Corrosion Inhibition, Inhibitor Environments, and the Role of Machine Learning
Abstract
1. Introduction
2. Recent Evolution of Inhibitor Assessment
3. Environments in Which Inhibitors Typically Perform
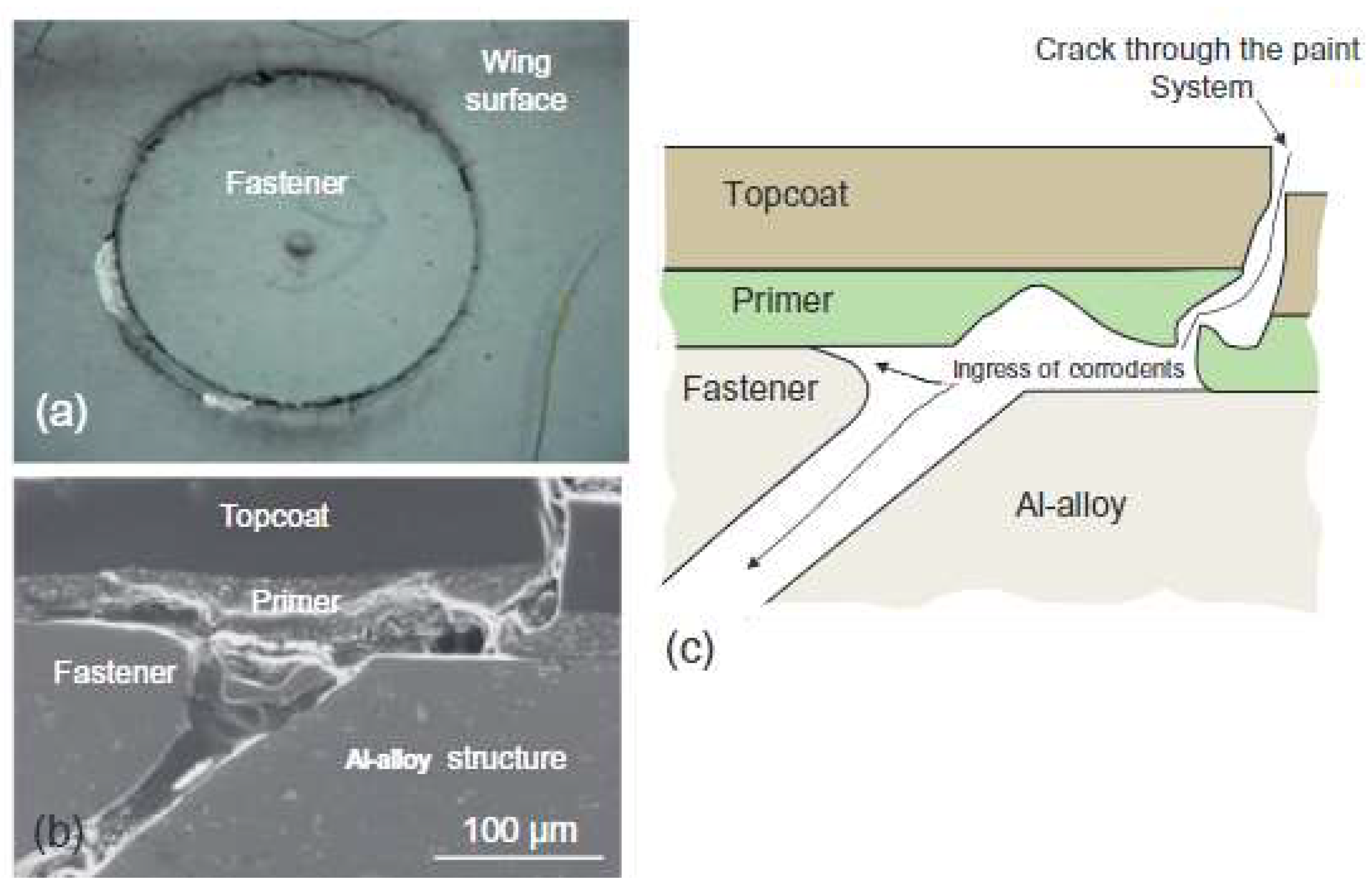
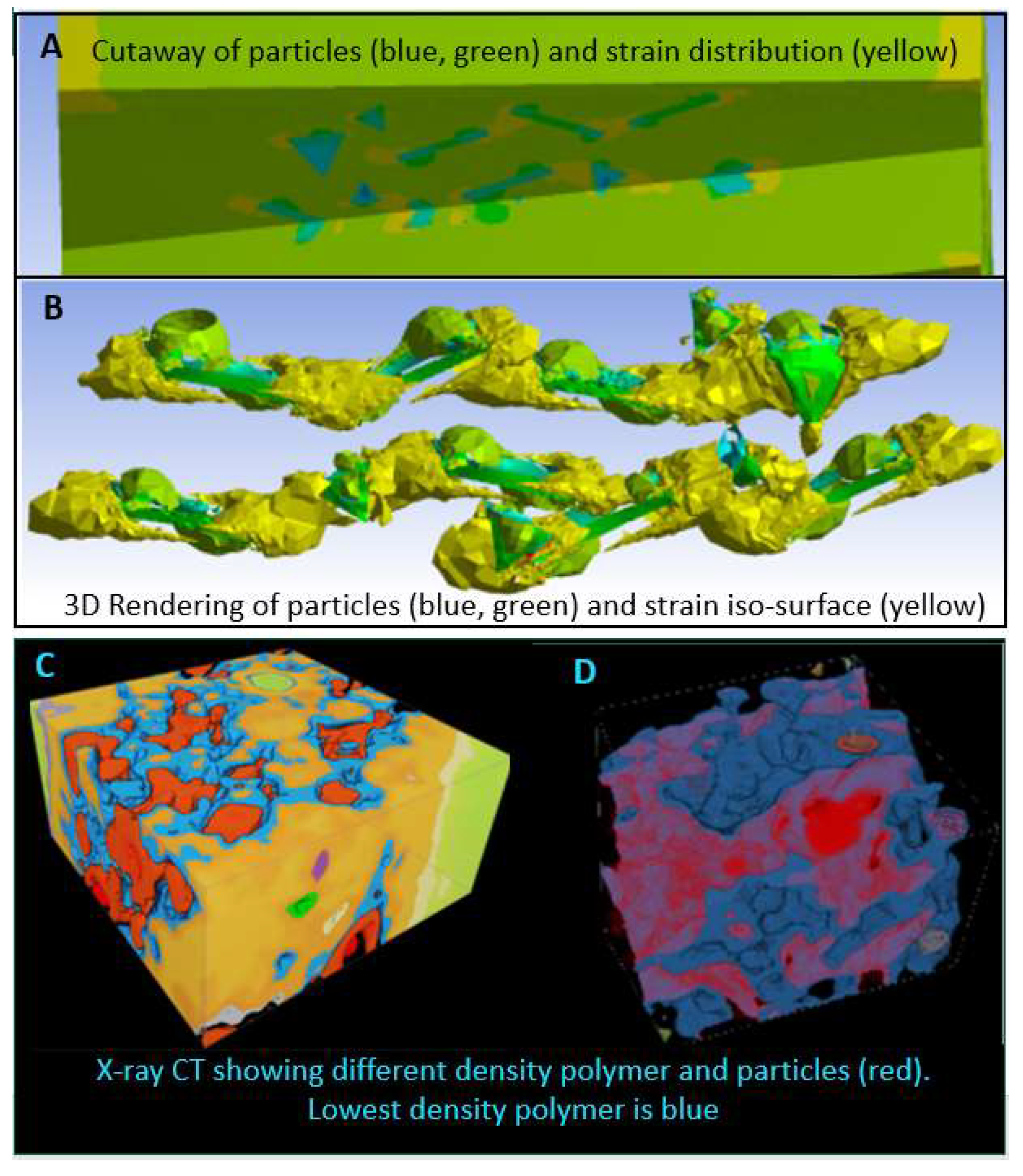
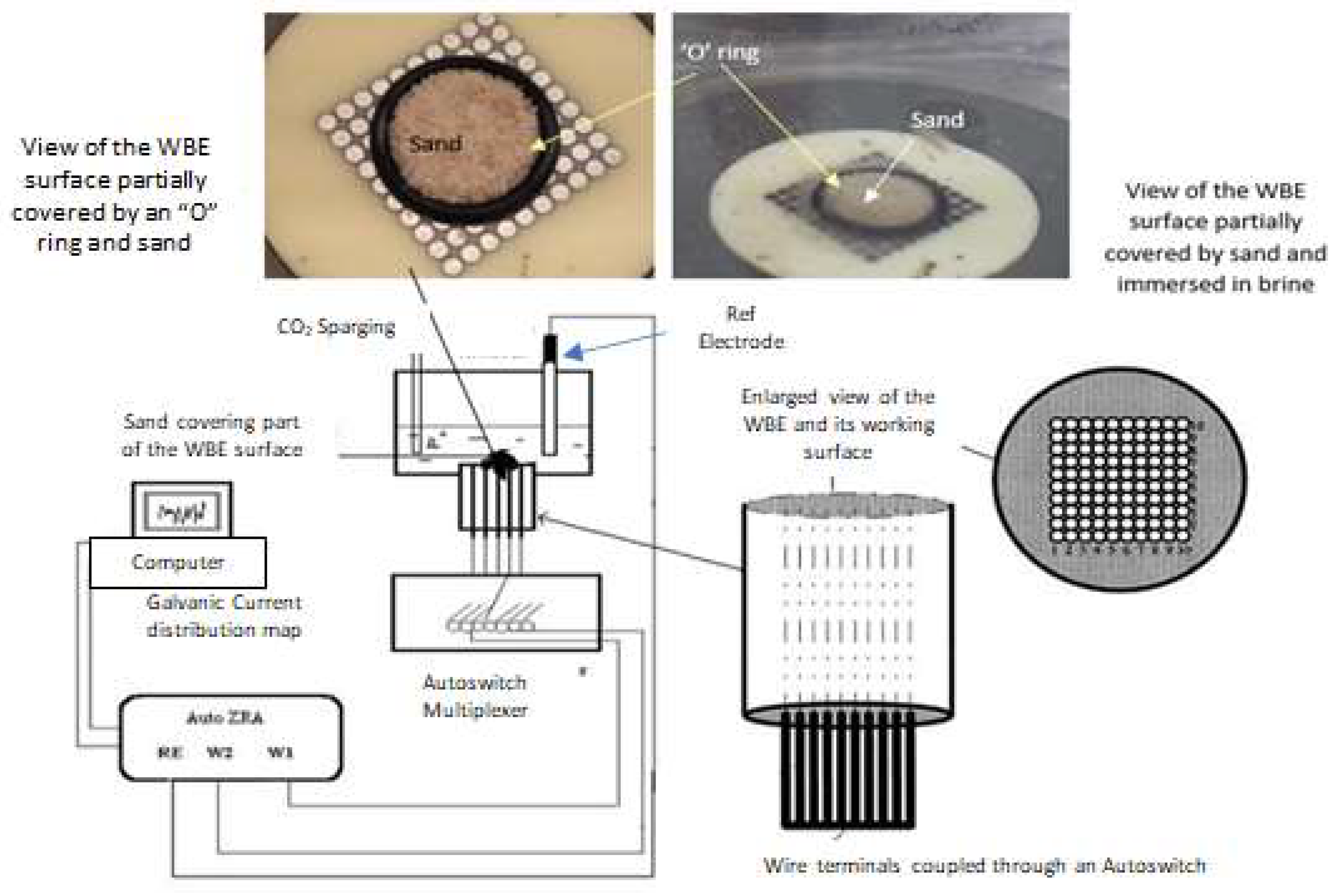
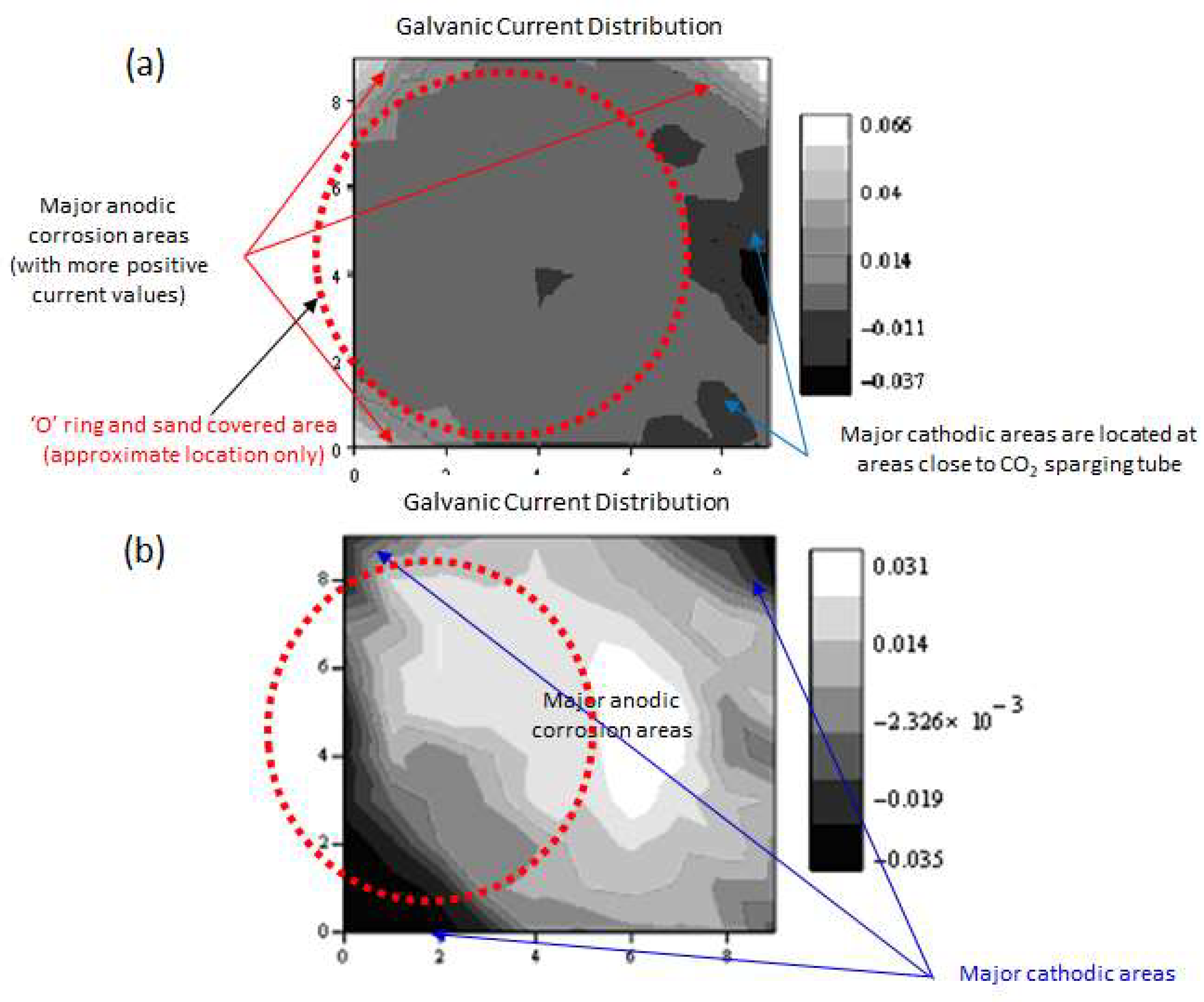
4. Localised Corrosion Environments and Attack
5. Machine Learning
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wernick, S.; Pinner, R.; Sheasby, P.G. The Surface Treatment and Finishing of Aluminium and Its Alloys, 5th ed.; ASM International: Materials Park, OH, USA, 1987. [Google Scholar]
- National Defence for Environmental Excellance. Alternatives fo Chrome Conversion Coatings on aluminium Alloys2024, 6061, 7075, and Ion Vapour deposited Aluminium on Steel; Engineering and Technical Services for Joint Group on Acquisution Pollution Prevention (JG-APP) Pilot Projects, Report HM-A-1-1, 1998; p. 154. Available online: https://p2infohouse.org/ref/05/04690.pdf (accessed on 1 September 2022).
- Sax, N.I. Dangerous Properties of Industrial Materials, 5th ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1979. [Google Scholar]
- Harvey, T.G.; Hardin, S.G.; Hughes, A.E.; Muster, T.H.; White, P.A.; Markley, T.A.; Corrigan, P.A.; Mardel, J.; Garcia, S.J.; Mol, J.M.C.; et al. The effect of inhibitor structure on the corrosion of AA2024 and AA7075. Corros. Sci. 2011, 53, 2184–2190. [Google Scholar] [CrossRef]
- Horner, L.; Meisel, K. Corrosion Inhibitors 23(1)—Does There Exist a Structure-Efficiency Relation in the Organic Inhibitors of Aluminium Corrosion? Wekst. Korros 1978, 29, 654–664. [Google Scholar] [CrossRef]
- Horner, L.; Schodel, D. Corrosion Inhibitors. Pt. 17-1. Importance of Pearson’s ‘Hard-Soft Concept’ with Respect to the Recognition of Relationships Between the Structure and Activity of Inhibitors of the Corrosion of Fe in NaCl Solution in the Presence of O. Werkst. Korros. 1974, 25, 711–720. [Google Scholar] [CrossRef]
- Horner, L.; Hinrichs, H. Identification of Mechanisms Common to the Inhibition of the Acid Corrosion of Iron, Zinc, Aluminum(and Raney Nickel). Werkst. Korros. 1971, 22, 867–869. [Google Scholar] [CrossRef]
- Taylor, S.R.; Chambers, B.D. The discovery of non-chromate corrosion inhibitors for aerospace alloys using high-throughput screening methods. Corros. Rev. 2007, 25, 571–590. [Google Scholar] [CrossRef]
- Taylor, S.R.; Chambers, B.D. Identification and characterization of nonchromate corrosion inhibitor synergies using high-throughput methods. Corrosion 2008, 64, 255–270. [Google Scholar] [CrossRef]
- Chambers, B.D.; Taylor, S.R. High-throughput assessment of inhibitor synergies on aluminum alloy 2024-T3 through measurement of surface copper enrichment. Corrosion 2007, 63, 268–276. [Google Scholar] [CrossRef]
- Muster, T.H.; Hughes, A.E.; Furman, S.A.; Harvey, T.; Sherman, N.; Hardin, S.; Corrigan, P.; Lau, D.; Scholes, F.H.; White, P.A.; et al. A rapid screening multi-electrode method for the evaluation of corrosion inhibitors. Electrochim. Acta 2009, 54, 3402–3411. [Google Scholar] [CrossRef]
- Garcia, S.J.; Muster, T.H.; Hughes, A.E.; White, P.A.; Lau, D.; Markley, T.; Harvey, T.; Sherman, N.; Hardin, S.; Corrigan, P.; et al. Validation of a fast scanning technique for corrosion inhibitor selection: Influence of cross-contamination on AA2024-T3. Surf. Interface Anal. 2010, 42, 205–210. [Google Scholar] [CrossRef]
- White, P.A.; Collis, G.E.; Skidmore, M.; Breedon, M.; Ganther, W.D.; Venkatesan, K. Towards materials discovery: Assays for screening and study of chemical interactions of novel corrosion inhibitors in solution and coatings. New J. Chem. 2020, 44, 7647–7658. [Google Scholar] [CrossRef]
- White, P.A.; Smith, G.B.; Harvey, T.G.; Corrigan, P.A.; Glenn, M.A.; Lau, D.; Hardin, S.G.; Mardel, J.; Markley, T.A.; Muster, T.H.; et al. A new high-throughput method for corrosion testing. Corros. Sci. 2012, 58, 327–331. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; White, P.; Hughes, A.E.; Sapper, E.D.; Cole, I. Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros. Sci. 2016, 106, 229–235. [Google Scholar] [CrossRef]
- White, P.A.; Hughes, A.E.; Furman, S.A.; Sherman, N.; Corrigan, P.A.; Glenn, M.A.; Lau, D.; Hardin, S.G.; Harvey, T.G.; Mardel, J.; et al. High-throughput channel arrays for inhibitor testing: Proof of concept for AA2024-T3. Corros. Sci. 2009, 51, 2279–2290. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Fernandez, M.; Breedon, M.; Cole, I.S.; Barnard, A.S. Modeling corrosion inhibition efficacy of small organic molecules as non-toxic chromate alternatives using comparative molecular surface analysis (CoMSA). Chemosphere 2016, 160, 80–88. [Google Scholar] [CrossRef]
- Würger, T.; Feiler, C.; Musil, F.; Feldbauer, G.B.V.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L.; Meißner, R.H. Data science based mg corrosion engineering. Front. Mater. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Galvão, T.L.P.; Ferreira, I.; Kuznetsova, A.; Novell-Leruth, G.; Song, C.; Feiler, C.; Lamaka, S.V.; Rocha, C.; Maia, F.; Zheludkevich, M.L.; et al. CORDATA: An open data management web application to select corrosion inhibitors. NPJ Mater. Degrad. 2022, 6, 48. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Smith, J.S.; Nebgen, B.T.; Zubatyuk, R.; Lubbers, N.; Devereux, C.; Barros, K.; Tretiak, S.; Isayev, O.; Roitberg, A.E. Approaching coupled cluster accuracy with a general-purpose neural network potential through transfer learning. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Feiler, C.; Mei, D.; Vaghefinazari, B.; Würger, T.; Meißner, R.H.; Luthringer-Feyerabend, B.J.C.; Winkler, D.A.; Zheludkevich, M.L.; Lamaka, S.V. In silico screening of modulators of magnesium dissolution. Corros. Sci. 2020, 163, 108245. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Esrafili, M.D.; Kazempour, A. Hybrid sol-gel coatings based on silanes-amino acids for corrosion protection of AZ91 magnesium alloy: Electrochemical and DFT insights. Prog. Org. Coat. 2019, 131, 191–202. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; Hughes, A.E.; Burden, F.R.; Barnard, A.S.; Harvey, T.G.; Cole, I. Towards chromate-free corrosion inhibitors: Structure–property models for organic alternatives. Green Chem. 2014, 16, 3349–3357. [Google Scholar] [CrossRef]
- Kokalj, A. Comments on the paper On the nature of inhibition performance of imidazole on iron surface by J.O. Mendes, E.C. da Silva, A.B. Rocha. Corros. Sci. 2013, 68, 286–289. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. The roles of mercapto, benzene, and methyl groups in the corrosion inhibition of imidazoles on copper: II. Inhibitor-copper bonding. Corros. Sci. 2015, 98, 457–470. [Google Scholar] [CrossRef]
- Kovačević, N.; Kokalj, A. DFT study of interaction of azoles with Cu(111) and Al(111) surfaces: Role of azole nitrogen atoms and dipole-dipole interactions. J. Phys. Chem. C 2011, 115, 24189–24197. [Google Scholar] [CrossRef]
- Poberžnik, M.; Chiter, F.; Milošev, I.; Marcus, P.; Costa, D.; Kokalj, A. DFT study of n-alkyl carboxylic acids on oxidized aluminum surfaces: From standalone molecules to self-assembled-monolayers. Appl. Surf. Sci. 2020, 525, 146156. [Google Scholar] [CrossRef]
- Neupane, S.; Losada-Pérez, P.; Tiringer, U.; Taheri, P.; Desta, D.; Xie, C.; Crespo, D.; Mol, A.; Milošev, I.; Kokalj, A.; et al. Study of Mercaptobenzimidazoles As Inhibitors for Copper Corrosion: Down to the Molecular Scale. J. Electrochem. Soc. 2021, 168, 051504. [Google Scholar] [CrossRef]
- Schiessler, E.J.; Würger, T.; Lamaka, S.V.; Meißner, R.H.; Cyron, C.J.; Zheludkevich, M.L.; Feiler, C.; Aydin, R.C. Predicting the inhibition efficiencies of magnesium dissolution modulators using sparse machine learning models. NPJ Comput. Mater. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Mišković-stanković, V.B.; Dražić, D.M.; Teodorović, M.J. Electrolyte penetration through epoxy coatings electrodeposited on steel. Corros. Sci. 1995, 37, 241–252. [Google Scholar] [CrossRef]
- Sousa, T.; Correia, J.; Pereira, V.; Rocha, M. Generative Deep Learning for Targeted Compound Design. J. Chem. Inf. Model. 2021, 61, 5343–5361. [Google Scholar] [CrossRef] [PubMed]
- Mercado, R.; Rastemo, T.; Lindelöf, E.; Klambauer, G.; Engkvist, O.; Chen, H.; Jannik Bjerrum, E. Graph networks for molecular design. Mach. Learn. Sci. Technol. 2021, 2, 025023. [Google Scholar] [CrossRef]
- Skerry, B.S.; Alavi, A.; Lindgren, K.I. Environmental and electrochemical test methods for the evaluation of protective organic coatings. J. Coat. Technol. 1988, 60, 97–106. [Google Scholar]
- Bierwagen, G.P.; He, L.; Li, J.; Ellingson, L.; Tallman, D.E. Studies of a new accelerated evaluation method for coating corrosion resistance—Thermal cycling testing. Prog. Org. Coat. 2000, 39, 67–78. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci, R.J., Jr.; Glover, C.F.; Kannan, B.; Walsh, Z.R.; Scully, J.R. A Review of Modern Assessment Methods for Metal and Metal-Oxide Based Primers for Substrate Corrosion Protection. Front. Mater. 2019, 6, 190. [Google Scholar] [CrossRef]
- Santucci, R.J., Jr.; Holleman, M.D.; Scully, J.R. Laboratory accelerated and field exposure testing of MgRP and MgORP on AA2024-T351: Chemical and electrochemical protection effects. Surf. Coat. Technol. 2020, 383, 125245. [Google Scholar] [CrossRef]
- Taheri, P.; Milošev, I.; Meeusen, M.; Kapun, B.; White, P.; Kokalj, A.; Mol, A. On the importance of time-resolved electrochemical evaluation in corrosion inhibitor-screening studies. Npj Mater. Degrad. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Abbas, M.; Shafiee, M. An overview of maintenance management strategies for corroded steel structures in extreme marine environments. Mar. Struct. 2020, 71, 102718. [Google Scholar] [CrossRef]
- Ganther, W.D.; Paterson, D.A.; Lewis, C.; Isaacs, P.; Galea, S.; Meunier, C.; Mangeon, G.; Cole, I.S. Monitoring Aircraft Microclimate and Corrosion. Procedia Eng. 2017, 188, 369–376. [Google Scholar] [CrossRef]
- Cole, I.S.; Marney, D. The science of pipe corrosion: A review of the literature on the corrosion of ferrous metals in soils. Corros. Sci. 2012, 56, 5–16. [Google Scholar] [CrossRef]
- Varela, F.; Tan, M.Y.; Forsyth, M. An overview of major methods for inspecting and monitoring external corrosion of on-shore transportation pipelines. Corros. Eng. Sci. Technol. 2015, 50, 226–235. [Google Scholar] [CrossRef]
- Russo, S.; Sharp, P.K.; Dhamari, R.; Mills, T.B.; Hinton, B.R.W.; Clark, G.; Shankar, K. The influence of the environment and corrosion on the structural integrity of aircraft materials. Fatigue Fract. Eng. Mater. Struct. 2009, 32, 464–472. [Google Scholar] [CrossRef]
- Rafla, V.N.; Scully, J.R. Galvanic couple behavior between AA7050-T7451 and stainless steel in a fastener arrangement assessed with coupled multi-electrode arrays under atmospheric exposure conditions. Corrosion 2019, 75, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.; Curtin, T.; Merrill, M.; Kim, M.; Friedersdorf, F.; Adey, R. Atmospheric corrosion measurements to improve understanding of galvanic corrosion of aircraft. In NACE International Corrosion Conference Proceedings; NACE International: Houston, TX, USA, 2018. [Google Scholar]
- Banerjee, P.C.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium implants: Prospects and challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E. Recent Advances in Smart Self-healing Polymers and Composites; Woodhead Publishing: Cambridge, UK, 2015; pp. 211–241. [Google Scholar] [CrossRef]
- Hughes, A.E.; Ho, D.; Forsyth, M.; Hinton, B.R.W. Towards replacement of chromate inhibitors by rare earth systems. Corros. Rev. 2007, 25, 591–605. [Google Scholar] [CrossRef]
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Visser, P.; Terryn, H.; Mol, J.M.C. Aerospace coatings. In Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2016; Volume 233, pp. 315–372. [Google Scholar]
- Nichols, M.; Tardiff, J. Automotive Coatings. Act. Prot. Coat. 2016, 233, 373–384. [Google Scholar] [CrossRef]
- Popov, K.I.; Kovaleva, N.E.; Rudakova, G.Y.; Kombarova, S.P.; Larchenko, V.E. Recent state-of-the-art of biodegradable scale inhibitors for cooling-water treatment applications (Review). Therm. Eng. 2016, 63, 122–129. [Google Scholar] [CrossRef]
- Emad, S.G.R.; Morsch, S.; Hashimoto, T.; Liu, Y.; Gibbon, S.R.; Lyon, S.B.; Zhou, X. Leaching from coatings pigmented with strontium aluminium polyphosphate inhibitor pigment- evidence for a cluster-percolation model. Prog. Org. Coat. 2019, 137, 105340. [Google Scholar] [CrossRef]
- Emad, S.G.R.; Zhou, X.; Morsch, S.; Lyon, S.B.; Liu, Y.; Graham, D.; Gibbon, S.R. How pigment volume concentration (PVC) and particle connectivity affect leaching of corrosion inhibitive species from coatings. Prog. Org. Coat. 2019, 134, 360–372. [Google Scholar] [CrossRef]
- Kopeć, M.; Rossenaar, B.D.; van Leerdam, K.; Davies, A.N.; Lyon, S.B.; Visser, P.; Gibbon, S.R. Chromate ion transport in epoxy films: Influence of BaSO4 particles. Prog. Org. Coat. 2020, 147, 105739. [Google Scholar] [CrossRef]
- Laird, J.S.; Visser, P.; Ranade, S.; Hughes, A.E.; Terryn, H.; Mol, J.M.C. Li leaching from Lithium Carbonate-primer: An emerging perspective of transport pathway development. Prog. Org. Coat. 2019, 134, 103–118. [Google Scholar] [CrossRef]
- Kendig, M.W.; Buchheit, R.G. Corrosion inhibition of aluminum and aluminum alloys by soluble chromates, chromate coatings, and chromate-free coatings. Corrosion 2003, 59, 379–400. [Google Scholar] [CrossRef]
- Hughes, A.E.; Chen, F.F.; Yang, Y.S.; Cole, I.S.; Sellaiyan, S.; Carr, J.; Lee, P.D.; Thompson, G.E.; Xiao, T.Q. Revealing intertwining organic and inorganic fractal structures in polymer coatings. Adv. Mater. 2014, 26, 4504–4508. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.; Forsyth, M.; Tan, M.Y.J. The initiation and propagation of coating morphological and structural defects under mechanical strain and their effects on the electrochemical behaviour of pipeline coatings. Prog. Org. Coat. 2017, 110, 62–77. [Google Scholar] [CrossRef]
- Ranade, S.D.; Hughes, T.; Yang, S.; Forsyth, M.; Li, J.; Wang, H.; Tan, M.Y. Visualizing the impact of mechanical strain and the environment on pipeline coatings from a three dimensional perspective. Prog. Org. Coat. 2018, 122, 45–55. [Google Scholar] [CrossRef]
- G170-06(2020)e1; Standard Guide for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors in the Laboratory. ASTM: West Conshohocken, PA, USA, 2020.
- G208-12(2020); Standard Practice for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors Using Jet Impingement Apparatus. ASTM: West Conshohocken, PA, USA, 2020.
- G184-06(2020)e1; Standard Practice for Evaluating and Qualifying Oil Field and Refinery Corrosion Inhibitors Using Rotating Cage. ASTM: West Conshohocken, PA, USA, 2020.
- G202-12(2020); Standard Test Method for Using Atmospheric Pressure Rotating Cage. ASTM: West Conshohocken, PA, USA, 2020.
- Mercer, A.D. Test methods for corrosion inhibitors: Report prepared for the European Federation of Corrosion Working Party on Inhibitors. Br. Corros. J. 1985, 20, 61–70. [Google Scholar] [CrossRef]
- NACE Task Group T-1D-34; Technical Committee Report, Laboratory Test Methods for Evaluating Oil field Corrosion Inhibitors; NACE: Houston, TX, USA, 1996.
- Muster, T.H.; Hughes, A.E.; Thompson, G.E. Cu Distributions in Aluminium Alloys, 1st ed.; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Blanc, C.; Gastaud, S.; Mankowski, G. Mechanistic studies of the corrosion of 2024 aluminum alloy in nitrate solutions. J. Electrochem. Soc. 2003, 150, B396–B404. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Boger, R.K.; Donohue, M.W. Copper dissolution phenomena in Al-Cu and Al-Cu-Mg alloys. In Corrosion and Corrosion Control in Saltwater Environments; Shifler, D.A., Natishan, P.M., Tsuru, T., Ito, S., Eds.; Electro Chemical Society: Pennington, NJ, USA, 2000; Volume 99, pp. 205–212. [Google Scholar]
- Joma, S.; Sancy, M.; Sutter, E.M.M.; Tran, T.T.M.; Tribollet, B. Incongruent dissolution of copper in an Al-Cu assembling. Influence of local pH changes. Surf. Interface Anal. 2013, 45, 1590–1596. [Google Scholar] [CrossRef]
- Scully, J.R.; Young, G.A., Jr. The effects of Temper, Test Temperature, and Alloyed Copper on the Hydrogen Controlled Crack Growth Rate of an Al-Zn-Mg-(Cu) Alloy. In Proceedings of the Corrosion 2000, Orlando, FL, USA, 26–31 March 2000; p. 00368. [Google Scholar]
- Fredriksson, W.; Malmgren, S.; Gustafsson, T.; Gorgoi, M.; Edström, K. Full depth profile of passive films on 316L stainless steel based on high resolution HAXPES in combination with ARXPS. Appl. Surf. Sci. 2012, 258, 5790–5797. [Google Scholar] [CrossRef]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-induced physicochemical alterations of the native surface oxide film on 316L austenitic stainless steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Tan, Y.; Huang, Y.; Mansfeld, F. Testing and analysis techniques in rare earth inhibitor research. Rare Earth-Based Inhib. 2014, 38–83. [Google Scholar] [CrossRef]
- Bazli, M.; Zhao, X.L.; Bai, Y.; Singh Raman, R.K.; Al-Saadi, S.; Haque, A. Durability of pultruded GFRP tubes subjected to seawater sea sand concrete and seawater environments. Constr. Build. Mater. 2020, 245, 118399. [Google Scholar] [CrossRef]
- G61-86(2018); Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM: West Conshohocken, PA, USA, 2018.
- Williams, G.; McMurray, H.N.; Worsley, D.A. Cerium(III) inhibition of corrosion-driven organic coating delamination studied using a scanning Kelvin probe technique. J. Electrochem. Soc. 2002, 149, B154–B162. [Google Scholar] [CrossRef]
- McMurray, H.N.; Williams, D.; Williams, G.; Worsley, D. Inhibitor pretreatment synergies demonstrated using a scanning Kelvin probe technique. Corros. Eng. Sci. Technol. 2003, 38, 112–118. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of corrosion inhibition of AA2024 by rare-earth compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Vyas, B. Scanning electrode techniques in corrosion. In Proceedings of the Tri-Service Corrosion Conference, Colorado Springs, CO, USA, 5 November 1980; pp. 191–214. [Google Scholar]
- Zou, F.; Thierry, D.; Isaacs, H.S. A high-resolution probe for localized electrochemical impedance spectroscopy measurements. J. Electrochem. Soc. 1997, 144, 1957–1965. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Karavai, O.V.; Ferreira, M.G.S. Influence of inhibitor addition on the corrosion protection performance of sol-gel coatings on AA2024. Prog. Org. Coat. 2008, 63, 352–361. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simoes, A.M.; Carmezim, M.J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl. Surf. Sci. 2007, 253, 6922–6931. [Google Scholar] [CrossRef]
- Hoseinieh, S.M.; Homborg, A.M.; Shahrabi, T.; Mol, J.M.C.; Ramezanzadeh, B. A Novel Approach for the Evaluation of Under Deposit Corrosion in Marine Environments Using Combined Analysis by Electrochemical Impedance Spectroscopy and Electrochemical Noise. Electrochim. Acta 2016, 217, 226–241. [Google Scholar] [CrossRef]
- Tan, Y.; Fwu, Y.; Bhardwaj, K. Electrochemical evaluation of under-deposit corrosion and its inhibition using the wire beam electrode method. Corros. Sci. 2011, 53, 1254–1261. [Google Scholar] [CrossRef]
- NACE Task Group TG 380. Under Deposit Corrosion Testing and Mitigation Methods; NACE: Houston, TX, USA, 2009; p. 28. [Google Scholar]
- de Reus, J.A.M.; Hendriksen, E.L.J.A.; Wilms, M.E.; Al-Habsi, Y.N.; Durnie, W.H.; Gough, M.A. Test methodologies and field verification of corrosion inhibitors to address under deposit corrosion in oil and gas production systems. In Proceedings of the NACE 2005, Houston, TX, USA, 3–7 April 2005. [Google Scholar]
- Cordell, D.; Rosemarin, A.; Schroder, J.J.; Smit, A.L. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.J.; Muster, T.H.; Ozkanat, O.; Sherman, N.; Hughes, A.E.; Terryn, H.; de Wit, J.H.W.; Mol, J.M.C. The influence of pH on corrosion inhibitor selection for 2024-T3 aluminium alloy assessed by high-throughput multielectrode and potentiodynamic testing. Electrochim. Acta 2010, 55, 2457–2465. [Google Scholar] [CrossRef]
- Rayment, T.; Davenport, A.J.; Dent, A.J.; Tinnes, J.-P.; Wiltshire, R.J.K.; Martin, C.; Clark, G.; Quinn, P.; Mosselmans, J.F.W. Characterisation of salt films on dissolving metal surfaces in artificial corrosion pits via in situ synchrotron X-ray diffraction. Electrochem. Commun. 2008, 10, 855–858. [Google Scholar] [CrossRef]
- Ghahari, S.M.; Davenport, A.J.; Rayment, T.; Suter, T.; Tinnes, J.P.; Padovani, C.; Hammons, J.A.; Stampanoni, M.; Marone, F.; Mokso, R. In situ synchrotron X-ray micro-tomography study of pitting corrosion in stainless steel. Corros. Sci. 2011, 53, 2684–2687. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Luo, C.; Kawano, K.; Thompson, G.E.; Hughes, A.E.; Skeldon, P.; Withers, P.J.; Marrow, T.J.; Sherry, A.H. Nanotomography for understanding materials degradation. Scr. Mater. 2010, 63, 835–838. [Google Scholar] [CrossRef]
- Hashimoto, T.; Curioni, M.; Zhou, X.; Mancuso, J.; Skeldon, P.; Thompson, G.E. Investigation of dealloying by ultra-high-resolution nanotomography. Surf. Interface Anal. 2013, 45, 1548–1552. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Yang, S.; Wang, J.; Li, J.; Glenn, A.M.; Xu, W.; Tan, M.Y. A critical insight into lack-of-fusion pore structures in additively manufactured stainless steel. Addit. Manuf. 2021, 38, 101762. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Yang, S.; Li, J.; Xu, W.; Gibson, I.; Tan, M.Y. Two and three-dimensional characterisation of localised corrosion affected by lack-of-fusion pores in 316L stainless steel produced by selective laser melting. Corros. Sci. 2020, 165, 108394. [Google Scholar] [CrossRef]
- Knight, S.P.; Salagaras, M.; Wythe, A.M.; De Carlo, F.; Davenport, A.J.; Trueman, A.R. In situ X-ray tomography of intergranular corrosion of 2024 and 7050 aluminium alloys. Corros. Sci. 2010, 52, 3855–3860. [Google Scholar] [CrossRef]
- Knight, S.P.; Salagaras, M.; Trueman, A.R. The study of intergranular corrosion in aircraft aluminium alloys using X-ray tomography. Corros. Sci. 2011, 53, 727–734. [Google Scholar] [CrossRef]
- Strehblow, H.H. Nucleation and Repassivation of Corrosion Pits for Pitting on Iron and Nickel. Mater. Corros. 1976, 27, 792–799. [Google Scholar] [CrossRef]
- Sazou, D.; Pagitsas, M. Non-linear dynamics of the passivity breakdown of iron in acidic solutions. Chaos Solitons Fractals 2003, 17, 505–522. [Google Scholar] [CrossRef]
- Enerhaug, J.; Steinsmo, U.M.; Grong, Ø.; Hellevik, L.R. Dissolution and repassivation kinetics of a 12.3Cr-2.6Mo-6.5Ni super martensitic stainless steel: A comparative study. J. Electrochem. Soc. 2002, 149, B256–B264. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Growth of corrosion pits on stainless steel in chloride solution containing dilute sulphate. Corros. Sci. 1992, 33, 1885–1897. [Google Scholar] [CrossRef]
- Galvele, J.R. Transport processes and mechanism of pitting of metals. J. Electrochem. Soc. 1976, 123, 464–474. [Google Scholar] [CrossRef]
- Sugie, Y.; Motooka, T.; Fujii, S. Early growth of single pits in weak acidic chloride media and anion effects on solubility of salt in the pit anolyte. Corrosion 1994, 50, 513–515. [Google Scholar] [CrossRef]
- Popova, K.; Prošek, T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals 2022, 12, 171. [Google Scholar] [CrossRef]
- Cole, I.S. Atmospheric corrosion. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands; ISBN 9780444527882.
- Policastro, S.A.; Anderson, R.M.; Hangarter, C.M. Estimating the Effect that Interactions Between Chemical Reactions and Environmental Influences Have on the Corrosivity of the Electrolyte. In Minerals, Metals and Materials Series; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Schaller, R.F.; Jove-Colon, C.F.; Taylor, J.M.; Schindelholz, E.J. The controlling role of sodium and carbonate on the atmospheric corrosion rate of aluminum. NPJ Mater. Degrad. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Sridhar, N.; Dunn, D.S. In situ study of salt film stability in simulated pits of nickel by Raman and electrochemical impedance spectroscopies. J. Electrochem. Soc. 1997, 144, 4243–4253. [Google Scholar] [CrossRef]
- Kuo, H.C.; Landolt, D. Rotating disc electrode study of anodic dissolution or iron in concentrated chloride media. Electrochim. Acta 1975, 20, 393–399. [Google Scholar] [CrossRef]
- Andrade, C.; Garcés, P.; Martínez, I. Galvanic currents and corrosion rates of reinforcements measured in cells simulating different pitting areas caused by chloride attack in sodium hydroxide. Corros. Sci. 2008, 50, 2959–2964. [Google Scholar] [CrossRef]
- Song, W.; Martin, H.J.; Hicks, A.; Seely, D.; Walton, C.A.; Lawrimore Ii, W.B.; Wang, P.T.; Horstemeyer, M.F. Corrosion behaviour of extruded AM30 magnesium alloy under salt-spray and immersion environments. Corros. Sci. 2014, 78, 353–368. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Cho, J.H. In Situ X-Ray Microprobe Study of Salt Layers during Anodic Dissolution of Stainless Steel in Chloride Solution. J. Electrochem. Soc. 1995, 142, 1111–1118. [Google Scholar] [CrossRef]
- Szymanski, R.; Jamieson, D.N.; Hughes, A.E.; Mol, A.; van der Zwaag, S.; Ryan, C.G. Filiform corrosion imaged beneath protection layers on Al alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 190, 365–369. [Google Scholar] [CrossRef]
- Mol, J.M.C.; Hughes, A.E.; Hinton, B.R.W.; van der Zwaag, S. A morphological study of filiform corrosive attack on chromated and alkaline-cleaned AA2024-T351 aluminium alloy. Corros. Sci. 2004, 46, 1201–1224. [Google Scholar] [CrossRef]
- King, P.C.; Cole, I.S.; Corrigan, P.A.; Hughes, A.E.; Muster, T.H.; Thomas, S. FIB/SEM study of AA2024 corrosion under a seawater drop, part II. Corros. Sci. 2012, 55, 116–125. [Google Scholar] [CrossRef]
- King, P.C.; Cole, I.S.; Corrigan, P.A.; Hughes, A.E.; Muster, T.H. FIB/SEM study of AA2024 corrosion under a seawater drop: Part I. Corros. Sci. 2011, 53, 1086–1096. [Google Scholar] [CrossRef]
- Pride, S.T.; Scully, J.R.; Hudson, J.L. Metastable pitting of aluminum and criteria for the transition to stable pit growth. J. Electrochem. Soc. 1994, 141, 3028–3040. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Wilmott, M.; Luo, J.L. Transition criterion of metastable pitting towards stability for carbon steel in chloride solutions. Br. Corros. J. 1999, 34, 280–284. [Google Scholar] [CrossRef]
- Trueman, A.R. Determining the probability of stable pit initiation on aluminium alloys using potentiostatic electrochemical measurements. Corros. Sci. 2005, 47, 2240–2256. [Google Scholar] [CrossRef]
- Burstein, G.T.; Pistorius, P.C.; Mattin, S.P. The nucleation and growth of corrosion pits on stainless steel. Corros. Sci. 1993, 35, 57–62. [Google Scholar] [CrossRef]
- Koushik, B.G.; Van den Steen, N.; Terryn, H.; Van Ingelgem, Y. Investigation of the importance of heat transfer during thin electrolyte formation in atmospheric corrosion using a novel experimental approach. Corros. Sci. 2021, 189, 109542. [Google Scholar] [CrossRef]
- Saberi, L.; Amiri, M. Modeling Atmospheric Corrosion under Dynamic Thin Film Electrolyte. J. Electrochem. Soc. 2021, 168, 081506. [Google Scholar] [CrossRef]
- Muster, T.H.; Neufeld, A.K.; Cole, I.S. The protective nature of passivation films on zinc: Wetting and surface energy. Corros. Sci. 2004, 46, 2337–2354. [Google Scholar] [CrossRef]
- Muster, T.H.; Bradbury, A.; Trinchi, A.; Cole, I.S.; Markley, T.; Lau, D.; Dligatch, S.; Bendavid, A.; Martin, P. The atmospheric corrosion of zinc: The effects of salt concentration, droplet size and droplet shape. Electrochim. Acta 2011, 56, 1866–1873. [Google Scholar] [CrossRef]
- Mikulskis, P.; Alexander, M.R.; Winkler, D.A. Toward Interpretable Machine Learning Models for Materials Discovery. Adv. Intell. Syst. 2019, 1, 1900045. [Google Scholar] [CrossRef]
- Fronzi, M.; Isayev, O.; Winkler, D.A.; Shapter, J.G.; Ellis, A.V.; Sherrell, P.C.; Shepelin, N.A.; Corletto, A.; Ford, M.J. Active Learning in Bayesian Neural Networks for Bandgap Predictions of Novel Van der Waals Heterostructures. Adv. Intell. Syst. 2021, 3, 2100080. [Google Scholar] [CrossRef]
- Winkler, D.A. Sparse QSAR modelling methods for therapeutic and regenerative medicine. J. Comput.-Aided Mol. Des. 2018, 32, 497–509. [Google Scholar] [CrossRef]
- Winkler, D.A. Predicting the Performance of Organic Corrosion Inhibitors. Metals 2017, 7, 553. [Google Scholar] [CrossRef]
- Le, T.; Epa, V.C.; Burden, F.R.; Winkler, D.A. Quantitative Structure–Property Relationship Modeling of Diverse Materials Properties. Chem. Rev. 2012, 112, 2889–2919. [Google Scholar] [CrossRef] [PubMed]



| Method | Method of Assessment | Application |
|---|---|---|
| Single-metal multi-electrodes | Corrosion current | Used in aqueous systems to assess a range of inhibitors |
| Wire beam electrode | Corrosion current | Used in electrolytes, but can also assess performances in structural configurations |
| Linear polarisation resistance | Resistance | Coatings |
| Multimetal electrodes | Corrosion current | Used for assessment as a function of pH and inhibitor concentration |
| Wells | Optical analysis of the level of corrosion | Multiple inhibitor assessment (individually or in combination) and pH on a single metal |
| Flow through channels | Optical analysis of the channel after testing and chemical analysis | Used to assess corrosion vs. time on single metal and multiple inhibitors limited by the number of channels |
| Quantum Mechanical | Machine Learning |
|---|---|
| Highest occupied molecular orbital (HOMO) Lowest unoccupied molecular orbital (LUMO) Electron affinity Ionisation potential Electronegativity Chemical potential Chemical hardness Fundamental gap | A11 number of tertiary N atoms A31 number of secondary sulphur atoms Topological distances between atoms X and Y, e.g., B0n[X-Y], representing the presence/absence or frequency of occurrence of an X and Y n bonds apart Topological frequencies between atoms X and Y, i.e., F0n[X-Y] HOMT aromaticity index based on the length of the conjugated pathway C-XXX: numerous descriptors describing the number of different types of groups nCconj: number of non-aromatic conjugated C(sp2) groups nCp: terminal primary C(sp2) groups nTriazoles—number of triazoles nBenzene—number of benzene rings |
| Metal | Pit Electrolyte | Reference |
|---|---|---|
| Ni | NiCl2·6H2O salt film/sat electrolyte | [92,110] |
| Fe | FeCl2·6H2O salt film/sat electrolyte | [92,111] |
| SS316L | FeCl2·6H2O salt film/sat electrolyte | [92] |
| SS304 | FeCl2, FeSO4 (probably with waters of hydration) | [105] |
| Steel bar (reo) | Anolyte FeCl2 (pH 2.7), catholyte NaOH (pH 13) | [112] |
| AM30 (Mg alloy) | MgCl2·MgOHCl 1 | [113] |
| Fe | 4.25 ± 0.05 M for FeCl2 | [111] |
| Austenitic SS | 3.5 M Fe2+, 1.1 M Cr3+, and 0.5 M Ni2+ | [114] |
| AA2024 | >20 wt% Cl− and Al(OH)3 in a filiform head | [115] |
| AA2024 | Strong chloride environment with O and Al in pits for filiform | [94,116] |
| AlCuMg2 | Al-hydroxide gel with Mg, chloride, and Cu ions under seawater droplet (S-phase embedded in AA2024-T3) | [117,118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, A.E.; Winkler, D.A.; Carr, J.; Lee, P.D.; Yang, Y.S.; Laleh, M.; Tan, M.Y. Corrosion Inhibition, Inhibitor Environments, and the Role of Machine Learning. Corros. Mater. Degrad. 2022, 3, 672-693. https://doi.org/10.3390/cmd3040037
Hughes AE, Winkler DA, Carr J, Lee PD, Yang YS, Laleh M, Tan MY. Corrosion Inhibition, Inhibitor Environments, and the Role of Machine Learning. Corrosion and Materials Degradation. 2022; 3(4):672-693. https://doi.org/10.3390/cmd3040037
Chicago/Turabian StyleHughes, Anthony E., David A. Winkler, James Carr, P. D. Lee, Y. S. Yang, Majid Laleh, and Mike Y. Tan. 2022. "Corrosion Inhibition, Inhibitor Environments, and the Role of Machine Learning" Corrosion and Materials Degradation 3, no. 4: 672-693. https://doi.org/10.3390/cmd3040037
APA StyleHughes, A. E., Winkler, D. A., Carr, J., Lee, P. D., Yang, Y. S., Laleh, M., & Tan, M. Y. (2022). Corrosion Inhibition, Inhibitor Environments, and the Role of Machine Learning. Corrosion and Materials Degradation, 3(4), 672-693. https://doi.org/10.3390/cmd3040037







