An Evaluation of the Efficacy and Safety of TAMIXAM®, Based on Hyaluronic Acid and Tamarind Seed Extract, for Esophageal Mucosal Protection from Acid Insult
Abstract
1. Introduction
2. Results
2.1. Safety Assessment
2.1.1. Cytotoxicity Test
2.1.2. Oral Mucosa Irritation Test
2.1.3. Skin Sensitization Test—Guinea Pig Maximization Test (GPMT)
2.2. Efficacy Assessment
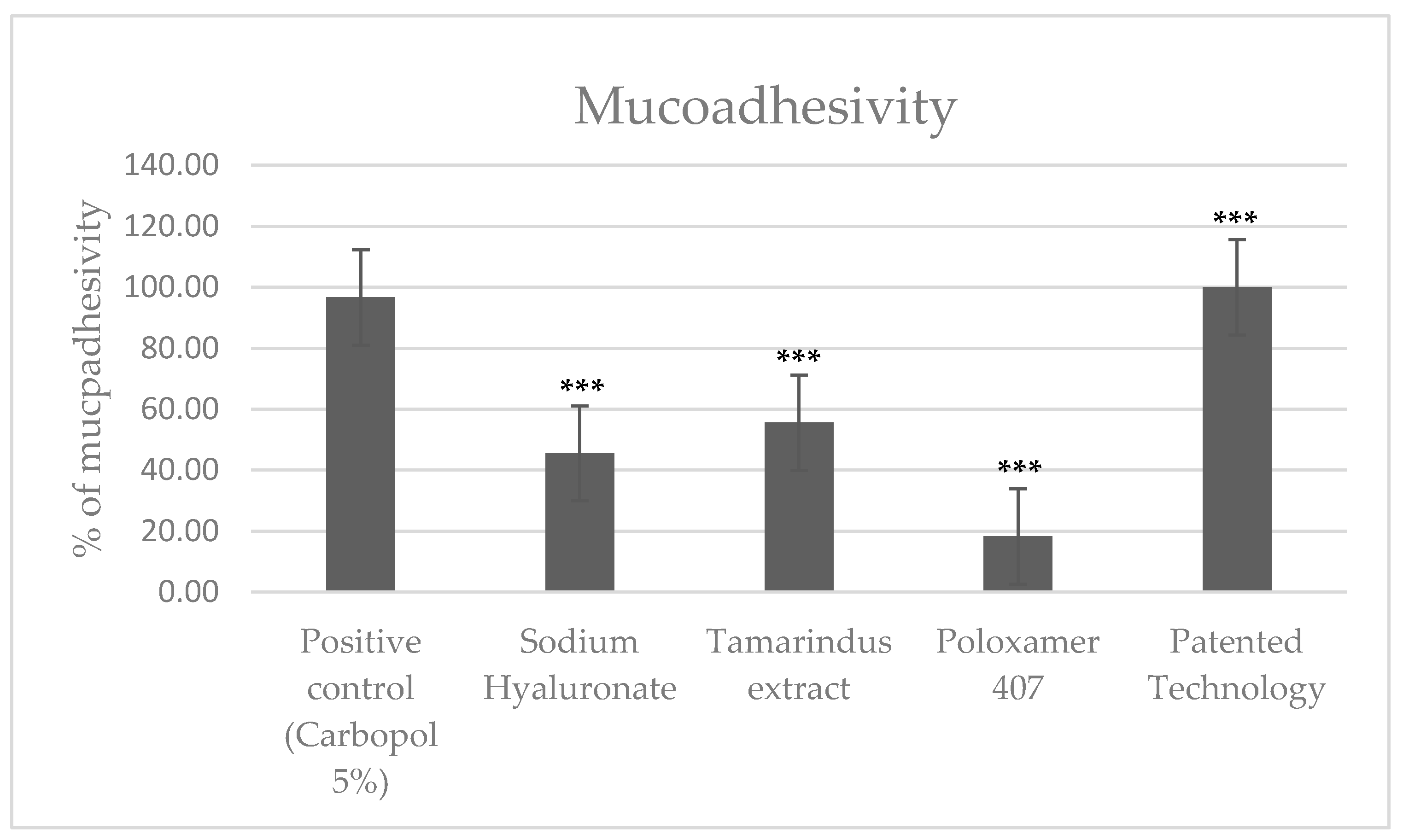
2.2.1. Mucoadhesion Test
2.2.2. Viability Test
2.2.3. TEER Measurement
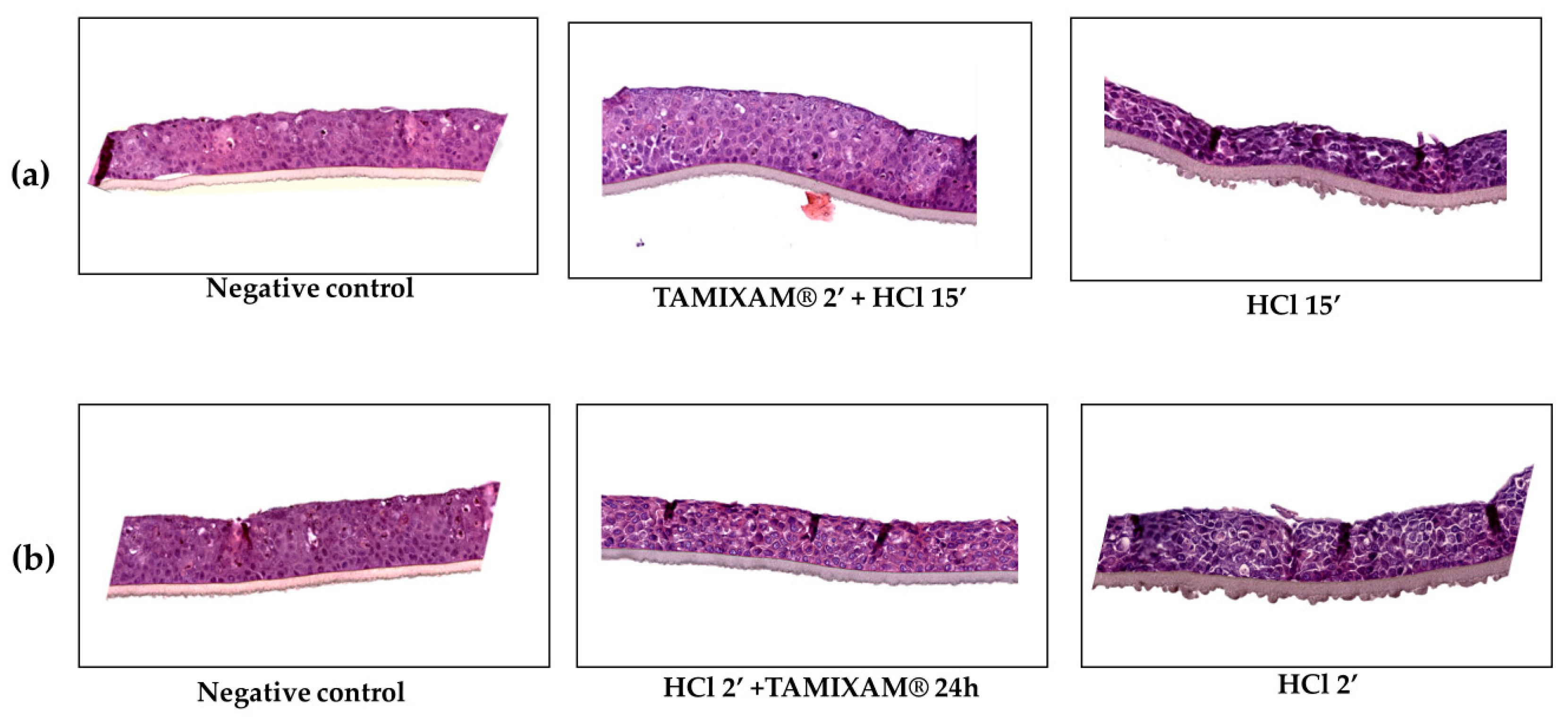
2.2.4. Morphological Analysis
3. Materials and Methods
3.1. Safety Assessment
3.1.1. Cell Cultures
3.1.2. Animals
3.1.3. Tested Sample
3.1.4. Cytotoxicity Test
3.1.5. Oral Mucosa Irritation Test
3.1.6. Skin Sensitization Tests—Guinea Pig Maximization Test (GPMT)
3.2. Efficacy Assessment
3.2.1. Materials
3.2.2. Mucoadhesion Assay
3.2.3. Experimental Conditions
3.2.4. Viability Assessment
3.2.5. TEER Measurement
3.2.6. Morphological Analysis
3.3. Data Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badillo, R.; Francis, D. Diagnosis and Treatment of Gastroesophageal Reflux Disease. World J. Gastrointest. Pharmacol. Ther. 2014, 5, 105–112. [Google Scholar] [CrossRef]
- Dent, J.; Armstrong, D.; Delaney, B.; Moayyedi, P.; Talley, N.J.; Vakil, N. Symptom Evaluation in Reflux Disease: Workshop Background, Processes, Terminology, Recommendations, and Discussion Outputs. Gut 2004, 53 (Suppl. 4), iv1–iv24. [Google Scholar] [CrossRef][Green Version]
- Gnessi, L.; Bacarea, V.; Marusteri, M.; Piqué, N. Xyloglucan for the Treatment of Acute Diarrhea: Results of a Randomized, Controlled, Open-Label, Parallel Group, Multicentre, National Clinical Trial. BMC Gastroenterol. 2015, 15, 153. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.-U.-D.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-Oesophageal Reflux Disease (GORD): Systematic Review with Meta-Analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.C. The Integrity of the Esophageal Mucosa. Balance between Offensive and Defensive Mechanisms. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Nordenstedt, H.; Pedersen, N.L.; Lagergren, J.; Ye, W. Lifestyle Factors and Risk for Symptomatic Gastroesophageal Reflux in Monozygotic Twins. Gastroenterology 2007, 132, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ament, P.W.; Dicola, D.B.; James, M.E. Reducing Adverse Effects of Proton Pump Inhibitors. Am. Fam. Physician 2012, 86, 66–70. [Google Scholar] [PubMed]
- Pellegatta, G.; Spadaccini, M.; Lamonaca, L.; Craviotto, V.; D’Amico, F.; Ceriotti, L.; Meloni, M.; Repici, A. Evaluation of Human Esophageal Epithelium Permeability in Presence of Different Formulations Containing Hyaluronic Acid and Chondroitin Sulphate. Med. Devices Evid. Res. 2020, 13, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.T.; Nuñez, A.; Strahan, G.D.; Chau, H.K.; White, A.K.; Marais, J.P.J.; Hom, K.; Vakkalanka, M.S.; Di, R.; Yam, K.L.; et al. Cranberry Xyloglucan Structure and Inhibition of Escherichia Coli Adhesion to Epithelial Cells. J. Agric. Food Chem. 2015, 63, 5622–5633. [Google Scholar] [CrossRef] [PubMed]
- Pecora, T.M.G.; Parisi, O.I.; Bertin, W.; Ragazzo, B.; Dattilo, M.; Scigliano, N.; Malivindi, R.; Amone, F.; Puoci, F. Barrier Effect and Wound Healing Activity of the Medical Device REF-FTP78 in the Treatment of Gastroesophageal Reflux Disease. Sci. Rep. 2022, 12, 6136. [Google Scholar] [CrossRef] [PubMed]
- Malivindi, R.; Patitucci, F.; Prete, S.; Dattilo, M.; Leonetti, A.E.; Scigliano, N.; Parisi, O.I.; Puoci, F. Efficacy and Safety Assessment of PIMIN050 Raft-Forming System as Medical Device Based on Citrus Sinensis and Crassostrea Gigas for the Management of Gastroesophageal Reflux Disease. J. Drug Deliv. Sci. Technol. 2022, 78, 103986. [Google Scholar] [CrossRef]
- Kapadia, C.J.; Mane, V.B. Raft-Forming Agents: Antireflux Formulations. Drug Dev. Ind. Pharm. 2007, 33, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Yadav, S.; Ahuja, M.; Dilbaghi, N. Synthesis, Characterization and Evaluation of Thiolated Tamarind Seed Polysaccharide as a Mucoadhesive Polymer. Carbohydr. Polym. 2012, 90, 1543–1549. [Google Scholar] [CrossRef]
- Strickland, F.M.; Kuchel, J.M.; Halliday, G.M. Natural Products as Aids for Protecting the Skin’s Immune System against UV Damage. Cutis 2004, 74, 24–28. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- ISO 10993-10:2010; Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization. ISO: Geneva, Switzerland, 2010.
- Uchida, M.; Fukazawa, T.; Yamazaki, Y.; Hashimoto, H.; Miyamoto, Y. A Modified Fast (4 Day) 96-Well Plate Caco-2 Permeability Assay. J. Pharmacol. Toxicol. Methods 2009, 59, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Alexea, O.; Bacarea, V.; Piqué, N. The Combination of Oligo- and Polysaccharides and Reticulated Protein for the Control of Symptoms in Patients with Irritable Bowel Syndrome: Results of a Randomised, Placebo-Controlled, Double-Blind, Parallel Group, Multicentre Clinical Trial. United Eur. Gastroenterol. J. 2015, 4, 455–465. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltes, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef]
- Nolan, A.; Baillie, C.; Badminton, J.; Rudralingham, M.; Seymour, R.A. The Efficacy of Topical Hyaluronic Acid in the Management of Recurrent Aphthous Ulceration. J. Oral Pathol. Med. 2006, 35, 461–465. [Google Scholar] [CrossRef]
- Kapoor, P.; Sachdeva, S.; Sachdeva, S. Topical Hyaluronic Acid in the Management of Oral Ulcers. Indian J. Dermatol. 2011, 56, 300. [Google Scholar] [CrossRef]
- Ialenti, A.; Rosa, M. Hyaluronic Acid Modulates Acute and Chronic Inflammation. Agents Actions 1994, 43, 44–47. [Google Scholar] [CrossRef]
- Lauder, R.M. Chondroitin Sulphate: A Complex Molecule with Potential Impacts on a Wide Range of Biological Systems. Complement. Ther. Med. 2009, 17, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Pecora, T.M.G.; Ragazzo, B.; Bertin, W.; Ragonese, A.; Mascagni, M.; Maffei, P.; Pignatello, R. Rheological Behavior of a New Mucoadhesive Oral Formulation Based on Sodium Chondroitin Sulfate, Xyloglucan and Glycerol. J. Funct. Biomater. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
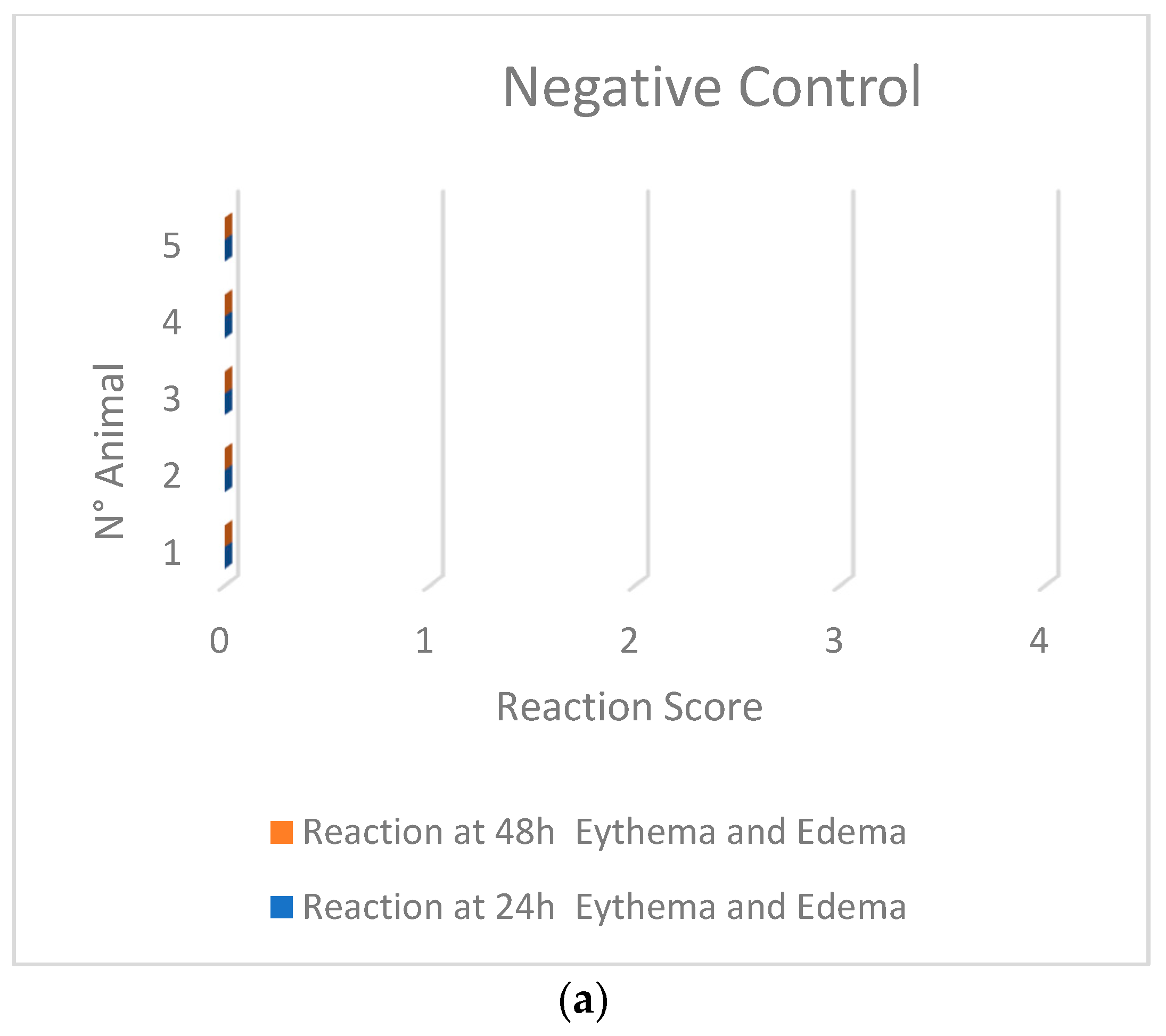
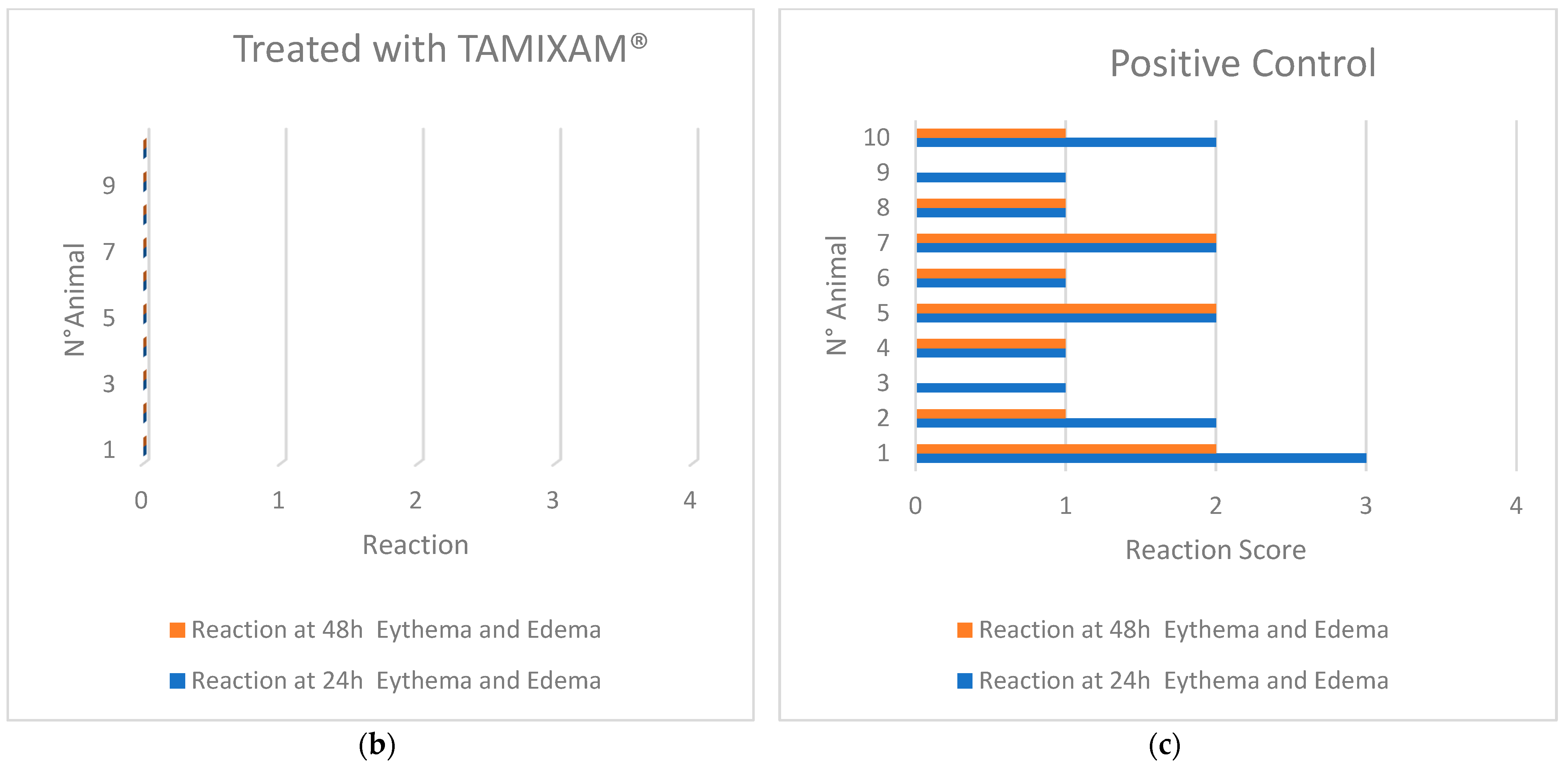

| Sample | Mean Score after 24 h | Mean Score after 48 h |
|---|---|---|
| Positive control | 4 | 4 |
| Negative control | 0 | 0 |
| MEM control | 0 | 0 |
| Undiluted extract | 2 | 4 |
| Diluted extract 1:5 | 0 | 0 |
| Diluted extract 1:10 | 0 | 0 |
| N° Hamster | Epithelium | Leukocyte Infiltration | Vascular Congestion | Edema | Total Score |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 |
| Mean score | 0.00 |
| Sample | Absorbance l = 450nm | Mucoadhesivity (%) | DEV. ST. | Ttest Referred to Negative Control | Ttest Referred to Positive Control | |||
|---|---|---|---|---|---|---|---|---|
| I | II | III | MEAN | |||||
| Negative Control (NaCl) | 3.88 | 3.75 | 3.71 | 3.78 | 0.00% | 0.0889 | ||
| Positive control (Carbopol 5%) | 0.11 | 0.09 | 0.18 | 0.13 | 96.65% | 3.836 × 10−7 | ||
| Sodium Hyaluronate | 1.99 | 2.11 | 2.08 | 2.06 | 45.50% | 0.06245 | 1.051 × 10−5 | 3.303 × 10−7 |
| Tamarindus extract | 1.68 | 1.78 | 1.58 | 1.68 | 55.56% | 0.1 | 1.089 × 10−5 | 1.668 × 10−5 |
| Poloxamer 407 | 3.11 | 3.04 | 3.12 | 3.09 | 18.25% | 0.043589 | 0.00027 | 1.19 × 10−7 |
| Patented Technology | 0.00 | 0.00 | 0.00 | 0.00 | 100.00% | 4.05 × 10−24 | 2.035 × 10−7 | 0.0071786 |
| Negative Control | HCl 15′ | “TAMIXAM®” 2′+ HCl 15′ | HCl 2′ | HCl 2′+ “TAMIXAM®” 24 h | |
|---|---|---|---|---|---|
| TEER t0 (ohm * cm2) ± dev.st | 98.33 ± 1.53 | 97.66 ± 2.52 | 96.33 ± 0.6 | 96.66 ± 0.6 | 96.33 ± 2.0.8 |
| TEER t1 (ohm * cm2) ± dev.st | 97.66 ± 1.53 | 39.33 ± 3.78 *** | 94.01 ± 1.11 * | 61.33 ± 4.16 *** | 73.66 ± 3.05*** |
| % reduction in TEER from t0 to t1 | 0.68% | 59.72% | 2.41% | 36.55% | 23.53% |
| Negative Control | HCl 15′ | TAMIXAM® 2′ + HCl 15′ | HCl 2′ + TAMIXAM® 24 h | HCl 2′ | |
|---|---|---|---|---|---|
| Cellular Degeneration | - | +++ | + | ++ | ++ |
| Necrosis | - | +++ | - | + | ++ |
| Erosion | - | ++ | - | + | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, M.F.; Pulitano, G.; Bagnulo, A.; Buriani, G.; Di Maio, U.; Amone, F.; Nobile, V.; Malivindi, R. An Evaluation of the Efficacy and Safety of TAMIXAM®, Based on Hyaluronic Acid and Tamarind Seed Extract, for Esophageal Mucosal Protection from Acid Insult. Gastrointest. Disord. 2024, 6, 202-213. https://doi.org/10.3390/gidisord6010015
Motta MF, Pulitano G, Bagnulo A, Buriani G, Di Maio U, Amone F, Nobile V, Malivindi R. An Evaluation of the Efficacy and Safety of TAMIXAM®, Based on Hyaluronic Acid and Tamarind Seed Extract, for Esophageal Mucosal Protection from Acid Insult. Gastrointestinal Disorders. 2024; 6(1):202-213. https://doi.org/10.3390/gidisord6010015
Chicago/Turabian StyleMotta, Marisa Francesca, Giuseppe Pulitano, Antonino Bagnulo, Giampaolo Buriani, Umberto Di Maio, Fabio Amone, Vincenzo Nobile, and Rocco Malivindi. 2024. "An Evaluation of the Efficacy and Safety of TAMIXAM®, Based on Hyaluronic Acid and Tamarind Seed Extract, for Esophageal Mucosal Protection from Acid Insult" Gastrointestinal Disorders 6, no. 1: 202-213. https://doi.org/10.3390/gidisord6010015
APA StyleMotta, M. F., Pulitano, G., Bagnulo, A., Buriani, G., Di Maio, U., Amone, F., Nobile, V., & Malivindi, R. (2024). An Evaluation of the Efficacy and Safety of TAMIXAM®, Based on Hyaluronic Acid and Tamarind Seed Extract, for Esophageal Mucosal Protection from Acid Insult. Gastrointestinal Disorders, 6(1), 202-213. https://doi.org/10.3390/gidisord6010015







