Abstract
This paper presents comprehensive studies of pitting corrosion, which precedes the appearance of fistulas in galvanized steel pipelines of hot and cold water supply systems. Corroded galvanized pipes taken out from water supply systems within their operation and scale samples were the subject of this research. The current work continues the research on one of the four structural elements of tubercles—the dense layer. The corrosion of the zinc coating and the steel base of pipes inside the tubercles led to a gradual increase in the concentration of a solution containing components of the corroding metal (zinc and iron cations) and anions in water (mainly chlorides and sulfates). To explain the corrosion under the tubercles, their dense layer was compared with an anion exchange membrane with selective properties, which provided the primary concentration of the salt solution in the structure of the tubercles with a significant increase in the concentration of aggressive anions compared to the source water. The formation of fistulas in the cavity leads to a secondary concentration of solution inside the tubercle, mainly consisting of iron chloride. At the same time, due to the hydrolysis of the formed iron salts and a decrease in pH, the corrosion rate increases and becomes independent of external conditions. This article summarizes ten years of experience in examining corrosion of steel pipes from external and internal water supply systems.
Keywords:
corrosion; scale; pitting; scale structure; fistula; steel galvanized pipes; hot water supply system 1. Introduction
Galvanized steel pipes in water supply systems demonstrate unpredictable operation with regard to corrosion development, especially in the case of hot water supply systems (HWSSs). There is evidence of galvanized pipes having high corrosion resistance and a long (up to fifty years) service life, but a large number of articles describe abnormally rapid corrosion with the appearance of fistulas within three to five years from the start of the pipes’ operation [1,2,3,4,5,6,7,8]. There are also examples showing that galvanized steel pipes in HWSSs fail within 18 months [9]. Another research study reveals degradation of the thickness of the zinc coating on samples of galvanized pipes within 12 months, which were obtained from the HWSSs of Moscow, Russia [10]. The most intensive degradation of the zinc coating occurred within the first few months of operation; however, the nature of this intensity remained unclear. Thus, in the first, second, and third months, the thickness of the zinc coating decreased by 17, 9, and 6%, respectively, while in the following 9 months, it decreased by 1%. This research also presents the quantitative assessment of the corrosion rate of galvanized pipes in the HWSS. In the experiment, the effect of phosphates and silicates on ulcerative corrosion of galvanized pipes caused by copper ions was investigated. Studies were carried out on sections of galvanized pipes with a diameter of 12.2 mm at a temperature of 65 °C and water circulation at a speed of 0.2 m/s. The water had the following characteristics: electrical conductivity of 20 °C at a rate of 50–62 mSm/m; pH of 7.3–7.5; chloride and sulfate contents of 1.36–1.88 mmol/L and 0.7–1.32 mmol/L, respectively; phosphate content of 5 mg/L in terms of P2O5; silicate content of 18–22.2 mg/L; and total hardness of 2.4–2.94 mmol/L. After 150 days of experimentation, the average corrosion rate of galvanized pipes was 1.1 g/m2∙day (0.046 g/m2∙h or 0.05 mm/year), and in water with copper ions, it was 1.4 g/m2∙day (0.058 g/m2∙h or 0.064 mm/year). This means that the presence of copper in water accelerates the corrosion of galvanized steel [10].
References [11,12,13] present long-term study results of the effect of operational parameters on accelerated corrosion of galvanized pipes. A lab-scale bench was assembled to estimate parameters of HWSSs that have a major impact on the corrosion rate. The bench included two identical pipelines with sections with different directions of water flow: horizontal, vertical downward flow, and vertical upward flow.
Figure 1 shows micrographs of the scale formed on the surface of galvanized pipe after 70 and 224 days in an HWSS. A flow of hot water was 30 L/h, with a temperature of 50 (+/−2 °C) and a velocity of 6 cm/s. For comparison, Figure 1 also shows micrographs of a new pipe with traces of corrosion after being exposed for 25 h to hot water.
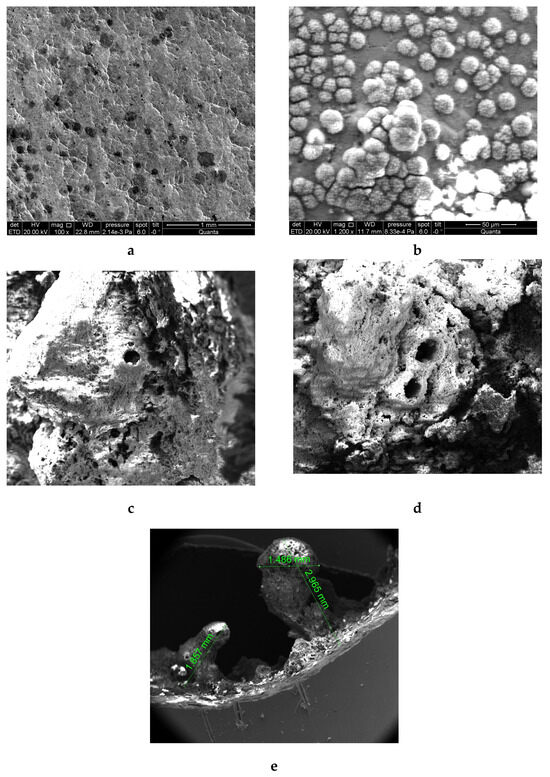
Figure 1.
Microphotographs of the progressive degradation of zinc coating of galvanized steel pipes in HWSS: (a) inner surface of a new pipe DN 20: (b) inner surface of a pipe after 25 h in hot water at a temperature of 50 °C; (c,d) the formation of a tubercle after 70 days of bench experiment (pipe DN 15) [12]; (e) the same after 224 days of bench experiment [13].
Figure 1 shows that corrosion of the zinc coating develops unevenly and has an ulcerative character with the formation of anode and cathode areas. Similarly to uncoated steel pipes, tubercles on the pipe surface consist mostly of oxides and zinc carbonate. This makes corrosion running intensive, with hydrogen depolarization and the release of hydrogen (see round holes on the surface of the scale, Figure 1c,d).
The structure of a tubercle in uncoated pipes includes (from the side of the water flow) four elements: a loose surface layer, a thin dense inner layer creating a rigid frame of the tubercle, a loose core containing corrosion products or a cavity filled with a solution of salts, and a corroding base. In several research studies, a mechanism of tubercle formation has been described, but there is no explanation for how the structure of the tubercle influences further corrosion [14,15,16,17,18,19,20].
The salt content in tap water is one of the key factors of accelerated corrosion of galvanized pipes, as was observed by many researchers [21,22,23]. However, the overall composition of water should not be ignored, especially the chloride content [21,24,25,26,27]. To assess the corrosive properties of water, the Langelier, Risner, and Larson indices have been introduced [28]. Based on the values of pH, salinity, hardness, calcium, chloride, sulfate, alkalinity, and temperature, these indices estimate the probability of intense corrosion. In the case of Moscow, these indices show a weak corrosive activity of the tap water. However, the practical experience of abnormally rapid corrosion investigation of galvanized pipes in HWSS may contradict the conclusion based on theoretical indices. Thus, observed facts of corrosion have to be explained from the corrosion mechanism viewpoint.
In recent years, a growing number of research papers have a focus on the pitting corrosion of stainless steels. A review by Soltis [29], which was devoted to the theory of pitting corrosion in metals, concluded that the development of stable pitting is determined by a chemical composition and high salt concentration inside. Pistorius and Burstein [30], after conducting studies of pitting corrosion on stainless-steel samples, established the range of concentration values of the solution (3–5 mol/L) inside the pitting, which precedes the beginning of the growth of stable pitting. In [31], the authors demonstrated that for stainless-steel type 304, to prevent repassivation, the concentration of metal cations in solution should be higher than 75–80% saturation of the chloride salt. If the concentration of metal cations is less than 75% saturation (i.e., below 3 M), the anolyte is not aggressive enough to support rapid dissolution of the alloy, and repassivation is inevitable. The aggressive anolyte in the growing pitting forms due to a low pH and high concentration of chlorides. However, in the conducted studies, the authors initially worked with high-concentration salt solutions without discussing how the required salt concentration appears at the metal–solution interface, which is especially important when water has a lower salinity.
The determination of the solution concentration inside the tubercles was first noted in [32]. This paper reveals the study of the structural and crystallographic composition of corrosion tubercles under similar conditions to the environment in the pipeline. During the experiment, the authors examined the bound water, i.e., water that surrounds and partially fills the tubercles in the pipes. Scale samples from cast-iron pipes removed from the water supply system (DN 80–120) were also examined. It was shown that the total salt content in the bound water was 3257.66 mg/L, with the concentration of chlorides 13.8 times higher than in the tap water. However, during sampling, the solution may be diluted with the tap water in the pipe. In addition, the research gave no explanation of how the solution concentration increases inside the tubercles.
In [33], the authors proposed a method to study the salt solution inside the tubercles from uncoated steel pipes and described the mechanism for how the salt concentration grows. A similar approach was used within current research in case of corrosion of galvanized pipes. The main objective of this study is to show the evolution of the corrosion damage of galvanized steel pipes, from the appearance of rusty water to the formation of fistulas. Current research also focuses on the mechanism for the occurrence and development of stable pitting corrosion under the tubercles.
2. Materials and Methods
Dried scale samples from corroded galvanized pipes were the subject of this study. The experiment began with extracting tubercles from two damaged pipes in which fistulas appeared. The second part was to analyze the soluble components of the scale contained in the tubercles. The inner surface of fragments of galvanized steel pipe DN 100 is shown in Figure 2a. The second pipe had a diameter of 80 mm (Figure 2b). Fistulas appeared within the third year of operation. Corrosion scale on the pipe surface had the form of irregularly shaped tubercles measuring 5 × 5–7 × 7 mm; the height of the tubercles was 5–12 mm.

Figure 2.
Samples of steel galvanized pipes: (a) DN100; (b–d) DN80.
The pipes were taken out from the HWSSs of residential buildings after 3–5 years of operation due to the appearance of fistulas. The structure of the majority of tubercles included a dense layer, a core (filled with either an amorphous sediment (Figure 2a,b) or a solution of salts (Figure 2c,d)), and a corroding base. The tubercles were friable and could be easily removed mechanically; the dense layer is less rigid and has a thickness of approximately 0.5 mm.
The method is based on the assumption that the hollow tubercle at the time of pipe extraction was filled with a solution of salts, whose concentration was determined by a combination of external and internal factors (Figure 2). The photograph demonstrates that before removing the pipe the tubercle had a closed contour around the entire perimeter, while the dense shell ensured no direct contact between the two fluids—the inner solution and the flowing hot water. After extraction and further exposure of the pipe sample to atmospheric conditions, the water from the tubercle gradually evaporated with salt deposition on the inner surface of the tubercle. The distilled water was used to extract salts from the tubercles (desorption) by penetrating into the pores, dissolving these salts, and forming a mixed solution.
Standard techniques were used to study the composition of desorbed solutions. The content of chlorides, calcium, and magnesium was determined by titration with standard solutions; iron, sulfates, and nitrates were measured with a Lange 5000 spectrophotometer (Hach, Loveland, CO, USA); and pH was measured with a Hanna HI 2215 pH meter (Hanna Instruments, Woonsocket, RI, USA). Micrographs of the sediment were obtained using a Quanta FEI 250 (FEI, Hillsboro, Oregon, USA) electron microscope in the secondary electron mode at an accelerating voltage of 20–25 kV.
The experiments were carried out in the following sequence. A sample of the tubercles was placed in a measuring cylinder with known volume of distilled water V1. The overall volume of water and the scale sample in the cylinder were immediately measured (V2). Then, after 48 h, the overall volume of water in the cylinder was re-measured (V3), which decreased due to the filling of pores in the tubercle structure. Based on these data, the density of the tubercle material was calculated, as well as the volume of water that was in the scale, equal to the volume of the pores: V4 = V2 − V3. Within 48 h, due to the diffusion of salts from the tubercles, they were evenly dissolved in distilled water, forming a multicomponent solution.
In addition to the quantitative analysis of the mineralization of the solution, the composition of desorbed salts was evaluated. For this purpose, 9 mL of solution (V3) was evaporated on an aluminum foil substrate in a drying oven at a temperature of 80 °C. The dry sediment was studied using a Quanta FEI 250 scanning electron microscope and an EDAX energy dispersion spectral analyzer (Gatan, Pleasanton, CA, USA).
3. Results
3.1. Desorption of Salts from Tubercles
Table 1 provides data on the study of the composition and concentration of salts found in tubercles extracted from non-galvanized steel pipes [33].

Table 1.
Sorption of distilled water by the scale.
Table 2 shows the calculated values of ion concentrations in solution inside the tubercles. The values were determined by multiplying the salt concentration in a water sample after dissolution and desorption of salts from the tubercles by the concentration coefficient K, found as a quotient of V1 by V4. Desorption of salts from the tubercles was performed once.

Table 2.
Calculated composition of the solution inside the tubercles.
The maximum salt concentration in the desorbed solution (the sum of cations) for galvanized pipes was 463 mEq/L, which is 36.9% lower than in the desorbed solution extracted from the tubercles of uncoated steel pipes (729 mEq/L). According to the values of the acidity and the concentration coefficient, the pH values of the solutions located directly in the pores of the tubercles at the time when the pipeline operation stopped were calculated.
Table 2 confirms that in the structure of the tubercles, there was a multicomponent solution of salts. The total concentration of solution significantly exceeds the salt content in the water in the pipeline. The water quality indicators in HWSS, from which the pipes (Figure 2b–d) were extracted, are shown below:
- pH = 7.4;
- Hardness = 4.2 mEq/L;
- Calcium = 3.2 mEq/L;
- Alkalinity = 3.4 mEq/L;
- Chlorides = 1.67 mEq/L;
- Sulfates = 0.85 mEq/L;
- Iron < 0.05 mg/L;
- Salinity = 338 mg/L.
Water quality data for pipes (Figure 2a) are missing.
For sample 5, the chloride content in the desorbed solution was 166 times higher than the chloride content in the source water, and the sulfate content was 246 times higher, respectively. In turn, the ratio of chloride concentrations to sulfates was 1.75. The values of the anion content in the desorbed solution for galvanized pipes and pipes without a protective coating are significantly different (Table 2). A higher rate of corrosion of non-galvanized pipes at temperatures up to 25 °C depends on the concentration that was established inside the tubercles (not the salt concentration in the tap water). The observed salt concentration in the desorbed solutions was significantly lower than the experimentally established limit for the occurrence of pitting corrosion in stainless steels [30,31].
3.2. Spectral Analysis of Desorbed Solution
The microphotograph of the sediment and the quantitative results of the spectral analysis are shown in Figure 3. These data demonstrate that the main components of the solution were chlorides, sulfates, zinc, and calcium. The contribution of the other elements is estimated at 0.5–1.5%. The ratio of Cl/SO4 anions was 2.44. The dense layer in non-galvanized pipes exhibits higher selectivity for chloride transfer compared to sulfates (Table 2), which is clearly related to the crystal structure of the dense layer.

Figure 3.
Microphotograph of the scale and results of spectral analysis (of the area inside the red square).
Figure 4 schematically represents the tubercle structure to explain the role of a dense layer in the formation of a high concentration of solution inside the tubercle. After the tubercles have formed, the surface of the metal underneath becomes the anode, and the outer surface of the dense layer becomes the cathode. The total concentration (C2) of the solution inside the tubercle increases in proportion to the transferred current (which is confirmed by experimental data). Due to this, the transfer of anions (chlorides and sulfates) through a dense layer to the anode (i.e., inside the tubercle) and iron cations (from the tubercle into the external solution) becomes unbalanced. Such imbalance occurs when a dense layer has selective properties and allows only anions to pass through (similar to an anion exchange membrane or solid electrolyte in the presence of fixed cations in the membrane structure).
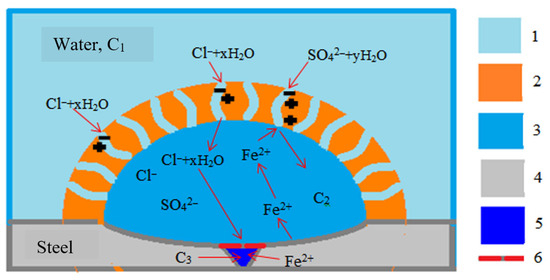
Figure 4.
Schematic representation of a tubercle: 1—an external solution (tap water); 2—a dense porous layer forming the structure of the tubercle and with selective properties; 3—the core of the tubercle (see Figure 2d); 4—the steel base of the pipe; 5—fistula; 6—porous coating of pitting; “+”—fixed charges (cations); “−”—counter-ions (anions); C1, C2, C3—concentrations of solutions in the tap water, in the core and in the fistula, respectively (C3 >> C2 >> C1).
Figure 5 clearly shows a dense layer and a loose core, whose main components are iron, zinc, and oxygen (cations); sulfates and chlorides act as anions. The content of sulfates exceeds the content of chlorides, which indicates the different nature of the permeability of the dense layer to anions depending on the sample under study (see Table 2). The dense layer is firmly bound to the pipe wall, while the core has a loose structure and shrinks when drying. Figure 5 also shows that, despite to the dimensions of the tubercle, the pipe wall has minor damage. At the same time, there are no signs of the transition to more destructive corrosion with the formation of fistulas.
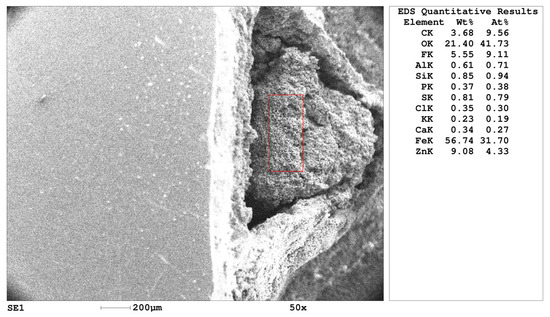
Figure 5.
Microphotograph and spectral analysis (of the area inside the red square) of the scale composition in a tubercle in a galvanized pipe (DN15) extracted from the HWSS.
Within the growth of the tubercle, the concentration of the solution in it increases due to the transfer of hydrated anions through a dense layer and the intake of iron cations. However, this concentration does not reach the values necessary for stable pitting corrosion, i.e., 3–5 M. Over time, the permeability of the dense layer decreases for both anions and oxygen; the growth of the tubercle stops, and the corrosion turns from conditionally uniform to pitting. Afterwards, the pitting corrosion becomes the main mechanism of localized metal destruction.
Analysis of a large number of tubercle samples removed from pipes (similarly to Figure 5) revealed that a salt solution at the core–metal boundary, after drying, forms hemispherical crystalline elements (Figure 6). The scale crystallization from a saturated solution of iron salts occurred after the pipe was taken out of operation and exposed to atmospheric oxygen.
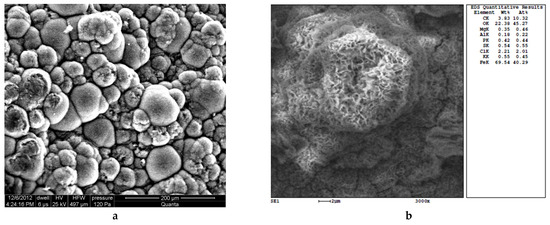
Figure 6.
A microphotograph of the base of the tubercle (copying the metal surface at the time of pipe removal (a)). The sediment was extracted from an uncoated steel pipe (DN 900). Micrography and spectral analysis of the growing pitting (b).
Figure 6a confirms that pitting corrosion develops along grain boundaries or in the presence of intermetallic inclusions [34]. At the same time, the shape of some crystallites differs from hemispherical and becomes closer to tubular. Figure 6b shows a fragment of the pitting that has an oblong shape with a decreasing diameter. According to the chemical composition, chlorides represent most of the anions.
Further development of pitting corrosion under the tubercles occurs with a significant decrease in oxygen content and an increased salt content (mainly salts of iron), which is hydrolyzed to form hydrogen and hydroxyl ions. The autocatalytic nature of corrosion is ensured by the presence of the anode and cathode directly in the growing pitting, a high concentration of electrolyte, and an acidic environment. In this case, the bottom of the pitting acts as an anode, and the walls act as a cathode. Hydrogen ions become a depolarizer, which are reduced to hydrogen gas at the cathode. Due to hydrolysis, the volume of water in the pitting decreases, which leads to a further increase in the concentration of the internal solution (C3). At high concentrations, iron salts and hydroxyl ions form an insoluble scale of Fe(OH)2, which precipitates directly into the pitting volume (Figure 7).
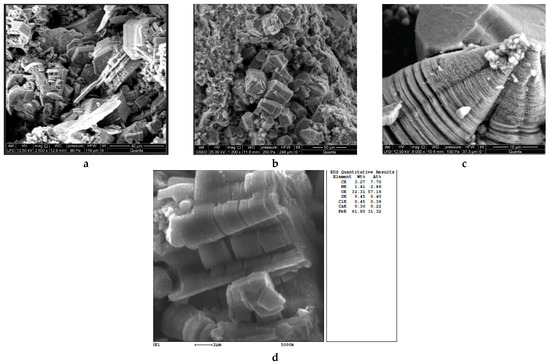
Figure 7.
Microphotographs of crystalline replicas formed in the cavities of the pitting: (a–d)—uncoated steel pipes; (c)—galvanized pipe from the HWSS.
Figure 7 shows crystal structures with a variety of shapes, including a conical shape that copies the shape of the recess in the metal. The random location of single crystals indicates their mutual movement inside the tubercles during the corrosion.
Figure 8 shows typical photographs of cross-cutting damage to the pipe wall in the form of fistulas formed due to corrosion of galvanized steel [34] and non-galvanized steel pipes. The tubercles with intense internal corrosion were directly above the fistulas.

Figure 8.
(a) Through-wall damage in the lower part of the NPS3 galvanized steel pipe (with an outer diameter of 3.5 inches) in the dry pipe system [35]; (b) uncoated steel pipe (DN150) from the heating system.
A fistula is a defect appeared within the corrosion in the form of a funnel-shaped depression in the pipe wall. The formation of a fistula is provided by a combination of the effects on the metal surface by the multiple pits located inside the limited area under a dense layer of tubercle. The dissolution of the metal is accompanied by the growth of cavities and the formation of crystalline replicas of pitting (Figure 7). The fistulas shown in Figure 8 can be estimated by their shape and depth. The three circles should be highlighted, which differ in color (Figure 8). The outer circle represents the contour of the tubercle and coincides with the undisturbed surface of the pipe. The middle circle is the boundary of the intense pitting corrosion. The third circle bounds the actual hole (fistula). Before the appearance of the fistula, the upper part of the depression under the tubercle looks like a half-ellipse or hemisphere. The second part of the recess has the shape of a truncated cone. In case of corrosion of the galvanized pipe, the zinc coating is already destroyed when the fistula is formed.
4. Discussion
4.1. Growth Mechanism of Solution Concentration in the Tubercles
Electrochemical corrosion is accompanied by the appearance of an electric field, in which an orderly movement of electrons and ions to electrodes of opposite charge is observed. In this case, a metal with a damaged passive layer becomes the anode, and a large area of passive metal becomes the cathode [36,37]. The current is provided by the transfer of electrons through the metal of the pipes and ions of the corroded metal through the solution: partially into the water flowing through the pipes, which leads to a deterioration in the quality of tap water, as well as into the composition of the resulting dense layer. In this case, the transfer of anions and cations ensures the electrical neutrality of the solution.
An equilibrium concentration of iron salts C2 (Figure 4) was established inside the tubercles, corresponding to the density of the corrosive current and the structure of the layer forming the shell of the tubercle. Moreover, the high concentration of the solution inside the tubercle is associated with the concentration of fixed charges in the dense layer. Fixed ions (cations) are embedded in the voids of the crystal lattice of a dense layer, forming metal–cationic complexes. In this case, the properties of the dense layer should be similar to ion-exchange membranes or solid solutions of electrolytes. The solution concentration inside the tubercle (C2) cannot exceed the concentration of fixed ions; otherwise, an electromotive force occurs, which may lead to iron diffusion from the tubercles. The selectivity of a dense layer can be demonstrated in charge (anion/cation) and in the composition and number of electrons they lose (simple/complex, chloride/sulfate). The selectivity of the dense layer regarding the iron ions is ensured by their electrostatic repulsion from the similarly charged layer.
The accumulation of iron ions (and other cations contained in the metal structure) leads to the appearance of a supersaturated solution and scale deposition in the core structure.
4.2. The Role of Pitting Corrosion, Which Leads to Fistula Formation
Pitting corrosion is the most dangerous from the point of view of the operation of steel pipelines, both galvanized and uncoated, which, in some cases, ends with the formation of fistulas. However, literature analysis demonstrates that pitting corrosion occurs at higher concentrations of electrolyte than those obtained in the experiment (Table 2), i.e., Cpit must exceed C2.
When studying pitting corrosion, many authors note that narrow and deep pores appear in the metal, and the depth of penetration into the metal exceeds the diameter of the pitting. In this case, the dimensions of the pitting are measured in microns with various shapes depending on the local conditions and whether the pitting contains a salt film or not. Vignal et al. [38] and Frankel [36] conclude that the pitting can be hemispherical with polished inner surfaces, while in the absence of a salt film, the pitting has an irregular shape.
A solid salt film on the pitting surface has been noted in numerous studies of corrosion in stainless steels. Frankel demonstrated a solid salt film that can form on the surface of the pitting while the ion concentration drops to the saturation value, and mass transfer limits the growth rate of the pitting [36]. Pitting cavities can be filled with corrosion products and form caps over them, sometimes creating tubercles. Although the pitting may take a variety of shapes, for steel and many alloys, it typically has the shape of a cone, or hemisphere [37].
Many authors confirmed that pitting corrosion has an autocatalytic nature [38,39,40], which is associated with local changes in conditions. Angal [39] described the growth of pitting in the marine environment as an autocatalytic process “in which Fe2+ ions attract negative Cl− ions and, through hydrolysis, create a porous Fe(OH)2 cap over the pitting. This process forms a self-propagating system in which increased acidity in the pitting cavity increases the corrosion of steel walls” [39]. Pistorius and Burstein [41] note that metastable growth requires a perforated cap over the pitting to provide an additional barrier to diffusion, allowing the aggressive anolyte of the pitting to be preserved. Therefore, in order to achieve conditions for stable pitting corrosion, the process of concentrating the solution inside the tubercle must continue, and the initial concentration for continued corrosion is the solution inside the tubercle (C2), which reaches a critical value C3 = Cpit.
It should be assumed that pitting corrosion in stainless and carbon steels may work via a similar mechanism [42,43,44,45]. The role of the coating over the pitting (Figure 4, pos. 6) demands further study; however, it acts also as a selective barrier that increases the transfer of chlorides.
4.3. Formation of the Fistula in Galvanized and Uncoated Steel Pipes
Despite numerous studies of the mechanism of pitting corrosion, there is a lack of description of how pitting turns into fistulas. First, this is due to the incommensurability of sizes, since the area of a fistula is at least tenfold greater than the area of an individual pitting, and the thickness of the metal is many times higher than the pitting size. Roberge et al. concluded that the real consequences of pitting depend on the thickness of the metal and the penetration rate [36]. It was found that the penetration rate decreases if there is greater pitting. This is because the closely spaced pitting must share an accessible adjacent cathode region that controls the corrosive current [46,47]. The current research attempts to present this process in detail, using the results of research and observations of real objects. The most probable process of pitting corrosion in real conditions is the sequential-layered repetition of metal destruction from the undamaged inner surface under the tubercle to the outer surface of the pipe.
As follows from Table 2, in uncoated steel pipes, a higher total salt concentration is observed in the scale than in galvanized pipes. The chloride content in the tubercles from uncoated pipes is much higher than the concentration of chlorides in the scale from galvanized pipes. These differences are most likely a consequence of the different crystallographic properties of the dense layer. In uncoated steel pipes, the dense layer is monocrystalline and consists mainly of magnetite. The dense layer of galvanized pipes has a polycrystalline structure that includes both iron and zinc oxides, which leads to the formation of a heterogeneous and less selective structure with respect to sulfates.
Another difference is the shape of the crystal structures formed in the pitting. In uncoated steel pipes, pits have a cylindrical or hexagonal shape, constant or slightly tapering in length (Figure 7a,b), which meets the conditions of their formation at an almost constant density of the corrosive current. In galvanized steel pipes, the pitting replicas have a conical shape (Figure 7c), which corresponds to an initially high current density that decreases over time at a high rate. These differences are also related to the temperature conditions in which corrosion deposits formed in the pipes in question. In the case of uncoated pipes, scale was removed from the outdoor cold water supply system, and corrosion occurred over a long period, while the galvanized pipe removed from the HWSS underwent accelerated corrosion.
The shape of the crystal structures is also related to the effect of the anionic composition of water on pitting corrosion. Thus, in [48], the results of experiments and simulations showed that in water that contain chlorides, nickel anodes locally corrode and form narrow and deep pitting, while Br ions form shallow and wide pitting. Combined corrosion with Cl− and Br− contents led to a mixed morphology of pitting. This means that at the last stage of corrosion in galvanized pipes, with the formation of conical elements under the tubercles and before fistulas appear, only chlorides represent the anionic composition of the solution. On the one hand, this explains why galvanized pipes are more resistant to corrosion than uncoated pipes (the concentration of electrolyte under the bumps is lower), and on the other hand, how corrosion of galvanized pipes accelerates under unfavorable operating conditions. Table 2 demonstrates that the sulfate content at the first stage of concentration is almost equal to the chloride content, which witnesses the inhibitory effect and leads to the formation of flat depressions in the metal, and that at the second stage, only chlorides are involved in corrosion with the formation of narrow and deep pitting. At the same time, the selective properties of the coating (see Figure 4, pos. 6) formed over the pitting cannot be denied, as is observed in the corrosion of stainless steels.
4.4. Inhibitors of Corrosion
One of the possible ways to protect galvanized pipes is to use corrosion inhibitors. Phosphates and silicates of sodium are typically used as corrosion inhibitors for HWSSs, individually and in the form of various compositions. To protect zinc from corrosion, a new combined method was proposed, including superhydrophobization of its surface and the addition of small amounts (1.0 mM) of various corrosion inhibitors to an aggressive medium in the form of a solution of 0.1 mM sodium chloride [49]. However, this paper contains no specific recommendations on how to apply this method to protect galvanized pipes in water supply systems.
In [50], the authors declare that a radical method to reduce the internal corrosion to a minimum level is to eliminate the pathways of oxygen entering the mains water. The authors also pointed out that the compositions proposed today based on phosphonic acids and zinc salts are effective inhibitors of carbonate deposits. However, anti-corrosion measures are still required. The disadvantages of corrosion inhibitors include their high cost and deterioration in the quality of tap water (pH changes, increased phosphate content), as well as the lack of regulatory documents on how these inhibitors should be applied at the initial stage. The experience of corrosion inhibitor application shows a certain effect, but corrosion continues, albeit at a slower rate.
The model of a dense layer as a selective membrane or metal–ion complex provides targeted intervention in the corrosion mechanism by replacing fixed cations in the structure of the dense layer with anions. Many authors also noted the selective properties of metal–ion complexes formed during corrosion. For example, the adsorption and incorporation of oxyanions (for example, CrO4−2 and MoO4−2) lead to the transformation of the anion-selective outer passive layer of stainless steel into cation-selective phases that can repel Cl− ions from the surface and prevent the penetration of aggressive anions into the film [51,52,53,54,55].
4.5. Trouble-Free Operation Conditions for Galvanized Pipe Water Supply Systems
The examples of abnormally rapid corrosion of galvanized steel pipes demonstrate their vulnerability to corrosion. However, there are cases when galvanized pipes have longer service than the standard period. One article [5] presents the results of the HWSS survey in several residential buildings conducted in 2018–2019. Cold and hot water pipes were replaced in 1998. Despite the service life of about 30 years and the presence of welded joints, the pipelines are in working condition.
The experience of HWSS inspection in various buildings, also mounted from galvanized pipes, shows that intense corrosion develops locally, while the main parts of the pipes work without accidents for a long time. Clearly, this does not happen by chance but has a completely scientific explanation. That is the formation of a protective layer on the surface of the zinc coating during operation due to zinc alloying with iron ions released during corrosion of the steel base of galvanized pipes. According to [27], the effect of alloying can be negative, leading to accelerated corrosion, or positive, increasing corrosion resistance due to the accumulation of a more electropositive component on the surface. The mechanism of influence of the alloying components of zinc coatings on their corrosion resistance has not been sufficiently studied in this case. The alloying component increases the density of the corrosion product layer; on the other hand, the authors suggest that the alloying component reduces the electrical conductivity of the corrosion product layer [27].
The main causes of accelerated corrosion of galvanized steel pipes in hot water systems that should be avoided are as follows:
- An extended period, when tenants start living in new buildings, coinciding with the periods of intensive corrosion of new pipes;
- Accumulation of corrosion products in main pipelines due to low water flow rates resulting from differences in actual and estimated water consumption by consumers;
- A large number of valves, filters, balancing valves, expansion joints, and towel rails made of materials (brass and stainless steel) with different electrode potentials;
- U- or T-shaped vertical sections in the pipe network, which may lead to local oxygen concentration gradients at low velocities of water movement in the pipes;
- Lack of available methods for monitoring the condition of pipelines.
5. Conclusions
A study of the evolution of abnormally rapid corrosion of galvanized steel pipes in hot water supply systems from the first days of operation to the appearance of fistulas allows for drawing the following conclusions:
- Corrosion of the new zinc coating occurs intensively during the first year of operation. At the second stage, the corrosion of the base metal (carbon steel) begins. In this case, numerous tubercles are formed. The primary concentration of the internal solution occurs inside the tubercles, due to the transfer of hydrated anions through a dense layer with selective properties, with a salt content significantly exceeding the salt concentration in the source tap water.
- At the third stage, the growth of tubercles stops, and the corrosion continues under the tubercle and becomes autocatalytic, i.e., independent of external factors.
- The condition for the fistula formation is pitting corrosion on the metal surface under the tubercle, accompanied by a secondary increase in the concentration of iron salts in the pitting, low pH, and sufficient volume of water inside the tubercle dissociating into H+ and OH ions.
- The presence of water is a necessary condition for the oxidation of corroding iron ions, and its volume must be electrochemically commensurate with the thickness of the remaining metal. In case of lack of water, individual pitting and, in general, tubercles become passive. At this stage, the role of chlorides in the development of pitting corrosion is increasing.
- The main causes of pitting corrosion of galvanized pipes in hot water systems are high concentrations of oxygen and chloride ions in the tap water, elevated temperatures, violations of construction and operation standards, structural features of water supply systems, and the presence of intermetallic inclusions in steel pipes.
Author Contributions
Conceptualization, V.C. and N.M.; methodology, V.C., and N.M.; software, N.M. and I.G.; validation, V.C.; formal analysis, V.C. and N.M.; investigation, V.C. and N.M.; resources, V.C. and N.M.; data curation, V.C.; writing—original draft preparation, V.C.; writing—review and editing, N.M.; visualization, N.M. and I.G.; supervision, N.M.; project administration, N.M.; funding acquisition, N.M. and I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Moscow State University of Civil Engineering (grant for fundamental scientific research, project no. 07-661/130).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HWSS | Hot water supply system |
| CHU | Central heating unit |
References
- Delaunois, F.; Tosar, F.; Vitry, V. Corrosion behaviour and biocorrosion of galvanized steel water distribution systems. Bioelectrochemistry 2014, 97, 110–119. [Google Scholar] [CrossRef]
- Andrianov, A.P.; Chukhin, V.A. Morphology, composition and formation condition analysis of corrosive deposits in water pipes. Wat. Ecol. 2016, 3, 18–34. [Google Scholar]
- Andrianov, A.P.; Chukhin, V.A. Reasons of corrosion of steel pipes in hot water supply systems. Wat. Mag. 2015, 3, 36–38. [Google Scholar]
- Chukhin, V.A.; Andrianov, A.P. Analysis of factors of corrosion of galvanized steel pipes in hot water supply systems. Sanit. Tech. Heat Suppl. Vent. 2018, 1, 54–58. [Google Scholar]
- Chukhin, V.A.; Andrianov, A.P. Accelerated corrosion of galvanized steel pipes in hot water supply systems. Sanit. Tech. Heat Suppl. Vent. 2019, 7, 22–30. [Google Scholar]
- Makisha, N.; Chukhin, V. Determination of Corrosion Rate in Galvanized Pipes in Centralized Hot Water Supply Systems. Appl. Sci. 2023, 13, 10564. [Google Scholar] [CrossRef]
- Marjanowski, J.; Ostrowski, J. Electrochemical Protection Against Corrosion Processes in Hot Tap Water Installations; C.B.W. UNITEX Ltd.: Gdansk, Poland, 2015. [Google Scholar]
- Pawlowski, B.; Krawczik, J.; Pala, P. The Premature Deterioration of Zinc-coated Steel Pipes in Water Distribution System. Int. J. Mater. Mech. Eng. 2013, 2, 42–47. [Google Scholar]
- Podporin, A.V.; Tsvetkova, L.I.; Chernikov, N.A. Application of steel pipes in water supply systems of Saint Petersburg. Bullet. Civ. Eng. 2019, 6, 252–256. [Google Scholar]
- Reizin, B.L.; Strizhevskiy, I.V.; Sazonov, R.P. Corrosion Protection of Hot Water Supply Systems; Stroiizdat: Moscow, Russia, 1986; p. 112. [Google Scholar]
- Chukhin, V.A.; Andrianov, A.P.; Hurgin, R.E. Experimental study of pitting corrosion mechanism in hot water supply systems. Int. J. Corros. Scale Inhibit. 2022, 11, 1679–1691. [Google Scholar]
- Andrianov, A.P.; Chukhin, V.A. Investigation of the initial stage of pitting corrosion of galvanized pipes in hot water supply systems. Sys. Technol. 2021, 1, 9–14. [Google Scholar]
- Chukhin, V.A.; Andrianov, A.P. Formation mechanism of iron tubercles during corrosion of water supply pipes. Int. J. Corros. Scale Inhib. 2022, 11, 812–830. [Google Scholar] [CrossRef]
- Sander, A.; Berghult, B.; Elfstroem Broo, A.; Lind Johansson, E.; Hedberg, T. Iron corrosion in drinking water distribution systems—The effect of pH, calcium and hydrogen carbonate. Corros. Sci. 1996, 38, 443–455. [Google Scholar] [CrossRef]
- Clarke, B.H.; Aguilera, A.M. Microbiologically Influenced Corrosion in Fire Sprinkler Systems Automatic Sprinkler Systems, Handbook 2007. Available online: https://www.cityofwoodland.gov/DocumentCenter/View/3634/MIC-Information-?bidId= (accessed on 25 July 2025).
- Sarin, P.; Snoeyink, V.; Lytle, D.; Kriven, W. Iron corrosion scales: Model for scale growth, iron release, and colored water formation. J. Environ. Eng. 2004, 30, 364–373. [Google Scholar] [CrossRef]
- Gerke, T.L.; Maynard, J.B.; Schock, M.R.; Lytle, D.L. Physiochemical characterization of five iron tubercles from a single drinking water distribution system: Possible new insights on their formation and growth. Corros. Sci. 2008, 50, 2030–2039. [Google Scholar] [CrossRef]
- Ray, R.I.; Lee, J.S.; Little, B.J.; Gerke, T. The anatomy of tubercles: A corrosion study in a fresh water estuary. Mater. Corros. 2010, 61, 993–999. [Google Scholar] [CrossRef]
- Herro, H.M. MIC Myths—Does Pitting Cause MIC? In Proceedings of the Corrosion 1998. San Diego, CA, USA, 22–27 March 1998. Technical Paper No. 278. [Google Scholar]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Kriven, W.M.; Clement, J.A. Physico-chemical characteristics of corrosion scales in old iron pipes. Water Res. 2001, 35, 2961–2969. [Google Scholar] [CrossRef]
- Smith, F.; Brownlie, F.; Hodgkiess, T.; Toumpis, A.; Pearson, A.; Galloway, A.M. Effect of salinity on the corrosive wear behaviour of engineering steels in aqueous solutions. Wear 2020, 462–463, 203515. [Google Scholar] [CrossRef]
- Hasan, B. Effect of salt content on the corrosion rate of steel pipe in turbulently flowing solutions. Al Nahrain J. Eng. Sci. 2010, 13, 66–73. [Google Scholar]
- Hu, J.; Dong, H.; Xu, Q.; Ling, W.; Qu, J.; Qiang, Z. Impacts of water quality on the corrosion of cast iron pipes for water distribution and proposed source water switch strategy. Water Res. 2018, 129, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Soltis, J.; Krouse, D.; Laycock, N. Localized dissolution of iron in buffered and non-buffered chloride containing solutions. Corros. Sci. 2011, 53, 2152–2160. [Google Scholar] [CrossRef]
- Lytle, D.A.; Tang, M.; Francis, A.T.; O’Donnell, A.J.; Newton, J.L. The effect of chloride, sulfate and dissolved inorganic carbon on iron release from cast iron. Water Res. 2020, 183, 116037. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Sun, X.; Zan, B.; Liang, M. Chloride corrosion behavior on heating pipeline made by AISI 304 and 316 in reclaimed water. RSC Adv. 2021, 11, 38765–38773. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Zakharyevich, D.A.; Galin, R.G.; Batmanova, T.V.; Zhivulin, V.E.; Ulyanov, M.N.; Fazlitdinova, A.G.; Zhizhin, E.V.; Koroleva, A.V.; Kasatkin, I.A.; et al. Corrosion of diffusion zinc coatings in neutral chloride solutions. Int. J. Corros. Scale Inhib. 2024, 13, 337–356. [Google Scholar] [CrossRef]
- McNeill, L.; Edwards, M. Iron Pipe Corrosion in Distribution Systems. Am. Water Works. Assoc. 2001, 93, 88–100. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Metastable pitting corrosion of stainless steel and the transition to stability. Philosoph. Transact. A 1992, 341, 531–559. [Google Scholar] [CrossRef]
- Gaudet, G.T.; Mo, W.T.; Hatton, T.A.; Tester, J.W.; Tilly, J.; Isaacs, H.S.; Newman, R.C. Mass transfer and electrochemical kinetic interactions in localized pitting corrosion. AIChE J. 1986, 32, 949–958. [Google Scholar] [CrossRef]
- Swietlik, J.; Raczyk-Stanisławiak, U.; Piszora, P.; Nawrocki, J. Corrosion in drinking water pipes: The importance of green rusts. Water Res. 2012, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chukhin, V.; Andrianov, A.; Makisha, N. Pitting Corrosion of Steel Pipes in Water Supply Systems: An Influence of Shell-like Layer. Appl. Sci. 2024, 14, 7189. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Su, P.; Fuller, D.B. Corrosion and Corrosion Mitigation in Fire Protection Systems; Research Technical Report No. 0003040794; FM Global: Boston, MA, USA, 2014. [Google Scholar]
- Frankel, G.S. Pitting corrosion of metals. A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Roberge, P.R. Corrosion Engineering: Principles and Practice; McGraw-Hill: New York, NY, USA, 2008; p. 754. [Google Scholar]
- Vignal, V.; Voltz, C.; Thiébaut, S.; Demésy, M.; Heintz, O.; Guerraz, S. Pitting Corrosion of Type 316L Stainless Steel Elaborated by the Selective Laser Melting Method: Influence of Microstructure. J. Mater. Eng. Perform 2021, 30, 5050–5058. [Google Scholar] [CrossRef]
- Angal, R. Principles and Prevention of Corrosion; Alpha Science International: Oxford, UK, 2010; p. 262. [Google Scholar]
- Caines, S.; Khan, F.; Shirokoff, J. Analysis of pitting corrosion on steel under insulation in marine environments. J. Loss Prev. Process Ind. 2013, 26, 1466–1483. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Growth of corrosion pits on stainless steel in chloride solution containing dilute sulphate. Corros. Sci. 1992, 33, 1885–1897. [Google Scholar] [CrossRef]
- Ahammed, M.; Melchers, R.E. Reliability of underground pipelines subject to corrosion. J. Transp. Eng. 1994, 120, 989–1002. [Google Scholar] [CrossRef]
- Melchers, R. Pitting corrosion of mild steel in marine immersion environment—Part 2: Variability of maximum pit depth. Corros. Sci. Sect. 2004, 60, 937–944. [Google Scholar] [CrossRef]
- Melchers, R. Pitting corrosion of mild steel in marine immersion environment—Part 1: Maximum pit depth. Corros. Sci. Sect. 2004, 60, 824–836. [Google Scholar] [CrossRef]
- Melchers, R. Effect of immersion depth on marine corrosion of mild steel. Corros. Sci. Sect. 2005, 61, 895–906. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Metals; National Association of Corrosion Engineers: Houston, TX, USA, 1986; p. 417. [Google Scholar]
- Roberge, R. Forms of corrosion in pictures. In Welcome to Corrosion Doctors; Kingston Technical Software: Kingston, ON, Canada, 2013. [Google Scholar]
- Zhang, S.; Wang, Y.; Li, S.; Wang, Z.; Chen, H.; Yi, L.; Chen, X.; Yang, Q.; Xu, W.; Wang, A.; et al. Concerning the stability of seawater electrolysis: A corrosion mechanism study of halide on Ni-based anode. Nat. Commun. 2023, 14, 4822. [Google Scholar] [CrossRef] [PubMed]
- Redkina, G.V.; Kuznetsov, Y.I. Combined method of zinc protection in aqueous solutions with superhydrophobic coatings and corrosion inhibitors. Int. J. Corros. Scale Inhib. 2023, 12, 1863–1882. [Google Scholar] [CrossRef]
- Slepchenok, V.S.; Brusov, K.N. Internal corrosion in open networks of heat supply and ways for mitigation. News Heat Supply 2000, 3, 11. [Google Scholar]
- Choi, S.-H.; Yoo, Y.-R.; Kim, Y.-S. Semiconductive Tendency of the Passive Film Formed on Super Austenitic Stainless Steel SR-50A in Acidic or Alkaline Chloride Solutions. Crystals 2024, 14, 766. [Google Scholar] [CrossRef]
- Sakashita, M.; Sato, N. The effect of molybdate anion on the ion-selectivity of hydrous ferric oxide films in chloride solutions. Corros. Sci. 1977, 17, 473–486. [Google Scholar]
- Sato, N. Toward a more fundamental understanding of corrosion processes. Corros. Sci. 1989, 45, 354–368. [Google Scholar] [CrossRef]
- Brooks, A.R.; Clayton, C.R.; Doss, K.; Lu, Y.U. On the role of Cr in the passivity of stainless steel. J. Electrochem. Soc. 1987, 133, 2459–2464. [Google Scholar] [CrossRef]
- Clayton, C.R.; Lu, Y.U. A bipolar model of the passivity of stainless steel: The role of Mo addition. J. Electrochem. Soc. 1987, 133, 2465–2473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).