Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation
Abstract
1. Introduction
2. Computational Methods
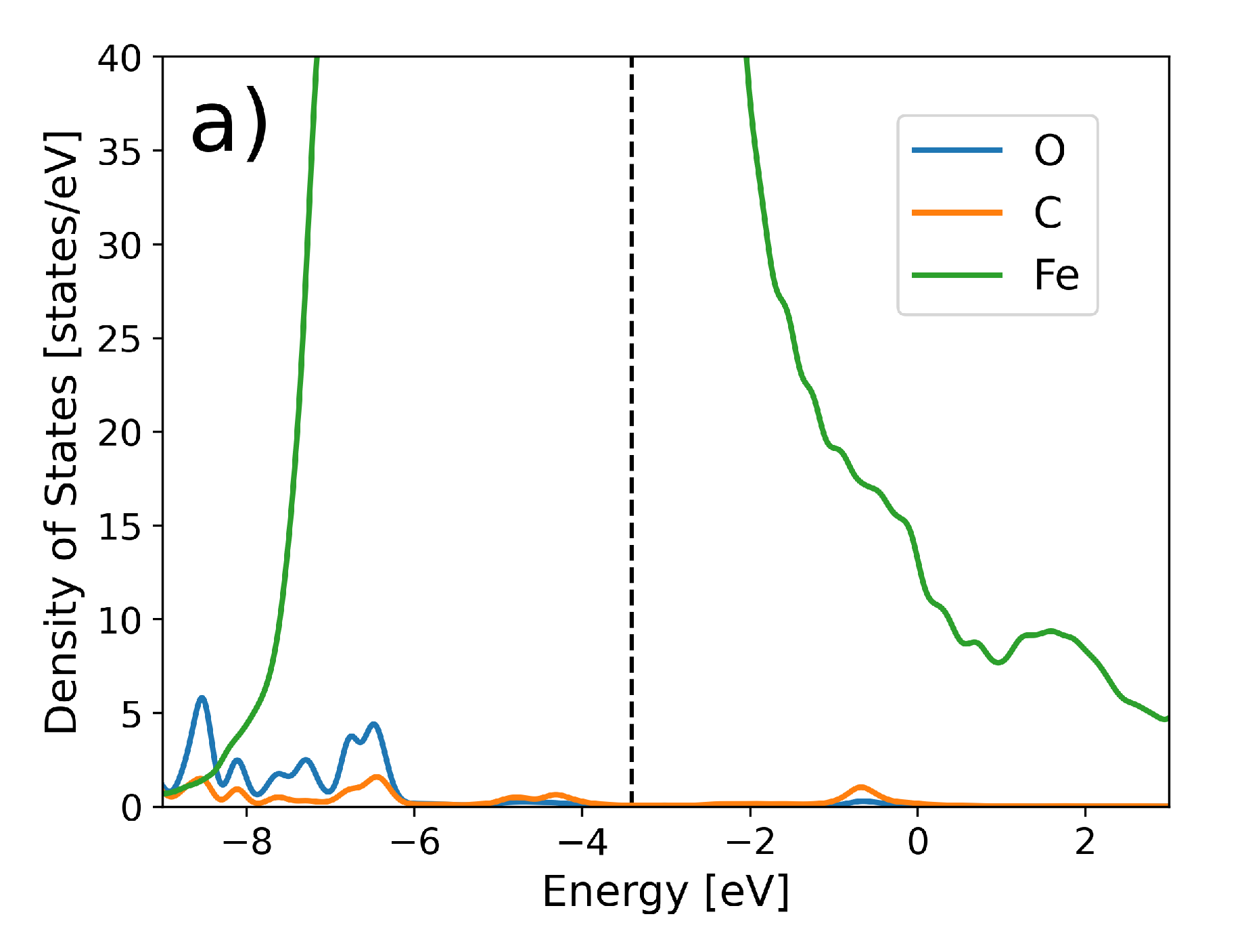
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulaiman, K.A.; Aljuhani, O.; Saleh, K.B.; Badreldin, H.A.; Harthi, A.A.; Alenazi, M.; Alharbi, A.; Algarni, R.; Harbi, S.A.; Alhammad, A.M.; et al. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: A propensity score matched study. Sci. Rep. 2021, 11, 17648. [Google Scholar]
- Li, N.; Zhao, G.; Wu, W.; Zhang, M.; Liu, W.; Chen, Q.; Wang, X. The efficacy and safety of vitamin c for iron supplementation in adult patients with iron deficiency anemia. JAMA Netw. Open 2020, 3, e2023644. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron absorption: Factors, limitations, and improvement methods. ACS Omega 2022, 7, 20441. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E. Ascorbic acid—The little-known antioxidant in woody plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and antioxidant effects of vitamin c in cancer in correspondence to its dietary and pharmacological concentrations. Oxid. Med. Cell. Longev. 2019, 2019, 1. [Google Scholar] [CrossRef]
- Morais, M.A.; Fonseca, K.S.; Medeiros, R.A.; Andrada, L.V.P.; de Aquino Saraiva, R.; Ferreira-Silva, S.L.; Lima, A.L.; Simões, A. Use of the abrasion technique in minimal processing as an alternative to increase purchase acceptability and minimize browning in yam. J. Sci. Food Agric. 2021, 102, 121. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, G.; Zhang, J.; Nau, W.M. Increased Antioxidant Reactivity of Vitamin C at Low pH in Model Membranes. J. Amer. Chem. Soc. 2002, 124, 11252. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys Acta Rev. Cancer 2012, 1826, 443. [Google Scholar] [CrossRef]
- Huang, J.; Agus, D.B.; Winfree, C.J.; Kiss, S.; Mack, W.J.; McTaggart, R.A.; Choudhri, T.F.; Kim, L.J.; Mocco, J.; Pinsky, D.J.; et al. Dehydroascorbic acid, a blood–brain barrier transportable form of vitamin c, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA 2001, 98, 11720. [Google Scholar] [CrossRef]
- Sekine, I.; Nakahata, Y.; Tanabe, H. The corrosion inhibition of mild steel by ascorbic and folic acids. Corros. Sci. 1988, 28, 987. [Google Scholar] [CrossRef]
- Jones, D. Principles and Prevention of Corrosion; MacMillan: New York, NY, USA, 1992. [Google Scholar]
- Arthur, D.E.; Jonathan, A.; Ameh, P.O.; Anya, C. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int. J. Ind. Chem. 2013, 4, 2. [Google Scholar] [CrossRef]
- Guedes, D.; Martins, G.R.; Jaramillo, L.Y.A.; Dias, D.S.B.; da Silva, A.J.R.; Lutterbach, M.T.S.; Reznik, L.Y.; Sérvulo, E.F.C.; Alviano, C.S.; Alviano, D.S. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Environments Containing Sonneratia caseolaris Leaf Extract. ACS Omega 2021, 6, 6893. [Google Scholar]
- Fontana, M.; Staehle, K. Advances in Corrosion Science and Technology; Plenum Press: New York, NY, USA, 1970; Volume 1. [Google Scholar]
- Stoyanova, A.; Petkova, G.; Peyerimhoff, S. Correlation between the molecular structure and the corrosion inhibiting effect of some pyrophthalone compounds. Chem. Phys. 2002, 279, 1. [Google Scholar] [CrossRef]
- Damaskin, B.; Petrii, O.; Batrakov, V. Adsorption; Plenum Press: New York, NY, USA, 1971. [Google Scholar]
- Shehata, O.S.; Korshed, L.A.; Attia, A. Corrosion Inhibitors, Principles and Recent Applications; InTech: London, UK, 2018. [Google Scholar]
- Chidiebere, M.A.; Oguzie, E.E.; Liu, L.; Li, Y.; Wang, F. Ascorbic acid as corrosion inhibitor for Q235 mild steel in acidic environments. J. Ind. Eng. Chem. 2015, 26, 182. [Google Scholar] [CrossRef]
- Ferreira, E.; Giacomelli, C.; Giacomelli, F.; Spinelli, A. Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565. [Google Scholar] [CrossRef]
- Mhlanga, N.; Mphahlele, K. Self-Standing Substrates; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 235–267. [Google Scholar]
- Verma, C.; Hussain, C.M.; Ebenso, E.E. Organic Corrosion Inhibitors: Synthesis, Characterization, Mechanism, and Applications; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Kchaou, M. An overview of green corrosion inhibitors for sustainable and environment friendly industrial development. J. Adhes. Sci. Technol. 2021, 35, 673. [Google Scholar] [CrossRef]
- Sruthi, G.; Shakeela, K.; Shanmugam, R.; Rao, G.R. The corrosion inhibition of stainless steel by ferrocene–polyoxometalate hybrid molecular materials—Experimental and first principles studies. Phys. Chem. Chem. Phys. 2020, 22, 3329. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Ferreira, I.; Kuznetsova, A.; Novell-Leruth, G.; Song, C.; Feiler, C.; Lamaka, S.V.; Rocha, C.; Maia, F.; Zheludkevich, M.L.; et al. CORDATA: An open data management web application to select corrosion inhibitors. NPJ Mater. Degrad. 2022, 6, 48. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Li, Y.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Comparison of the synergistic inhibition mechanism of two eco-friendly amino acids combined corrosion inhibitors for carbon steel pipelines in oil and gas production. Appl. Surf. Sci. 2022, 583, 152559. [Google Scholar] [CrossRef]
- Shehata, O.S.; Khorshed, L.A.; Mandour, H.S. Effect of Acetamide Derivative and its Mn-Complex as Corrosion Inhibitor for Mild Steel in sulphuric Acid. Egypt. J. Chem. 2017, 60, 243. [Google Scholar]
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. NPJ Mater. Degrad. 2022, 6, 5. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corr. Sci. 2019, 146, 134. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corr. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Santos, R.S.; Barbosa, T.S.; Mafra, M.A.; Ribeiro, A.; Sousa, F.; Andrade-Filho, T. Simulation of Iron Corrosion Inhibition by Biological Molecules Thymol and Carvacrol. Mater. Lett. 2022, 308, 131249. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Brandenburg, J.G.; Grimme, S. Accurate modeling of organic molecular crystals by dispersion-corrected density functional tight binding (DFTB). J. Phys. Chem. Lett. 2014, 5, 1785. [Google Scholar] [CrossRef]
- Zheng, G.; Witek, H.A.; Bobadova-Parvanova, P.; Irle, S.; Musaev, D.G.; Prabhakar, R.; Morokuma, K.; Lundberg, M.; Elstner, M.; Köhler, C.; et al. Parameter Calibration of Transition-Metal Elements for the Spin-Polarized Self-Consistent-Charge Density-Functional Tight-Binding (DFTB) Method: Sc, Ti, Fe, Co, and Ni. J. Chem. Theory Comput. 2007, 3, 1349. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; He, S.; Zhu, J.; Wei, L.; Zheng, S. A DFT-Based Model on the Adsorption Behavior of H2O, H+, Cl−, and OH− on Clean and Cr-Doped Fe(110) Planes. Coatings 2018, 8, 51. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitrică, T.; Dominguez, A.; et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33. [Google Scholar] [CrossRef]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe(110) surface using the DFTB method. RSC Adv. 2017, 7, 29042. [Google Scholar] [CrossRef]
- Zemann, J. Crystal structures, 2nd edition. Vol. 1by R. W. G. Wyckoff. Acta Crystallogr. 1965, 18, 139. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lin, Y. Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Tian, G.; Yuan, K. Performance and Mechanism of Alkylimidazolium Ionic Liquids as Corrosion Inhibitors for Copper in Sulfuric Acid Solution. Molecules 2021, 26, 4910. [Google Scholar] [CrossRef]
- Abdallah, M.; Soliman, K.A.; Jahdaly, B.A.A.; Al-Fahemi, J.H.; Hawsawi, H.; Altass, H.M.; Motawea, M.S.; Al-Juaid, S.S. Natural parsley oil as a green and safe inhibitor for corrosion of X80 carbon steel in 0.5 M H2SO4 solution: A chemical, electrochemical, DFT and MC simulation approach. RSC Adv. 2022, 12, 2959. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Sadeh, E.; Abdouss, M.; Alavi, A. Exploration of Sunflower Oil As a Renewable Biomass Source to Develop Scalable and Highly Effective Corrosion Inhibitors in a 15% HCl Medium at High Temperatures. ACS Appl. Mater. Interf. 2021, 13, 3119. [Google Scholar] [CrossRef]
- Zarrouk, A.; Zarrok, H.; Salghi, R.; Hammoutil, B.; Al-Deyab, S.; Touzanil, R.; Bouachrine, M.; Warad, I.; Hadda, T.B. A Theoretical Investigation on the Corrosion Inhibition of Copper by Quinoxaline Derivatives in Nitric Acid Solution. Int. J. Electrochem. Sci. 2012, 7, 6353. [Google Scholar] [CrossRef]
- Fang, J.; Li, J. Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J. Mol. Struc. THEOCHEM 2002, 593, 179. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, S. A Theoretical Investigation on the Inhibition Efficiencies of Some Schiff bases as Corrosion Inhibitors of Steel in Hydrochloric Acid. Int. J. Electrochem. Sci. 2012, 7, 11884. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Imidazole derivatives as corrosion inhibitors for copper: A DFT and reactive force field study. Corr. Sci. 2018, 142, 102. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H.S. Facile preparation of new hydrazone compounds and their application for long-term corrosion inhibition of N80 steel in 15% HCl: An experimental study combined with DFTB calculations. J. Mol. Liq. 2022, 347, 117952. [Google Scholar] [CrossRef]
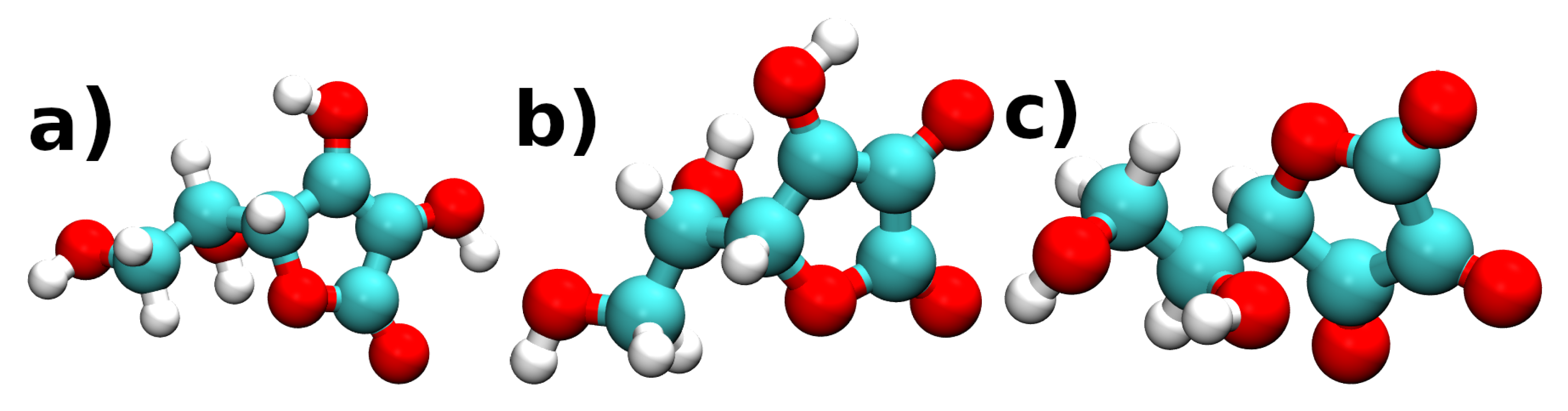
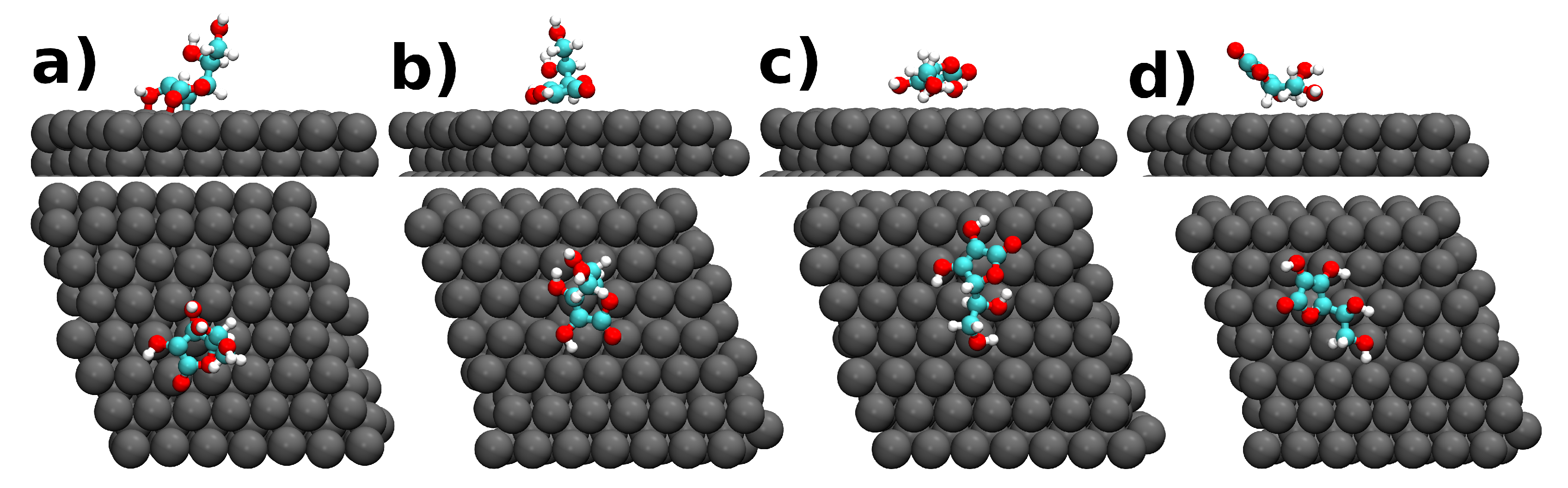
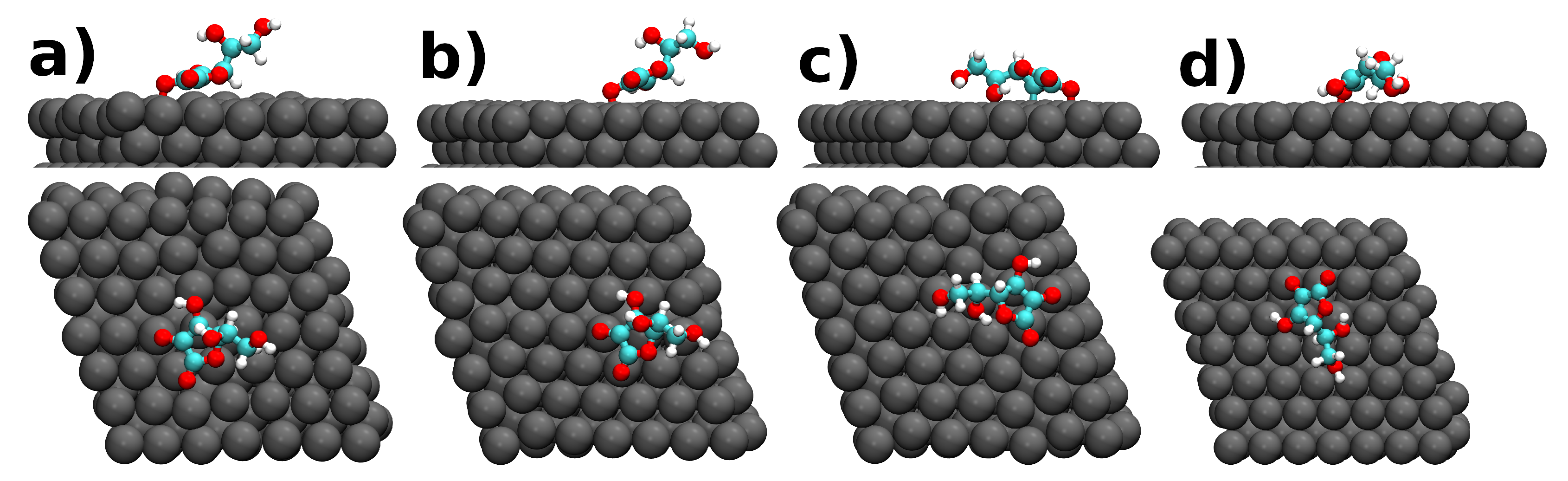
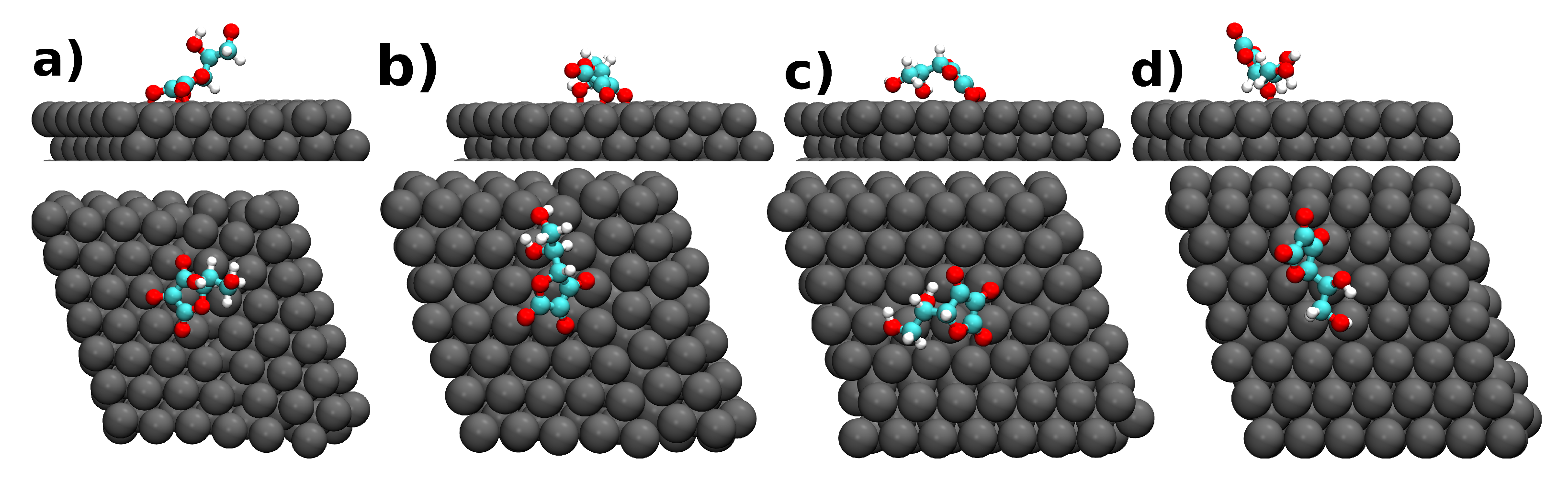

| Molecule@Fe(110) | (eV) | h (Å) | Q (e) |
|---|---|---|---|
| a) AA | −10.20 | 2.00 | −0.47 |
| b) AA | −6.47 | 2.23 | 0.20 |
| c) AA | −5.25 | 2.28 | 0.30 |
| d) AA | −3.25 | 2.28 | 0.20 |
| a) ASA | −9.00 | 1.96 | 0.82 |
| b) ASA | −7.63 | 1.92 | 0.77 |
| c) ASA | −12.25 | 2.00 | 0.77 |
| d) ASA | −12.80 | 1.87 | 0.92 |
| a) DHA | −11.66 | 1.95 | −0.40 |
| b) DHA | −11.88 | 1.88 | −0.34 |
| c) DHA | −15.99 | 1.90 | −0.46 |
| d) DHA | −12.80 | 1.86 | −0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, B.D.F.; Lage, M.R.; Santos, A.O.d.; Sousa, F.F.d.; Gester, R.; Stoyanov, S.R.; Andrade-Filho, T. Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation. Corros. Mater. Degrad. 2024, 5, 615-623. https://doi.org/10.3390/cmd5040029
Souza BDF, Lage MR, Santos AOd, Sousa FFd, Gester R, Stoyanov SR, Andrade-Filho T. Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation. Corrosion and Materials Degradation. 2024; 5(4):615-623. https://doi.org/10.3390/cmd5040029
Chicago/Turabian StyleSouza, Bruno D. F., Mateus R. Lage, Adenilson Oliveira dos Santos, Francisco Ferreira de Sousa, Rodrigo Gester, Stanislav R. Stoyanov, and Tarciso Andrade-Filho. 2024. "Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation" Corrosion and Materials Degradation 5, no. 4: 615-623. https://doi.org/10.3390/cmd5040029
APA StyleSouza, B. D. F., Lage, M. R., Santos, A. O. d., Sousa, F. F. d., Gester, R., Stoyanov, S. R., & Andrade-Filho, T. (2024). Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation. Corrosion and Materials Degradation, 5(4), 615-623. https://doi.org/10.3390/cmd5040029







