Vedolizumab as Rescue Therapy in Carboplatin-Gemcitabine-Induced Triggered Acute Severe Ulcerative Colitis Flare-Up
Abstract
:1. Introduction
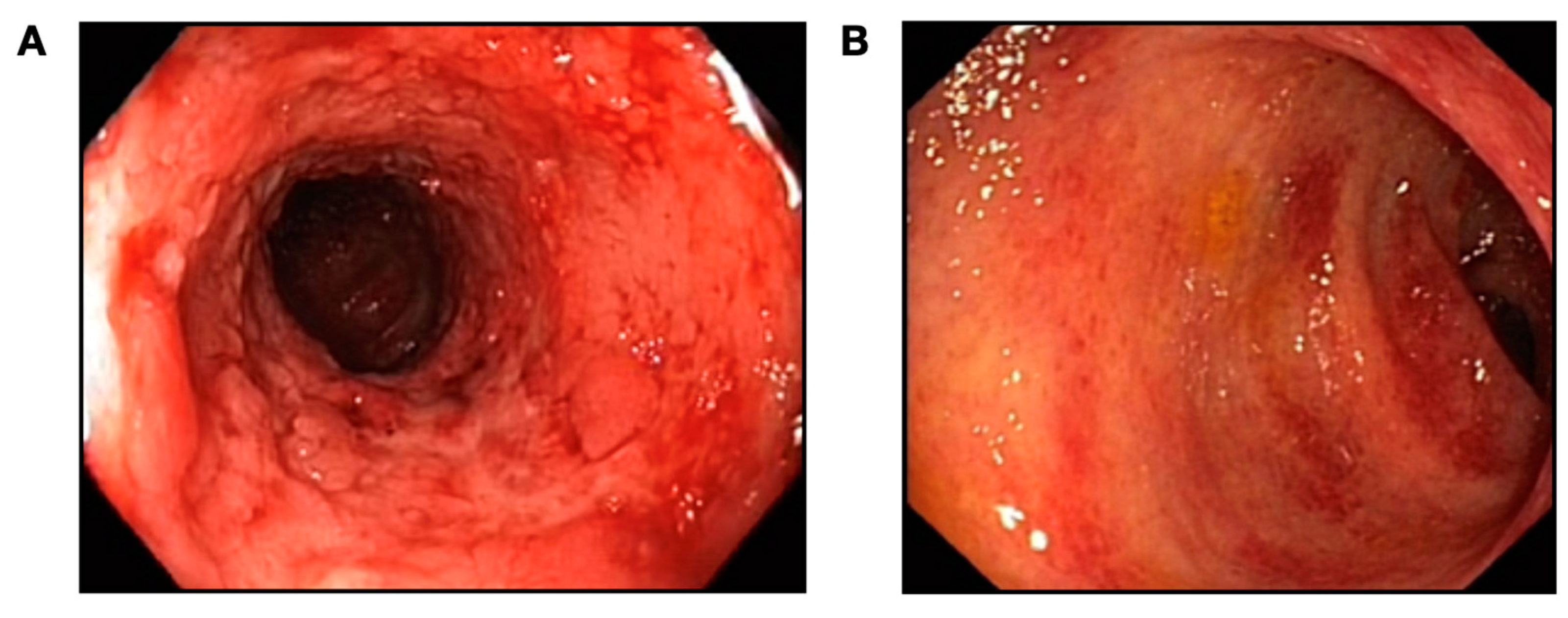
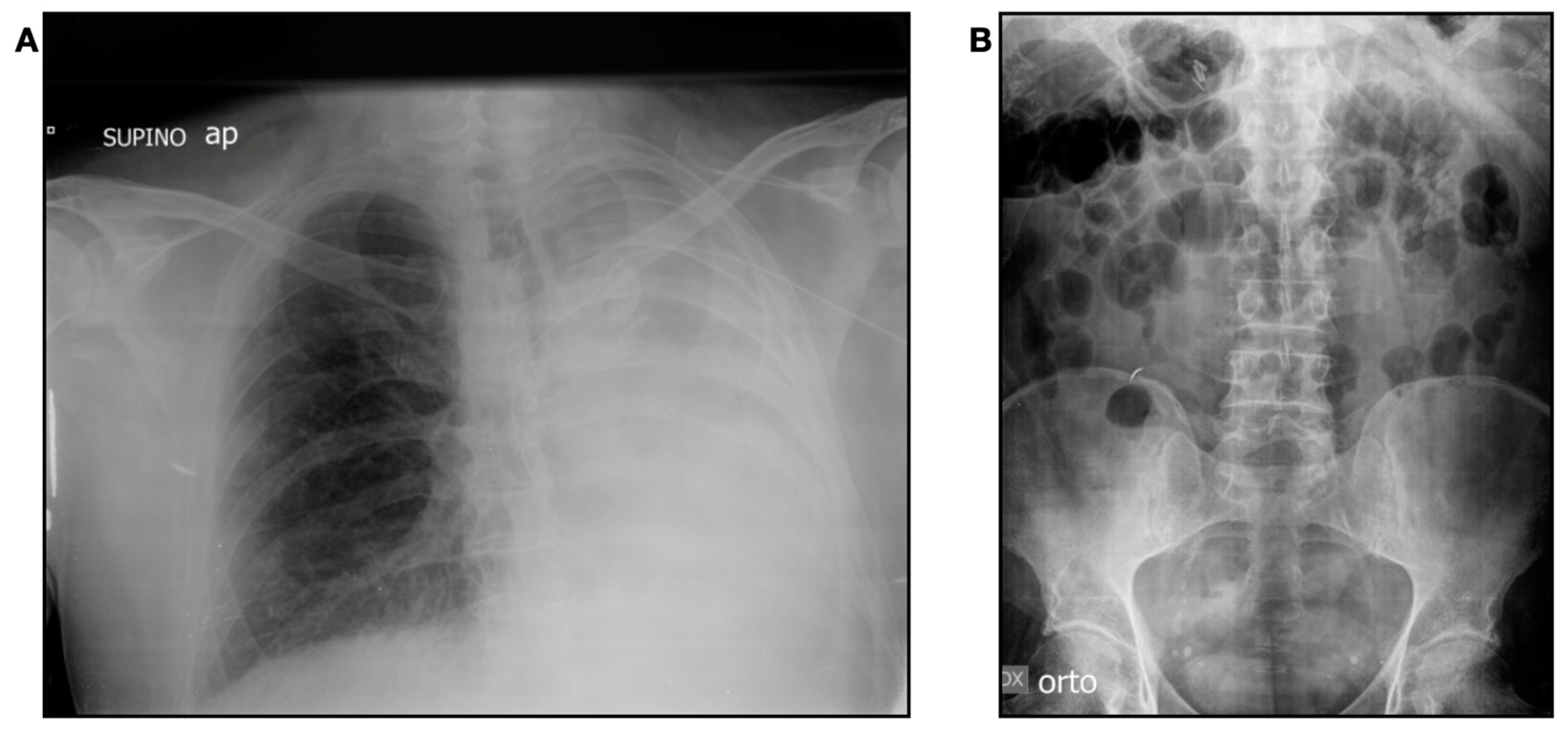
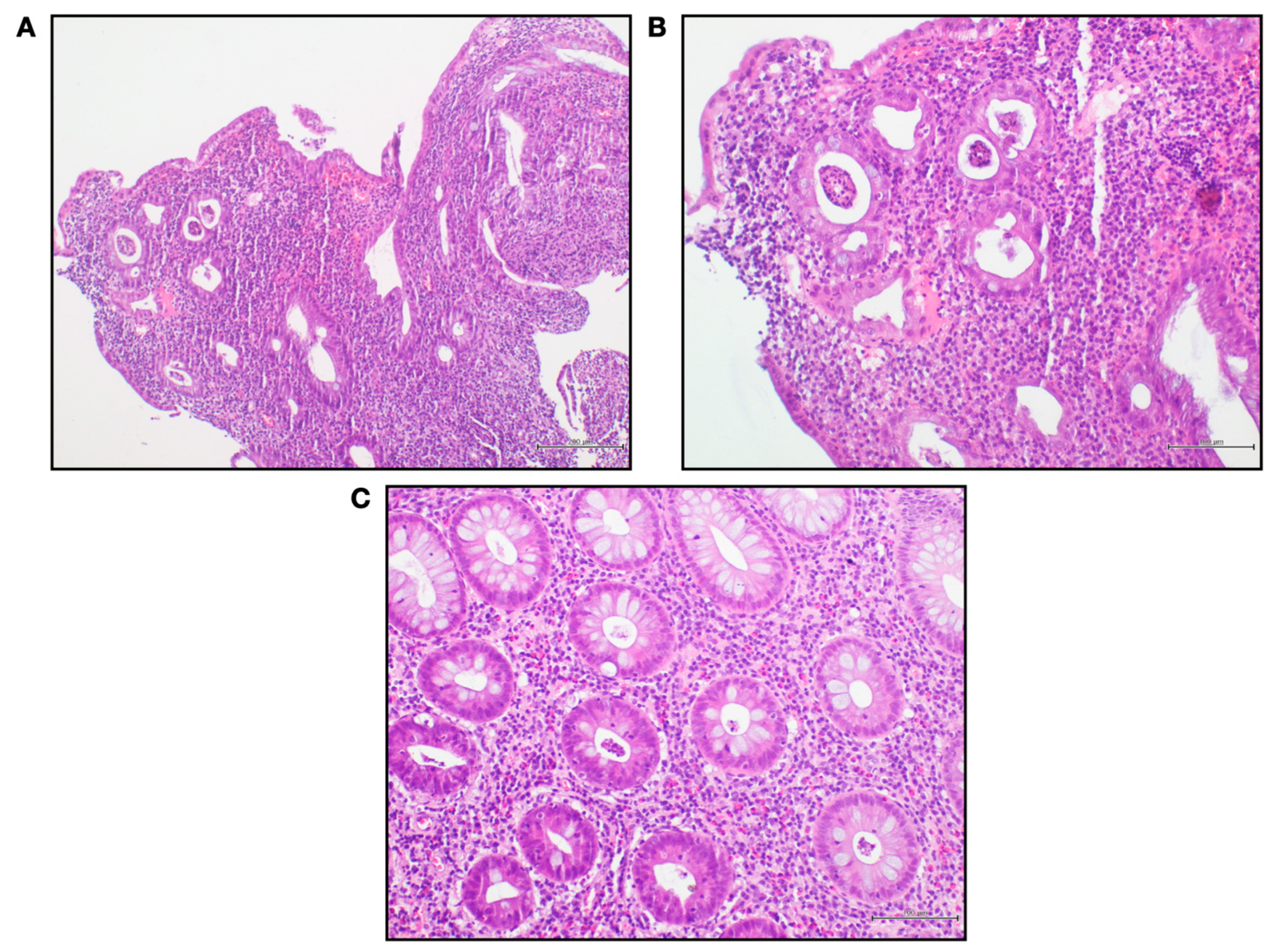
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedano, R.; Quera, R.; Simian, D.; Yarur, A.J. An Approach to Acute Severe Ulcerative Colitis. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef]
- Lasa, J.S.; Olivera, P.A.; Danese, S.; Peyrin-Biroulet, L. Efficacy and Safety of Biologics and Small Molecule Drugs for Patients with Moderate-to-Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 161–170. [Google Scholar] [CrossRef]
- Veisman, I.; Barzilay, O.; Bruckmayer, L.; Haj-Natour, O.; Kopylov, U.; Eliakim, R.; Ben-Horin, S.; Ungar, B. Association of Infliximab and Vedolizumab Trough Levels with Reported Rates of Adverse Events: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 4265. [Google Scholar] [CrossRef] [PubMed]
- Pouillon, L.; Rousseau, H.; Busby-Venner, H.; De Carvalho Bittencourt, M.; Choukour, M.; Gauchotte, G.; Zallot, C.; Danese, S.; Baumann, C.; Peyrin-Biroulet, L. Vedolizumab Trough Levels and Histological Healing during Maintenance Therapy in Ulcerative Colitis. J. Crohns Colitis 2019, 13, 970–975. [Google Scholar] [CrossRef]
- Plevris, N.; Jenkinson, P.W.; Chuah, C.S.; Lyons, M.; Merchant, L.M.; Pattenden, R.J.; Arnott, I.D.; Jones, G.R.; Lees, C.W. Association of Trough Vedolizumab Levels with Clinical, Biological and Endoscopic Outcomes during Maintenance Therapy in Inflammatory Bowel Disease. Frontline Gastroenterol. 2020, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ungar, B.; Malickova, K.; Hanžel, J.; Abu Arisha, M.; Paul, S.; Rocha, C.; Ben Shatach, Z.; Abitbol, C.M.; Haj Natour, O.; Selinger, L.; et al. Dose Optimisation for Loss of Response to Vedolizumab- Pharmacokinetics and Immune Mechanisms. J. Crohns Colitis 2021, 15, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro-Sanchez, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2021, 16, 2–17. [Google Scholar] [CrossRef]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, Z.; Tang, M.; Xiao, D.; Cai, P. Impact of Genetic Factors on Platinum-Induced Gastrointestinal Toxicity. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108324. [Google Scholar] [CrossRef] [PubMed]
- de Gara, C.J.; Gagic, N.; Arnold, A.; Seaton, T. Toxic Megacolon Associated with Anticancer Chemotherapy. Can. J. Surg. 1991, 34, 339–341. [Google Scholar]
- Marin-Acevedo, J.A.; Harris, D.M.; Burton, M.C. Immunotherapy-Induced Colitis: An Emerging Problem for the Hospitalist. J. Hosp. Med. 2018, 13, 413–418. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Mallepally, N.; Goldstein, R.; Chen, E.; Tang, T.; Dike, U.K.; Al-Asadi, M.; Westin, S.; Halperin, D.; Wang, Y. Gastrointestinal Toxic Effects in Patients with Cancer Receiving Platinum-Based Therapy. J. Cancer 2020, 11, 3144–3150. [Google Scholar] [CrossRef]
- Allen, V.A.; Manahan, K.J.; Geisler, J.P. Recurrent Pseudomembranous Colitis in an Ovarian Cancer Patient Undergoing Carboplatin Chemotherapy. Case Rep. Obstet. Gynecol. 2016, 2016, 7540302. [Google Scholar] [CrossRef]
- Elsayed, A.G.; Srivastava, R.; Pacioles, T.; Limjoco, T.; Tirona, M.T. Ischemic Colitis Associated with Paclitaxel and Carboplatin Combination. Case Rep. Oncol. 2017, 10, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Yoshikawa, I.; Kume, K.; Otsuki, M. Ischemic Colitis Associated with Paclitaxel and Carboplatin Chemotherapy. Am. J. Gastroenterol. 2003, 98, 231–232. [Google Scholar] [CrossRef]
- Resnik, E.; Lefevre, C.A. Fulminant Clostridium Difficile Colitis Associated with Paclitaxel and Carboplatin Chemotherapy. Int. J. Gynecol. Cancer 1999, 9, 512–514. [Google Scholar] [CrossRef]
- Osumi, H.; Ozaka, M.; Ishii, H.; Sasahira, N. Severe Ischemic Colitis after Treatment of Bile-Duct Cancer Using Gemcitabine and Cisplatin. Jpn. J. Clin. Oncol. 2015, 45, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohn’s Colitis 2022, 16, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Romeo, M.; Palladino, G.; Cipullo, M.; Iadanza, G.; Olivieri, S.; Zagaria, G.; De Gennaro, N.; Santonastaso, A.; et al. Quality of Bowel Preparation in Patients with Inflammatory Bowel Disease Undergoing Colonoscopy: What Factors to Consider? World J. Gastrointest. Endosc. 2023, 15, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Choy, M.C.; Seah, D.; Faleck, D.M.; Shah, S.C.; Chao, C.-Y.; An, Y.-K.; Radford-Smith, G.; Bessissow, T.; Dubinsky, M.C.; Ford, A.C.; et al. Systematic Review and Meta-Analysis: Optimal Salvage Therapy in Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Macaluso, F.S.; Cassata, N.; Citrano, M.; Orlando, A. Rescue Therapy with Intensive Vedolizumab Optimization in a Seventeen-Year-Old Girl with Acute Severe Ulcerative Colitis. Dig. Dis. Sci. 2021, 66, 2470–2471. [Google Scholar] [CrossRef]
- Sahu, P.; Kedia, S.; Vuyyuru, S.K.; Bajaj, A.; Markandey, M.; Singh, N.; Singh, M.; Kante, B.; Kumar, P.; Ranjan, M.; et al. Randomised Clinical Trial: Exclusive Enteral Nutrition versus Standard of Care for Acute Severe Ulcerative Colitis. Aliment. Pharmacol. Ther. 2021, 53, 568–576. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef]
- Gisbert, J.P.; García, M.J.; Chaparro, M. Rescue Therapies for Steroid-Refractory Acute Severe Ulcerative Colitis: A Review. J. Crohns Colitis 2023, 17, 972–994. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Juhl, C.B.; Chen, I.M.; Kellermann, L.; Nielsen, O.H. Immune Checkpoint Inhibitor–Induced Diarrhea and Colitis: Incidence and Management. A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2022, 109, 102440. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Wang, Y. Immune-Checkpoint Inhibitors Induced Diarrhea and Colitis: A Review of Incidence, Pathogenesis and Management. Curr. Opin. Gastroenterol. 2020, 36, 25–32. [Google Scholar] [CrossRef]
- Stojanovska, V.; Sakkal, S.; Nurgali, K. Platinum-Based Chemotherapy: Gastrointestinal Immunomodulation and Enteric Nervous System Toxicity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G223–G232. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chanona, E.; Trinchieri, G. The Role of Microbiota in Cancer Therapy. Curr. Opin. Immunol. 2016, 39, 75–81. [Google Scholar] [CrossRef]
- Goubet, A.-G.; Daillère, R.; Routy, B.; Derosa, L.; Roberti, P.M.; Zitvogel, L. The Impact of the Intestinal Microbiota in Therapeutic Responses against Cancer. C R. Biol. 2018, 341, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value | Normal Range |
|---|---|---|
| White blood cells | 8.21 × 103/μL | 4–11 × 103/μL |
| Red blood cells | 4.8 × 103/μL | 4.5–6 × 103/μL |
| Haemoglobin | 10.9 g/dL | 13–17.5 g/dL |
| Platelets | 400 fL | 150–450 fL |
| Blood glucose | 68 mg/dL | 70–100 mg/dL |
| Creatinine | 1.31 mg/dL | 0.67–1.17 mg/dL |
| Cholinesterase | 5926 U/L | 5320–12,920 U/L |
| Sodium | 141 mEq/L | 135–146 mEq/L |
| Potassium | 3.4 mEq/L | 3.5–5.3 mEq/L |
| Chlorine | 104 mEq/L | 98–111 mEq/L |
| Calcium | 8.7 mg/dL | 8.6–10.2 mg/dL |
| Lactate dehydrogenase | 297 U/L | 120–240 U/L |
| Blood iron | 30 μg/dL | 59–158 μg/dL |
| Transferrin | 249 mg/dL | 191–337 mg/dL |
| Ferritin | 50 ng/mL | 30–400 ng/mL |
| Creatine phosphokinase | 31 U/L | 60–190 U/L |
| C-reactive protein | 3 mg/dL | 0–1 mg/dL |
| ESR | 40 mm/h | 2–15 mm/h |
| Total proteins | 7.3 g/dL | 6.6–8.7 g/dL |
| Albumin | 4 g/dL | 3.5–5.5 g/dL |
| Aspartate aminotransferase | 15 U/L | 5–40 U/L |
| Alanine aminotransferase | 42 U/L | 5–41 U/L |
| C. difficile stool test | negative | negative |
| QuantiFERON test | negative | negative |
| Stool culture and faecal parasitological exam | negative | negative |
| TORCH complex, including HIV | negative | negative |
| CMV blood tests | negative | negative |
| Thyroid hormones | normal | normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, R.; Fasano, M.; Morgillo, F.; Palladino, G.; Vassallo, I.; Pirozzi, M.; Imperio, G.; Auletta, S.; Ventura, A.; Panarese, I.; et al. Vedolizumab as Rescue Therapy in Carboplatin-Gemcitabine-Induced Triggered Acute Severe Ulcerative Colitis Flare-Up. Gastrointest. Disord. 2023, 5, 367-375. https://doi.org/10.3390/gidisord5030030
Pellegrino R, Fasano M, Morgillo F, Palladino G, Vassallo I, Pirozzi M, Imperio G, Auletta S, Ventura A, Panarese I, et al. Vedolizumab as Rescue Therapy in Carboplatin-Gemcitabine-Induced Triggered Acute Severe Ulcerative Colitis Flare-Up. Gastrointestinal Disorders. 2023; 5(3):367-375. https://doi.org/10.3390/gidisord5030030
Chicago/Turabian StylePellegrino, Raffaele, Morena Fasano, Floriana Morgillo, Giovanna Palladino, Isabella Vassallo, Mario Pirozzi, Giuseppe Imperio, Salvatore Auletta, Andrea Ventura, Iacopo Panarese, and et al. 2023. "Vedolizumab as Rescue Therapy in Carboplatin-Gemcitabine-Induced Triggered Acute Severe Ulcerative Colitis Flare-Up" Gastrointestinal Disorders 5, no. 3: 367-375. https://doi.org/10.3390/gidisord5030030
APA StylePellegrino, R., Fasano, M., Morgillo, F., Palladino, G., Vassallo, I., Pirozzi, M., Imperio, G., Auletta, S., Ventura, A., Panarese, I., Federico, A., & Gravina, A. G. (2023). Vedolizumab as Rescue Therapy in Carboplatin-Gemcitabine-Induced Triggered Acute Severe Ulcerative Colitis Flare-Up. Gastrointestinal Disorders, 5(3), 367-375. https://doi.org/10.3390/gidisord5030030








