prior history of inflammatory disease and 6 patients with inadequate bowel preparation were excluded. This resulted in a total of 559 eligible subjects for analysis, distributed as follows: M: 330 (59%) and F: 229 (41%).
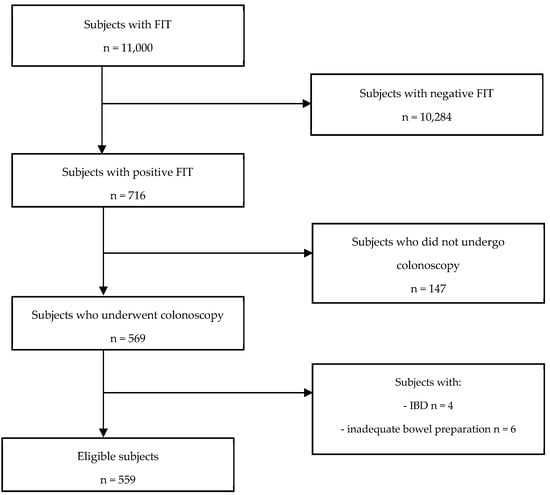
Figure 1.
Flow chart illustrating the selection of eligible study subjects.
Characteristics of the study population are summarized in Table 1. The median age of the study population was 62, and 59% were male subjects. Based on BMI categories, the percentage of overweight or obese individuals was 81.5%. The percentages of current or ex-smokers and alcohol drinkers were, respectively, 25.2% and 7.2%, with a higher ratio in male individuals.

Table 1.
Descriptive characteristics of the study population.
After the colonoscopy, 21 (3.8%) subjects were diagnosed with CRC, 27 (4.8%) showed at least one large polyp, 64 (11.4%) had at least one small polyp, 3 (0.5%) had diverticulosis, and 26 (4.7%) had hemorrhoids. A total of 418 (74.8%) patients did not show any colonoscopy findings. Higher percentages of CRC and large and small polyps have been recorded in males.
3.2. Fecal Hemoglobin Concentrations and Colonoscopy Findings
In Figure 2, the box plot shows the f-Hb concentrations according to the colonoscopy findings. There was no significant difference in f-Hb concentration between males and females (p = 0.90).
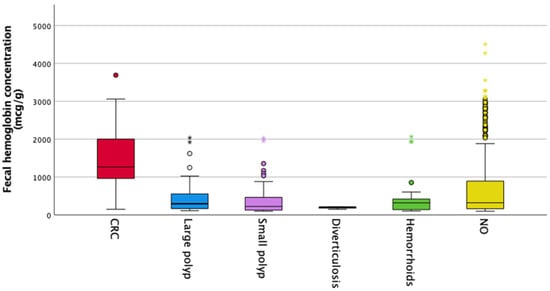
Figure 2.
f-Hb levels and colorectal diagnosis.
Figure 2 shows a boxplot for fecal hemoglobin concentrations according to the colonoscopy findings. The median f-Hb concentration thresholds were as follows: in colorectal cancer 1269 μg/mg, large polyp 269 μg/mg, small polyp 226 μg/g, diverticulosis 206 μg/g, hemorrhoid 320 μg/g, and 226 μg/g in patients without any findings.
Figure 3 shows that the ROC curve was used to estimate the effect of f-Hb in discriminating colorectal cancer from other benign colorectal diseases.
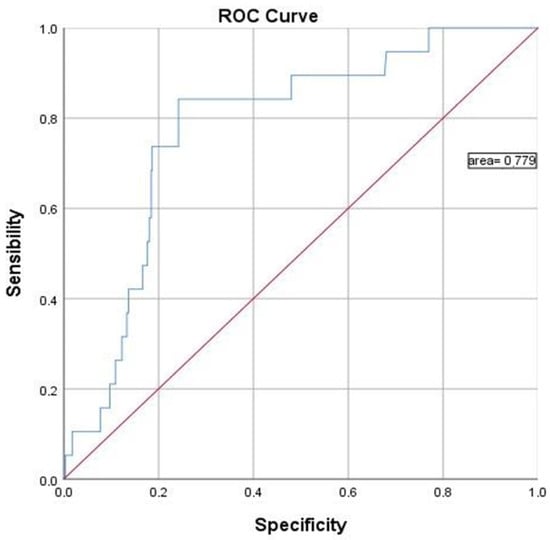
Figure 3.
The receiver operator curve (ROC) of the f-Hb concentration for cancer diagnosis.
The ROC curve used colorectal cancer as the endpoint for detection compared to normal, large polyp, small polyp, hemorrhoids, and diverticulosis. For the detection of colorectal cancer, the area under the curve (AUC) was 77.9%.
3.3. Fecal Hemoglobin Concentrations and Lifestyle Factors (Red Meat and Coffee Consumption as Well as BMI)
As shown in Figure 4, a higher median level of f-Hb was found in patients with red meat consumption Q3 compared to Q1 and Q2 in the category with CRC, despite there not being a statistically significant difference among groups (p = 0.742).
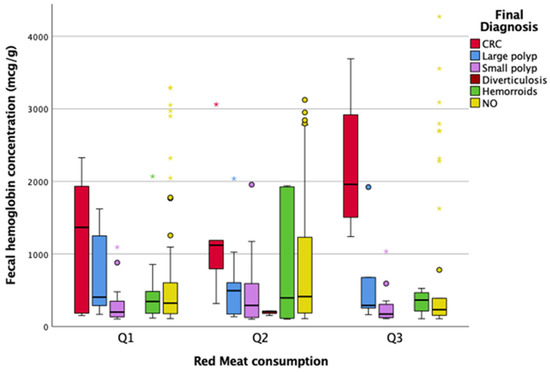
Figure 4.
Association between red meat consumption, f-Hb levels, and diagnosis. CRC, colorectal cancer.
Figure 5 illustrates that there is not a statistically significant difference between coffee consumption and f-Hb concentrations in the different groups (p = 0.697).
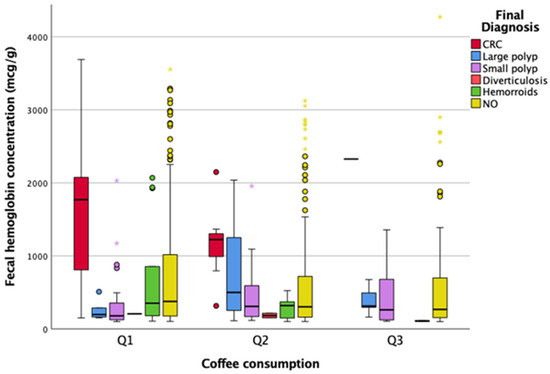
Figure 5.
Association between coffee consumption, f-Hb levels, and diagnosis. CRC, colorectal cancer.
Smoking, gender, age, BMI, red meat, and coffee intake were unrelated to the risk of all colorectal conditions, except for coffee consumption which showed a protective effect on small polyps, and for the male gender which was associated with an increased risk of large polyp.
As shown in Table 2, no association was recorded between different demographic and lifestyle factors and the colorectal conditions, except for the male gender which was associated with a higher risk of large polyps (p < 0.05) and lower coffee consumption that was associated with higher risk of small polyps (p < 0.05).

Table 2.
Association between factors and colorectal abnormalities.
Moving from a lower to a higher tensile of red meat consumption, there is an increase in colorectal cancer risk of 79.9%. Also, belonging to the male gender increased the risk of CRC threefold, and moving from a lower to a higher quartile of age increased the risk by 80%. Coffee consumption seemed to be protective against CRC, with a lower risk of −47% moving from Q1 to Q2. BMI was a risk factor for CRC, since moving from a lower category to a higher one increased the risk by +44%.
4. Discussion
This study explored for the first time in Bahrain the association between colorectal cancer or colorectal abnormalities and different dietary, lifestyle, and demographic factors. To the best of our knowledge, this study is the first of its kind in the Middle East to explore these associations. Furthermore, the association between f-Hb levels in relation to colonoscopy findings and a wide range of factors in a FIT-positive population have been considered.
The importance of FIT screening has been highlighted in a new review of CRC screening programs in 12 Middle Eastern countries, indicating invitation coverage of 30–100% and participation coverage of 7–68% [15]. The paucity of adherence could be explained by sociocultural barriers based on misconceptions and the cost-effectiveness of screening programs.
Incidence of CRC in Bahrain
In this study, we found that the percentage of CRC in a group of FIT-positive subjects in the Kingdom of Bahrain was 3.8%. The incidence was higher in males (4.5%) than females (2.6%). This is in line with previous studies that estimate the rates of occurrence in men to be 1.4–1.5-fold higher than that in women [2].
The higher rates of incidence in men can be attributed to many factors. One of the main factors regards their elevated sensitivity to environmental factors [2]. Men are thought to be more influenced by environmental factors than genetic factors in colorectal carcinogens (heritability of CRC is 45% in women and 28% in men). Men are also thought to be more exposed to environmental factors than women [2]. Women’s endogenous estrogen hormones are also thought to be protective against CRC [2].
Interestingly, there are countries regarded as having a low risk for CRC (Sweden and Finland) and others regarded as high risk (Norway) [2]. A study found that men who migrated from a high-risk CRC country to a low-risk country had decreased incidence rates [2]. Similarly, when men migrated from a low-risk CRC country to a high-risk country, their incidence rates increased [2].
In a pilot program of CRC screening with FIT in Qatar, 57 (4.5%) of 1242 healthy subjects between the ages of 45–74 years in three primary health care centers were found to be positive and referred for colonoscopy. Of these, 32 underwent colonoscopy, among whom 5 (15%) were found to have colorectal cancer [16].
The ethnicities of individuals after age standardization are also thought to influence CRC [2]. This may be attributable to the novel differences in genetic factors between the ethnicities, such as single nucleotide polymorphisms (SNPs) [2]. SNPs linked to CRC risk have been revealed in previous studies performed on black individuals [2].
In a previous study on a diverse group of an American population, the incidence and mortality of CRC was highest in black individuals (43.2 and 18.6 per 100,000 persons, respectively) and lowest in Asian people and Pacific Islanders (28.8 and 9.9 per 100,000 persons, respectively) [2].
As shown by John et al., colorectal neoplasms can be detected in primary health center-based population screening programs due to these findings. The number of positive results for CRC screening in Qatar and Bahrain is comparable to the numbers reported in other studies of high-risk countries [17], although our study showed a lower incidence of new detected cases in the sample screened.
Association between f-Hb concentration and CRC risk
This study showed that in the Bahraini population, screening for colorectal cancer revealed that FIT positivity was associated with CRC, showing an area under the curve (AUC) of 77.9%. This is the first data showing the FIT test accuracy in a large cohort of asymptomatic Bahraini population. F-Hb concentration was shown to be higher in the CRC group (1269 μg/mg) compared to all other cohorts. In accordance with our results, previous studies have shown an increase in f-Hb concentrations associated with an increased risk of advanced colorectal neoplasia [6,7,8].
Regardless of the main association between f-Hb concentration and CRC risk, this study also showed that f-Hb could be affected by different factors. Specifically, higher levels of f-Hb have been recorded in higher red meat consumers with colon cancer. We also noticed that in the cohort with colorectal cancer with lower consumption of red meat, the f-Hb levels were lower.
A recent publication reports that folate and vitamin B12 could be associated with f-Hb concentrations [6]. Vitamin B12 and folate deficiency can cause megaloblastic anemia, where red blood cells become larger than normal. This can lead to a variety of symptoms but does not directly relate to fecal hemoglobin.
Association between coffee or red meat consumption and CRC risk
This study showed an interesting trend. Higher red meat consumption increased the colorectal cancer risk by 79.9% (from Q1 to Q2 and Q3). There is worldwide evidence [18] about the association between red meat consumption and CRC cancer, but no specific reports on the Middle East. A second possible factor could be explained because, in Bahrain and Saudi Arabia, a 143.3% increase in the consumption of fat was observed from 1970 to 1997, accompanied by a 48.3% increase in calorie intake [19] with a preference for full-fat cheese with a strong link with a derangement of lipid profile [20].
Our study also showed that coffee consumption seemed to be protective against CRC, with a lower risk of −47% moving from Q1 to Q2 and Q3. A recent meta-analysis demonstrated the protective effects shown by decaffeinated coffee against CRC in both men and women, but different effects have been evidenced based on ethnicity [21]. In line with our results, a recent study revealed a reduction in CRC risk due to the daily intake of black tea and coffee among the Saudi population [22].
Alterations in the gut microbiome composition, known as gut dysbiosis, have been identified as a fundamental risk factor in the development and progression of colorectal cancer (CRC). Specific bacterial species have been associated with increased CRC risk, including Alistipes, Akkermansia, Parabacteroides, and Bacteroides. These bacteria may create a more favorable microenvironment for tumor development.
Diet, particularly the consumption of processed and unprocessed meat, as well as low fiber intake, can alter the gut microbiome and contribute to CRC risk. In contrast, a diet rich in fruits and vegetables may have a protective effect.
Association between BMI and CRC risk
Our study reveals that BMI was a risk factor for CRC since moving from a lower category to a higher one increased the risk by +44%.
A recent study by Althumiri et al. demonstrated that in Saudi Arabia the risk of colon cancer is 30% higher in patients with obesity, showing that findings were in agreement with our study and with literature from around the world. Our study further confirmed the association of obesity with the aforementioned conditions irrespective of the sociodemographic differences found between countries [23]. Also, in a study performed in Kuwait by Alsheridah in 2018, the data showed an association between colon cancer and high red meat consumption with obesity [24].
Limitations
One of the limitations of this study was the non-standardized method used to assess red meat and coffee consumption. Also, another critical factor was the assessment of smoking and alcohol consumption because of cultural reasons. In addition, this sample was heterogeneous, with most of the subjects overweight or obese. Furthermore, given that this is a retrospectively designed study, it inherently possesses the flaws of retrospective and observational studies. Additionally, the study was confined to patients who present with a positive FIT, and because patients with a negative FIT did not perform a colonoscopy, we do not obtain information on the pathology in those with an FIT level below the predetermined threshold.
Another limitation centers around the participation rates, highlighting the importance of spreading awareness about screening programs especially when non-invasive methods such as FIT are implemented.
Finally, there have been recent reports supporting the increased incidence of CRC in young adults in the last few years [25]. More worryingly, these cases of CRC early onset were not linked to prior family history. The increases have been suspected to arise from generational changes in lifestyle factors, diet, and environmental exposures [25]. Young adults in Bahrain were not a part of the study in this research. In addition, physical activity has not been measured.
5. Conclusions
This study highlighted the importance of high f-Hb concentration as a predictive biomarker of CRC. Although the study did not identify a specific factor associated with CRC development, red meat, coffee, and BMI must be further investigated as possible synergic risk factors. Future prospective studies should further investigate CRC development through standardized methods of red meat and coffee consumption assessments in a population of equal BMI ratios, and add young adults to the study population. Finally, genetic profiling and analysis may indicate a novel genetic component linked to increased or decreased CRC risk of development in Bahraini individuals.
Author Contributions
Conceptualization, O.S. and D.A.; methodology, S.P.; software, S.P.; formal analysis, S.P.; resources, A.F., S.A.-T., D.A., F.A. (Fidaa Alsaffar), E.J., F.A. (Faisal Abubaker), A.B., M.A., Z.M., S.H., H.M. and A.T.; data curation, O.S.; writing—original draft preparation, S.P.; writing—review and editing, M.R., A.F., S.A.-T., D.A., F.A. (Fidaa Alsaffar), E.J., F.A. (Faisal Abubaker), A.B., M.A., Z.M., S.H., H.M. and A.T.; supervision, A.F., S.A.-T., D.A., F.A. (Fidaa Alsaffar), E.J., F.A. (Faisal Abubaker), A.B., M.A., Z.M., S.H., H.M. and A.T.; project administration, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice, following approval from the Scientific Research and Development Directorate in KHUH (Ethic code reference number: 23-572).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG clinical guidelines: Colorectal cancer screening 2021. Off. J. Am. Coll. Gastroenterol.|ACG 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Nicolas, A.; Ferrandez, A.; Lanas, A. Colorectal cancer population screening programs worldwide in 2016: An update. World J. Gastroenterol. 2017, 23, 3632. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Fontham, E.T.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.; Fraser, C.G.; Carey, F.A.; McDonald, P.J.; Strachan, J.A.; Diament, R.H.; Balsitis, M.; Steele, R.J. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J. Clin. Pathol. 2013, 66, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.S.; Lin, Y.M.; Chang, H.C.; Chen, Y.H.; Chong, L.W.; Chen, C.H.; Lin, Y.S.; Yang, K.C.; Shih, C.H. Application of quantitative estimates of fecal hemoglobin concentration for risk prediction of colorectal neoplasia. World J. Gastroenterol. WJG 2013, 19, 8366. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kwon, M.J.; Kim, H.Y.; Lee, T.; Jeong, S.H.; Park, D.I.; Choi, K.; Jung, Y.S. Fecal hemoglobin concentration is useful for risk stratification of advanced colorectal neoplasia. Dig. Liver Dis. 2016, 48, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Al Awadhi, M.A.; Abulfateh, N.M.; Board, A. Drowning Epidemiology in Bahrain (2003–2015). Bahrain Med. Bull. 2018, 40, 11–13. [Google Scholar] [CrossRef]
- Al Awadhi, M.A.; Abulfateh, N.M.; Abu-Hassan, F.; Majida Ahmed Fikree Janahi, E.; Carlo, R. Cancer Incidence and Mortality in the Kingdom of Bahrain Statistics and Trends. Bah. Med. Bull. 2016, 38, 30–34. [Google Scholar]
- Al Shaikh, S.; Shubbar, A.S. Evaluation and Analysis of Colon Cancer in Kingdom of Bahrain: A Retrospective Study in a Tertiary Care Center. Mathews J. Cancer Sci. 2022, 7, 1–6. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Colorectal Cancer; American Institute for Cancer Research: Arlington, VA, USA, 2018. [Google Scholar]
- Elmusharaf, K.; Grafton, D.; Roberts, E. Prevention and Control of Non-Communicable Diseases in Bahrain: The Case for Investment; UNDP, WHO, UNIATF, GHC: Geneva, Switzerland, 2021. [Google Scholar]
- Musaiger, A.O.; Takruri, H.R.; Hassan, A.S.; Abu-Tarboush, H. Food-based dietary guidelines for the Arab Gulf countries. J. Nutr. Metab. 2012, 2012, 905303. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, C.; Blom, J.; Bulliard, J.L.; Garcia, M.; Hagoel, L.; Mai, V.; Patnick, J.; Rozjabek, H.; Senore, C.; Törnberg, S. Participation rates for organized colorectal cancer screening programmes: An international comparison. J. Med. Screen. 2015, 22, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Al Raya Newspaper Gulf Times (2015). PHCC to Launch Cancer Screening Program. 28 May 2015. Available online: http://portal.www.gov.qa/wps/portal/media-center/news/individualnews/phcctolaunchcancerscreeningprogram (accessed on 9 February 2017).
- John, A.; Al Kaabi, S.; Dweik, N.; Yakoub, R.; John, A.; Al Mohannadi, M.; Sharma, M.; Wani, H.; Butt, M.T.; Derbala, M.F.; et al. Emerging role for colorectal cancer screening in Asian countries. Trop. Gastroenterol. 2014, 35, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Musaiger, A.O. Diet and prevention of coronary heart disease in the Arab Middle East countries. Med. Princ. Pract. 2002, 11 (Suppl. 2), 9–16. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Hammad, L.H.; Mohamed, M.W.; Alromaihi, D.; Alhammadi, M.; Al-Khater, N.; Alchuban, A.R.; Aledrisy, M.A.; Ilyas, Z.; Alalwan, T.A.; et al. Cheese Intake Exhibits an Alteration of Glycolipid Profile and Impacts on Non-Alcoholic Fatty Liver in Bahraini Older Adults. Geriatrics 2022, 7, 75. [Google Scholar] [CrossRef]
- Sartini, M.; Bragazzi, N.L.; Spagnolo, A.M.; Schinca, E.; Ottria, G.; Dupont, C.; Cristina, M.L. Coffee consumption and risk of colorectal cancer: A systematic review and meta-analysis of prospective studies. Nutrients 2019, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Azzeh, F.S.; Alshammari, E.M.; Alazzeh, A.Y.; Jazar, A.S.; Dabbour, I.R.; el-Taani, H.A.; Obeidat, A.A.; Kattan, F.A.; Tashtoush, S.H. Healthy dietary patterns decrease the risk of colorectal cancer in the Mecca Region, Saudi Arabia: A case-control study. BMC Public Health 2017, 17, 607. [Google Scholar] [CrossRef]
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; Almubark, R.A.; BinDhim, N.F.; Alkhamaali, Z.; Alqahtani, S.A. Obesity in Saudi Arabia in 2020: Prevalence, distribution, and its current association with various health conditions. Healthcare 2021, 9, 311. [Google Scholar] [CrossRef]
- Alsheridah, N.; Akhtar, S. Diet, obesity and colorectal carcinoma risk: Results from a national cancer registry-based middle-eastern study. BMC Cancer 2018, 18, 1227. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).