Comparison of the Passive Behavior of NiTi and CoNiCrMo in Simulated Physiological Solutions
Abstract
1. Introduction
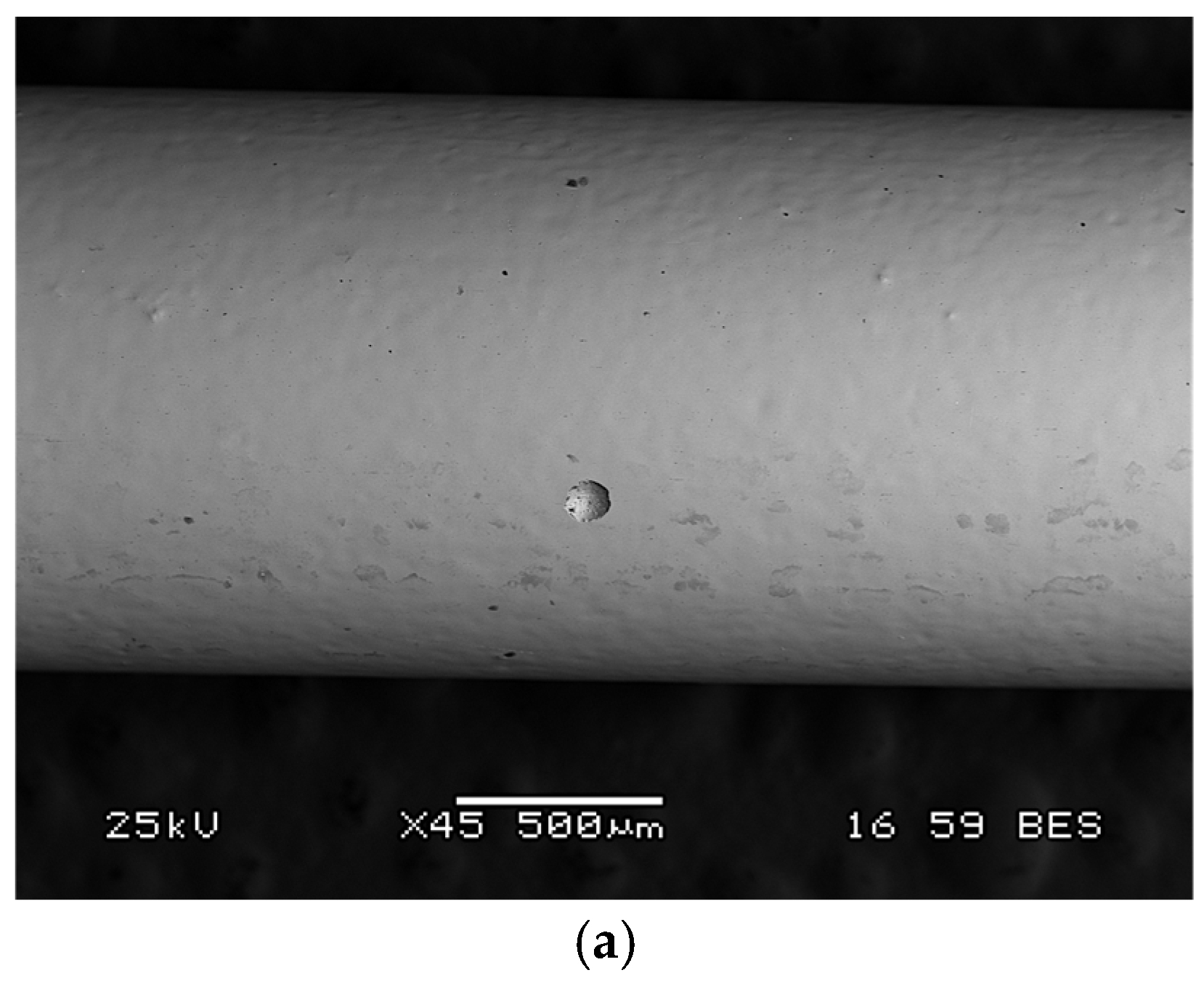
2. Surface Analysis
2.1. Oxide Film Composition
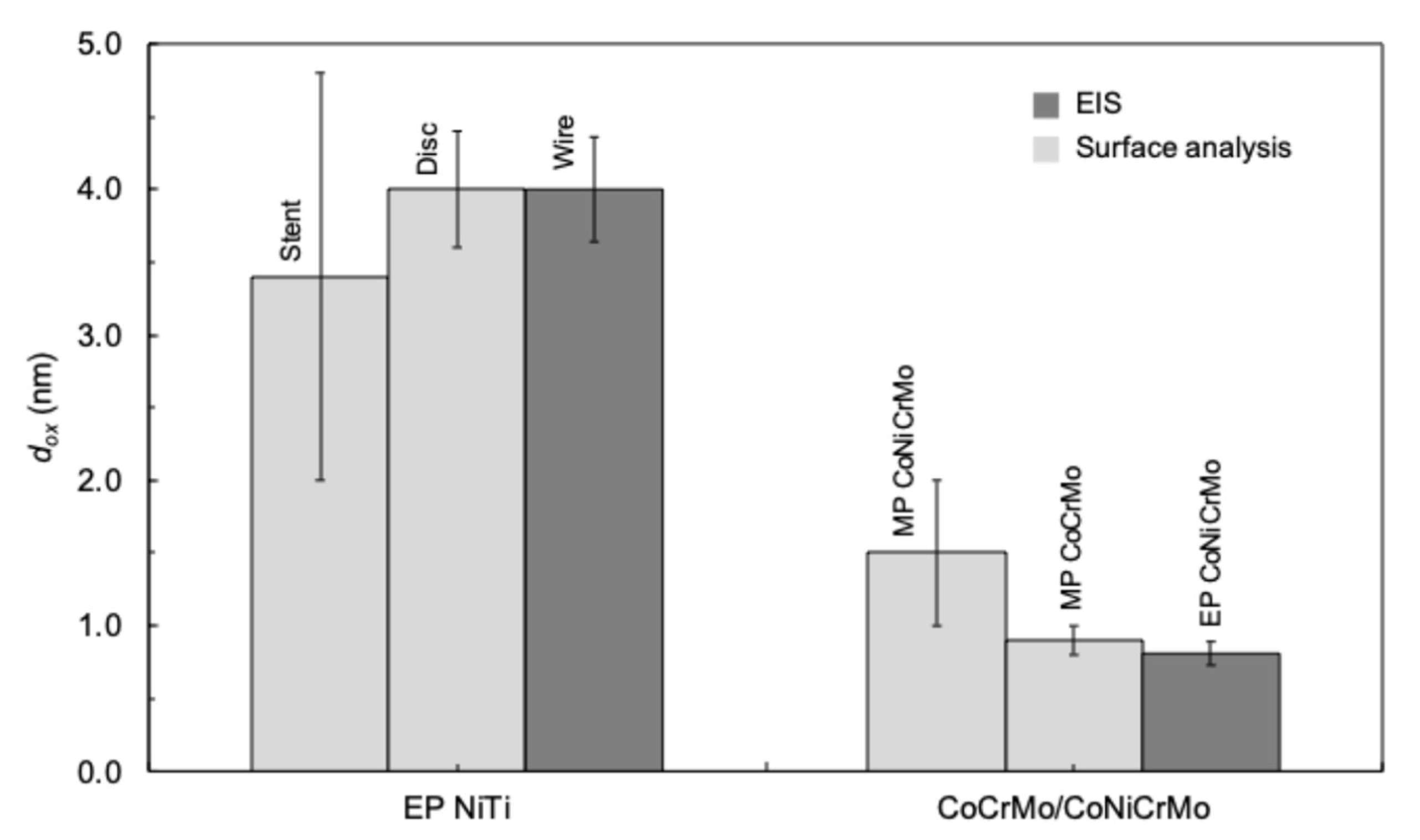
2.2. Oxide Film Thickness
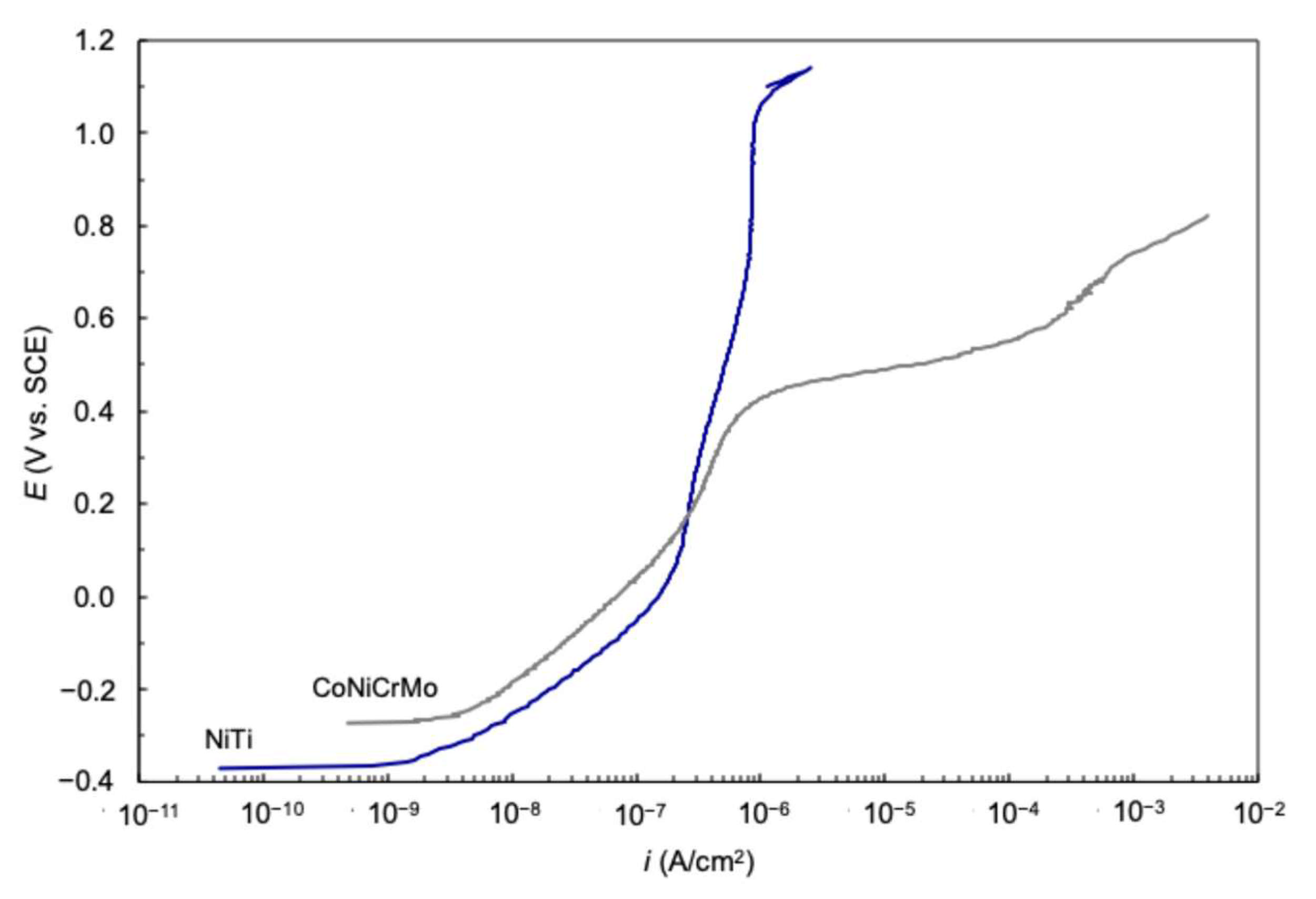
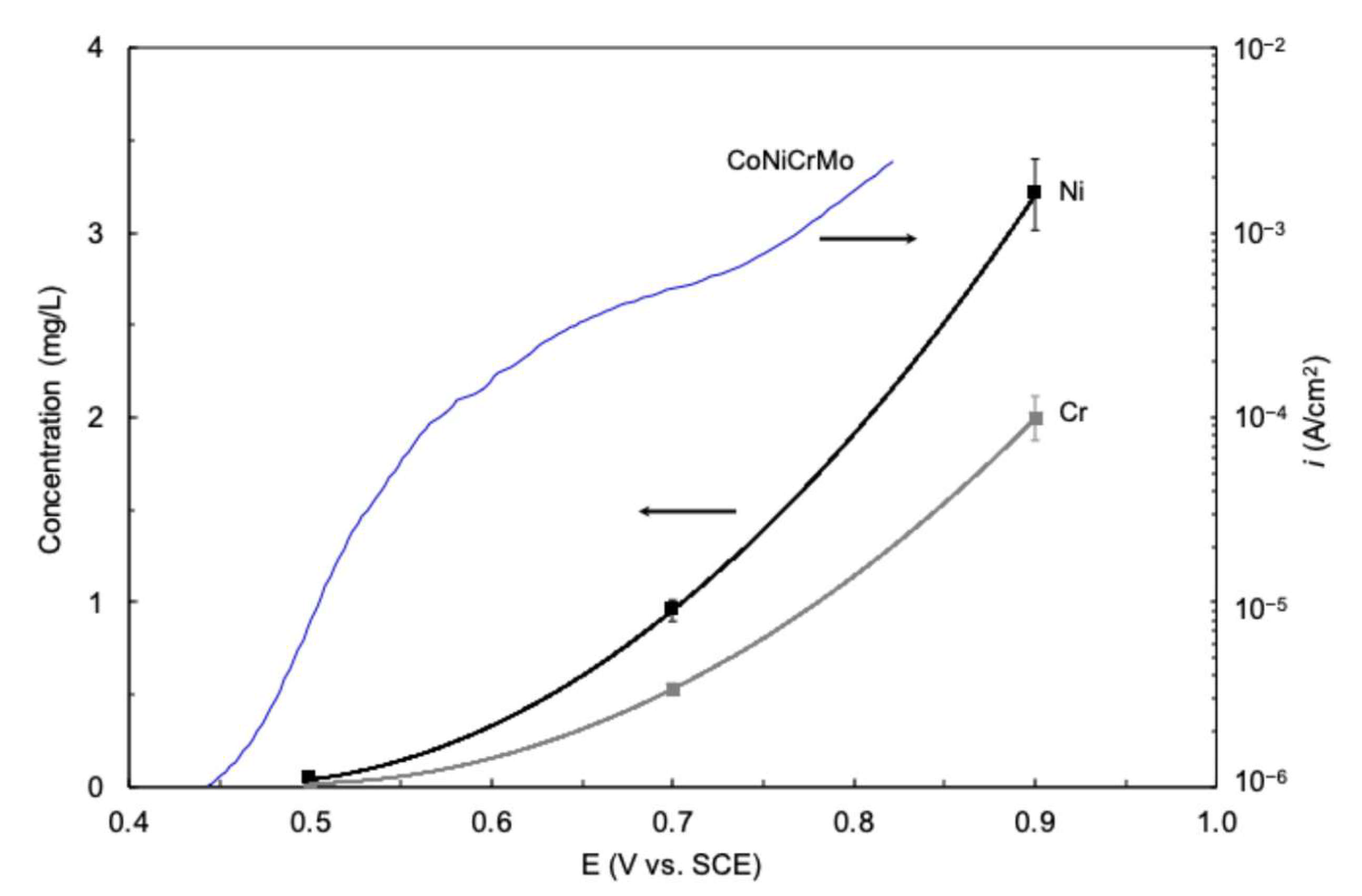
3. Passive Behavior
4. Model for Film Growth and Dissolution
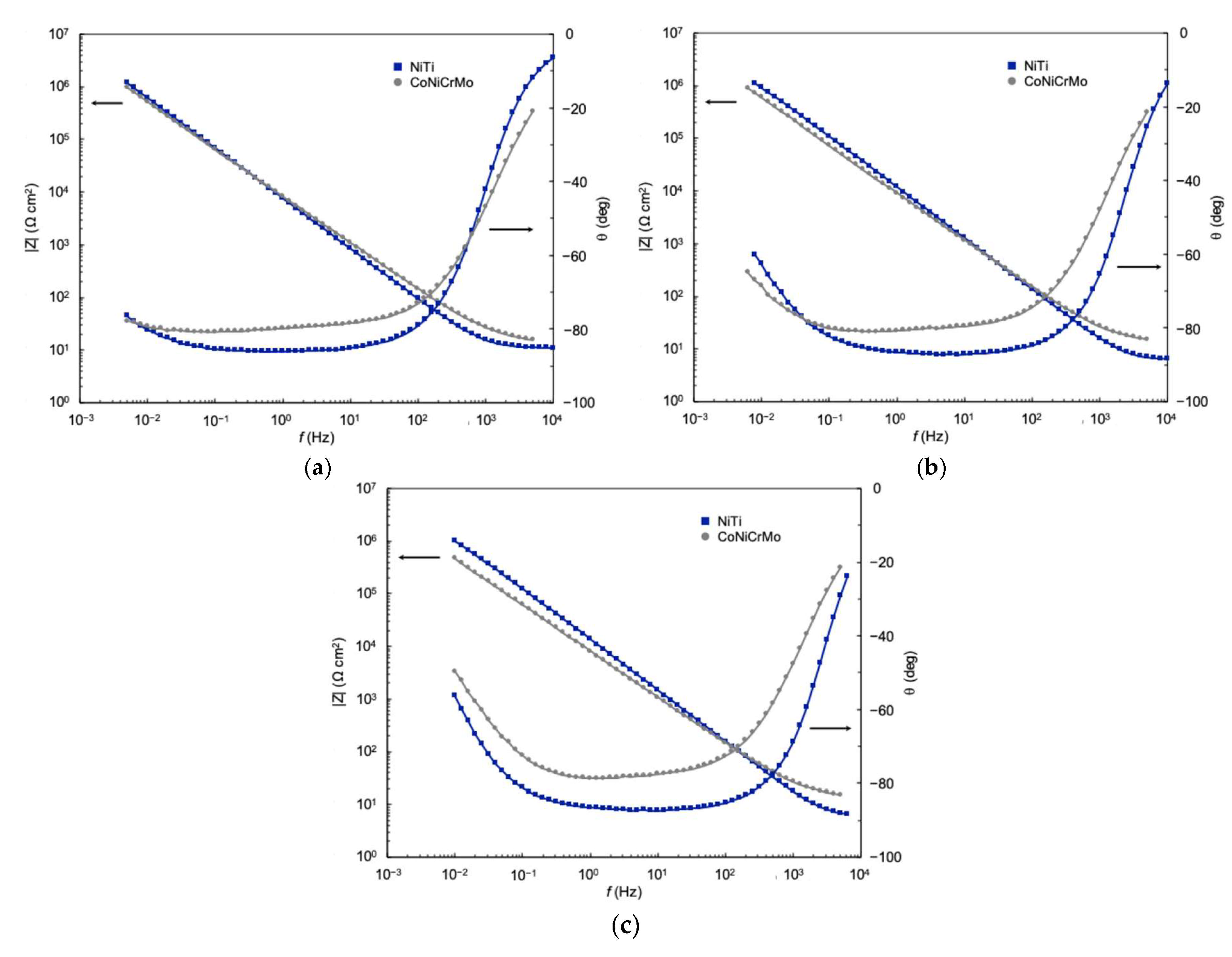
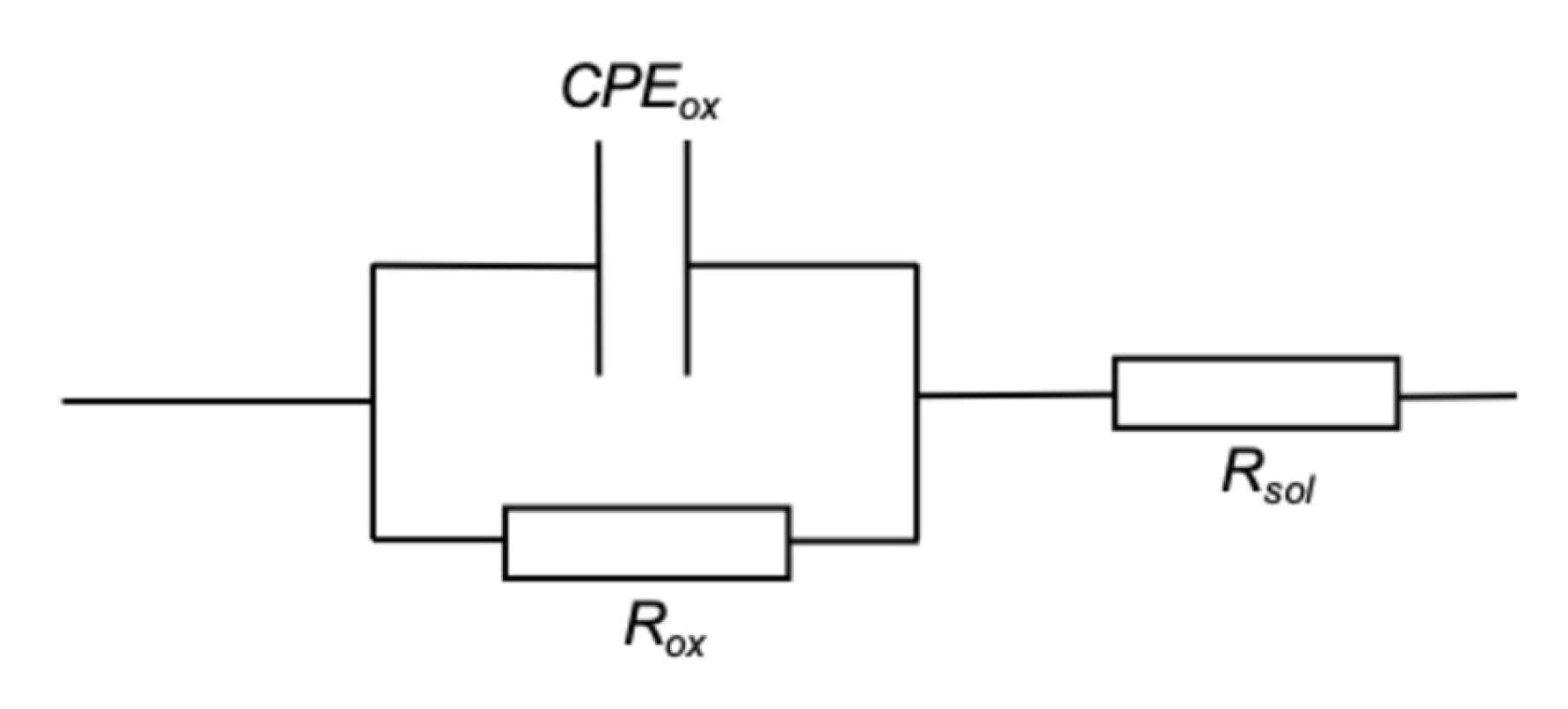
5. EIS Characterization
5.1. Oxide Thickness
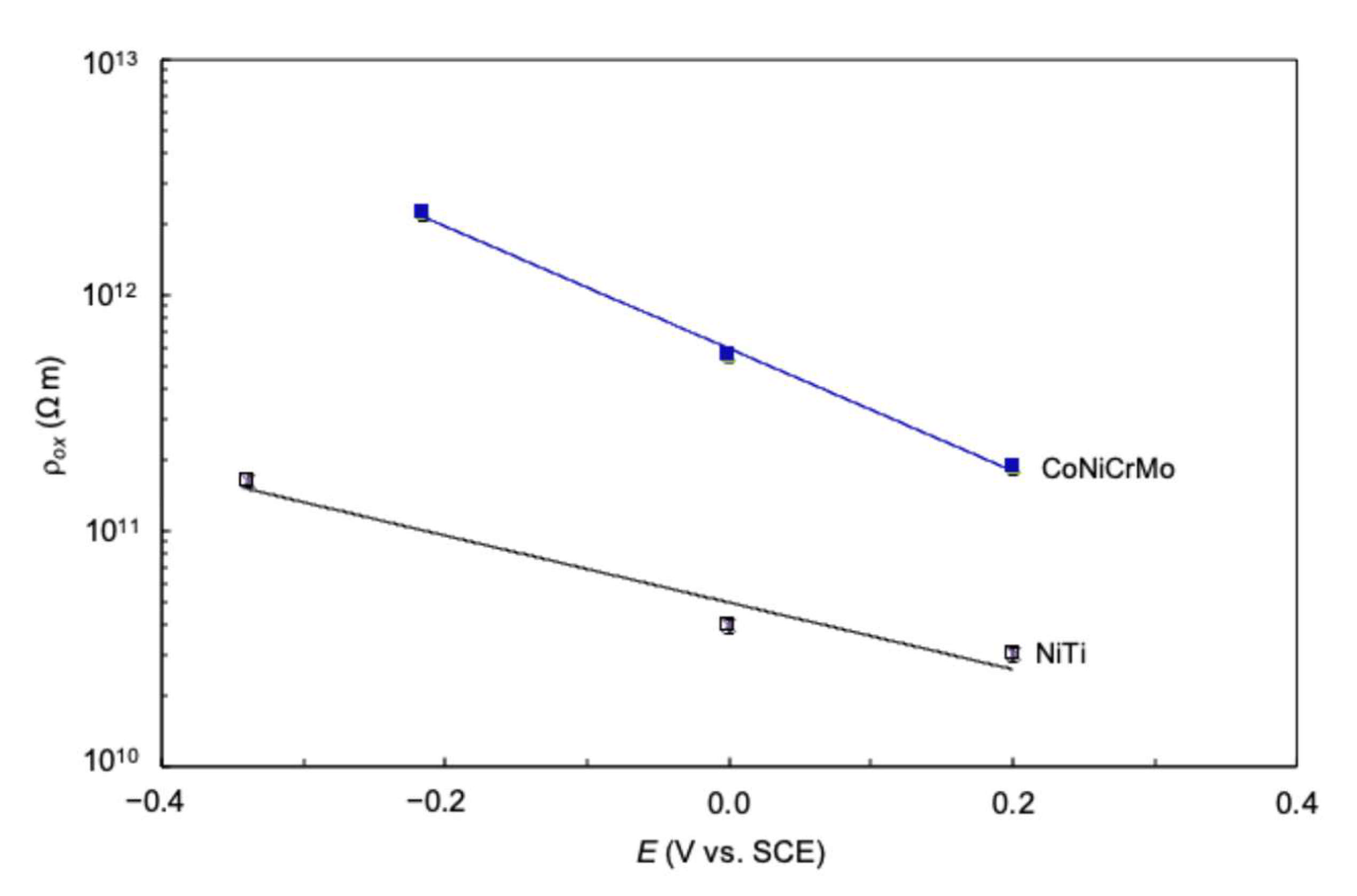
5.2. Oxide Resistivity
6. Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Pound, B.G. Susceptibility of nitinol to localized corrosion. J. Biomed. Mater. Res. Part A 2006, 77, 185–191. [Google Scholar] [CrossRef]
- Trepanier, C.; Tabrizian, M.; Yahia, L.H.; Bilodeau, L.; Piron, D.L. Effect of modification of oxide layer on NiTi stent corrosion resistance. J. Biomed. Mater. Res. 1998, 43, 433–440. [Google Scholar] [CrossRef]
- Thierry, B.; Tabrizian, M.; Trepanier, C.; Savadogo, O.; Yahia, L.H. Effect of surface treatment and sterilization processes on the corrosion behavior of NiTi shape memory alloy. J. Biomed. Mater. Res. 2000, 51, 685–693. [Google Scholar] [CrossRef]
- Trigwell, S.; Hayden, R.D.; Nelson, K.F.; Selvaduray, G. Effects of surface treatment on the surface chemistry of NiTi alloy for biomedical applications. Surf. Interface Anal. 1998, 26, 483–489. [Google Scholar] [CrossRef]
- Kocijan, A.; Milosev, I.; Pihlar, B. Cobalt-based alloys for orthopaedic applications studied by electrochemical and XPS analysis. J. Mater. Sci. Mater. Med. 2004, 15, 643–650. [Google Scholar] [CrossRef]
- Pound, B.G. The electrochemical behavior of nitinol in simulated physiological solutions. J. Biomed. Mater. Res. Part A 2008, 85, 1103–1113. [Google Scholar] [CrossRef]
- Pound, B.G. Electrochemical behavior of cobalt-chromium alloys in a simulated physiological solution. J. Biomed. Mater. Res. Part A 2010, 94, 93–102. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.-O.A.; Mischler, S. Passive and transpassive behavior of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Schmuki, P.; Virtanen, S.; Davenport, A.J.; Vitus, C.M. Transpassive dissolution of Cr and sputter-deposited Cr oxide studied by in situ x-ray near-edge spectroscopy. J. Electrochem. Soc. 1996, 143, 3997–4005. [Google Scholar] [CrossRef]
- Tabrizian, M.; Thierry, B.; Savadago, O.; Yahia, L.H. Surface characterization of sterilized electropolished NiTi shape memory alloy as biomaterials. In Proceedings of the Sensory Phenomena and Measurement Instrumentation for Smart Structures and Materials, Newport Beach, CA, USA, 1 March 1999; SPIE: Bellingham, WA, USA, 1999; Volume 3670, pp. 106–114. [Google Scholar]
- Pound, B.G.; Becker, C.H. Composition of surface films on nickel based superalloys. J. Electrochem. Soc. 1991, 138, 696–700. [Google Scholar] [CrossRef]
- Igual Muñoz, A.; Mischler, S. Interactive effects of albumin and phosphate ions on the corrosion of CoCrMo implant alloy. J. Electrochem. Soc. 2007, 154, C562–C570. [Google Scholar] [CrossRef]
- Pound, B.G. Pit initiation on nitinol in simulated physiological solutions. J. Biomed. Mater. Res. Part B 2018, 106, 1605–1610. [Google Scholar] [CrossRef]
- Pound, B.G. Pit initiation on biomedical alloys—A review. J. Biomed. Mater. Res. Part B 2024, 112, e35367. [Google Scholar] [CrossRef]
- Hu, R.; Ornberg, A.; Pan, J. Investigation of influence of small particles in MP35N on the corrosion resistance in synthetic biological environment. J. Electrochem. Soc. 2009, 156, C341–C344. [Google Scholar] [CrossRef]
- Pound, B.G. The passive behavior of biomedical alloys in simulated physiological solutions. J. Biomed. Mater. Res. Part B 2022, 110, 768–775. [Google Scholar] [CrossRef]
- Venugopalan, R.; Trepanier, C. Assessing the corrosion behavior of Nitinol for minimally-invasive device design. Minim. Invasive Ther. Allied Technol. 2000, 9, 67–74. [Google Scholar] [CrossRef]
- Trepanier, C.; Pelton, A.R. Effect of strain on the corrosion resistance of nitinol and stainless steel in simulated physiological environment. In Proceedings of the International Conference on Shape Memory and Superelastic Technologies, SMST-2003, Pacific Grove, CA, USA, 5–8 May 2003; pp. 393–398. [Google Scholar]
- Wever, D.J.; Veldhuizen, A.G.; de Vries, J.; Busscher, H.J.; Uges, D.R.A.; van Horn, J.R. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef]
- Ouerd, A.; Alemany-Dumont, C.; Normand, B.; Szunerits, S. Reactivity of CoCrMo alloy in physiological medium: Electrochemical characterization of the metal/protein interface. Electrochim. Acta 2008, 53, 4461–4469. [Google Scholar] [CrossRef]
- Alkhateeb, E.; Virtanen, S. Influence of surface self-modification in Ringer’s solution on the passive behavior of titanium. J. Biomed. Mater. Res. Part A 2005, 75, 934–940. [Google Scholar] [CrossRef]
- Seyeux, A.; Maurice, V.; Marcus, P. Oxide film growth kinetics on metals and alloys. I. Physical model. J. Electrochem. Soc. 2013, 160, C189–C196. [Google Scholar] [CrossRef]
- Lutton, K.; Scully, J.R. Kinetics of oxide growth of passive films on transition metals. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: New York, NY, USA, 2018; pp. 284–290. [Google Scholar]
- Leistner, K.; Toulemonde, C.; Diawara, B.; Seyeux, A.; Marcus, P. Oxide film growth kinetics on metals and alloys. II. Numerical simulation of transient behavior. J. Electrochem. Soc. 2013, 160, C197–C205. [Google Scholar] [CrossRef]
- Yeung, K.W.K.; Poon, R.W.Y.; Chu, P.K.; Chung, C.Y.; Liu, X.Y.; Lu, W.W.; Chan, D.; Chan, S.C.W.; Luk, K.D.K.; Cheung, K.M.C. Surface mechanical properties, corrosion resistance, and cytocompatibility of nitrogen plasma-implanted nickel–titanium alloys: A comparative study with commonly used medical grade materials. J. Biomed. Mater. Res. Part A 2007, 82, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ornberg, A.; Pan, J.; Herstedt, M.; Leygraf, C. Corrosion resistance, chemical passivation, and metal release of 35N LT and MP35N for biomedical material application. J. Electrochem. Soc. 2007, 154, C546–C551. [Google Scholar] [CrossRef]
- Schroeder, V. Evolution of the passive film on mechanically damaged nitinol. J. Biomed. Mater. Res. Part A 2009, 90, 1–17. [Google Scholar] [CrossRef]
- Ferreira, M.G.S.; Da Cunha Belo, M.; Hakiki, N.E.; Goodlet, G.; Montemor, M.F.; Simões, A.M.P. Semiconducting properties of oxide and passive films formed on AISI 304 stainless steel and Alloy 600. J. Braz. Chem. Soc. 2002, 13, 433–440. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Lopez, A.S.; Mirza-Rosca, J.; Vasilescu, C.; Ionita, D. Corrosion susceptibility of implant materials Ti-5Al-4V and Ti-6Al-4Fe in artificial extra-cellular fluids. Electrochim. Acta 2004, 49, 2113–2121. [Google Scholar] [CrossRef]
- Kolman, D.G.; Scully, J.R. Electrochemistry and passivity of Ti-15V-3Cr-3Al-3Sn β-titanium alloy in ambient temperature aqueous chloride solutions. J. Electrochem. Soc. 1994, 141, 2622–2641. [Google Scholar] [CrossRef]
- Weast, R.C. (Ed.) CRC Handbook of Chemistry and Physics, 64th ed.; CRC Press: New York, NY, USA, 1983–1984; p. E-55. [Google Scholar]
- Kwan, C.-P.; Chen, R.; Singisetti, U.; Bird, J.P. Electric-field dependent conduction mechanisms in crystalline chromia. Appl. Phys. Lett. 2015, 106, 112901. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Sushko, P.V.; Bowden, M.E.; Heald, S.M.; Papadogianni, A.; Tschammer, C.; Bierwagen, O.; Chambers, S.A. Defect compensation by Cr vacancies and oxygen interstitials in Ti4+-doped Cr2O3 epitaxial thin films. Phys. Rev. B 2016, 94, 155409. [Google Scholar] [CrossRef]
- McAleer, J.F.; Peter, L.M. Photocurrent spectroscopy of anodic oxide films on titanium. Faraday Disc. Chem. Soc. 1980, 70, 67–80. [Google Scholar] [CrossRef]
- Torresi, R.M.; Camara, O.R.; De Pauli, C.P. Influence of the hydrogen evolution reaction on the anodic titanium oxide film properties. Electrochim. Acta 1987, 32, 1357–1363. [Google Scholar] [CrossRef]
- Sekizawa, K.; Oh-ishi, K.; Morikawa, T. Photoelectrochemical water-splitting over a surface modified p-type Cr2O3 photocathode. Dalton Trans. 2020, 49, 659–666. [Google Scholar] [CrossRef] [PubMed]


| Alloy | ba′ (V) | icorr (nA/cm2) |
|---|---|---|
| NiTi | 0.16 ± 0.1 | 4.7 ± 0.2 |
| CoNiCrMo | 0.19 ± 0.1 | 3.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pound, B.G. Comparison of the Passive Behavior of NiTi and CoNiCrMo in Simulated Physiological Solutions. Corros. Mater. Degrad. 2025, 6, 4. https://doi.org/10.3390/cmd6010004
Pound BG. Comparison of the Passive Behavior of NiTi and CoNiCrMo in Simulated Physiological Solutions. Corrosion and Materials Degradation. 2025; 6(1):4. https://doi.org/10.3390/cmd6010004
Chicago/Turabian StylePound, Bruce G. 2025. "Comparison of the Passive Behavior of NiTi and CoNiCrMo in Simulated Physiological Solutions" Corrosion and Materials Degradation 6, no. 1: 4. https://doi.org/10.3390/cmd6010004
APA StylePound, B. G. (2025). Comparison of the Passive Behavior of NiTi and CoNiCrMo in Simulated Physiological Solutions. Corrosion and Materials Degradation, 6(1), 4. https://doi.org/10.3390/cmd6010004