Body Mass Index: An Unreliable Adiposity Indicator for Predicting Outcomes of Liver Transplantation Due to Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
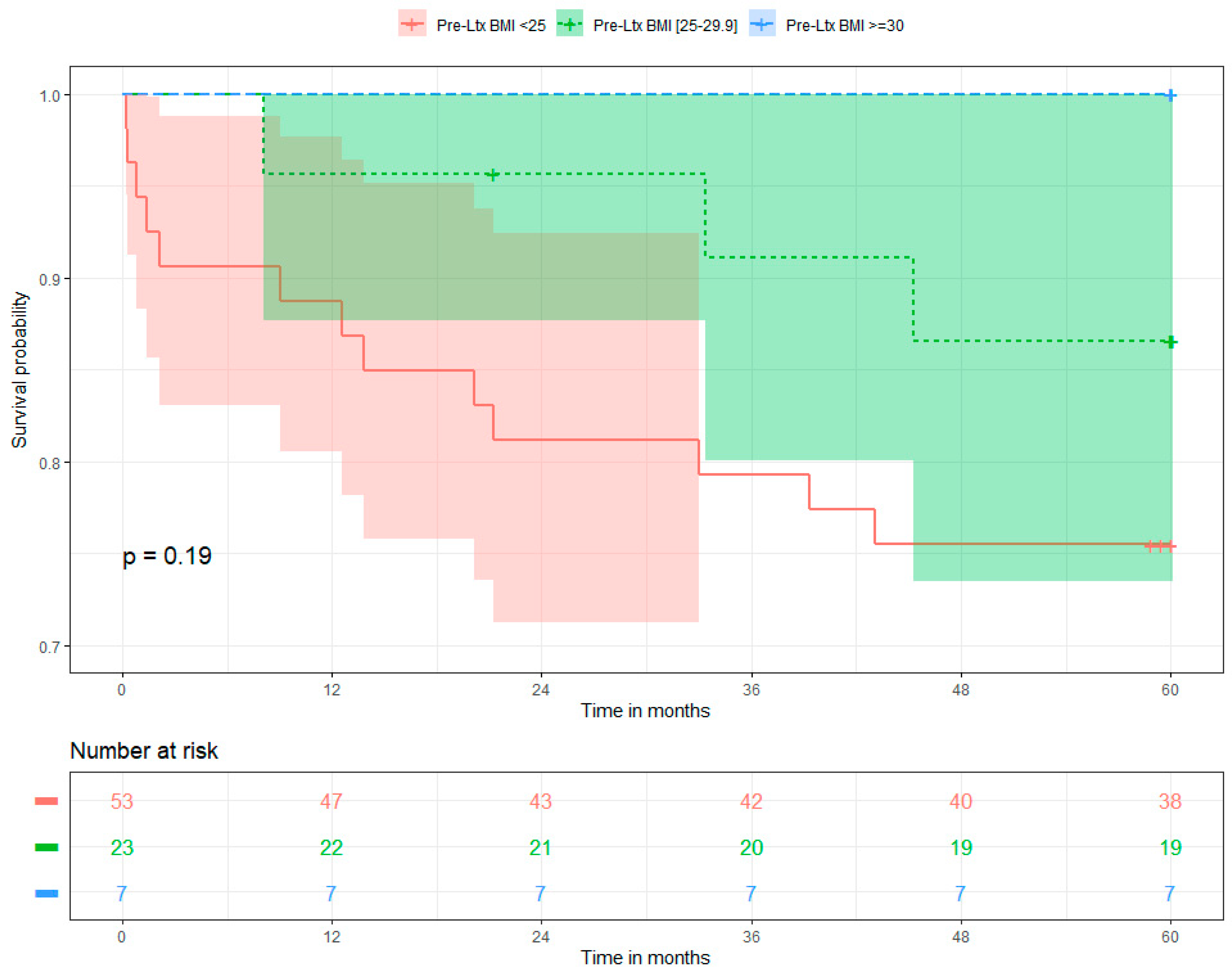
3.1. Five-Year Overall Survival and Determinants
3.2. Five-Year Recurrence-Free Survival and Determinants of HCC Recurrence
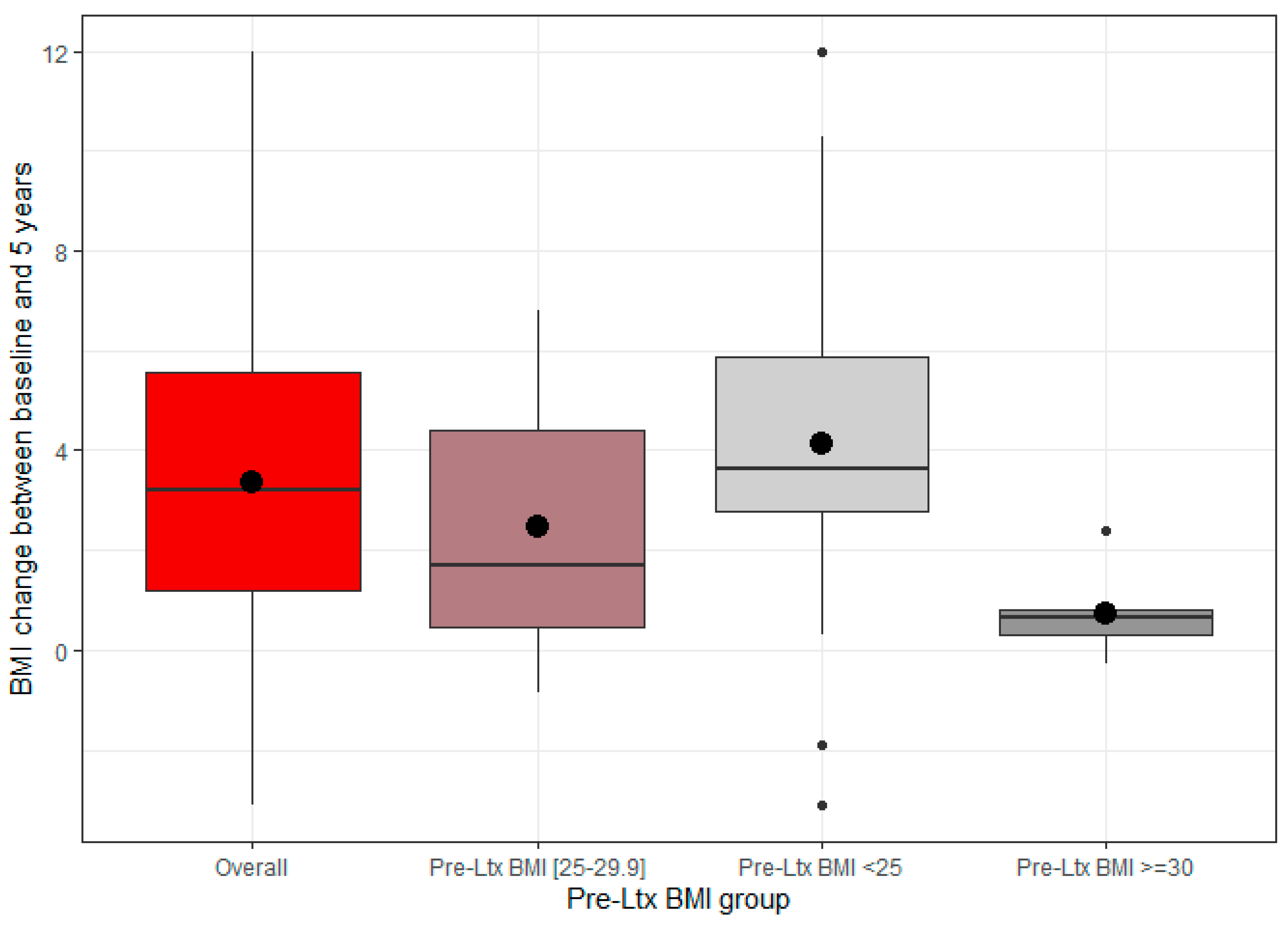
3.3. BMI Alterations over the Course of the Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Clavien, P.-A.; Lesurtel, M.; Bossuyt, P.M.; Gores, G.J.; Langer, B.; Perrier, A. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Puigvehí, M.; Hashim, D.; Haber, P.K.; Dinani, A.; Schiano, T.D.; Asgharpour, A.; Kushner, T.; Kakked, G.; Tabrizian, P.; Schwartz, M.; et al. Liver transplant for hepatocellular carcinoma in the United States: Evolving trends over the last three decades. Am. J. Transplant. 2020, 20, 220–230. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Halazun, K.J.; Najjar, M.; Abdelmessih, R.M.; Samstein, B.; Griesemer, A.D.; Guarrera, J.V.; Kato, T.; Verna, E.C.; Emond, J.C.; Brown, R.S. Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. 2017, 265, 557–564. [Google Scholar] [CrossRef]
- Mehta, N.; Heimbach, J.; Harnois, D.M.; Sapisochin, G.; Dodge, J.L.; Lee, D.; Burns, J.M.; Sanchez, W.; Greig, P.D.; Grant, D.R.; et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017, 3, 493. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.D.; Lee, M.; Jun, B.G.; Kim, T.S.; Suk, K.T.; Kang, S.H.; Kim, M.Y.; Cheon, G.J.; Kim, D.J.; et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018, 69, 1066–1073. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.M.; Stypułkowski, J.; Bik, E.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Grąt, K.; Patkowski, W.; Krawczyk, M. The Warsaw Proposal for the Use of Extended Selection Criteria in Liver Transplantation for Hepatocellular Cancer. Ann. Surg. Oncol. 2017, 24, 526–534. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Halazun, K.J.; Tabrizian, P.; Najjar, M.; Florman, S.; Schwartz, M.; Michelassi, F.; Samstein, B.; Brown Jr, R.S.; Emond, J.C.; Busuttil, R.W.; et al. Is it Time to Abandon the Milan Criteria? Ann. Surg. 2018, 268, 690–699. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Grąt, M.; Krasnodębski, M.; Krawczyk, M.; Stypułkowski, J.; Morawski, M.; Wasilewicz, M.; Lewandowski, Z.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Extremes of Liver Transplantation for Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 787. [Google Scholar] [CrossRef]
- Grat, M.; Stypulkowski, J.; Morawski, M.; Wronka, K.M.; Wasilewicz, M.; Lewandowski, Z.; Grat, K.; Wójcik, Z.; Patkowski, W.; Zieniewicz, K. Shadows Behind Using Simple Risk Models in Selection of Hepatocellular Carcinoma Patients for Liver Transplantation. Ann. Surg. 2020, 271, 1124–1131. [Google Scholar] [CrossRef]
- Pomfret, E.A.; Washburn, K.; Wald, C.; Nalesnik, M.A.; Douglas, D.; Russo, M.; Roberts, J.; Reich, D.J.; Schwartz, M.E.; Mieles, L.; et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplant. 2010, 16, 262–278. [Google Scholar] [CrossRef]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K., Jr.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef]
- Levi, D.M.; Tzakis, A.G.; Martin, P.; Nishida, S.; Island, E.; Moon, J.; Selvaggi, G.; Tekin, A.; Madrazo, B.L.; Narayanan, G.; et al. Liver Transplantation for Hepatocellular Carcinoma in the Model for End-Stage Liver Disease Era. J. Am. Coll. Surg. 2010, 210, 727–734. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef]
- de’Angelis, N.; Filippo, L.; Carra, M.; Azoulay, D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185. [Google Scholar] [CrossRef] [PubMed]
- El-Domiaty, N.; Saliba, F.; Karam, V.; Sobesky, R.; Ibrahim, W.; Vibert, E.; Pittau, G.; Amer, K.; Saeed, M.A.; Shawky, J.A.; et al. Impact of body mass index on hepatocellular carcinoma recurrence after liver transplantation through long-term follow-up. Hepatobiliary Surg. Nutr. 2021, 10, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Wong, C.; Ng, C.H.; Poh, C.W.; Jain, S.R.; Huang, D.Q.; Muthiah, M.D. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J. Clin. Med. 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Amado, V.; Rodríguez-Perálvarez, M.; Ferrín, G.; De la Mata, M. Selecting patients with hepatocellular carcinoma for liver transplantation: Incorporating tumor biology criteria. J. Hepatocell. Carcinoma 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef]
- Shiota, M.; Takeuchi, A.; Sugimoto, M.; Kashiwagi, E.; Dejima, T.; Kiyoshima, K.; Inokuchi, J.; Tatsugami, K.; Yokomizo, A.; Eto, M. The Differential Impact of Body Mass Index and the Feature of Metabolic Syndrome on Oncological Outcomes Following Different Surgical Procedures in Japanese Men with Prostate Cancer. Ann. Surg. Oncol. 2017, 24, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Kambara, Y.; Yuasa, N.; Takeuchi, E.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Okuno, M.; Miyata, K. Overweight or Obesity is an Unfavorable Long-Term Prognostic Factor for Patients who Underwent Gastrectomy for Stage II/III Gastric Cancer. World J. Surg. 2019, 43, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Hatano, E.; Okajima, H.; et al. Visceral Adiposity and Sarcopenic Visceral Obesity are Associated with Poor Prognosis After Resection of Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 3732–3740. [Google Scholar] [CrossRef]
- Karczewski, J.; Begier-Krasińska, B.; Staszewski, R.; Popławska, E.; Gulczynska-Elhadi, K.; Dobrowolska, A. Obesity and the Risk of Gastrointestinal Cancers. Dig. Dis. Sci. 2019, 64, 2740–2749. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef]
- Fujihara, S.; Mori, H.; Kobara, H.; Nishiyama, N.; Kobayashi, M.; Oryu, M.; Masaki, T. Metabolic Syndrome, Obesity, and Gastrointestinal Cancer. Gastroenterol. Res. Pract. 2012, 2012, 483623. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Gupta, A.; Das, A.; Majumder, K.; Arora, N.; Mayo, H.G.; Singh, P.P.; Beg, M.S.; Singh, S. Obesity is Independently Associated With Increased Risk of Hepatocellular Cancer–related Mortality. Am. J. Clin. Oncol. 2018, 41, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Shirabe, K.; Matsumoto, Y.; Yoshiya, S.; Muto, J.; Harimoto, N.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Nishie, A.; et al. Effect of Body Composition on Outcomes after Hepatic Resection for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2014, 21, 3063–3068. [Google Scholar] [PubMed]
- Utsunomiya, T.; Okamoto, M.; Kameyama, T.; Matsuyama, A.; Yamamoto, M.; Fujiwara, M.; Mori, M.; Aimitsu, S.; Ishida, T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 1553. [Google Scholar]
- Grąt, M.; Krawczyk, M.; Wronka, K.M.; Stypułkowski, J.; Lewandowski, Z.; Wasilewicz, M.; Krawczyk, P.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Ischemia-reperfusion injury and the risk of hepatocellular carcinoma recurrence after deceased donor liver transplantation. Sci. Rep. 2018, 8, 8935. [Google Scholar]
- Siegel, A.B.; Lim, E.A.; Wang, S.; Brubaker, W.; Rodriguez, R.D.; Goyal, A.; Jacobson, J.S.; Hershman, D.L.; Verna, E.C.; Zaretsky, J.; et al. Diabetes, Body Mass Index, and Outcomes in Hepatocellular Carcinoma Patients Undergoing Liver Transplantation. Transplantation 2012, 94, 539–543. [Google Scholar] [PubMed]
- Mathur, A.; Franco, E.S.; Leone, J.P.; Osman-Mohamed, H.; Rojas, H.; Kemmer, N.; Neff, G.W.; Rosemurgy, A.S.; Alsina, A.E. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB 2013, 15, 504–510. [Google Scholar]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M. and Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar]
- The Polish Transplant Society. Available online: https://p-t-t.org/artykul/zalecenia-dotyczace-leczenia-immunosupresyjnego-po-przeszczepieniu-narzadow-unaczynionych (accessed on 15 March 2024).
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef]
- Angin, Y.S.; Usta, S.; Ceylan, C.; Ince, V.; Isik, B.; Carr, B.I.; Yilmaz, S. Is Obesity a Risk Factor. for Recurrence in HCC Patients Who Undergo Liver Transplantation? J. Inonu Liver Transplant. Inst. 2024, 94–99. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Mazurak, V.C.; Ebadi, M.; Meza-Junco, J.; Sawyer, M.B.; Baracos, V.E.; Kneteman, N. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 2018, 67, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Shirai, H.; Yao, S.; Kamo, N.; Yagi, S.; Taura, K.; Okajima, H.; et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2019, 269, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Grąt, K.; Pacho, R.; Grąt, M.; Krawczyk, M.; Zieniewicz, K.; Rowiński, O. Impact of Body Composition on the Risk of Hepatocellular Carcinoma Recurrence After Liver Transplantation. J. Clin. Med. 2019, 8, 1672. [Google Scholar] [CrossRef]
- Ribatti, D.; Belloni, A.S.; Nico, B.; Di Comite, M.; Crivellato, E.; Vacca, A. Leptin–leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides 2008, 29, 1596–1602. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant Activation of the JAK/STAT, PI3K/AKT, and ERK Signaling Is Involved in Leptin-Mediated Promotion of Invasion and Migration of Hepatocellular Carcinoma Cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-F.; Tang, P.; Li, Q.; Yu, Z.-T. Obesity, adipokines and hepatocellular carcinoma. Int. J. Cancer 2013, 133, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.-S.; Zhang, C.; Chen, P.; Jin, S.-J.; Jiang, G.-Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 12870. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Rabindranath, M.; Chara, B.S.; Simonetto, D.A. Artificial intelligence, machine learning, and deep learning in liver transplantation. J. Hepatol. 2023, 78, 1216–1233. [Google Scholar] [PubMed]
- Calderaro, J.; Seraphin, T.P.; Luedde, T.; Simon, T.G. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1348–1361. [Google Scholar] [CrossRef]

| Variable | Overall (N = 83) | Pre-Ltx BMI < 25 kg/m2 (n = 53) | Pre-Ltx BMI [25–29.9 kg/m2] (n = 23) | Pre-Ltx BMI ≥ 30 kg/m2 (n = 7) | Statistical Test | p-Value |
|---|---|---|---|---|---|---|
| Sociodemographics | ||||||
| Age at transplantation (years) Median (Q1–Q3) Range | 57.01 (51.27–59.5) 32.85–69.75 | 56.58 (49.26–59.52) 32.85–69.75 | 56.42 (52.98–59.22) 45.15–64.5 | 57.62 (49.66–62.14) 42.1–69.14 | Kruskal–Wallis | 0.9195 |
| Male | 84.3% (n = 70) | 84.9% (n = 45) | 82.6% (n = 19) | 85.7% (n = 6) | Fisher | 1 |
| Excessive alcohol consumption | 12% (n = 10) | 9.4% (n = 5) | 17.4% (n = 4) | 14.3% (n = 1) | Fisher | 0.4828 |
| Indication for liver transplantation | ||||||
| HCV + HCC | 72.3% (n = 60) | 75.5% (n = 40) | 56.5% (n = 13) | 100% (n = 7) | Fisher | 0.062 |
| HBV + HCC | 9.6% (n = 8) | 9.4% (n = 5) | 13% (n = 3) | 0% (n = 0) | Fisher | 0.8527 |
| ALD + HCC | 6% (n = 5) | 5.7% (n = 3) | 8.7% (n = 2) | 0% (n = 0) | Fisher | 0.768 |
| PBC, AIH, PSC + HCC | 3.6% (n = 3) | 5.7% (n = 3) | 0% (n = 0) | 0% (n = 0) | Fisher | 0.6551 |
| MASH + HCC | 6% (n = 5) | 3.8% (n = 2) | 13% (n = 3) | 0% (n = 0) | Fisher | 0.3352 |
| Cryptogenic + HCC | 1.2% (n = 1) | 0% (n = 0) | 4.3% (n = 1) | 0% (n = 0) | Fisher | 0.3614 |
| HCC | 1.2% (n = 1) | 0% (n = 0) | 4.3% (n = 1) | 0% (n = 0) | Fisher | 0.3614 |
| Baseline features of the host | ||||||
| MELD score at transplantation Median (Q1–Q3) Range | 11 (9–14.5) 7–35 | 11 (10–15) 7–35 | 11 (8–13) 7–20 | 10 (10–15) 9–23 | Kruskal–Wallis | 0.6258 |
| AFP at transplant [ng/mL] Median (Q1–Q3) Range | 10.2 (4.6–31.75) 1.4–334 | 10.2 (4.6–20) 1.9–147.5 | 9.4 (5.65–59.5) 1.4–334 | 28.6 (8.85–34.55) 1.5–271 | Kruskal–Wallis | 0.461 |
| Neutrophil/lymphocyte ratio at transplant Median (Q1–Q3) Range | 2.28 (1.64–3.35) 0.89–11.5 | 2.29 (1.63–3.2) 0.94–11.5 | 2.78 (1.86–3.8) 1.04–10.42 | 1.87 (1.3–2.97) 0.89–5.57 | Kruskal–Wallis | 0.33 |
| AFP model Median (Q1–Q3) Range | 0 (0–0) 0–6 | 0 (0–1) 0–6 | 0 (0–1) 0–3 | 1 (0–1) 0–6 | Kruskal–Wallis | 0.6306 |
| Creatinine at baseline (mg/dL) Median (Q1–Q3) Range | 0.9 (0.75–1.1) 0.45–1.8 | 0.9 (0.8–1.1) 0.52–1.8 | 0.81 (0.7–1.1) 0.45–1.7 | 0.84 (0.72–1) 0.5–1 | Kruskal–Wallis | 0.651 |
| Diabetes mellitus at baseline | 30.1% (n = 25) | 22.6% (n = 12) | 47.8% (n = 11) | 28.6% (n = 2) | Fisher | 0.0922 |
| Pre-operative BMI Median (Q1–Q3) Range | 23.59 (21.49–26.24) 17.3–32.61 | 22.08 (20.98–23.37) 17.3–24.98 | 26.78 (25.88–27.49) 25.06–29.17 | 30.8 (30.46–31.04) 30.11–32.61 | Kruskal–Wallis | <0.001 |
| Bridging therapy before Ltx No treatment Radiofrequency ablation Resection Percutaneous ethanol injection | 84.3% (n = 70) 10.8% (n = 9) 3.6% (n = 3) 1.2% (n = 1) | 83% (n = 44) 11.3% (n = 6) 5.7% (n = 3) 0% (n = 0) | 87% (n = 20) 8.7% (n = 2) 0% (n = 0) 4.3% (n = 1) | 85.7% (n = 6) 14.3% (n = 1) 0% (n = 0) 0% (n = 0) | Fisher | 0.6317 |
| Donor and transplant variables | ||||||
| Donor age (years) Median (Q1–Q3) Range | 42 (32.5–50.5) 10–65 | 44 (32–53) 17–65 | 41 (33–45) 10–59 | 46 (35–47) 20–51 | ANOVA | 0.7662 |
| Cold ischaemia time (min) Median (Q1–Q3) Range | 385 (312.5–457.5) 200–730 | 385 (305–455) 200–730 | 360 (320–420) 255–720 | 430 (322.5–493.5) 245–560 | Kruskal–Wallis | 0.8395 |
| Warm ischaemia time (min) Median (Q1–Q3) Range | 42 (35–47) 28–175 | 41 (35–46) 28–175 | 45 (40–49) 30–70 | 40 (32.5–45) 30–54 | Kruskal–Wallis | 0.2758 |
| Immunosuppression at baseline | ||||||
| Steroids | 100% (n = 83) | 100% (n = 53) | 100% (n = 23) | 100% (n = 7) | Fisher | 1 |
| Tacrolimus | 95.2% (n = 79) | 98.1% (n = 52) | 87% (n = 20) | 100% (n = 7) | Fisher | 0.1478 |
| MMF | 94% (n = 78) | 96.2% (n = 51) | 91.3% (n = 21) | 85.7% (n = 6) | Fisher | 0.3352 |
| Cyclosporine | 4.8% (n = 4) | 1.9% (n = 1) | 13% (n = 3) | 0% (n = 0) | Fisher | 0.1478 |
| Everolimus | 2.4% (n = 2) | 0% (n = 0) | 4.3% (n = 1) | 14.3% (n = 1) | Fisher | 0.0535 |
| Tumour characteristics | ||||||
| Diameter of the largest tumour (cm) Median (Q1–Q3) Range | 2 (1.5–3) 0.5–8 | 2 (1.5–3) 0.5–7 | 2.5 (1.75–2.75) 0.7–6 | 3 (1.9–5.25) 1.5–8 | Kruskal–Wallis | 0.3232 |
| Number of nodules Median (Q1–Q3) Range | 1 (1–2) 1–4 | 1 (1–2) 1–4 | 1 (1–2) 1–3 | 1 (1–1) 1–2 | Kruskal–Wallis | 0.4546 |
| Fulfilment of Milan criteria | 83.1% (n = 69) | 83% (n = 44) | 87% (n = 20) | 71.4% (n = 5) | Fisher | 0.6605 |
| Microvascular invasion | 33.7% (n = 28) | 30.2% (n = 16) | 43.5% (n = 10) | 28.6% (n = 2) | Fisher | 0.5101 |
| Tumour differentiation | ||||||
| G1 | 20.5% (n = 17) | 20.8% (n = 11) | 26.1% (n = 6) | 0% (n = 0) | Fisher | 0.7669 |
| G2 | 73.5% (n = 61) | 71.7% (n = 38) | 69.6% (n = 16) | 100% (n = 7) | ||
| G3 | 4.8% (n = 4) | 5.7% (n = 3) | 4.3% (n = 1) | 0% (n = 0) | ||
| G4 | 1.2% (n = 1) | 1.9% (n = 1) | 0% (n = 0) | 0% (n = 0) | ||
| Time of follow-up (months) Median (Q1–Q3) Range | 60.03 (59.74–60.03) 0.2–60.16 | 60.03 (58.85–60.03) 0.2–60.07 | 60.03 (60.03–60.07) 5.72–60.16 | 60.03 (60.03–60.03) 30.44–60.07 | Kruskal–Wallis | 0.1144 |
| Variable | Estimate | OR | LCI | UCI | p-Value |
|---|---|---|---|---|---|
| Intercept | −2.726 | 0.066 | 0.003 | 0.438 | 0.018 |
| Presence of microvascular invasion | 3.008 | 20.239 | 2.891 | 426.549 | 0.010 |
| AFP level at transplant | 0.013 | 1.013 | 1.002 | 1.027 | 0.030 |
| Fulfilment of Milan criteria | −1.937 | 0.144 | 0.018 | 0.921 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka, K.; Czarnecka, P.; Tronina, O.; Bączkowska, T.; Durlik, M. Body Mass Index: An Unreliable Adiposity Indicator for Predicting Outcomes of Liver Transplantation Due to Hepatocellular Carcinoma. Gastrointest. Disord. 2024, 6, 607-621. https://doi.org/10.3390/gidisord6030040
Czarnecka K, Czarnecka P, Tronina O, Bączkowska T, Durlik M. Body Mass Index: An Unreliable Adiposity Indicator for Predicting Outcomes of Liver Transplantation Due to Hepatocellular Carcinoma. Gastrointestinal Disorders. 2024; 6(3):607-621. https://doi.org/10.3390/gidisord6030040
Chicago/Turabian StyleCzarnecka, Kinga, Paulina Czarnecka, Olga Tronina, Teresa Bączkowska, and Magdalena Durlik. 2024. "Body Mass Index: An Unreliable Adiposity Indicator for Predicting Outcomes of Liver Transplantation Due to Hepatocellular Carcinoma" Gastrointestinal Disorders 6, no. 3: 607-621. https://doi.org/10.3390/gidisord6030040
APA StyleCzarnecka, K., Czarnecka, P., Tronina, O., Bączkowska, T., & Durlik, M. (2024). Body Mass Index: An Unreliable Adiposity Indicator for Predicting Outcomes of Liver Transplantation Due to Hepatocellular Carcinoma. Gastrointestinal Disorders, 6(3), 607-621. https://doi.org/10.3390/gidisord6030040






