The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Exposure Conditions
2.3. In Situ Infrared Reflection Absorption Spectroscopy (IRRAS)
2.4. FTIR-FPA Imaging
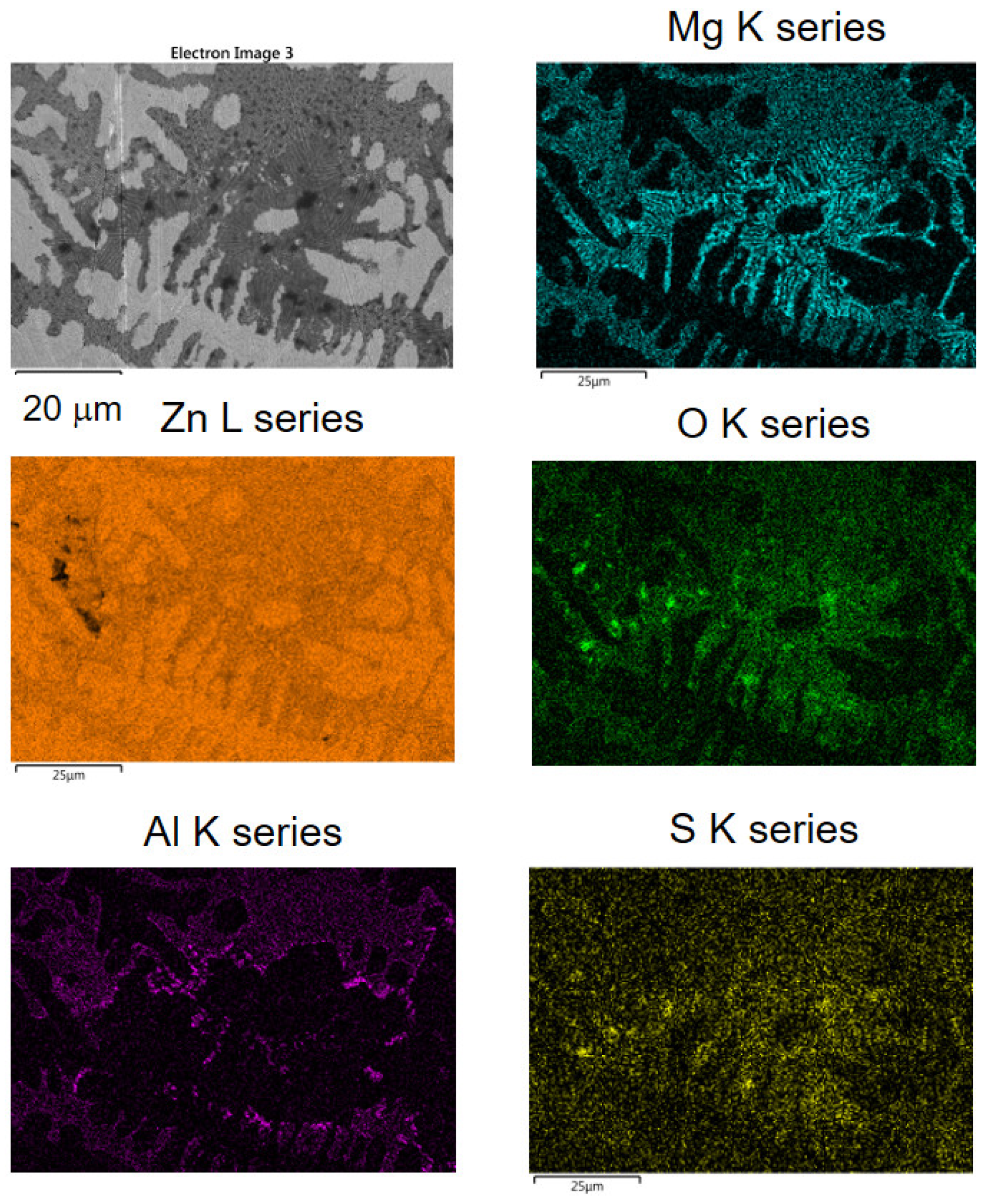
2.5. SEM-EDS
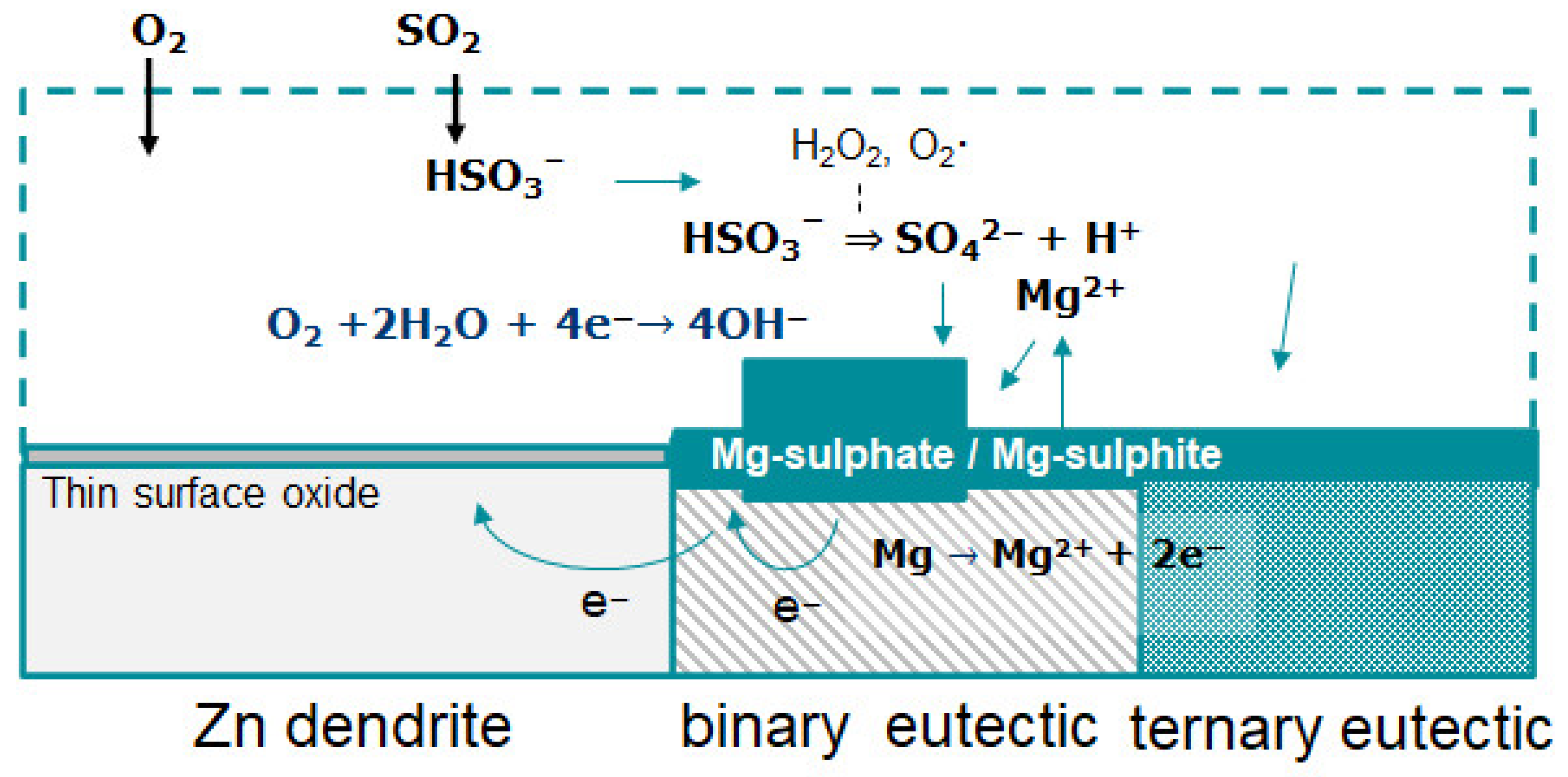
3. Results and Discussion
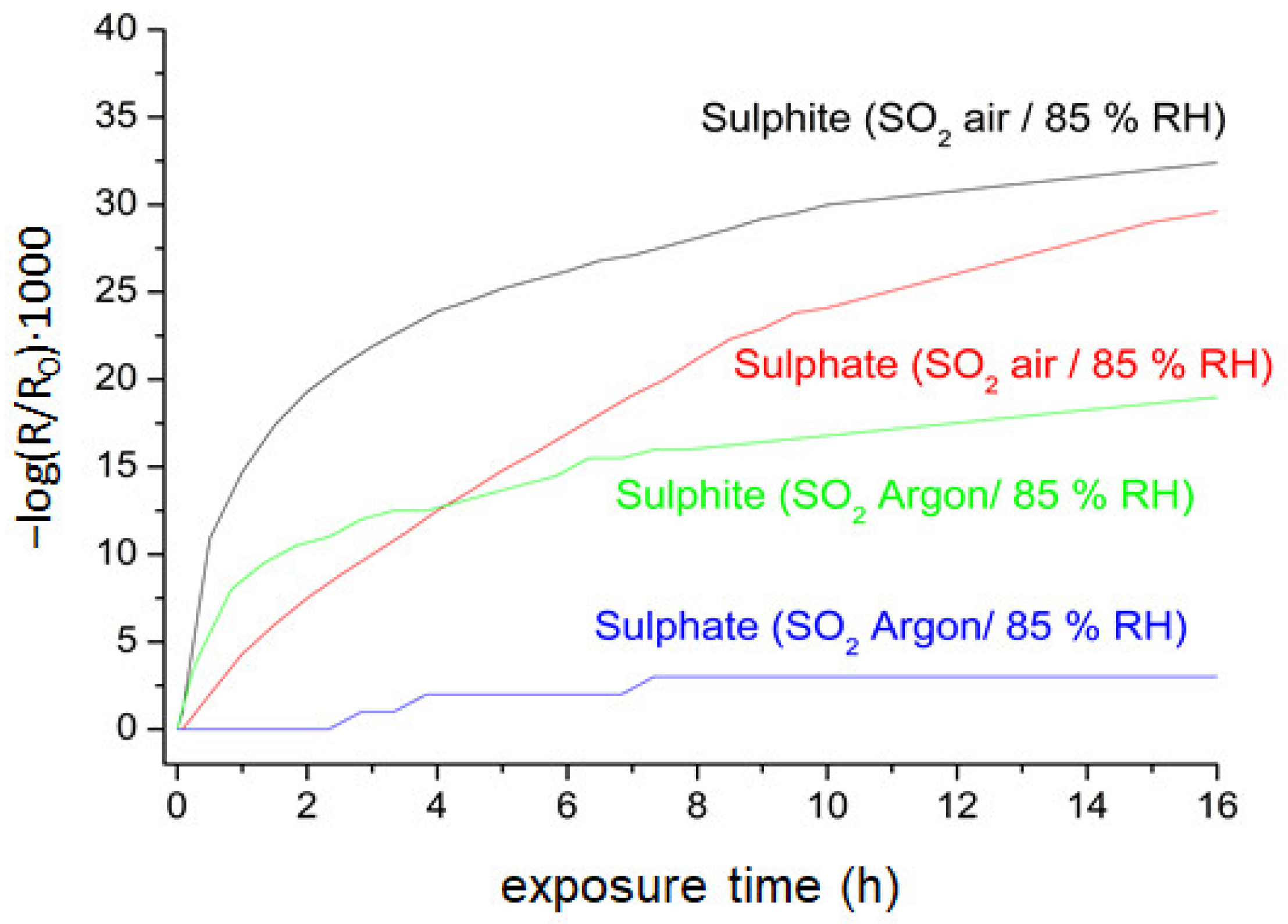
3.1. In Situ Infrared Reflection Absorption Spectroscopy (IRRAS)
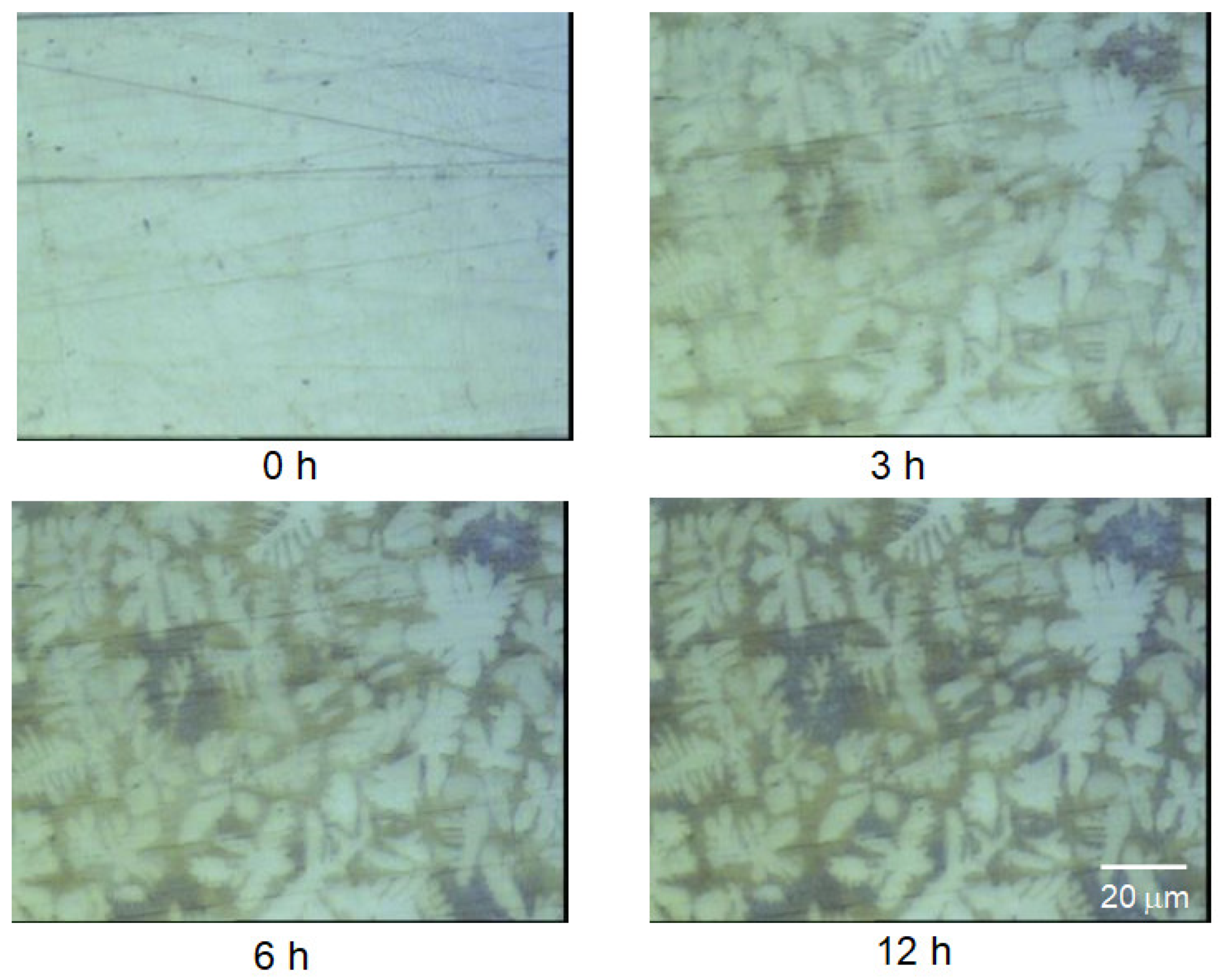
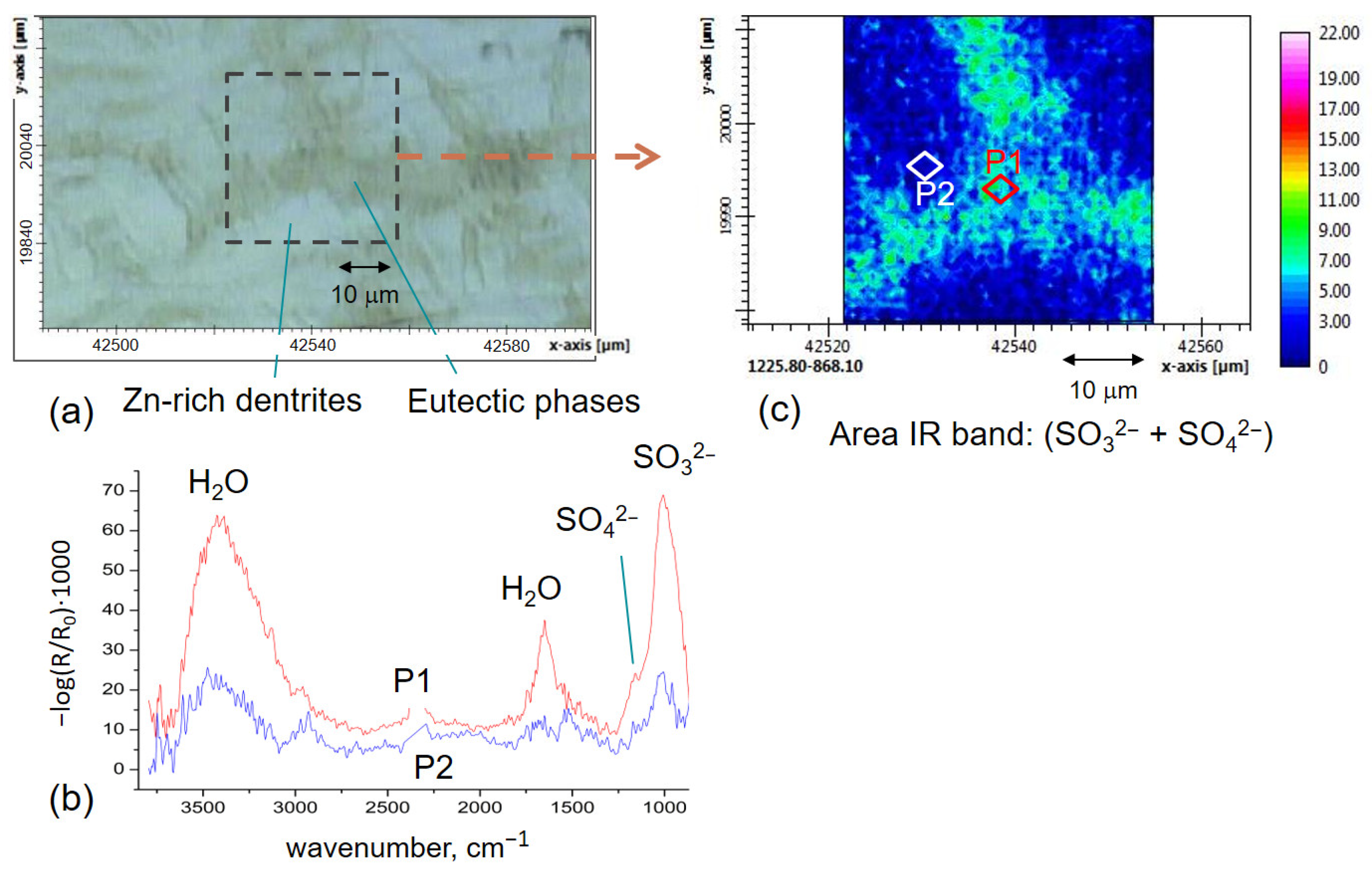
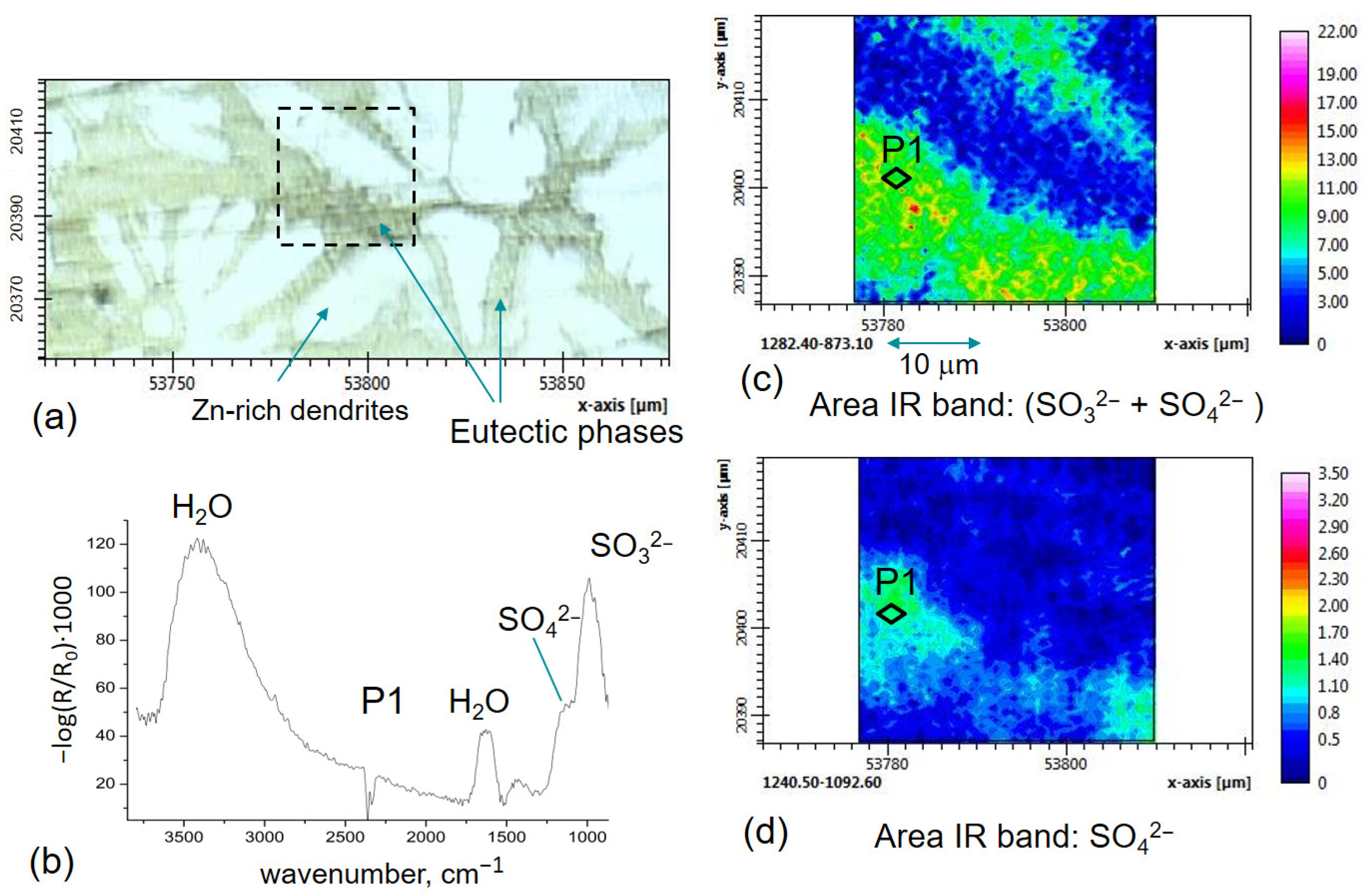
3.2. FTIR-ATR FPA Imaging and SEM-EDS Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schürz, S.; Luckeneder, G.H.; Preis, K.; Haunschmied, T.; Mori, G.; Kneissl, A.C. Corrosion behaviour of Zn–Al–Mg coated steel sheet in sodium chloride-containing environment. Corros. Sci. 2009, 51, 2355–2363. [Google Scholar] [CrossRef]
- Schürz, S.; Luckeneder, G.H.; Fleischanderl, M.; Mack, P.; Gsaller, H.; Kneissl, A.C.; Mori, G. Chemistry of corrosion products on Zn–Al–Mg alloy coated steel. Corros. Sci. 2010, 52, 3271–3279. [Google Scholar] [CrossRef]
- Prosek, T.; Larché, N.; Vlot, M.; Goodwin, F.; Thierry, D. Corrosion performance of Zn–Al–Mg coatings in open and confined zones in conditions simulating automotive applications. Mater. Corros. 2010, 60, 412–420. [Google Scholar] [CrossRef]
- Diler, E.; Rouvellou, B.; Rioual, S.; Lescop, B.; Vien, G.N.; Thierry, D. Characterization of corrosion products of Zn and Zn–Mg–Al coated steel in a marine atmosphere. Corros. Sci. 2014, 87, 111–117. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn (Mg, Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corros. Sci. 2015, 90, 472–481. [Google Scholar] [CrossRef]
- Tomandl, A.; Labrenz, E. The corrosion behavior of ZnAlMg alloys in maritime environments. Mater. Corros. 2016, 67, 1286–1293. [Google Scholar] [CrossRef]
- Volovitch, P.; Vu, T.N.; Allély, C.; Abdel, A.; Aal, A.A.; Ogle, K. Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn–Al–Mg coatings on steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Peltola, A.; Luxem, L.; Luckeneder, G.; Marchiaro, G.; Rohwerder, M. Corrosion performance of Zn–Mg–Al coated steel in accelerated corrosion tests used in the automotive industry and field exposures. Mater. Corros. 2013, 64, 969–978. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; Luckeneder, G.; Stellnberger, K.-H. Atmospheric corrosion of ZnAlMg coated steel during long term atmospheric weathering at different worldwide exposure sites. Corros. Sci. 2019, 148, 338–354. [Google Scholar] [CrossRef]
- Duchoslav, J.; Truglas, T.; Groiß, H.; Riener, C.K.; Arndt, M.; Stellnberger, K.H.; Luckeneder, G.; Angeli, G.; Stifter, D. Structure and chemistry of surface oxides on ZnMgAl corrosion protection coatings with varying alloy composition. Surf. Coat. Tech. 2019, 368, 51–58. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Persson, D.; Riener, C.K.; Luckeneder, G. Influence of microstructure of zinc-aluminium-magnesium alloy coated steel on the corrosion behavior in outdoor marine atmosphere. Surf. Coat. Technol. 2019, 374, 897–909. [Google Scholar] [CrossRef]
- Wint, N.; Cooze, N.; Searle, J.R.; Sullivan, J.H.; Williams, G.; McMurray, H.N.; Luckeneder, G.; Riener, C.J. The effect of microstructural refinement on the localized corrosion of model Zn-Al-Mg alloy coatings on steel. Electrochem. Soc. 2019, 166, C3147. [Google Scholar] [CrossRef]
- Sullivan, J.; Mehraban, S.; Elvins, J. In situ monitoring of the microstructural corrosion mechanisms of zinc–magnesium–aluminium alloys using time lapse microscopy. Corros. Sci. 2011, 54, 2208–2215. [Google Scholar] [CrossRef]
- Gu, T.; Liu, Y.; Zhang, C.P.; Wang, Z. Initial atmospheric corrosion of zinc-aluminum-magnesium coated steel and galvanized steel in regions of extremely cold and industrial climate. Mat. Chem. Phys. 2022, 291, 126686. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion mechanisms for zinc exposed to the atmosphere. J. Electrochem. Soc. 1989, 136, 193C–203C. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Springer: New York, NY, USA, 1996. [Google Scholar]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- De la Fuente, D.; Castano, J.G.; Morcillo, M. Long-term atmospheric corrosion of zinc. Corros. Sci. 2007, 49, 1420–1436. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Sydberger, T.; Vannerberg, N.G. The influence of the relative humidity and corrosion products on the adsorption of sulfur dioxide on metal surfaces. Corr. Sci. 1972, 12, 775–784. [Google Scholar] [CrossRef]
- Duncan, J.R.; Spedding, D.J. The effect of relative humidity on adsorption of sulphur dioxide on to metal surfaces. Corros. Sci. 1973, 13, 993–1001. [Google Scholar] [CrossRef]
- Duncan, J.R.; Spedding, D.J. The mode of initial reaction of SO2 at a metal surface. Corros. Sci. 1974, 14, 241–249. [Google Scholar] [CrossRef]
- Persson, D.; LeBozec, T.P.N.; Thierry, D.; Luckeneder, G. Initial SO2-induced atmospheric corrosion of ZnAlMg coated steel studied with in situ Infrared Reflection Absorption Spectroscopy. Corros. Sci. 2015, 90, 276–283. [Google Scholar] [CrossRef]
- Martin, L.R. SO2, NO and NO2 Oxidation Mechanisms: Atmospheric Considerations; Calvert, J.G., Ed.; Butterworth Publishers: Stoneham, MA, USA, 1984. [Google Scholar]
- Persson, D.; Thierry, D.; LeBozec, N.; Prosek, T. In situ infrared reflection spectroscopy studies of the initial atmospheric corrosion of Zn–Al–Mg coated steel. Corros. Sci. 2013, 72, 54–63. [Google Scholar] [CrossRef]
- Ito, M.; Ooi, A.; Tada, E.; Nishikata, A. In situ evaluation of carbon steel corrosion under salt spray test by electrochemical impedance spectroscopy. J. Electrochem. Soc. 2020, 167, 101508. [Google Scholar] [CrossRef]
- Similion, H.; Dolgikh, O.; Terryn, H.; Deconinck, J. Atmospheric corrosion modeling. J. Corros. Rev. 2014, 32, 73–100. [Google Scholar] [CrossRef]
- Zhong, X.; Schulz, M.; Wu, C.-H.; Rabe, M.; Erbe, A.; Rohwerder, M. Limiting current density of oxygen reduction under ultrathin electrolyte layers: From the micrometer range to monolayers. ChemElectroChem 2021, 8, 712–718. [Google Scholar] [CrossRef]
- Steiger, M.; Linnow, K.; Ehrhardt, D.; Rohde, M. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4–H2O and Na+–Mg2+–Cl−–SO42−–H2O systems with implications for Mars. Goechim. Cosmochim. Acta 2011, 75, 3600–3626. [Google Scholar] [CrossRef]
- Terraglio, F.P.; Manganelli, R.M. The absorption of atmospheric sulfur dioxide by water solutions. J. Air Pollut. Control. Assoc. 1967, 17, 403–406. [Google Scholar] [CrossRef]
- Boto, K.G.; Williams, L.F.G. Rotating disc electrode studies of zinc corrosion. J. Electroanal. Chem. 1977, 77, 1–20. [Google Scholar] [CrossRef]
- Wroblowa, H.S.; Qaderi, S.B. The mechanism of oxygen reduction on zinc. J. Electroanal. Chem. 1990, 295, 153–161. [Google Scholar] [CrossRef]
- Nayak, S.; Biedermann, P.U.; Erbe, A. Superoxide intermediate in the oxygen reduction on a zinc hydroxide model corrosion product. J. Chem. Phys. 2022, 157, 224702. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Edwards, J.O. Kinetics of the oxidation of sulfite by hydrogen peroxide in acidic solution. J. Phys. Chem. 1975, 79, 2096–2098. [Google Scholar] [CrossRef]
- Dafydd, H.; Worsley, D.A.; McMurray, H.N. The kinetics and mechanism of cathodic oxygen reduction on zinc and zinc–aluminium alloy galvanized coatings. Corros. Sci. 2005, 47, 3006–3018. [Google Scholar] [CrossRef]
- Yadav, P.; Nishikata, A.; Tsuru, T.J. Oxygen reduction mechanism on corroded zinc. Electroanal. Chem. 2005, 585, 142–149. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persson, D.; Thierry, D.; LeBozec, N. The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging. Corros. Mater. Degrad. 2023, 4, 503-515. https://doi.org/10.3390/cmd4030026
Persson D, Thierry D, LeBozec N. The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging. Corrosion and Materials Degradation. 2023; 4(3):503-515. https://doi.org/10.3390/cmd4030026
Chicago/Turabian StylePersson, Dan, Dominique Thierry, and Nathalie LeBozec. 2023. "The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging" Corrosion and Materials Degradation 4, no. 3: 503-515. https://doi.org/10.3390/cmd4030026
APA StylePersson, D., Thierry, D., & LeBozec, N. (2023). The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging. Corrosion and Materials Degradation, 4(3), 503-515. https://doi.org/10.3390/cmd4030026






