Abstract
In this work, the microstructure and degradation properties of a novel metal matrix composite composed of Mg with the addition of 1 vol. % hydroxyapatite nanopowder (Mg + 1 vol % nHAp) were evaluated. The composites in the form of discs produced using spark plasma sintering (SPS) were subjected to plastic deformation using a modified extrusion technique with an oscillating die located at the end of the extruder (called KoBo), which enables deformation without the preheating of the initial billet. The microstructure was analyzed using optical and scanning electron microscopy (SEM) with subsequent electron backscattered diffraction (EBSD) measurements. The corrosion properties were evaluated based on electrochemical and immersion tests. To assess early biological performance, cytotoxicity tests were performed. The addition of nHAp did not significantly change the corrosion rate; however, the subsequent plastic deformation greatly decreased it. Interestingly, the sample after plastic deformation without the preheating of the initial billet was characterized by the highest cell viability. Overall, the addition of nHAp improved the biological assessment of the extruded composite; however, during plastic deformation, due to the refinement of loosely adherent nHAp and the formation of bimodally distributed grain sizes, a high number of microgalvanic couples were formed, resulting in worse corrosion performance.
1. Introduction
Magnesium (Mg) and its alloys continue to attract considerable interest due to their status as the lightest structural metallic materials [1]. They hold significant promise in biomedical engineering because of their biodegradability, biocompatibility, and mechanical properties that are closely aligned with human bone [2]. When dissolving in the human body, Mg ions may support bone resorption processes, which makes Mg and its alloys attractive and under consideration as bioabsorbable materials [3]. When compared to Fe- and Ti-based commercially used implants, they do not show toxic behaviour and may compensate for the shielding effect [4,5]. However, the rapid degradation of Mg hinders its application in load-bearing applications [6]. Specifically, in bone implants, the accumulation of hydrogen at the implant–bone interface compromises Mg’s mechanical integrity and exposes its limited bioactivity [7,8]. Incorporating elements such as zinc (Zn), manganese (Mn), zirconium (Zr), calcium (Ca), and trace amounts of low-toxicity rare-earth (RE) elements into Mg alloys significantly enhances their corrosion resistance. Another proposed method is to fabricate composites by combining Mg with biocompatible and bioresorbable reinforcement materials [9]. Mg-based metal matrix composites (MMCs) often exhibit superior mechanical properties, enhanced low density, improved dimensional stability, better corrosion resistance, and damping properties [10].
Powder metallurgy is a widely utilized method for the production of Mg-based composites [11,12,13]. Other methods include stir casting [14] and metal additive manufacturing [15]. These methods directly influence the microstructure and properties of the composites, including their mechanical properties, corrosion resistance, and biological behaviour [16]. Among these, powder metallurgy facilitates the uniform distribution of reinforcement within the matrix while maintaining low manufacturing temperatures and costs [17].
Mg-based MMCs can be fabricated by incorporating various types of reinforcement materials. Li et al. [18] studied the behaviour of carbon nanotube (CNT)-reinforced Mg-based composites. They used powder metallurgy to produce the composites and carried out compression tests in different orientations. The yield strengths of these composites increased by 35–129% compared to pure Mg; however, when using CNTs with high aspect ratios as reinforcement, achieving the uniform distribution of CNTs in Mg becomes challenging, particularly at elevated CNT weight percentages. Silicon carbide (SiC) is another important reinforcement in Mg-based MMCs, and over the years, different sizes and concentrations of SiC have been used [19]. Some other reinforcement materials include alumina (Al2O3), graphene nanoplatelets, boron carbide (B4O), titanium (Ti) and its alloys, and nanohydroxyapatite (nHA), which have been tested to improve the mechanical properties of Mg-based composites [20].
From a biological perspective, it is crucial to improve the integration and functionality of Mg-based MMCs, ensuring they support the regeneration of bone tissue and minimize adverse reactions within the body. Since nHA is the most important reinforcement ceramic material when used in biomedical applications, its addition may be crucial in the production of novel absorbable Mg-based implants [21]. nHA is the most stable form of calcium phosphate bio-ceramics, and it plays a key role in enhancing bioactivity and osteoconductivity, allowing for quicker integration with surrounding bone tissue. Additionally, it has been reported that nHA particles have the potential to inhibit the growth of certain cancer cells [22]. According to Lim et al. [23], the best bioactivity was achieved by incorporating 10 wt% HA into the Mg-Ca alloy, within the range of 5 wt% to 15 wt% HA. Xu et al. [24] found that incorporating higher amounts of HAp may negatively impact the bioactivity or mechanical properties of magnesium alloys, and the detrimental effects of increased HAp content on the mechanical properties of the magnesium matrix are primarily due to particle agglomeration and the uneven distribution of the reinforcement within the matrix. Jayasathyakawin et al. [25] studied the effect of 9 wt.% nHA on the mechanical properties of sintered Mg3Al. Vickers hardness was recorded to improve significantly from 28 VHN to 60 VHN. In terms of the requirements of biodegradable materials for clinical applications, the composite Mg3Zn0.5Zr containing 1 wt% HAp particles evidently could be a promising bone fixation material [26]. Khodaei et al. [27] added 10 wt% nHA particles to Mg; the results showed that the compressive strength improved by 27%. In another study, combinations of Mg with 2 and 4 wt% nHA showed optimal properties; for example, the yield strength doubled compared to pure Mg, the plateau stress increased by three to four times, the corrosion rate decreased by 35–40%, and the composite had a similar strength and elastic modulus to human cancellous bones [28]. All these studies support the idea that a lower amount of nHA has the best effects on the properties of Mg-based composites.
In our study, we moved a step forward and made an attempt to use plastic deformation without the preheating of the initial billet (extrusion with an oscillating die, called KoBo) to significantly improve the mechanical and biological properties of Mg with the addition of 1 vol.% hydroxyapatite nanopowder (nHAp). KoBo involves deforming metals at lower temperatures, ultimately producing high-strength materials with enhanced properties [29]. Therefore, in this study, we sintered Mg-1 vol.% nHA and extruded the alloy without the preheating of the initial billet, which has not been used so far for hcp-structured metallic composites. The results showed improvements in the biological and mechanical properties of the composites, but the subsequent plastic deformation caused a significant increase in the corrosion rate.
2. Materials Preparation
Mg-based composites were fabricated using the high-purity atomized Mg powder Atomised Magnesium Powder Société pour la Fabrication du Magnésium (SFM, UNS 1418 with a particle size of 25–80 μm, Martigny, Switzerland) and hydroxyapatite nanopowder (Sigma Aldrich, <200 nm particle size, Poznan, Poland). Pure Mg powder was mixed with 1 vol% nHAp using a turbula mixer for 3 h, as shown in Figure 1a. To obtain better homogeneity of the powders, steel balls were used with a 1:1 ratio of balls to powder. Next, samples were produced using spark plasma sintering (SPS) [30]. The powder mixtures were placed in a cylindrical graphite die with an inner diameter of 40 mm between two graphite punches, as shown in Figure 1b. The powder was then heated up to 500 °C at a rate of 30–40 °C/min. Upon reaching this temperature, a final pressure of 40 MPa was applied and maintained for 10 min, resulting in the production of round specimens with a diameter of 40 mm. As a reference material, pure Mg was sintered. The SPS-ed composite was then subjected to extrusion with an oscillating die (KoBo), which enables deformation of Mg alloys at high deformation ratios without preheating of the initial billet (Figure 1c). The sample was extruded at a remarkable force of 150 tons with an extrusion speed of 0.2 mm/s, and the die was rotated at angle of ± 8° with a 5 Hz frequency. The final diameter of the sample was 5 mm, resulting in an 8:1 extrusion ratio.
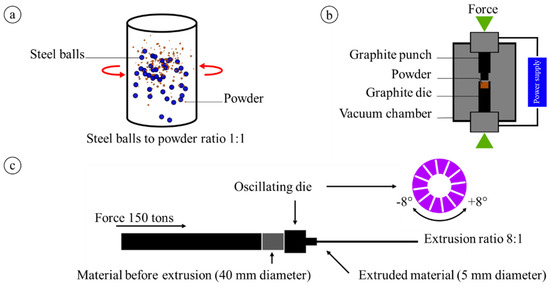
Figure 1.
Schematic of materials’ preparation: (a) powder preparation using turbula mixing, (b) spark plasma sintering (SPS) used for sintering, and (c) extrusion with an oscillating die (KoBo).
3. Methodology
3.1. Powder Characterization
The particle size distribution (PSD) was measured using a laser diffraction analyser, Horiba LA950, in a water suspension. The powder morphology and chemical analyses were performed using a scanning electron microscope (FE-SEM, Hitachi SU70, Tokyo, Japan) equipped with an energy-dispersive spectrometer (EDS).
3.2. Microstructure Characterization
To reveal the optical microstructure of the samples, the sintered and extruded composites were cut, mounted with resin, and grinded with 320-, 600-, 1200-, and 2500-grit sand paper. Polishing was performed with water-free diamond suspensions (3 µm and 1 µm) and glycol-based lubricant. Samples were etched using reagent based on picric acid, and the optical images were made using an optical microscope (Axio Scope A.1 Zeiss, Warsaw, Poland). To achieve a comprehensive description of the microstructure formed during sintering, the samples were prepared for further observations by ion milling in an Ar (IM Hitachi 4000, Hitachi Ltd., Tokyo, Japan). The electron backscatter diffraction (EBSD) scans were then conducted using a SEM (Hitachi SU70, Hitachi Ltd., Tokyo, Japan) equipped with an EBSD detector (Bruker, Berlin, Germany). The EBSD recorded data with a step size of 200 nm, and the grain sizes were determined using Quantax Esprit Bruker microanalysis software (https://www.bruker.com/en/products-and-solutions/elemental-analyzers/eds-wds-ebsd-SEM-Micro-XRF/software-esprit-family.html). The crystallographic orientations of the grains were displayed in the form of inverse pole figure (IPF) maps, where different colours represented the orientation of a specific sample direction within a crystal frame.
Vickers hardness testing machine was used to evaluate the microhardness of the samples with the maximum load of 200 g. An Innovatest® (Maastricht, The Netherlands) Falcon 500 micro indenter was used [31]. A total of 10 indents were applied on each sample in a line, with the distance among dents set to 400 μm.
3.3. Corrosion Studies
The corrosion behaviour of the materials was evaluated using electrochemical measurements, while the corrosion rate was determined based on the hydrogen release method. In this research, all samples were tested with open circuit potential (OCP) and potentiodynamic polarization (PDP) starting from 0.5 V below EOCP to 1.5 V vs. Ref with a scan rate of 5 mV. After PDP testing, Tafel extrapolation was performed and characteristic parameters including corrosion potential (Ecorr), corrosion current density (icorr), and corrosion rate (CR) were calculated. The tests were conducted at ambient temperature in 0.1 M NaCl solution using a Gamry 600+ potentiostat. A three-electrode setup was used, with the sample as a working electrode, Pt as a counter electrode (CE), and Ag/AgCl as a reference electrode. The corrosion rate was calculated using the hydrogen release method test in 0.1 M NaCl solution for 1 h, as described in [32]. Prior to the immersion, samples were ground up to #4000 SiC paper and rinsed in ethanol for 30 s. Then, they were immersed in freshly prepared solution using 50 mL per 1 cm2 of the sample surface. Samples were placed into the beaker, and the volume of hydrogen gas was measured through PVC tubes connected to the burette. Based on the amount of hydrogen, the mass loss of the samples was calculated, and this allowed us to calculate the corrosion rate according to [33]. To ensure reproducibility, three samples from each material were tested. Using the amount of released hydrogen, the corrosion rate was calculated. After immersion tests, the corroded surfaces were analysed after the removal of corrosion product by chemical immersion in water-based CrO3 solution, as specified in Pałgan et al. [34].
3.4. Early Biological Assessment
The cytotoxicity of Mg and the composites was evaluated using an extract assay according to ISO 10993-5 norm, as described in [35]. Prior to tests, the samples were cleaned for 15 min in acetone and 15 min in ethanol, dried in a laminar flow cabinet, and exposed to UV light (30 min for each side). The extracts were prepared by immersing the samples in Dulbecco’s Modified Eagle Medium (DMEM) at a surface area/volume equal to 2.4 cm2/mL (we reduced the ratio by 20% to ensure complete immersion of the samples) and incubating them at 37 °C in an atmosphere containing 5% CO2 and ~90% humidity for a duration of 24 h. As a control, medium without any samples was also incubated in the same conditions. L929 cells were cultured in DMEM with the addition of 10% fetal bovine serum (FBS), 1% penicillin and streptomycin (PS), and 2 mM L-glutamine. Cells were seeded in 96-well plates and allowed to adhere overnight. On the next day, DMEM extracts were supplemented with FBS, added to the wells with cells, and incubated for 24 h. The effect of soluble corrosion products on cell viability was evaluated using qualitative microscopic observations of cell morphology and quantified using an MTS assay. Cell morphology was visualized using a Primovert inverted microscope and Zen 3.6 software (Zeiss, Poland). For the MTS assay, the extracts were replaced by 100 μL of fresh DMEM w/o FBS, to which 20 μL of MTS was added. After incubation for 1 h in cell culture conditions, the absorbance was measured at λ = 490 nm using a Fluorostar Omega plate reader (BMG Labtech, Ortenberg, Germany). The results were statistically analysed using a one-way ANOVA and Tukey–Kramer pair-wise comparisons (KyPlot, version 2.0 beta 12).
4. Results
4.1. Powder Characterization
Pure Mg has the highest number of particles in the range of 45–70 μm, as shown in Figure 2. After nHAp powder was mixed with pure Mg, no significant change was observed, except that the average particle size shifted slightly towards higher values. Mg powder particles were highly spherical, and the nHAp particles appeared to have an irregular, granular morphology which agglomerated and forming clusters, having a less uniform shape and more developed surface, as shown in Figure 3.
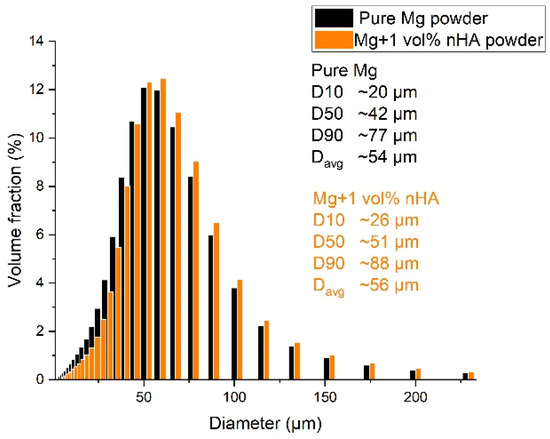
Figure 2.
Particle size distribution of pure Mg and Mg + 1 vol% nHAp powder after turbula mixing.
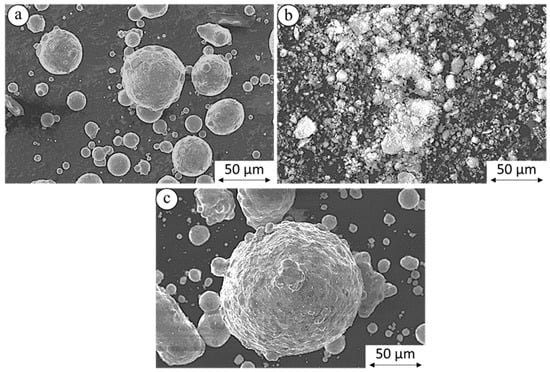
Figure 3.
SEM images of powder particles: (a) pure Mg, (b) hydroxyapatite nanopowder (nHAp), and (c) Mg+1 vol% nHAp after turbula mixing.
4.2. Microstructure Characterization of the Composites
The microstructure of the composite Mg + 1 vol.% nHAp consisted of clearly visible Mg powder particles (marked by the yellow arrows, Figure 4a), in which sintering was limited due to the oxide shell formation. Inside each particle, grain boundaries were observed (marked by the red arrows, Figure 4a,b). The nHAp particles were located mainly between Mg particles (white arrows Figure 4a,b). The subsequent plastic deformation, which was conducted without preheating of the initial billet, led to significant grain refinement (Figure 4c,d). No more particle boundaries and grain boundaries were recognized. As a result of plastic deformation, the nHAp particles were also refined (Figure 4d).
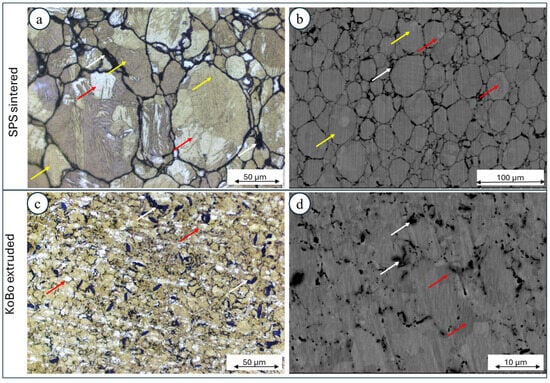
Figure 4.
The characterization of the microstructure of the SPS-ed Mg + 1 vol.% nHAp composite ((a) optical image, (b) SEM image) SPS, band Mg + 1 vol.% nHAp composite produced using SPS with subsequent extrusion using KoBo ((c) optical image, (d) SEM image) (yellow arrows—powder particles, red arrows—grains, white arrows—nHAp).
The randomly oriented grains were formed in the composite sintered using SPS, while after extrusion without preheating of the initial billet, the orientation of the grains changed, and they were mainly oriented towards <10> and <20>, Figure 5a,b. It should be underlined that extrusion using KoBo without preheating of the initial billet refined the grain size 6.5-fold, from 20 µm to 4 µm (Figure 5e,f). This is a very atypical phenomenon for hexagonally crystalized metals; however, an additional die located at the end of the extruder caused that material enter to the state of plastic flow, which enabled such a significant reduction of grains. In the case of SPS-ed material, all grains were misoriented by more than 15°, and high-angle grain boundaries (HAGBs) were formed (Figure 5c). A significant change was observed for the plastically deformed alloy, where despite HAGBs, low-angle grain boundaries (LAGBs) were also observed, suggesting that a substructure formed (Figure 5d). This in turn is a clear indication that, typical of Mg, dynamic recrystallization (DRX) processes occurred [31]. As a result of the addition of ceramic filler, the microhardness values of the composites were higher than those measured for pure Mg (Figure 6).
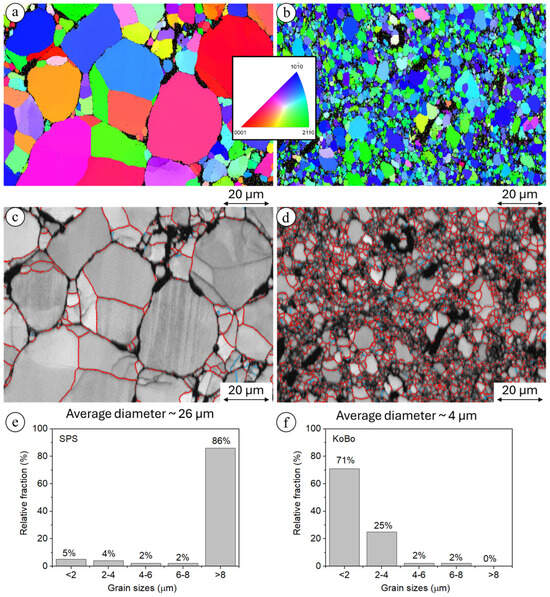
Figure 5.
The EBSD data obtained from the measurements of the Mg+nHAp; (a) IPF map for the composite produced using SPS, (b) IPF map for the composite produced using SPS with subsequent extrusion using KoBo, (c) grain boundaries characteristic of the composite produced using SPS, (d) grain boundaries characteristic of the composite produced using SPS with subsequent extrusion using KoBo, (e) grain size distribution for Mg + nHAp produced using SPS, (f) grain size distribution for Mg + nHAp produced using SPS with subsequent extrusion using KoBo.
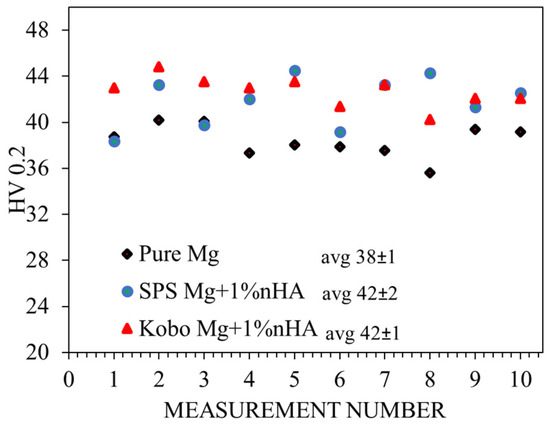
Figure 6.
Microhardness profiles for the investigated materials.
4.3. Corrosion Performance
The open circuit potential (EOCP) evolution recorded in 0.1 M NaCl during 1 h of immersion for the investigated materials is shown in Figure 7a. Among the analysed materials, the most stable one is that for pure Mg (around −1.53 V/Ref). A rapid increase of EOCP from −1.56 V/Ref to −1.51 V/Ref during the first 15 min of immersion was observed for the SPS-ed sample, and it started to gradually decrease, exhibiting a value of −1.52 V/Ref at the end of the immersion time. Such a situation favours passivation reactions. A different situation was observed for the plastically deformed material; herein, a rapid decrease of the initial values of EOCP from around −1.52 V/Ref to −1.54 V/Ref was noticed, indicating an initial reduction of the passive layer.
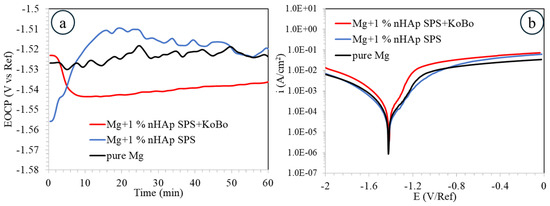
Figure 7.
Results obtained from the electrochemical testing in 0.1M NaCl for pure Mg, the SPS sintered composite, and the SPS + KoBo extruded composite: (a) open circuit potential evaluation, (b) potentiodynamic curves.
The shape of the potentiodynamic curves followed the same trend in very similar current density and potential value ranges. The anodic branches indicated quick dissolution of the passive layers formed on the materials with some slight inflection point around the potential value of −1.48 V/Ref. The calculated values of corrosion current density, icorr = 58 ± 4 µA/cm2, varied, with the lowest being for the SPS-ed sample; this shows that the addition of the nHAp may decrease the corrosion rate of pure Mg by improving passivation reactions. The highest icorr = 113 ± 4 µA/cm2 was calculated for the samples after plastic deformation (Table 1).

Table 1.
Electrochemical parameters extrapolated using the Tafel method for the investigated materials (icorr—corrosion current density, Ecorr—corrosion potential, and corrosion rate in mpy).
Since we are aware that Tafel extrapolation is not the ideal method through which to calculate the corrosion rate for Mg alloys, we also decided to perform measurements using the hydrogen release method. As shown in Table 2, the results fully agree with the ones obtained from the electrochemical measurements, showing a highest corrosion rate of 2132 ± 203 mpy for plastically deformed composite and a lowest rate for the SPS-ed sintered sample (95 ± 23 mpy). The corrosion rate of pure Mg is always in the middle of the trend.

Table 2.
Corrosion rate calculated for the investigated materials based on hydrogen release testing.
The corrosion damage formed on the SPS-ed samples was mostly observed in the form of pits developing on the surface. They were formed in the areas where the nHAp was built into the microstructure (Figure 8a). The SEM observations show that the severe corrosion propagated around Mg particles where an oxygen shell was formed (in Figure 8b,c, some dissolved Mg particles can be seen in the magnified SEM image). A different kind of corrosion damage was observed for the samples after plastic deformation. The corrosion was distributed more uniformly across the surface (Figure 8d). The deep empty areas in Figure 8e correspond with the places where nHAp was embedded. Based on Figure 8e,f, one can notice that microgalvanic corrosion also occurred; however, it did not spread throughout the full depth of the material, as in the case of the SPS-ed sample. The magnified image shows typical corrosion development across the hcp-structured Mg alloys, as previously observed for KoBo-extruded Mg-4Li-3Al-1Zn [36].
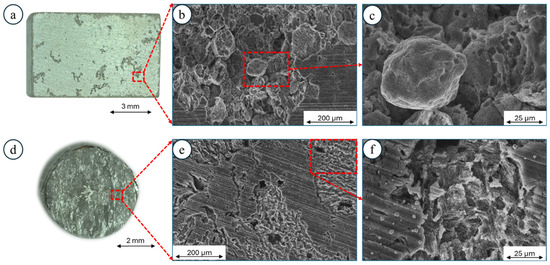
Figure 8.
Characterization of corrosion damage using optical microscopy and SEM after chemical removal of corrosion products: (a–c) Mg + 1 vol.% nHAp composite sintered using SPS, (d–f) Mg + 1 vol.% nHAp composite produced using SPS with subsequent extrusion using KoBo.
4.4. Cytotoxic Effect of Corrosion Products
As Mg-based composites are envisioned for biomedical applications, we investigated their cytotoxicity by performing in vitro test on extracts. As depicted in Figure 9, cell density was lower in wells with Mg-based extracts compared to the negative control. Moreover, the majority of cells cultured in wells with pure Mg and the Mg + 1 vol.% nHAp SPS-ed composite exhibited a round morphology. This was further confirmed by quantitative analysis of cell viability using the MTS assay (Figure 9e). Indeed, the viability was significantly lower for all of the Mg-based materials than for the negative control and the extraction vessel control (EV). However, additional processing of the composite through plastic deformation using a modified extrusion technique (KoBo) seemed to increase their corrosion rate. Although no significant differences were measured in cell viability, qualitative analysis of the morphology of L929 fibroblasts confirmed this trend: cell density was higher in the Mg + SPS + KoBo group than in the Mg and Mg + SPS groups; moreover, the well-spread cells outnumbered the round ones in the former group.
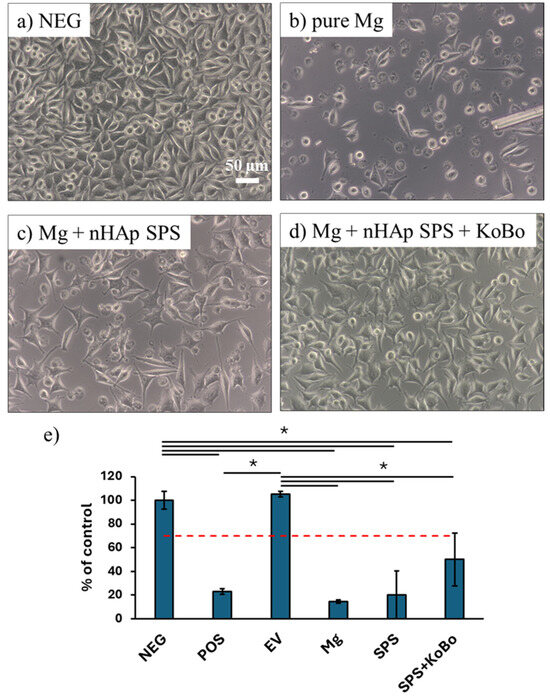
Figure 9.
Evaluation of the cytotoxicity. (a–d) Morphology of L929 fibroblasts cultured in complete cell culture medium (negative control, NEG) and cultured with extract-based media for 24 h. (e) Quantitative assessment of viability of L929 cells determined by means of the MTS assay. The red dashed line depicts the cytotoxicity threshold, and solid lines indicate the statistical differences between the tested groups. POS—positive control, i.e., complete medium supplemented with DMSO; EV—extraction vessel, i.e., medium incubated w/o samples. *—p < 0.05.
5. Discussion
In this work, the effect of hydroxyapatite nanopowder (nHAp) was investigated in terms of optimization of the degradation and the biological properties of pure Mg produced using SPS. Next, the role of plastic deformation at high extrusion ratios without preheating of the initial billet was also evaluated. The sintered composite did not undergo full crystallization, and particles of Mg powders still existed in the matrix. It is commonly known that the Mg particles are covered by an oxide shell, and it would be very hard to choose conditions that assure the full diffusion of oxygen during sintering. A similar phenomenon was observed by Nakahata et al. [37] and by Khalil et al. [38], regardless of the conditions of sintering.
The nHAp was located mainly in the interparticle areas. As a result of subsequent plastic deformation, the fully recrystallized microstructure that formed was characterized by refined grains; notably, the size of the nHAp changed and underwent significant reduction, leading to a more uniform distribution of the ceramic filler in the Mg matrix. Such a situation is favourable for improving biological responses. It has previously been proven that the shape and the distribution of nHAp in the Mg matrix have a significant impact on corrosion behaviour. For example, Razavi et al. [39] found that spherical nHAp performs better than needle-shaped nHAp. In our case, the corrosion behaviour was instead related to the size and distribution of nHAp. As proven by the SEM observations compiled with microstructural ones, the corrosion attack started between loosely adherent nHAp particles and the Mg matrix, where the corrosion solution was easily reached, resulting in dropout of nHAp through the dissolution of the surrounding Mg matrix. Additionally, the microstructure with bimodal grain size distribution enhanced the quick dissolution of the Mg matrix, lowering the corrosion resistance of the KoBo-deformed alloy. In the mentioned system, the big primary grain interiors are cathodes, and they force small recrystallized grains and grain boundaries to corrode severely. Such a corrosion mechanism has been previously proven in our other research projects when analyzing Mg-based alloys [40]. From a commercial use perspective, the cell viability of bioabsorbable Mg composites may lead to several beneficial situations; however, their corrosion rate needs to be further evaluated in terms of its adjustability for use as a biodegradable material.
6. Conclusions
Based on the above research, the following conclusions can be drawn:
- It is possible to plastically deform Mg + 1 vol. % nHAp at a high extrusion ratio (R = 8:1) via extrusion without preheating of the initial billet. During deformation, a significant reduction in grain size occurs, alongside refinement of the ceramic filler. This leads to a more uniform distribution of nHAp in the Mg matrix.
- The addition of nHAp improves cell viability. Simultaneously, its higher refinement after KoBo deformation and its bimodally distributed grain sizes accelerate the degradation of the plastically deformed samples.
- Since an implant working in the human body should possess essential strength to support fractures and stabilize the area of a given fracture, the subsequent plastic deformation of Mg+1 vol.% HAp may have a detrimental effect on its application. Independently, microstructural changes leading to an improvement in cell viability may cause poor cell adhesion.
Author Contributions
Conceptualization, A.D.; methodology, A.D., K.K. and J.I.; validation, A.D., R.Z. and J.M.; investigation, K.K. and Z.H.; resources, M.T., R.Z. and D.K.; data curation, A.D., J.I., Z.H., K.K. and J.M.; writing—original draft preparation, A.D., Z.H. and J.I.; writing—review and editing, A.D., K.K., J.I. and J.M.; supervision, J.M. and R.Z.; funding acquisition, M.T. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dubey, A.; Lahiri, D. In Vitro Biodegradation and Biocompatibility of Mg–HA-Based Composites for Orthopaedic Applications: A Review. J. Indian Inst. Sci. 2019, 99, 303–327. [Google Scholar] [CrossRef]
- Lane, J.M.; Mait, J.E.; Unnanuntana, A.; Hirsch, B.P.; Shaffer, A.D.; Shonuga, O.A. Materials in Fracture Fixation. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 219–235. ISBN 978-0-08-055294-1. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Junker, R.; Bronkhorst, E.M.; Jansen, J.A. Bone regeneration related to calcium phosphate-coated implants in osteoporotic animal models: A meta-analysis. Tissue Eng. Part B Rev. 2012, 18, 383–395. [Google Scholar] [CrossRef]
- Suh, J.S.; Suh, B.C.; Bae, J.H.; Kim, Y.M. Machine learning-based design of biodegradable Mg alloys for load-bearing implants. Mater. Des. 2023, 225, 111442. [Google Scholar] [CrossRef]
- Haghshenas, M. Mechanical characteristics of biodegradable magnesium matrix composites: A review. J. Magnes. Alloys 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Khalil, K.A. A new-developed nanostructured Mg/HAp nanocomposite by high frequency induction heat sintering process. Int. J. Electrochem. Sci. 2012, 7, 10698–10710. [Google Scholar] [CrossRef]
- Arora, G.S.; Saxena, K.K.; Mohammed, K.A.; Prakash, C.; Dixit, S. Manufacturing Techniques for Mg-Based Metal Matrix Composite with Different Reinforcements. Crystals 2022, 12, 945. [Google Scholar] [CrossRef]
- Ghasali, E.; Bordbar-Khiabani, A.; Alizadeh, M.; Mozafari, M.; Niazmand, M.; Kazemzadeh, H.; Ebadzadeh, T. Corrosion behavior and in-vitro bioactivity of porous Mg/Al2O3 and Mg/Si3N4 metal matrix composites fabricated using microwave sintering process. Mater. Chem. Phys. 2019, 225, 331–339. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Improving mechanical properties of magnesium using nano-yttria reinforcement and microwave assisted powder metallurgy method. Compos. Sci. Technol. 2007, 67, 2657–2664. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.A.; Mohan, K.S.S.; Rabeeh, V.P.M.; Joseph, M.A.; Mubarak Ali, M.; Hanas, T. Hot rolled Mg-Ca/nHA composite for biodegradable implant material—A novel approach. Mater. Today Commun. 2023, 35, 106235. [Google Scholar] [CrossRef]
- Ali, F.; Kalva, S.N.; Koç, M. Additive Manufacturing of Polymer/Mg-Based Composites for Porous Tissue Scaffolds. Polymers 2022, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.R.; Dumpala, R. Magnesium-Based Composites for Degradable Implant Applications. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Oxford, UK, 2021; pp. 770–780. ISBN 978-0-12-819731-8. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Cheng, Y.; Gong, D.; Wang, W. Microstructure, mechanical, corrosion properties and cytotoxicity of beta-calcium polyphosphate reinforced ZK61 magnesium alloy composite by spark plasma sintering. Mater. Sci. Eng. C 2019, 99, 1035–1047. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B. Compression behavior of magnesium/carbon nanotube composites. J. Mater. Res. 2013, 28, 1877–1884. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gao, X.Y.; Nie, K.B.; Li, Y.N.; Deng, K.K. Different effects of SiC dimensions on the microstructure and mechanical properties of magnesium matrix composites. Mater. Sci. Eng. A 2022, 847, 143273. [Google Scholar] [CrossRef]
- Sambasivam, S.; Shirbhate, S.; Abed, A.S.; Patil, P.P.; Khan, I.; Singh, R.; Kansal, L.; Awasthi, A. Significance of reinforcement in Mg-based MMCs for various applications: A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Kavasi, R.M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In vitro biocompatibility assessment of nano-hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Lim, P.N.; Lam, R.N.; Zheng, Y.F.; Thian, E.S. Magnesium-calcium/hydroxyapatite (Mg-Ca/HA) composites with enhanced bone differentiation properties for orthopedic applications. Mater. Lett. 2016, 172, 193–197. [Google Scholar] [CrossRef]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Jayasathyakawin, S.; Ravichandran, M.; Ismail, S.O.; Srinivasan, D. Effects of hydroxyapatite addition on the microstructure and mechanical properties of sintered magnesium matrix composites. Mater. Today Commun. 2023, 35, 105582. [Google Scholar] [CrossRef]
- Sun, J.; Chen, M.; Cao, G.; Bi, Y.; Liu, D.B.; Wei, J. The effect of nano-hydroxyapatite on the microstructure and properties of Mg–3Zn–0.5Zr alloy. J. Compos. Mater. 2014, 48, 825–834. [Google Scholar] [CrossRef]
- Khodaei, M.; Nejatidanesh, F.; Shirani, M.J.; Iyengar, S.; Sina, H.; Savabi, O. Magnesium/Nano-hydroxyapatite Composite for Bone Reconstruction: The Effect of Processing Method. J. Bionic Eng. 2020, 17, 92–99. [Google Scholar] [CrossRef]
- Moradi, E.; Ebrahimian-Hosseinabadi, M.; Khodaei, M.; Toghyani, S. Magnesium/nano-hydroxyapatite porous biodegradable composite for biomedical applications. Mater. Res. Express 2019, 6, 75408. [Google Scholar] [CrossRef]
- Bochniak, W.; Ostachowski, P.; Korbel, A.; Łagoda, M. Potential of the KOBO extrusion process for nonferrous metals in the form of solids and chips. Int. J. Adv. Manuf. Technol. 2023, 127, 733–750. [Google Scholar] [CrossRef]
- Dobkowska, A.; Kruszewski, M.J.; Ciftci, J.; Morończyk, B.; Zgłobicka, I.; Zybała, R.; Żrodowski, Ł. Microstructure and Corrosion of Mg-Based Composites Produced from Custom-Made Powders of AZ31 and Ti6Al4V via Pulse Plasma Sintering. Materials 2024, 17, 1602. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-cie, B.; Aydee, M.; Garcia, G. Effect of High Deformation without Preheating on Microstructure and Corrosion of Pure Mg. Metals 2024, 14, 949. [Google Scholar] [CrossRef]
- Dobkowska, A.; Żrodowski, Ł.; Chlewicka, M.; Koralnik, M.; Adamczyk-Cieślak, B.; Ciftci, J.; Morończyk, B.; Kruszewski, M.; Jaroszewicz, J.; Kuc, D.; et al. A comparison of the microstructure-dependent corrosion of dual-structured Mg-Li alloys fabricated by powder consolidation methods: Laser powder bed fusion vs pulse plasma sintering. J. Magnes. Alloys 2022, 10, 3553–3564. [Google Scholar] [CrossRef]
- ASTM B90/B90M-12; Standard Specification for Magnesium-Alloy Sheet and Plate. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Pałgan, D.; Dobkowska, A.; Zielińska, A.; Drozdenko, D.; Máthis, K.; Święszkowski, W. The Role of LPSO Structures in Corrosion Resistance of Mg-Y-Zn Alloys. Crystals 2022, 12, 1723. [Google Scholar] [CrossRef]
- Zgłobicka, I.B.; Dobkowska, A.; Zielińska, A.; Borucinska, E.; Kruszewski, M.J.; Zybała, R.; Płociński, T.; Idaszek, J.; Jaroszewicz, J.; Paradowski, K.; et al. In-depth analysis of the influence of bio-silica filler (Didymosphenia geminata frustules) on the properties of Mg matrix composites. J. Magnes. Alloys 2023, 11, 2853–2871. [Google Scholar] [CrossRef]
- Dobkowska, A.; Koralnik, M.; Adamczyk-Cieślak, B.; Kuc, D.; Chromiński, W.; Kubasek, J.; Mizera, J. The Effect of Extrusion Ratio on the Corrosion Resistance of Ultrafine-Grained Mg-4Li-3Al-Zn Alloy Deformed Using Extrusion with a Forward-Backward Oscillating Die. J. Mater. Eng. Perform. 2022, 31, 8932–8939. [Google Scholar] [CrossRef]
- Nakahata, I.; Tsutsumi, Y.; Kobayashi, E. Mechanical Properties and Corrosion Resistance of Magnesium—Hydroxyapatite Composites Fabricated. Metals 2020, 10, 1314. [Google Scholar] [CrossRef]
- Khalil, K.A.; Almajid, A.A. Effect of high-frequency induction heat sintering conditions on the microstructure and mechanical properties of nanostructured magnesium/hydroxyapatite nanocomposites. Mater. Des. 2012, 36, 58–68. [Google Scholar] [CrossRef]
- Razavi, M.; Huang, Y. Effect of hydroxyapatite (HA) nanoparticles shape on biodegradation of Mg/HA nanocomposites processed by high shear solidification/equal channel angular extrusion route. Mater. Lett. 2020, 267, 127541. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk–Cieślak, B.; Kuc, D.; Hadasik, E.; Płociński, T.; Ura-Bińczyk, E.; Mizera, J. Influence of bimodal grain size distribution on the corrosion resistance of Mg–4Li–3Al–1Zn (LAZ431). J. Mater. Res. Technol. 2021, 13, 346–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).