Abstract
Functional gastrointestinal disorders (FGIDs), such as irritable bowel syndrome, functional constipation, and functional dyspepsia, have had a high prevalence over the past few years. Recent evidence suggests that functional foods and bioactive compounds, such as probiotics and phytochemicals, may have a positive effect in treating the symptoms of the above diseases. In this systematic review study, 32 published studies were selected with the use of comprehensive scientific databases, according to PRISMA guidelines, with emphasis on recent interventional studies that reflect the effect of probiotics and selected phytochemicals on the improvement of FGID symptoms. The bioactive compounds in the selected studies were administered to patients either in capsule form or in enriched food products (yogurt, juice, etc.). According to the results, there is a correlation between the consumption of probiotics and phytochemicals, such as polyphenols, and the relief of symptoms in selected gastrointestinal disorders. Enriching foods that are regularly consumed by the population, such as fruit juices, yogurt, and cheese, with ingredients that may have a positive effect on gastrointestinal disorders, could be a possible novel goal for the management of these diseases. However, further evidence is required for the role of probiotics and phytochemicals in FGIDs to be fully understood.
1. Introduction
Over the years, several scientific efforts were initiated to understand the relationship between humans and microbial communities. Improvements in the throughput and accuracy of the DNA sequencing of the genomes of microbial communities that are associated with human samples, complemented by analyses of transcriptomes, proteomes, metabolomes, and immunomes, together with mechanistic experiments in model systems, have vastly improved our ability to understand the structure and function of the microbiome in both diseased and healthy states of humans [1].
The microbiome is increasingly recognized as a critical component in human development, health, and well-being. Research into the microbiome, the indigenous microbial communities (microbiota), and the host environments that they inhabit, has changed clinicians’ ideas about microbes in human health and disease. Perhaps the most radical change is the realization that most of the microbes that inhabit our bodies have a crucial role of benefiting the entire host–microbe system [2]. The human microbiome contains an enormous population of prokaryotes, viruses, and eukaryotes. Different microbial communities occupy different habitats in the human body. We rely on these microbes to aid in nutrition, to combat pathogens, and to modulate our immune systems [3].
The human gastrointestinal tract hosts more than 100 trillion bacteria and archaea, which together make up the gut microbiota. The human gut microbiota co-evolved with humans to achieve a symbiotic relationship, leading to physiological homeostasis [4]. Generally, the intestinal microbiome is dominated by members of the following divisions: Bacteroidetes and Firmicutes [5]. However, the intestinal microbiome probably varies in different periods of human life, as it is a dynamic system that can be affected by several genetic and environmental factors. Among the environmental factors, dietary habits play a key role in the modulation of gut microbiota composition. A particular diet may promote the growth of specific bacterial strains, driving hosts to a consequent alteration of fermentative metabolism, with a direct effect on intestinal pH, which can be responsible for the development of pathogenic flora [6].
Mounting evidence has highlighted the prominent influence of the gut mutualistic bacterial communities on human health. Microbial colonization occurs alongside immune system development and plays a role in intestinal physiology [7]. Disruption of the symbiosis between the human body and its microbiome may be unfavorable and associated with the development of the diverse diseases, known as functional gastrointestinal disorders (FGIDs). FGIDs share definable clinical features but have no characteristic morphology or biomarkers that enable diagnosis. Diagnosis of an FGID requires identifying symptoms-based criteria and excluding other specific conditions that have similar clinical presentations, by physical examination, laboratory studies, and imaging [8]. FGID pathophysiology is, therefore, complex. It involves bidirectional dysregulation of gut–brain interaction (via the gut–brain axis), as well as microbial dysbiosis within the gut, altered mucosal immune function, visceral hypersensitivity, and abnormal gastrointestinal motility [9].
Dietary patterns may have a role in the treatment of FGIDs. Dietary components also play a beneficial role beyond basic nutrition, leading to the development of the functional food concept and nutraceuticals [10]. Probiotics and phytochemicals are two of the most well-characterized dietary bioactive compounds. The beneficial effects of probiotics mainly rely on their influence on gut microbiota composition and their ability to generate fermentation products (short-chain fatty acids, enterolactone, etc.) with diverse biological roles. Phytochemicals are bioactive non-nutrient plant compounds, which have raised interest because of their potential effects as antioxidants, anti-estrogenics, anti-inflammatories, immunomodulators, and anti-carcinogenics. However, the bioavailability and effects of phytochemicals depend greatly on their transformation by the components of gut microbiota [11].
According to the World Health Organization’s definition, probiotics are the living bacteria that confer health benefits on a host when administered in adequate amounts [12,13]. Some commonly used probiotics today are gram-positive species, such as the Lactobacillus and Bifidobacterium, as well as some gram-negative, most notably Escherichia coli Nissle 1917 [12]. The health-promoting benefits of probiotics include preventing the colonization or curbing the growth of pathogenic bacteria, enhancing epithelial barrier functions, stimulating the host immune response, and modulating the inflammatory gene expression in the gut [14]. Many of these microorganisms are part of the normal human gut flora, where they live in a symbiotic relationship. The potential for probiotics to modulate the gut microbiome, and thus to correct dysbiosis, support the use of probiotics in the treatment of gastrointestinal (GI) conditions [15,16].
Phytochemicals are biologically active, naturally occurring chemical compounds found in plants, which provide health benefits for humans as medicinal ingredients and nutrients [17]. Recently, it has been clearly shown that they also have roles in the protection of human health when their dietary intake is significant [18]. It is easy to incorporate phytochemicals into the daily diet, as they are abundant in foods such as fruits, vegetables, nuts, and whole grains [19]. The exact classification of phytochemicals has not yet been determined, because of their diverse forms and structures. Classically, phytochemicals have been classified as primary or secondary metabolites, depending on their role in plant metabolism. As primary metabolites, phytochemicals can be common sugars, amino acids, proteins, purines and pyrimidines of nucleic acids, chlorophylls, etc. As secondary metabolites, they can be considered as plant chemicals, including alkaloids, terpenes, flavonoids, lignans, plant steroids, curcumins, saponins, phenolics, and glucosides [20]. Phytochemicals that are considered to be secondary metabolites, are non-nutritive bioactive chemical compounds produced by plants. They are called non-nutritive because they are synthesized by plants, only in specific ways, and not by way of energy metabolism, catabolic metabolism, or anabolic metabolism [21,22].
Polyphenols are a diverse class of plant secondary metabolites, often associated with the color, taste, and defense mechanisms of fruit and vegetables. The gut microbiota plays a critical role in transforming dietary polyphenols into absorbable biologically active species, acting on the estimated 95% of dietary polyphenols that reach the colon [23]. In turn, polyphenols modulate gut microbiota beyond the “prebiotic-like” effect, promoting the growth of beneficial microorganisms such as Akkermansia spp. and Faecalibacterium spp. Given the relevance of polyphenols/gut microbiota interactions in health, different gut microbial ecologies can lead to various health effects after polyphenol intake [24].
The present systematic review intends to critically investigate in depth the recent scientific evidence and to present any potential link between diet and functional gastrointestinal disorders. In addition, this review explores the possible efficacy of probiotics and phytochemicals in FGIDs treatment. A further understanding of the interaction between probiotics and gut microbiota is of high importance in order for nutritional policies and nutritional education to be enhanced.
2. Results
Recent studies have investigated the effect of probiotic and phytochemical consumption on human health. These bioactive compounds seem to play an important role in the regulation of gut microbiota and, more specifically, in regard to the Bacteroidetes and Firmicute concentration ratio. Therefore, patients with FGIDs, including irritable bowel syndrome, functional dyspepsia, and functional constipation, can benefit from the consumption of these bioactive compounds to treat and reduce their symptoms (Figure 1).
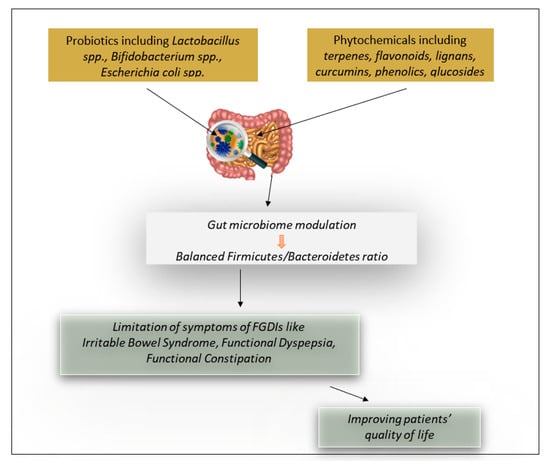
Figure 1.
The effect of probiotics and phytochemicals on functional gastrointestinal disorders (FGIDs).
2.1. Irritable Bowel Syndrome (IBS)
Irritable Bowel Syndrome (IBS) is a common gastrointestinal condition characterized by chronic or recurrent abdominal pain associated with altered bowel habits. Despite the high prevalence of IBS, which is currently estimated at 5–20% of the population [25], the exact pathophysiology of IBS is not clear [26]. According to the updated Rome III criteria, IBS is a clinical diagnosis and presents as one of the three predominant subtypes: (1) IBS with constipation (IBS-C); (2) IBS with diarrhea (IBS-D); and (3) mixed IBS (IBS-M) Former ROME definitions referred to IBS-M as alternating IBS (IBS-A). Across these IBS subtypes, the presentation of symptoms may vary among patients and change over time. Patients report that the most distressing symptoms are abdominal pain, straining, myalgias, urgency, bloating, and feelings of serious illness [27]. Treatments that target alterations in gut microbiota may be beneficial for patients with IBS [28]. Effective pharmacological therapies for IBS exist, but the duration of most treatment trials is less than 6 months, and so the long-term efficacy of these therapies is unclear [29,30].
Dietary habits and bioactive compounds of several foods may have a positive effect on the composition of human gut microbiota. A class of bioactive compounds that are associated with the treatment of intestinal diseases are probiotics. In particular, probiotics are possible therapeutic factors associated with modification of the gut microbiome for the alleviation of IBS symptoms [31]. As presented in Table 1, numerous studies aimed to investigate the association between probiotics consumption and irritable bowel syndrome.

Table 1.
Studies that summarize the efficacy of probiotics in treating irritable bowel syndrome.
2.1.1. Efficacy of Probiotics in Irritable Bowel Syndrome
A systematic review and meta-analysis of random clinical trials examined 23 randomized controlled trials to understand whether probiotics are effective therapies for IBS global symptoms. According to the inclusion criteria of the study, the duration of therapy was at least 7 days and the diagnosis of IBS was based on either a physician’s opinion or symptoms-based diagnostic criteria, supplemented by the results of investigations to exclude organic disease. Subjects were required to be followed up for at least one week. Nineteen trials used a combination of probiotics, eight trials used Lactobacillus, three trials used Bifidobacterium, two trials used E. coli, one trial used Streptococcus, one trial used Saccharomyces, and one trial used either Lactobacillus or Bifidobacterium. In the majority of the trials, probiotics were administered in the form of a capsule. In others, beverages enriched with probiotic strains were used. Specifically, several studies used rose hip drink, fruit drink, malted drink, fermented milk, or a milk- based drink. According to the results, probiotics had beneficial effects on global IBS symptoms (abdominal pain, bloating, and flatulence), or exclusively on abdominal pain, so they could be characterized as effective treatments for IBS. However the, individual species and strains that are the most beneficial remain unclear [32].
Results from a second meta-analysis were in accordance with the above analysis. This second systematic review and meta-analysis demonstrated that combinations of probiotics, or specific species and strains of probiotics, appear to have beneficial effects in IBS in terms of their effects on global IBS symptoms and abdominal pain. Specifically, the seven-strain combination of three Bifidobacterium, three Lactobacillus, and one Streptococcus were associated with significant improvements in global symptoms. Among individual probiotics, Lactobacillus plantarum, Escherichia coli, and Streptococcus faecium also had beneficial effects on global symptoms. The probiotics in the included studies were administered in the form of a drug.
Moreover, in this meta-analysis, randomized placebo-controlled trials examining the effect of at least 7 days of probiotics in adult patients (over the age of 16 years) with IBS were eligible for inclusion. The diagnosis of IBS, based on either a physician’s opinion or symptoms-based diagnostic criteria, were supplemented by the results of investigations to exclude organic disease, where the studies deemed this necessary. Subjects were required to be followed up for at least 1 week [33].
Another interesting meta-analysis based on 15 independent studies came to similar conclusions. The fifteen selected trials included the use of the criteria of Rome II, Rome III, the International Classification of Health Problems in Primary Care, and the World Organization of Family Doctors. The quality score of the included trials were assessed and reported according to Jadad quality scoring and all trials had high quality, with scores ranging from 3 to 5. A total of 1793 patients with diarrhea-predominant IBS (D-IBS), constipation-predominant IBS (C-IBS), and alternative IBS (A-IBS) were included. The probiotics in the included studies were administered in the form of a drug. According to the results, probiotics reduced the pain and symptoms of IBS disease. As in the above studies, there were results that reflected improvement in global symptoms, or exclusively in bloating or flatulence or the stability of intestinal microbiota. Moreover, the results demonstrated the beneficial effects of probiotics for IBS patients, in relation to pain assessment analyses that showed that probiotics significantly reduced pain severity after 8 weeks and 10 weeks of administration. However, the reduction rate was rather higher at week 8 than week 10, suggesting reduced effectiveness with the long-term consumption of probiotics [34].
Another meta-analysis compared the efficacy of a single probiotic, Bifidobacterium infantis 35624, to the efficacy of a probiotic blend in patients with irritable bowel syndrome. This study included randomized controlled trials that used the Rome criteria I, II, or III for the diagnosis of IBS, and engaged adult participants. Moreover, the subjects had to be followed up for more than 1 week. The probiotics in the included studies were administered in the form of a drug and the duration of the studies was 4 weeks or 8 weeks. According to the results, treatment with the single probiotic B. infantis did not impact abdominal pain, bloating/distention, or bowel habit satisfaction among IBS patients. However, patients who received composite probiotics containing B. infantis experienced significantly reduced abdominal pain and bloating/distention. These data led to the conclusion that composite probiotics containing B. infantis might be an effective therapeutic option for IBS patients, significantly alleviating the symptoms of IBS without significant adverse effects [35].
Another systematic review accorded with the above results. The search identified 35 studies, of which 11 met the inclusion criteria and were included in the systematic review. Patients were both males and females, except for one study that examined only female participants. According to the inclusion criteria, studies had to be double- or triple-blinded and the diagnosis of IBS was based on the Rome III or Rome IV criteria. The probiotics in the included studies were given in the form of a drug and the examining period ranged from 60 days to 16 weeks. According to the results, seven studies (63.6%) reported that supplementation with probiotics for IBS patients, compared to a placebo, significantly improved symptoms, while the remaining four studies (36.4%) did not report any significant improvement in symptoms after probiotic supplementation. Of note, three of the trials evaluated the effect of a monostrain supplement, while the remaining eight trials used a multistrain probiotic. Overall, the beneficial effects were more distinct in the trials using multistrain supplements, with an intervention of 8 weeks or more, suggesting that multistrain probiotics supplemented over a period of time have the potential to improve IBS symptoms [36].
Finally, a randomized, placebo-controlled study focused on the efficacy of the strains Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in alleviating the symptoms of irritable bowel syndrome. This study included a 2-week run-in period, during which regular bowel habits were reported, and an 8-week intervention period, consisting of a total of four clinic visits. Participants were aged 18 years or older and were IBS-diagnosed according to the Rome III criteria. The probiotics in the included studies were administered in the form of a capsule. Each probiotic capsule contained 10 × 109 colony forming units (CFU) of either freeze-dried B. longum or L. paracasei, with potato starch and magnesium stearate as excipients. The placebo contained only potato starch and magnesium stearate. Both L. paracasei and B. longum supplementation improved the subjects’ quality of life in terms of emotional well-being and social functioning. In conclusion, L. paracasei and B. longum may reduce the severity of GI symptoms and improve the psychological well-being of individuals with certain IBS subtypes [37].
2.1.2. Efficacy of Phytochemicals in Treating Irritable Bowel Syndrome
A meta-analysis of collected data from twenty-three randomized controlled trials examined the efficacy and safety of biophenol-rich nutraceuticals in adults with irritable bowel syndrome. Participants in the selected studies were human adults aged ≥18 years with IBS. The types of studies were parallel, or crossover randomized controlled trials (RCTs) and the studies were included if the participants in the intervention group were treated with an orally consumed biophenol-rich nutraceutical with no coadministration of any test product or therapy beyond standard care for more than 1-week. In most of the studies, biophenol-rich nutraceuticals were administered in the form of a capsule. Other studies used aloe vera gel, natural mastiha tablets, fresh juice, or blended essential oils. The studies lasted from 2 to 12 weeks. According to the results, biophenol-rich nutraceuticals may be an effective and safe adjuvant treatment for the management of IBS, with higher certainty of evidence for peppermint oil [38], as further discussed below.
A meta-analysis that specifically examined the efficacy and safety of peppermint oil in patients with IBS was in accordance with the above results. Randomized placebo-controlled trials with a minimum treatment duration of 2 weeks were included in this meta-analysis. Patients had to be IBS-diagnosed according to the criteria of Rome I or II. Specifically, nine studies that evaluated 726 patients were identified and peppermint oil was found to be significantly superior to the placebo for global improvement of IBS symptoms and improvement of abdominal pain. Although peppermint oil patients were significantly more likely to experience an adverse event, such events were mild and transient in nature. The most reported adverse event was heartburn. It is concluded that peppermint oil is a safe and effective short-term treatment for IBS [39].
2.2. Functional Dyspepsia (FD)
Functional dyspepsia is one of the most prevalent functional gastrointestinal disorders. Functional dyspepsia comprises three subtypes with presumed different pathophysiologies and etiologies: postprandial distress syndrome (PDS), epigastric pain syndrome (EPS), and a subtype with overlapping PDS and EPS features [40]. Functional dyspepsia affects up to 16% of otherwise healthy individuals in the general population. The pathophysiology remains incompletely understood, but it is probably related to disordered communication between the gut and the brain, leading to motility disturbances, visceral hypersensitivity, and alterations in gastrointestinal microbiota, mucosal and immune function, and CNS processing. A genetic predisposition is probable but less evident than in other functional gastrointestinal disorders, such as irritable bowel syndrome (IBS) [41].
Eating behaviors, irregular meal patterns, and moderate-to-fast eating rates are significantly associated with functional dyspepsia [42]. Many patients recognize meals as the main triggering factor; thus, dietary manipulations often represent the first-line management strategy in this cohort of patients. Nonetheless, little quality evidence has been produced regarding the relationship between specific foods and/or macronutrients and the onset of FD symptoms, resulting in non-standardized nutritional approaches. As a result, most dietary advices are empirical and often lead to exclusion diets [43]. The role of probiotics and phytochemicals in the management of FD is currently unclear, due to the small number of RCTs available to assess the efficacy and safety of those bioactive compounds in patients with FD. However, the role of probiotics and phytochemicals is suggested as a way of treatment, due to the positive effect they may have on gut microbiota.
2.2.1. Efficacy of Probiotics in Functional Dyspepsia
Clinical trials have been implemented to study the efficacy of probiotics in functional dyspepsia (Table 2). A recent single-centered study, with a randomized, double-blind, placebo-controlled, and parallel-group design with open-label extension, showed that Bacillus coagulans MY01 and Bacillus subtilis MY02 spore-forming probiotics were efficacious and safe in the treatment of functional dyspepsia. In this exploratory study, scientists showed the efficacy and safety of capsules with B coagulans MY01 and B subtilis MY02 spore-forming probiotics in patients with functional dyspepsia, compared with a placebo, consumed twice a day for 8 weeks. Specifically, reduced PDS scores were noted for patients with functional dyspepsia, using probiotics versus a placebo. The effects of probiotics on PDS and EPS symptoms were corroborated for the key individual symptoms of the daily diary, compared with a placebo. Moreover, spore-forming probiotics reduced the proportion of positive glycocholic acid breath tests in patients with functional dyspepsia who were on proton-pump inhibitors, suggesting a reduction of small intestinal bacterial overgrowth. Finally, treatment with spore-forming probiotics was well tolerated. Despite the high prevalence of functional dyspepsia, participants experienced potentially beneficial immune and microbial changes, which could provide insights into possible underlying mechanisms as future predictors or treatment targets [44].

Table 2.
Studies that summarize the efficacy of probiotics in treating functional dyspepsia.
A meta-analysis study examined data for 400 patients with functional dyspepsia who participated to clinical trials. The probiotic group had an improvement in functional dyspepsia symptoms compared to the placebo group. As was stated in the first study, probiotics seemed to be effective treatments for FD. However, the individual species and strains that are most beneficial remained unclear [45].
Moreover, a pilot study was conducted in order to examine the role of probiotics in the treatment of functional dyspepsia. It was a small-scale preliminary study that lasted 7 days. Eight subjects with functional dyspepsia were examined. According to the protocol, the participants had to include in their diets extra-virgin oil, enriched with probiotics. Despite the short period of consumption of the enriched olive oil, a significant improvement in dyspeptic symptoms was observed in subjects receiving it [46].
2.2.2. Efficacy of Phytochemicals in Treating Functional Dyspepsia
Several studies aimed to investigate the association between phytochemicals consumption and functional dyspepsia. The latter pilot study also examined the role of probiotics in the treatment of functional dyspepsia using extra-virgin oil enriched with antioxidants. In this case, the results were encouraging. However larger studies could better elucidate the role of antioxidants in treating functional dyspepsia [46].
Another randomized, double-blind, and placebo-controlled study examined the efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection. Patients with functional dyspepsia were divided into three groups, with 44 individuals in each group (placebo, 16 mg, or 40 mg astaxanthin, respectively). In general, no curative effect of astaxanthin was found in functional dyspepsia patients. A significantly greater reduction of reflux symptoms was detected in patients treated with the highest dose of the natural antioxidant astaxanthin. The response was more pronounced in H. pylori-infected patients [47].
2.3. Functional Constipation (FC)
Constipation is a heterogeneous, polysymptomatic, multifactorial disease that has several subtypes, such as acute or transient constipation, slow-transit constipation, and functional constipation [48]. Functional constipation (FC) is a very common disorder in children and adults, for which the resolution of symptoms with the currently available therapies is difficult to achieve. The symptoms of the disease vary, but mainly comprise a group of functional disorders that present difficulties in defecation. These disorders include infrequent bowel movements or seemingly incomplete evacuation, hard or lumpy stools, excessive straining, sensation of incomplete evacuation or blockage, and, in some instances, the use of manual maneuvers to facilitate evacuation. In recent years, attention has focused on the use of probiotics, which have proven to be effective in the management of various gastrointestinal disorders [49].
2.3.1. Efficacy of Probiotics in Treating Functional Constipation
Table 3 summarizes the recent data from clinical trials on the effect of probiotics in treating functional constipation. A randomized clinical trial studied the efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation. Twenty constipated patients participated in the study and each patient was required to consume 180 g per day of ordinary artichokes, or artichokes enriched with Lactobacillus paracasei IMPC 2.1, for 15 days (daily dose of 2 × 1010 CFU). This trial showed a positive effect on symptoms in constipated patients after an intake of probiotic-enriched artichokes [50].

Table 3.
Studies that summarize the efficacy of probiotics in treating functional constipation.
A study in a larger population took place some years later and agreed with the above results. In this randomized, double-blind, placebo-controlled study, the participants were 180 patients aged 18 to 75, who were required to consume chocolate enriched with probiotics (3.0 × 108 CFU/g Streptococcus thermophilus MG510 and 1.0 × 108 CFU/g Lactobacillus plantarum LRCC5193) or a placebo, daily for 4 weeks, and who were followed up for a 4-week washout period without intervention. According to the results, probiotics significantly ameliorated stool consistency in patients with chronic constipation. Moreover, the beneficial effect of L. plantarum on stool consistency remained after the probiotic supplementation was discontinued [51].
Another randomized controlled trial investigated the clinical efficacy of a multi-strain probiotic product on bowel habits and on the microbial profile in participants with functional constipation. The participants received a placebo or the probiotic product (1.5 × 1010 CFU/day), consisting of Lactobacillus acidophilus DDS-1, Bifidobacterium animalis subsp. lactis UABla-12, Bifidobacterium longum UABl-14 and Bifidobacterium bifidum UABb-10 over 4 weeks. According to the results, the probiotic helped to modulate bowel function earlier than the placebo. Specifically, a normalization of stool frequency and consistency was observed, with most participants achieving a normalized profile after 1 week. However, no significant differences were observed in symptomology [52].
A meta-analysis of randomized controlled trials that examined the effect of probiotics on functional constipation in adults was in accordance with the above results. That study examined 14 records (1182 patients), and the participants were adult populations with functional chronic constipation as defined by clinical symptoms, a physician’s opinion, or the Rome I, II, or III criteria. Probiotics were administered in tablet, powder, capsule, softgel, or fortified food forms and the examining period ranged from 14 days to 8 weeks. According to the results, the probiotics significantly reduced whole gut transit time and increased stool frequency, and this was significant for Bifidobacterium lactis but not for Lactobacillus casei Shirota. Moreover, the probiotics improved stool consistency, and this was significant for Bifidobacterium lactis but not for L. casei Shirota [53].
Finally, several studies that used a probiotic blend or individual probiotic strains did not indicate a difference in symptomology or stool consistency between those who consumed the above probiotics and the controls. After a post hoc analysis, all studies showed improved patient outcomes after specific periods of probiotic consumption [54,55,56].
2.3.2. Efficacy of Phytochemicals in Treating Functional Constipation
A randomized controlled trial examined how the polyphenol-rich mango (Mangifera indica L.) ameliorates functional constipation symptoms in adults, beyond the equivalent amount of fiber. The 4-week consumption of mango fruit (300 g), or the equivalent amount of fiber, was investigated in otherwise healthy human volunteers with chronic constipation, who were randomly assigned to either group. Blood and fecal samples and digestive wellness questionnaires were collected at the beginning and end of the study. As presented in Table 4, the results showed that mango consumption significantly improved constipation status (stool frequency, consistency, and shape) and increased gastrin levels and fecal concentrations of short chain fatty acid (valeric acid), while lowering endotoxin and interleukin 6 concentrations in plasma [57].

Table 4.
Studies that summarize the efficacy of phytochemicals in treating irritable bowel syndrome, functional dyspepsia, and functional constipation.
Unfortunately, no additional studies have been found that examine the role of polyphenols or phytochemicals in treating functional constipation, and that meet the inclusion criteria.
3. Discussion
According to the above revised human studies, the present review clarifies the correlation between diet and gastrointestinal malfunctions and, in particular, the possible beneficial effects that bioactive compounds, such as probiotics and phytochemicals, may have in the treatment of functional gastrointestinal disorders. Specifically, the present study, summarizing the recent literature, provides significant information and identifies microorganisms whose synergy can lead to the effective control of FGIDs. In addition, cases of foods enriched with phytochemicals are equally helpful in leading to conclusions about the association of bioactive compounds with gastrointestinal diseases.
It has become increasingly evident in recent years that gut microbiome and the brain communicate in a bidirectional manner, with each possibly affecting the other’s functions [58]. FGIDs are thought to result from the interaction of altered gut physiology and psychological factors via the gut-brain axis, where brain and gut symptoms reciprocally influence each other’s expression [59]. Complex mechanisms underlying the disturbances in the bidirectional communication between the gastrointestinal tract and the brain have a vital role in pathogenesis and are key to our understanding of the disease phenomenon [60]. In order to understand these complex disorders, scientific data was collected with emphasis on clinical trials. However, more and larger scale human studies are required to identify the possible therapeutic targets of FGIDs. This systematic review study examined the possible treatment of FGIDs through enriched foods or supplements high in probiotics or phytochemical compounds, without underlying molecular mechanisms that may have a role in the pathogenesis of functional gastrointestinal disorders.
Irritable bowel syndrome is directly related to gut microbiota and studies have shown differences in the composition of gut microbiota when comparing IBS patients with healthy control subjects [61,62,63]. Moreover, some studies confirmed that IBS is associated with a decrease in the stability and biodiversity of gut microbiota [64,65]. For these reasons, methods of treatment focus on the effect and differentiation of the intestinal microbiota. One proposed method is fecal microbiota transplantation, which has the goals of reducing IBS symptoms 3 months after FMT, reducing the dysbiosis index, and changing the intestinal bacterial profile [66]. Other well-known methods are dietary education with a low fermentable oligosaccharide, disaccharide, and polyol (FODMAP) diet [67] and increasing physical activity in order to improve GI symptoms in IBS [68]. Treatment of IBS with enriched functional foods has not been more widely established, but is a method that seems to be effective. As shown in the above studies, combinations of probiotic strains have been shown to be quite effective in reducing symptoms. The main strains that have been studied, either individually or in synergy, are Lactobacillus, Bifidobacterium, Escherichia, Saccharomyces, and Streptococcus.
Polyphenols may also have a therapeutic benefit in treating irritable bowel syndrome. They might be applicable in preventing IBS and improving IBS symptoms, mainly through suppressing the inflammatory signaling pathways, which nowadays are known as a novel platform for IBS management [69]. The data from clinical trials are very limited; hence, many large-scale clinical trials, such as epidemiological studies, should be performed to confirm the gastroprotective activity of polyphenols and their metabolites before making recommendations.
Functional dyspepsia, consisting of epigastric pain syndrome and postprandial distress syndrome, is a prevalent functional gastrointestinal disorder. Currently, only limited treatment options are available and conflicting results have been reported in regard to efficacy [70]. According to the recent American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) guidelines for dyspepsia, patients with Helicobacter pylori (H. pylori) need to eradicate dyspepsia as a first treatment option [71]. On the other hand, prokinetic agents, as therapy for FD, showed significant efficacy in several clinical trials performed either in Japan or Europe [72]. Recent topics also propose acupuncture [73], electrical stimulation [74], gastric peroral endoscopic myotomy [75,76,77], and meal and lifestyle modification [78,79], as non-pharmacological treatments.
From the bibliographic data, it follows that combination of pharmacological and non-pharmacological treatment options are anticipated for functional dyspepsia. Consequently, non-pharmacological treatment options are increasingly being explored. In this context, several studies have demonstrated differences in the commensal bacterial community between patients with FD and healthy control subjects, while other studies have shown that intestinal dysbiosis might be associated with the severity of the disease’s symptoms [80]. According to several randomized controlled trials, probiotics and antioxidants may have a beneficial effect in reducing the severity of symptoms and improving the intestinal microbiota in patients with functional dyspepsia. A larger number of studies, and mainly clinical studies based on enriched foods and not on dietary supplements, is necessary. The aim of further studies will be to obtain safe data about the use of dietary probiotics, antioxidants, or broader phytochemicals in functional foods, in order to treat functional dyspepsia in a non-pharmacοlogical way.
Constipation is a common functional problem of the digestive system and may occur secondary to diet, drugs, endocrine diseases, metabolic diseases, neurological diseases, psychiatric disorders, or gastrointestinal obstruction. When there is no secondary cause, constipation is diagnosed as functional constipation. The first steps that should be taken to relieve symptoms are diet and lifestyle modifications; if those steps are unsuccessful, laxative therapy should be initiated [81]. Recent research into intestinal diseases and gut microbiota has gradually revealed a connection between constipation and the disturbance of intestinal flora, providing a theoretical basis for microbial treatment for chronic constipation. Microbial treatment mainly includes probiotic preparations such as probiotics, prebiotics, symbiotics, and fecal microbiota transplantation (FMT) [82]. The efficacy of probiotics in improving clinical symptoms, improving stool frequency and consistency, and altering fecal microbiota was investigated in many clinical trials with patients with functional constipation. Selected strains of Lactobacillus and Bifidobacterium have been studied in enriched foods and it has been shown that they are able to ameliorate constipation-related symptoms. Scientific data on the association between polyphenols or phytochemicals and functional dyspepsia are still limited. However, there is evidence of a positive effect of polyphenols on the constipation status of patients.
Adherence to a healthy diet, such as the Mediterranean diet, may be beneficial for microbial gut composition, with a possible preventive effect on the pathophysiology of gastrointestinal diseases [78]. In addition, the development of functional foods enriched with bioactive compounds, such as probiotics and phytochemicals, could be a novel approach that may contribute, as part of a balanced diet, to the improvement of biochemical indices that correlate with chronic diseases [79].
4. Methods
The following databases were searched: MEDLINE (PubMed), ScienceDirect, and Google Scholar. Combinations of specific keywords, such as “irritable bowel syndrome” or “functional dyspepsia” or “functional constipation”, and “probiotics” or “phytochemicals”, were used. This review was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [83].
The final revised selection of the articles was performed by specific inclusion criteria, including (a) recent articles published mainly in the last decade, (b) articles that presented human clinical trials and epidemiological studies (observational, cross-sectional, and cohort), meta-analyses and analytical studies, and (c) studies that aimed to evaluate diet-based interventions and to profile the fecal microbiome of each study sample. Human studies with non-reliable study designs or inaccurate findings were excluded from the present systematic review, so that valid research data were cited.
As presented in Figure 2, from an initial total of 1080 studies that were retrieved, 167 were selected. Finally, 32 articles were used for the revision process, according to the above inclusion criteria.
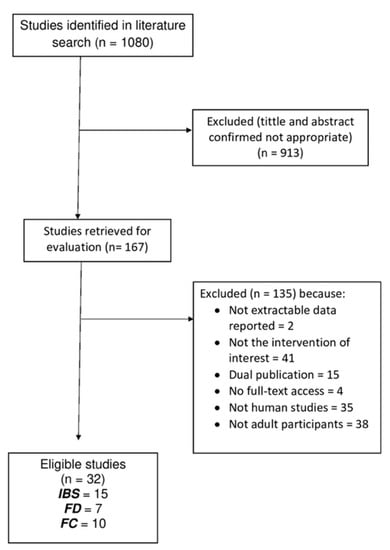
Figure 2.
Flow diagram of studies evaluated in the review.
5. Conclusions
It is clear from the above literature review that the relationship between probiotics and phytochemicals and gut microbiota and the treatment of gastrointestinal disorders is of great interest. The data show a correlation between the consumption of the above bioactive compounds and the relief of symptoms in selected gastrointestinal disorders. Although there are numerous studies on the effect of probiotics and phytochemicals on FGIDs, the mechanisms of action of these bioactive compounds are not yet known. There is a lack of knowledge about the potential synergy of bioactive compounds aimed at preventing or treating gastrointestinal disorders. More specialized studies on the mechanisms of action would be very helpful for future innovations in the field of food science. Moreover, most studies examined the use of a capsule containing the above compounds, while studies examining enriched foods are limited. Clinical studies examining the role of foods enriched with probiotics and phytochemicals in the treatment of gastrointestinal disorders present a future challenge. Enriching foods that are regularly consumed, such as fruit juices, yogurt, and cheese, with different strains of probiotics and different sources of phytochemicals that may have a positive effect on gut microbiota and on gastrointestinal disorders, could be a new goal. The above innovations need to be supported by a number of clinical and epidemiological studies in order to draw valid and safe conclusions about the role of functional foods in the treatment of gastrointestinal disorders.
Funding
This research is part of the project, “Infrastructure of Microbiome Applications in Food Systems-FoodBiomes”, which is co-funded by the European Regional Development Fund (ERDF), under the Operational Program “Competitiveness, Entrepreneurship and Innovation-EPANEK 2014–2020”, Call 111 “Support for Regional Excellence”.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Maria Kapsokefalou for useful discussions and comments on the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Young, V.B. The Role of the Microbiome in Human Health and Disease: An Introduction for Clinicians. BMJ 2017, 356, j831. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An Ecological and Evolutionary Perspective on Human-Microbe Mutualism and Disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- Mondot, S.; de Wouters, T.; Doré, J.; Lepage, P. The Human Gut Microbiome and Its Dysfunctions. Dig. Dis. 2013, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The Role of Diet on Gut Microbiota Composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Functional Gastrointestinal Disorders: Advances in Understanding and Management—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33049221/ (accessed on 15 December 2021).
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35 (Suppl. 1), 5–13. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of Functional Food Components on Gut Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Hosseini, A.; Nikfar, S.; Abdollahi, M. Are Probiotics Effective in Management of Irritable Bowel Syndrome? Arch. Med. Sci. 2012, 8, 403–405. [Google Scholar] [CrossRef]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and Probiotic Overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, Examples, and Specific Applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizock, B.A. Probiotics. Dis. Mon. 2015, 61, 259–290. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U. Clinical Uses of Probiotics. Medicine 2016, 95, e2658. [Google Scholar] [CrossRef]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Phys. 2017, 96, 170–178. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J. Phytochemicals: Guardians of Our Health. J. Am. Diet Assoc. 1997, 97, S199–S204. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Forsch. Komplementmed. 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Probst, Y.; Guan, V.; Kent, K. Dietary Phytochemical Intake from Foods and Health Outcomes: A Systematic Review Protocol and Preliminary Scoping. BMJ Open 2017, 7, e013337. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and Their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of Gut Microbiota with Dietary Polyphenols and Consequences to Human Health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of Gut Microbiota with Functional Food Components and Nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Saha, L. Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence-Based Medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Sultan Qaboos Univ. Med. J. 2020, 20, e13–e24. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Talley, N.J.; Spiegel, B.M.R.; Foxx-Orenstein, A.E.; Schiller, L.; Quigley, E.M.M.; Moayyedi, P. Effect of Fibre, Antispasmodics, and Peppermint Oil in the Treatment of Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. BMJ 2008, 337, a2313. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Talley, N.J.; Schoenfeld, P.S.; Quigley, E.M.M.; Moayyedi, P. Efficacy of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. Gut 2009, 58, 367–378. [Google Scholar] [CrossRef]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, Prebiotics, and Low FODMAP Diet for Irritable Bowel Syndrome—What Is the Current Evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic Review with Meta-Analysis: The Efficacy of Prebiotics, Probiotics, Synbiotics and Antibiotics in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of Probiotics in Irritable Bowel Syndrome: Updated Systematic Review with Meta-Analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Ni, H.; Asche, C.V.; Kim, M.; Walayat, S.; Ren, J. Efficacy of Bifidobacterium Infantis 35624 in Patients with Irritable Bowel Syndrome: A Meta-Analysis. Curr. Med. Res. Opin. 2017, 33, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Rasmussen, S.H.; Asiller, Ö.Ö.; Lied, G.A. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus Paracasei HA-196 and Bifidobacterium Longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 1159. [Google Scholar] [CrossRef] [Green Version]
- Giang, J.; Lan, X.; Crichton, M.; Marx, W.; Marshall, S. Efficacy and Safety of Biophenol-Rich Nutraceuticals in Adults with Inflammatory Gastrointestinal Diseases or Irritable Bowel Syndrome: A Systematic Literature Review and Meta-Analysis. Nutr. Diet 2021, 1–18. [Google Scholar] [CrossRef]
- Khanna, R.; MacDonald, J.K.; Levesque, B.G. Peppermint Oil for the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2014, 48, 505–512. [Google Scholar] [CrossRef]
- Enck, P.; Azpiroz, F.; Boeckxstaens, G.; Elsenbruch, S.; Feinle-Bisset, C.; Holtmann, G.; Lackner, J.M.; Ronkainen, J.; Schemann, M.; Stengel, A.; et al. Functional Dyspepsia. Nat. Rev. Dis. Primers 2017, 3, 17081. [Google Scholar] [CrossRef]
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional Dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef]
- Duboc, H.; Latrache, S.; Nebunu, N.; Coffin, B. The Role of Diet in Functional Dyspepsia Management. Front. Psychiatry 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and Functional Dyspepsia: Clinical Correlates and Therapeutic Perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Slaets, H.; De Paepe, K.; Ceulemans, M.; Wetzels, S.; Geboers, K.; Toth, J.; Thys, W.; Dybajlo, R.; Walgraeve, D.; et al. Efficacy and Safety of Spore-Forming Probiotics in the Treatment of Functional Dyspepsia: A Pilot Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 784–792. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.M.; Wang, X.; Xie, J.; Li, X.; Ma, J.; Wang, F.; Tang, X. Efficacy of Prebiotics and Probiotics for Functional Dyspepsia: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19107. [Google Scholar] [CrossRef]
- Ianiro, G.; Pizzoferrato, M.; Franceschi, F.; Tarullo, A.; Luisi, T.; Gasbarrini, G. Effect of an Extra-Virgin Olive Oil Enriched with Probiotics or Antioxidants on Functional Dyspepsia: A Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2085–2090. [Google Scholar]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the Natural Antioxidant Astaxanthin in the Treatment of Functional Dyspepsia in Patients with or without Helicobacter Pylori Infection: A Prospective, Randomized, Double Blind, and Placebo-Controlled Study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Rattanakovit, K.; Patcharatrakul, T. Diagnosis and Management of Chronic Constipation in Adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. [Google Scholar] [CrossRef]
- Scarpato, E.; Coppola, V.; Staiano, A. Probiotics and Applications to Constipation; Academic Press: Cambridge, MA, USA, 2019; pp. 193–196. ISBN 978-0-12-814468-8. [Google Scholar]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; De Candia, S.; Russo, F. Randomised Clinical Trial: Efficacy of Lactobacillus Paracasei-Enriched Artichokes in the Treatment of Patients with Functional Constipation--a Double-Blind, Controlled, Crossover Study. Aliment. Pharmacol. Ther. 2012, 35, 441–450. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cha, J.M.; Oh, J.K.; Tan, P.L.; Kim, S.H.; Kwak, M.S.; Jeon, J.W.; Shin, H.P. Probiotics Ameliorate Stool Consistency in Patients with Chronic Constipation: A Randomized, Double-Blind, Placebo-Controlled Study. Dig. Dis. Sci. 2018, 63, 2754–2764. [Google Scholar] [CrossRef]
- Martoni, C.J.; Evans, M.; Chow, C.-E.T.; Chan, L.S.; Leyer, G. Impact of a Probiotic Product on Bowel Habits and Microbial Profile in Participants with Functional Constipation: A Randomized Controlled Trial. J. Dig. Dis. 2019, 20, 435–446. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The Effect of Probiotics on Functional Constipation in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Mazlyn, M.M.; Nagarajah, L.H.-L.; Fatimah, A.; Norimah, A.K.; Goh, K.-L. Effects of a Probiotic Fermented Milk on Functional Constipation: A Randomized, Double-Blind, Placebo-Controlled Study. J. Gastroenterol. Hepatol. 2013, 28, 1141–1147. [Google Scholar] [CrossRef]
- Ibarra, A.; Latreille-Barbier, M.; Donazzolo, Y.; Pelletier, X.; Ouwehand, A.C. Effects of 28-Day Bifidobacterium Animalis Subsp. Lactis HN019 Supplementation on Colonic Transit Time and Gastrointestinal Symptoms in Adults with Functional Constipation: A Double-Blind, Randomized, Placebo-Controlled, and Dose-Ranging Trial. Gut Microbes 2018, 9, 236–251. [Google Scholar] [CrossRef] [Green Version]
- Airaksinen, K.; Yeung, N.; Lyra, A.; Lahtinen, S.J.; Huttunen, T.; Shanahan, F.; Ouwehand, A.C. The Effect of a Probiotic Blend on Gastrointestinal Symptoms in Constipated Patients: A Double Blind, Randomised, Placebo Controlled 2-Week Trial. Benef. Microbes 2019, 10, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Venancio, V.P.; Kim, H.; Sirven, M.A.; Tekwe, C.D.; Honvoh, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenol-Rich Mango (Mangifera indica L.) Ameliorate Functional Constipation Symptoms in Humans beyond Equivalent Amount of Fiber. Mol. Nutr. Food Res. 2018, 62, e1701034. [Google Scholar] [CrossRef] [PubMed]
- Relationship between the Gut Microbiome and Brain Function—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29701810/ (accessed on 15 December 2021).
- De Palma, G.; Collins, S.M.; Bercik, P. The Microbiota-Gut-Brain Axis in Functional Gastrointestinal Disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional Gastrointestinal Disorders and Gut-Brain Axis: What Does the Future Hold? World J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An Irritable Bowel Syndrome Subtype Defined by Species-Specific Alterations in Faecal Microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional Dysbiosis within the Gut Microbiota of Patients with Constipated-Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Shukla, R.; Ghoshal, U.; Dhole, T.N.; Ghoshal, U.C. Fecal Microbiota in Patients with Irritable Bowel Syndrome Compared with Healthy Controls Using Real-Time Polymerase Chain Reaction: An Evidence of Dysbiosis. Dig. Dis. Sci. 2015, 60, 2953–2962. [Google Scholar] [CrossRef]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Artacho, A.; Garrigues, V.; Ortiz, V.; Ponce, J.; Latorre, A.; Moya, A. Instability of the Faecal Microbiota in Diarrhoea-Predominant Irritable Bowel Syndrome. FEMS Microbiol. Ecol. 2013, 86, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Salgado, P.; Ponce, M.; Ponce, J.; Garrigues, V.; Latorre, A.; Moya, A. Structural Alterations of Faecal and Mucosa-Associated Bacterial Communities in Irritable Bowel Syndrome. Environ. Microbiol. Rep. 2012, 4, 242–247. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-Term Irritable Bowel Syndrome Symptom Control with Reintroduction of Selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef]
- Physical Activity Improves Symptoms in Irritable Bowel Syndrome: A Randomized Controlled Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21206488/ (accessed on 15 December 2021).
- Natural Polyphenols for the Prevention of Irritable Bowel Syndrome: Molecular Mechanisms and Targets; a Comprehensive Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31273572/ (accessed on 15 December 2021).
- Masuy, I.; Van Oudenhove, L.; Tack, J. Review Article: Treatment Options for Functional Dyspepsia. Aliment. Pharmacol. Ther. 2019, 49, 1134–1172. [Google Scholar] [CrossRef] [Green Version]
- Moayyedi, P. Helicobacter Pylori Eradication for Functional Dyspepsia: What Are We Treating?: Comment on “Helicobacter Pylori Eradication in Functional Dyspepsia”. Arch. Intern. Med. 2011, 171, 1936–1937. [Google Scholar] [CrossRef]
- Tomita, T.; Oshima, T.; Miwa, H. New Approaches to Diagnosis and Treatment of Functional Dyspepsia. Curr. Gastroenterol. Rep. 2018, 20, 55. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, J.; Sun, X.; Zeng, F.; Li, Y.; Wu, X.; Li, J.; Zhao, L.; Chang, X.-R.; Liu, M.; et al. Electroacupuncture for Patients with Refractory Functional Dyspepsia: A Randomized Controlled Trial. Neurogastroenterol. Motil. 2018, 30, e13316. [Google Scholar] [CrossRef]
- Davis, B.R.; Sarosiek, I.; Bashashati, M.; Alvarado, B.; McCallum, R.W. The Long-Term Efficacy and Safety of Pyloroplasty Combined with Gastric Electrical Stimulation Therapy in Gastroparesis. J. Gastrointest. Surg. 2017, 21, 222–227. [Google Scholar] [CrossRef]
- Malik, Z.; Kataria, R.; Modayil, R.; Ehrlich, A.C.; Schey, R.; Parkman, H.P.; Stavropoulos, S.N. Gastric Per Oral Endoscopic Myotomy (G-POEM) for the Treatment of Refractory Gastroparesis: Early Experience. Dig. Dis. Sci. 2018, 63, 2405–2412. [Google Scholar] [CrossRef]
- Xue, H.B.; Fan, H.Z.; Meng, X.M.; Cristofaro, S.; Mekaroonkamol, P.; Dacha, S.; Li, L.Y.; Fu, X.L.; Zhan, S.H.; Cai, Q. Fluoroscopy-Guided Gastric Peroral Endoscopic Pyloromyotomy (G-POEM): A More Reliable and Efficient Method for Treatment of Refractory Gastroparesis. Surg. Endosc. 2017, 31, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Mekaroonkamol, P.; Dacha, S.; Wang, L.; Li, X.; Jiang, Y.; Li, L.; Li, T.; Shahnavaz, N.; Sakaria, S.; LeVert, F.E.; et al. Gastric Peroral Endoscopic Pyloromyotomy Reduces Symptoms, Increases Quality of Life, and Reduces Health Care Use For Patients With Gastroparesis. Clin. Gastroenterol. Hepatol. 2019, 17, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, K.R.; Talley, N.J.; Walker, M.M.; Burrows, T.L. Food and Functional Dyspepsia: A Systematic Review. J. Hum. Nutr. Diet 2018, 31, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Feinle-Bisset, C.; Vozzo, R.; Horowitz, M.; Talley, N.J. Diet, Food Intake, and Disturbed Physiology in the Pathogenesis of Symptoms in Functional Dyspepsia. Am. J. Gastroenterol. 2004, 99, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Gkolfakis, P.; Papanikolaou, I.S.; Mathur, R.; Pimentel, M.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Gut Microbiota Dysbiosis in Functional Dyspepsia. Microorganisms 2020, 8, 691. [Google Scholar] [CrossRef]
- Shin, J.E.; Park, K.S.; Nam, K. Chronic Functional Constipation. Korean J. Gastroenterol. 2019, 73, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhu, Q.; Qu, X.; Qin, H. Microbial Treatment in Chronic Constipation. Sci. China Life Sci. 2018, 61, 744–752. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).