Different Clinical Features of Idiopathic Achalasia in Various Countries
Abstract
1. Introduction
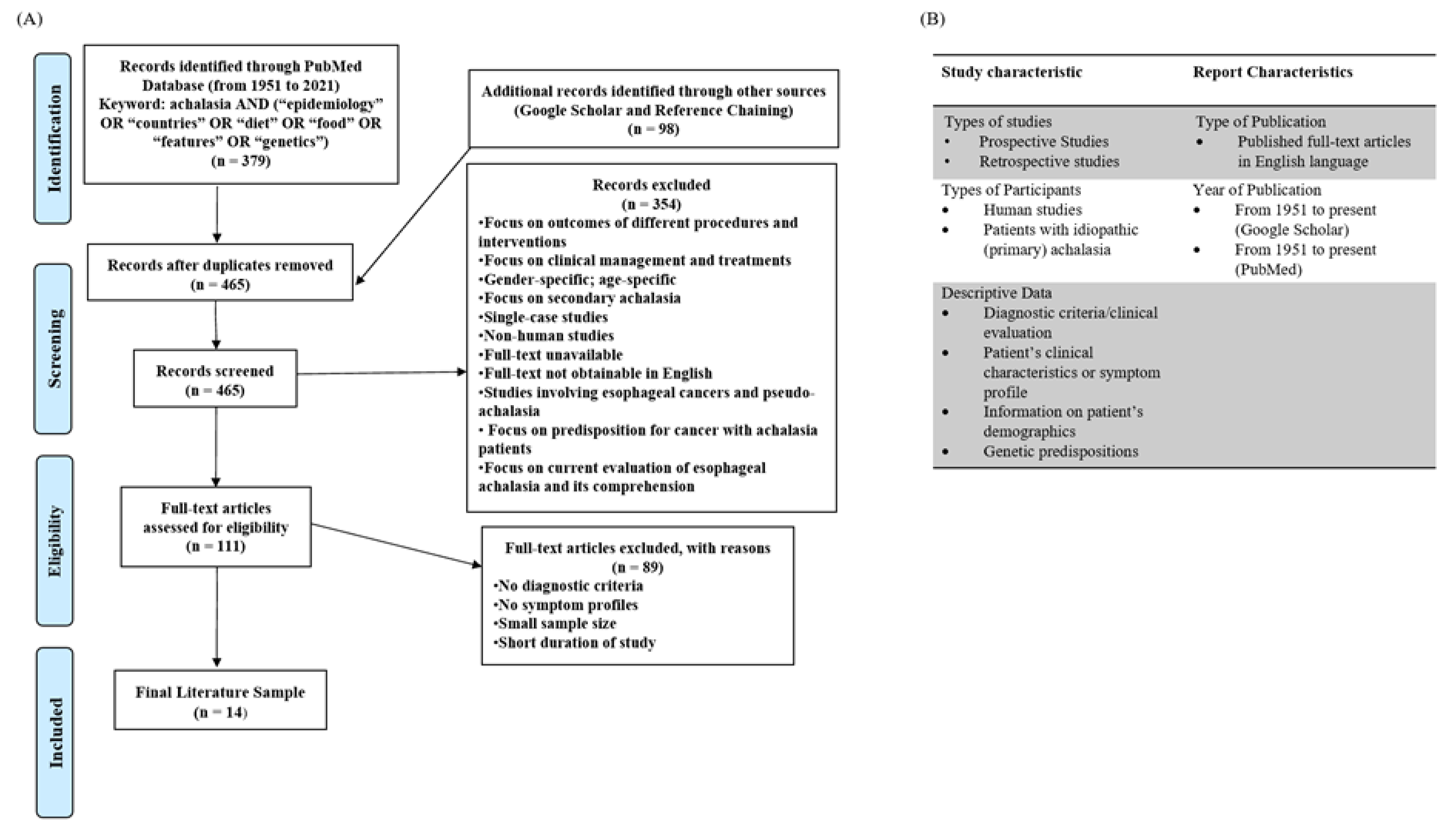
2. Methods
2.1. Selection Process
2.2. Dietary Composition Data
3. Results
3.1. Clinical Characteristics
3.2. Dietary Compositions
3.3. Genetics
4. Discussion
4.1. Clinical Features and Diet
4.2. Climate
4.3. Genetics
4.4. Limitations/Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gockel, H.R.; Schumacher, J.; Gockel, I.; Lang, H.; Haaf, T.; Nöthen, M.M. Achalasia: Will genetic studies provide insights? Hum. Genet. 2010, 128, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Daschakraborty, S.B.; Singh, R. Pathogenesis of achalasia cardia. World J. Gastroenterol. 2012, 18, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E. Achalasia-an update. J. Neurogastroenterol. Motil. 2010, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Abu Baker, F.; Pellicano, R.; Khoury, T. Diagnosis and Management of Achalasia: Updates of the Last Two Years. J. Clin. Med. 2021, 10, 3607. [Google Scholar] [CrossRef]
- Kalantari, M.; Hollywood, A.; Lim, R.; Hashemi, M. Mapping the experiences of people with achalasia from initial symptoms to long-term management. Health Expect. 2021, 24, 131–139. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Pouyez, C.; Neshkova, E.; von Renteln, D.; Bouin, M. Management of Esophageal Achalasia in Quebec. J. Clin. Med. Res. 2019, 11, 682–689. [Google Scholar] [CrossRef][Green Version]
- Sinan, H.; Tatum, R.P.; Soares, R.V.; Martin, A.V.; Pellegrini, C.A.; Oelschlager, B.K. Prevalence of respiratory symptoms in patients with achalasia. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2011, 24, 224–228. [Google Scholar] [CrossRef]
- Adeyemo, A.O.; Lawal, O.; Ojelade, A. Achalasia of the esophagus: Reflections upon a clinical study of 33 cases. J. Natl. Med. Assoc. 1987, 79, 65–71. [Google Scholar]
- Ahmed, A.; Yusufu, L.M.; Ukwenya, Y.A.; Khalid, L.; Garba, E.S. Surgical management of achalasia in Zaria, Northern Nigeria. S. Afr. J. Surg. 2008, 46, 48–51. [Google Scholar]
- Ezemba, N.; Ekwunife, C.N.; Eze, J.C. Achalasia in Nigeria. Current Status. Chirurgia 2007, 20, 125–129. [Google Scholar]
- Tebaibia, A.; Boudjella, M.A.; Boutarene, D.; Benmediouni, F.; Brahimi, H.; Oumnia, N. Incidence, clinical features and para-clinical findings of achalasia in Algeria: Experience of 25 years. World J. Gastroenterol. 2016, 22, 8615–8623. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Medani, S.; Abdallah, T.M.; Gasim, G.I. Clinical Utility of Esophageal manometry in the patients with dysphagia—Experience from Sudan. Int. J. Health Sci. 2016, 10, 522–531. [Google Scholar] [CrossRef]
- Ng, K.Y.; Li, K.F.; Lok, K.H.; Lai, L.; Ng, C.H.; Li, K.K.; Szeto, M.L. Ten-year review of epidemiology, clinical features, and treatment outcome of achalasia in a regional hospital in Hong Kong. Hong Kong Med. J. 2010, 16, 362–366. [Google Scholar]
- Jeon, H.H.; Kim, J.H.; Youn, Y.H.; Park, H.; Conklin, J.L. Clinical Characteristics of Patients with Untreated Achalasia. J. Neurogastroenterol. Motil. 2017, 23, 378–384. [Google Scholar] [CrossRef]
- Aljebreen, A.M.; Samarkandi, S.; Al-Harbi, T.; Al-Radhi, H.; Almadi, M.A. Efficacy of pneumatic dilatation in Saudi achalasia patients. Saudi J. Gastroenterol. 2014, 20, 43. [Google Scholar] [CrossRef]
- Jain, M.; Baijal, N.; Srinivas, M.; Baijal, R.; Pratap, N.; Bachkaniwala, V.; Ganesh, P.; Venkataraman, J. Retrospective study on symptoms and treatment modalities used and short-term follow up of achalasia cardia in Indian setting. JGH Ope Open Access J. Gastroenterol. Hepatol. 2020, 4, 856–859. [Google Scholar] [CrossRef]
- Ahmed, W.U.; Qureshi, H.; Maher, M.; Arif, A. Achalasia in a gastroenterology unit of Karachi. J. Pak. Med. Assoc. 2008, 58, 661–664. [Google Scholar]
- Birgisson, S.; Richter, J.E. Achalasia in Iceland, 1952–2002: An Epidemiologic Study. Dig. Dis. Sci. 2007, 52, 1855–1860. [Google Scholar] [CrossRef]
- Farrukh, A.; DeCaestecker, J.; Mayberry, J.F. An epidemiological study of achalasia among the South Asian population of Leicester, 1986–2005. Dysphagia 2008, 23, 161–164. [Google Scholar] [CrossRef]
- Farrokhi, F.; Vaezi, M.F. Idiopathic (primary) achalasia. Orphanet J. Rare Dis. 2007, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Torres-Landa, S.; Valdovinos, M.Á.; Coss-Adame, E.; Martín Del Campo, L.A.; Torres-Villalobos, G. New insights into the pathophysiology of achalasia and implications for future treatment. World J. Gastroenterol. 2016, 22, 7892–7907. [Google Scholar] [CrossRef] [PubMed]
- De León, A.R.; de la Serna, J.P.; Santiago, J.L.; Sevilla, C.; Fernández-Arquero, M.; de la Concha, E.G.; Nuñez, C.; Urcelay, E.; Vigo, A.G. Association between idiopathic achalasia and IL23R gene. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 734-e218. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moon, S.; Popkin, B.M. The nutrition transition in South Korea. Am. J. Clin. Nutr. 2000, 71, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T.; Sbeit, W.; Pellicano, R.; Mari, A. Gastric peroral endoscopic myotomy for gastroparesis: A spark of hope. Minerva Gastroenterol. 2021, 67, 171–172. [Google Scholar] [CrossRef] [PubMed]

| Author | Country | Region | Population (N) | Symptoms | Total Percentage (%) |
|---|---|---|---|---|---|
| Pouyez et al. (2019) [7] | Canada | North America | (169) |
| (98) (83) (78) (40) (23) (23) (16) |
| Sinan et al. (2011) [8] | United States | (165) | |||
| Adeyemo et al. (1987) [9] | Nigeria | West Africa | (33) |
| (98) (72) (42) (28) (26) (20) (14) (14) |
| Ahmed, A et al. (2008) [10] | Nigeria | (47) | |||
| Ezemba et.al (2007) [11] | Nigeria | (43) | |||
| Tebaibia et al. (2016) [12] | Algeria | North Africa | (1256) |
| (99) (83) (70) (25) (22) |
| Abbas et al. (2016) [13] | Sudan | Northeast Africa | (51) |
| (100) (65) (44) (43) (26) (22) |
| Ng et al. (2010) [14] | Hong Kong | East Asia | (32) |
| (100) *(59) (51) (47) (3) |
| Jeon et.al (2017) [15] | South Korea | (64) | |||
| Aljebreen et al. (2014) [16] | Saudi Arabia | Western Asia | (29) |
| (100) (56) (45) (24) (21) |
| Jain et al. (2020) [17] | India | Southern Asia | (452) |
| (94) (80) (54) (35) (30) |
| Ahmed et al. (2008) [18] | Pakistan | (46) | |||
| Birgisson et al. (2007) [19] | Iceland | Europe | (62) |
| (99) (48) (47) (39) (11) (8) |
| Farrukh et al. (2008) [20] | United Kingdom | (14) |
| Commodity Group | Region with Highest Weight Consumption |
|---|---|
| Oils/Fat | North America (24.39%) |
| Sugar | North America (14.98%) |
| Starchy roots | West Africa (22.81%) |
| Cereals and Grains | Southern Asia (54.2%) |
| Fruits and Vegetables | North Africa (10.29%) |
| Dairy and Eggs | Northeast Africa (11.25%) |
| Meat | Europe (13.94%) |
| Pulses | Southern Asia (5.12%) |
| Alcohol | Europe (4.47%) |
| Others | Western Asia (3.38%) |
| Commodity Group | Region with Lowest Weight Consumption |
|---|---|
| Oils/Fat | Northeast Africa (10.10%) |
| Sugar | West Africa (3.89%) |
| Starchy roots | East Asia (1.18%) |
| Cereals and Grains | North America (22.19%) |
| Fruits and Vegetables | West Africa (4.41%) |
| Dairy and Eggs | West Africa (0.89%) |
| Meat | Southern Asia (1.29%) |
| Pulses | Southern Asia (5.12%) |
| Alcohol | Western Asia (0.00%) |
| Others | Western Africa (0.52%) |
| Candidate Genes | Function | Isozymes | Chromosome Location |
|---|---|---|---|
| Nitric Oxide Synthase [21] | Synthesizes nitric oxide | Neuronal NOS (NOS1) | chromosome 12q24.2 |
| Inducible NOS (NOS2) | chromosome 17q11.2-q12 | ||
| Endothelial NOS (NOS3) | chromosome 7q36 | ||
| Vasoactive intestinal polypeptide (VIPR1) gene [21] | A small neuropeptide that acts as a neurotransmitter with anti-inflammatory properties. This is found in the myenteric plexus to regulate relaxation of the LES and distal esophagus | chromosome 3p22 | |
| Interleukin-23 Receptor (IL-23R) gene [23] | A Type I transmembrane protein that is expressed by Th17 cells. These are associated with inflammatory and chronic autoimmune disorders | chromosome 1p31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeung, A.; Benmerzouga, I. Different Clinical Features of Idiopathic Achalasia in Various Countries. Gastrointest. Disord. 2022, 4, 56-65. https://doi.org/10.3390/gidisord4020007
Yeung A, Benmerzouga I. Different Clinical Features of Idiopathic Achalasia in Various Countries. Gastrointestinal Disorders. 2022; 4(2):56-65. https://doi.org/10.3390/gidisord4020007
Chicago/Turabian StyleYeung, Amy, and Imaan Benmerzouga. 2022. "Different Clinical Features of Idiopathic Achalasia in Various Countries" Gastrointestinal Disorders 4, no. 2: 56-65. https://doi.org/10.3390/gidisord4020007
APA StyleYeung, A., & Benmerzouga, I. (2022). Different Clinical Features of Idiopathic Achalasia in Various Countries. Gastrointestinal Disorders, 4(2), 56-65. https://doi.org/10.3390/gidisord4020007





