The Biological Clock Influenced by Burnout, Hormonal Dysregulation and Circadian Misalignment: A Systematic Review
Abstract
1. Introduction
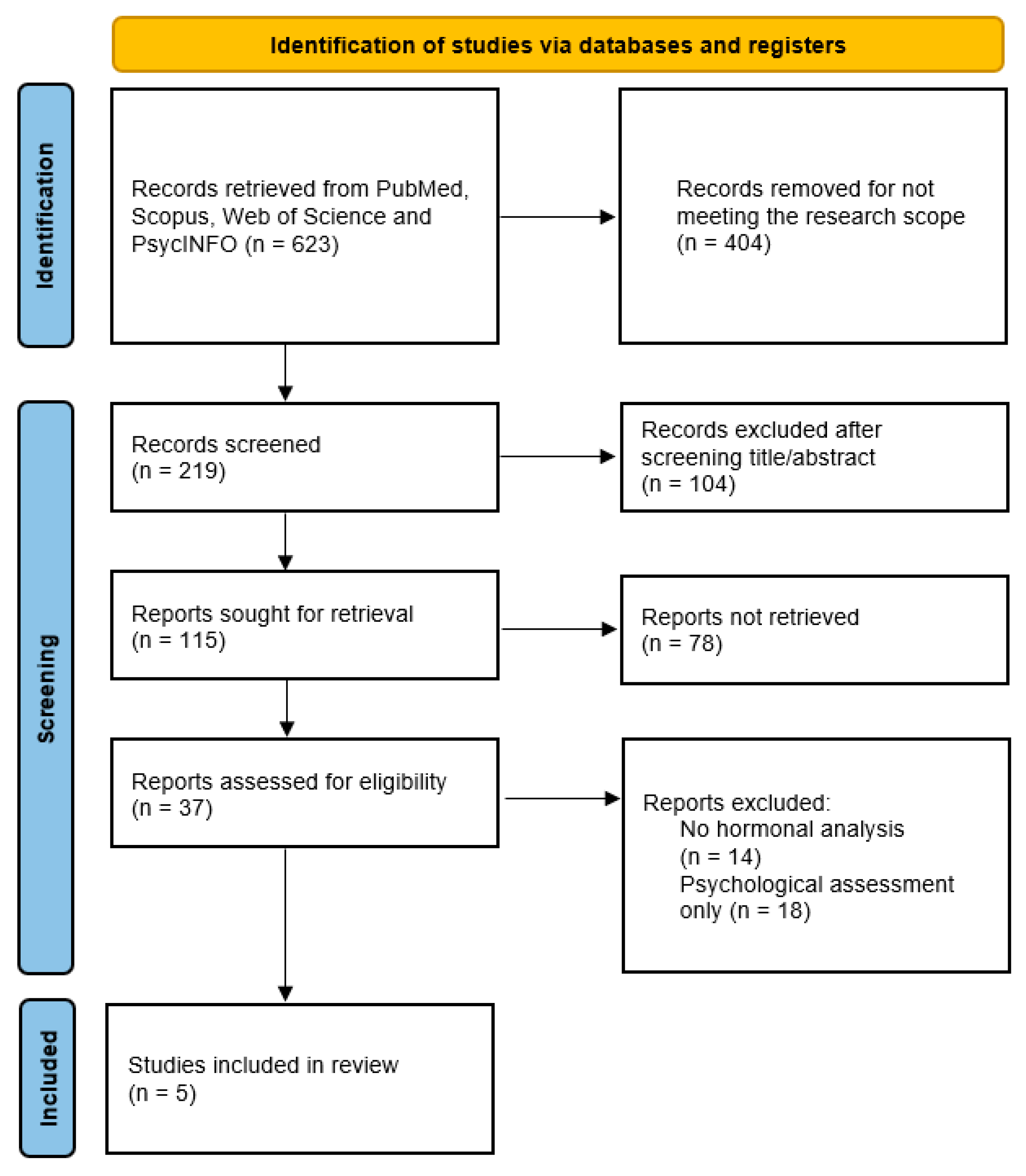
2. Methods
3. Results and Discussion
Excluded Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a.m. | anterior meridian |
| p.m. | post meridian |
| DYMERS | Dysregulation of Mood, Energy, and Social Rhythms Syndrome |
| HPA | Hypothalamus–Pituitary–Adrenal |
| MBI | Maslach Burnout Inventory |
| NOS | Newcastle–Ottawa Scale |
| SAM | Sympathetic-Adrenomedullary System |
References
- Nash, C. Dysregulation of mood, energy, and social rhythms syndrome (DYMERS) and burnout: A scoping review in healthcare professionals. J. Clin. Med. 2025, 14, 1035. [Google Scholar] [CrossRef]
- Loredana-Sabina, P.; Perri, D.; Vlad, B.A.; Ciubara, A.; Marilena, M.; Virginia, M. The Effects of Blue Light in Modern Society? BRAIN Broad Res. Artif. Intell. Neurosci. 2019, 10, 5–11. [Google Scholar] [CrossRef]
- Kakiashvili, T.; Leszek, J.; Rutkowski, K. The medical perspective on burnout. Int. J. Occup. Med. Environ. Health 2013, 26, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Ramin, C.; Devore, E.E.; Wang, W.; Pierre-Paul, J.; Wegrzyn, L.R.; Schernhammer, E.S. Night shift work at specific age ranges and chronic disease risk factors. Occup. Environ. Med. 2015, 72, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Freudenberger, H.J. Staff burn-out. J. Soc. Issues 1974, 30, 159–165. [Google Scholar] [CrossRef]
- Maslach, C.; Jackson, S.E. The measurement of experienced burnout. J. Occup. Behav. 1981, 2, 99–113. [Google Scholar] [CrossRef]
- Maslach, C.; Schaufeli, W.B.; Leiter, M.P. Job burnout. Annu. Rev. Psychol. 2001, 52, 397–422. [Google Scholar] [CrossRef]
- World Health Organization. Burn-Out an “Occupational Phenomenon”: International Classification of Diseases (ICD-11); World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/mental_health/evidence/burn-out/en/ (accessed on 28 June 2025).
- Ghahramani, S.; Bagheri Lankarani, K.; Yousefi, M.; Heydari, K.; Shahabi, S.; Azmand, S. A systematic review and meta-analysis of burnout among healthcare workers during COVID-19. Front. Psychiatry 2021, 12, 758849. [Google Scholar] [CrossRef] [PubMed]
- Macaron, M.M.; Segun-Omosehin, O.A.; Matar, R.H.; Beran, A.; Nakanishi, H.; Than, C.A.; Abulseoud, O.A. A systematic review and meta-analysis on burnout in physicians during the COVID-19 pandemic: A hidden healthcare crisis. Front. Psychiatry 2023, 13, 1071397. [Google Scholar] [CrossRef]
- Jonsdottir, I.H.; Sjörs Dahlman, A. Mechanisms in endocrinology: Endocrine and immunological aspects of burnout: A narrative review. Eur. J. Endocrinol. 2019, 180, R147–R158. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Damian, A.C.; Neagu, C. Burnout and neuroendocrine-immune pathways. Rom. J. Morphol. Embryol. 2021, 62, 13–18. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Adrenal fatigue: A systematic review. BMC Endocr. Disord. 2016, 16, 48. [Google Scholar] [CrossRef]
- Edwards, D.; Heufelder, A.; Zimmermann, A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms—Results of an open-label study. Phytother. Res. 2012, 26, 1220–1225. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized, double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Metlaine, A.; Sauvet, F.; Gomez-Merino, D.; Boucher, T.; Elbaz, M.; Delafosse, J.Y.; Leger, D.; Chennaoui, M. Sleep and biological parameters in professional burnout: A psychophysiological characterization. PLoS ONE 2018, 13, e0190607. [Google Scholar] [CrossRef]
- Bagheri Hosseinabadi, M.; Ebrahimi, M.H.; Khanjani, N.; Biganeh, J.; Mohammadi, S.; Abdolahfard, M. The effects of amplitude and stability of circadian rhythm and occupational stress on burnout syndrome and job dissatisfaction among irregular shift-working nurses. J. Clin. Nurs. 2019, 28, 1868–1878. [Google Scholar] [CrossRef]
- Ungur, A.P.; Bârsan, M.; Socaciu, A.I.; Râjnoveanu, A.G.; Ionuț, R.; Goia, L.; Procopciuc, L.M. A narrative review of burnout syndrome in medical personnel. Diagnostics 2024, 14, 1971. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Panagioti, M.; Esmail, A.; Agius, R.; Van Tongeren, M.; Bower, P. Factors Associated With Burnout and Stress in Trainee Physicians: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2013761. [Google Scholar] [CrossRef] [PubMed]
- Glandorf, H.L.; Madigan, D.J.; Kavanagh, O.; Mallinson-Howard, S.H. Mental and physical health outcomes of burnout in athletes: A systematic review and meta-analysis. Int. Rev. Sport Exerc. Psychol. 2023, 18, 372–416. [Google Scholar] [CrossRef]
- Rotenstein, L.S.; Torre, M.; Ramos, M.A.; Mata, D.A. Prevalence of burnout among physicians: A systematic review. JAMA 2018, 320, 1131–1150. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Duffy, J.F.; Czeisler, C.A. Effect of light on human circadian physiology. Sleep Med. Clin. 2009, 4, 165–177. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 May 2025).
- Popescu, C.M.; Marina, V.; Avram, G.; Cristescu Budala, C.L. Spectrum of Magnetic Resonance Imaging Findings in Acute Pediatric Traumatic Brain Injury—A Pictorial Essay. J. Multidiscip. Healthc. 2024, 17, 2921–2934. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Tremblay, G.M. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, E.; Reely, K.; Dowdy, K.; Seth, N.; McGrade, P.; Jones, C.; Horan, M.J.; Provenzano, N.; Ramirez, P.; Hammonds, K.; et al. A Systematic Review of Burnout and Its Relation to Work-Life Balance and Scheduling among United States Physicians. Southwest Respir. Crit. Care Chron. 2020, 8, 40–46. Available online: https://pulmonarychronicles.com/index.php/pulmonarychronicles/article/view/663 (accessed on 12 May 2025). [CrossRef]
- Dijk, D.-J.; Duffy, J.F. Novel Approaches for Assessing Circadian Rhythmicity in Humans: A Review. J. Biol. Rhythms 2020, 35, 421–438. [Google Scholar] [CrossRef] [PubMed]


| Study Title | Authors | Study Type | Sample Size | Key Findings |
|---|---|---|---|---|
| Dysregulation of Mood, Energy, and Social Rhythms Syndrome (DYMERS) [1] | Nash, C. (2025) | Scoping Review | 600 healthcare workers | Irregular social and biological rhythms contribute to burnout symptoms, with disrupted sleep and circadian misalignment. |
| Burnout Among Healthcare Workers During the COVID-19 Pandemic [9] | Ghahramani, S. et al. (2021) | Systematic Review & Meta-Analysis | 52 studies | Burnout prevalence was 52%, with nurses and physicians at the highest risk. Contributing factors included workload and emotional exhaustion. |
| A Systematic Review and Meta-Analysis on Burnout in Physicians During the COVID-19 Pandemic: A Hidden Healthcare Crisis [10] | Macaron, M.M. et al. (2023) | Systematic Review & Meta-Analysis | 45 studies | Burnout prevalence was 54.6% (95% CI: 46.7–62.2%), with frontline physicians more affected than second-line colleagues (OR = 1.64, 95% CI: 1.13–2.37). |
| Endocrine and Immunological Aspects of Burnout [11] | Jonsdottir, I.H., & Sjörs Dahlman, A. (2019) | Narrative Review | Multiple studies reviewed | HPA axis dysfunction and immune suppression are linked to burnout, leading to increased physical health risks. |
| A Narrative Review of Burnout Syndrome in Medical Personnel [18] | Ungur, A.P. et al. (2024) | Narrative Review | Multiple studies reviewed | Synthesizes evidence on burnout among medical personnel and emphasizes hormonal and psychosomatic mechanisms relevant to clinical practice. |
| Factors Associated with Burnout and Stress in Trainee Physicians [19] | Zhou, A.Y. et al. (2020) | Systematic Review & Meta-Analysis | 30 studies | Workload, training environment, and inadequate support systems were identified as the main factors influencing burnout among trainee physicians. |
| Mental and physical health outcomes of burnout in athletes [20] | Glandorf, H.L. et al. (2023) | Systematic Review & Meta-Analysis | 54 studies; 13,976 athletes | Athlete burnout associated with increased negative mental health, decreased positive mental health; mixed evidence on physical health outcomes |
| Prevalence of Burnout Among Physicians [21] | Rotenstein, L.S. et al. (2018) | Systematic Review | 40 studies | Burnout prevalence varied widely (0% to 80.5%), highlighting the need for standardized assessment methods. |
| Criterion | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study Design | Empirical studies, systematic reviews, and meta-analyses | Non-peer-reviewed sources, conference abstracts, opinion pieces |
| Population | Individuals diagnosed with burnout, healthcare professionals | Studies without hormonal biomarker analysis |
| Outcomes | Cortisol, melatonin, immune markers, metabolic indicators | Psychological outcomes only |
| Language | English or widely accessible languages | Non-English studies without translation |
| Publication Year | 2000–2024 | Studies published before 2000 |
| Study | Sample Size | Biomarkers Assessed | Key Findings |
|---|---|---|---|
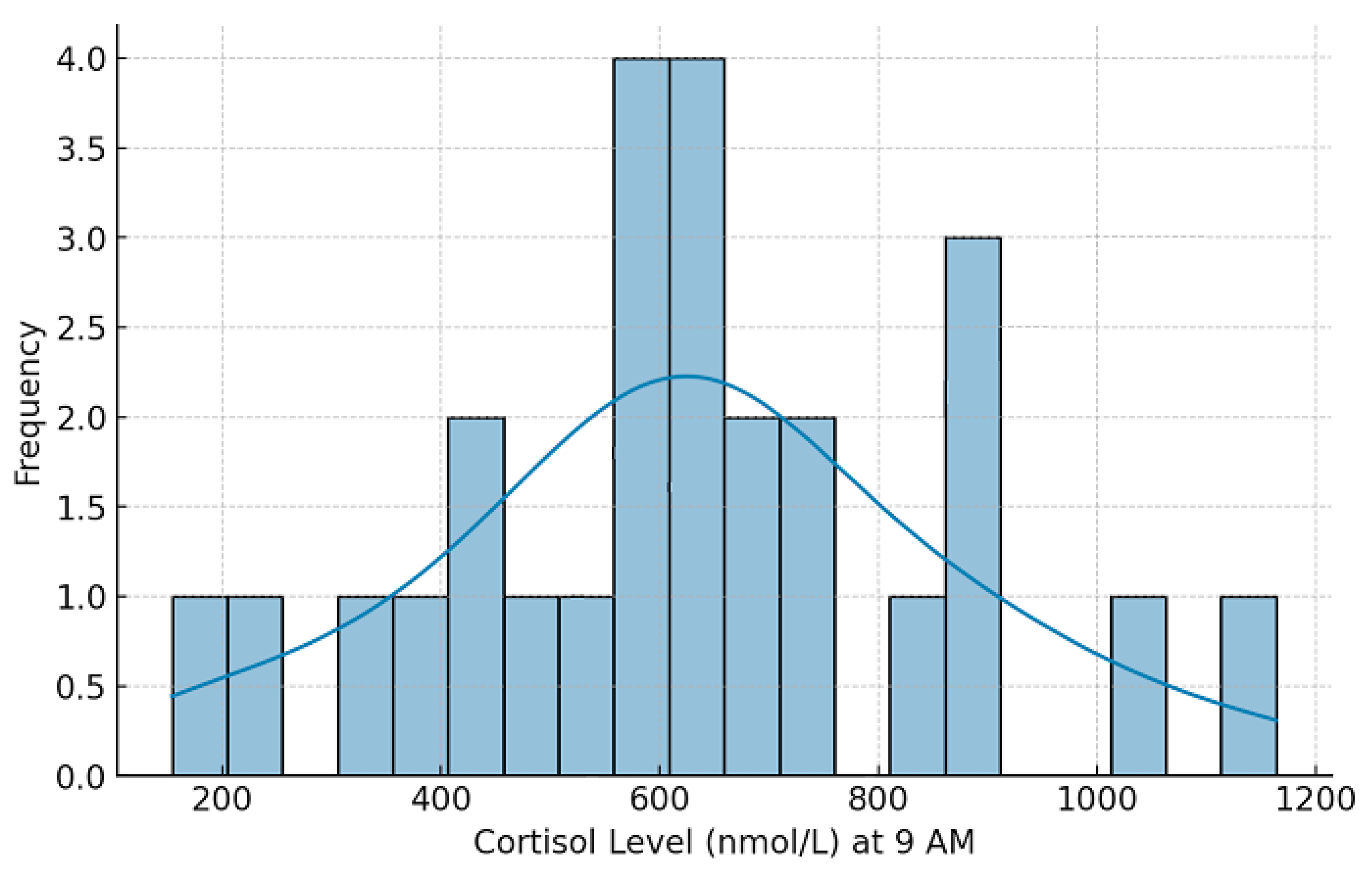
| Jonsdottir & Sjörs Dahlman (2019) [11] | 1200 | Cortisol, HPA axis | Burnout associated with blunted cortisol response |
| Ciobanu et al. (2021) [12] | 800 | Cortisol, immune markers | Neuroendocrine alterations linked to immune dysfunction |
| Cadegiani & Kater (2016) [13] | 750 | Cortisol, metabolic markers | No direct evidence for adrenal fatigue but strong metabolic impact |
| Nash (2025) [1] | 600 | Melatonin, circadian rhythms | Shift work disrupts melatonin secretion in burnout cases |
| Kakiashvili et al. (2013) [3] | 1000 | Cardiovascular markers | Burnout increases cardiovascular disease risk |
| Factors | Burnout Score Correlation | Interpretation |
|---|---|---|
| Physical Symptoms | 0.81 (strong positive) | Increased physical symptoms are strongly associated with burnout severity, indicating significant physiological impacts (cardiac, digestive, headaches, musculoskeletal, immune). |
| Emotional Exhaustion | 0.90 (strong positive) | Emotional exhaustion is a primary contributor to burnout, closely related to physiological stress. |
| Depersonalization | 0.82 (strong positive) | Higher depersonalization levels correlate strongly with increased burnout, emphasizing psychological distancing as a stress-coping mechanism. |
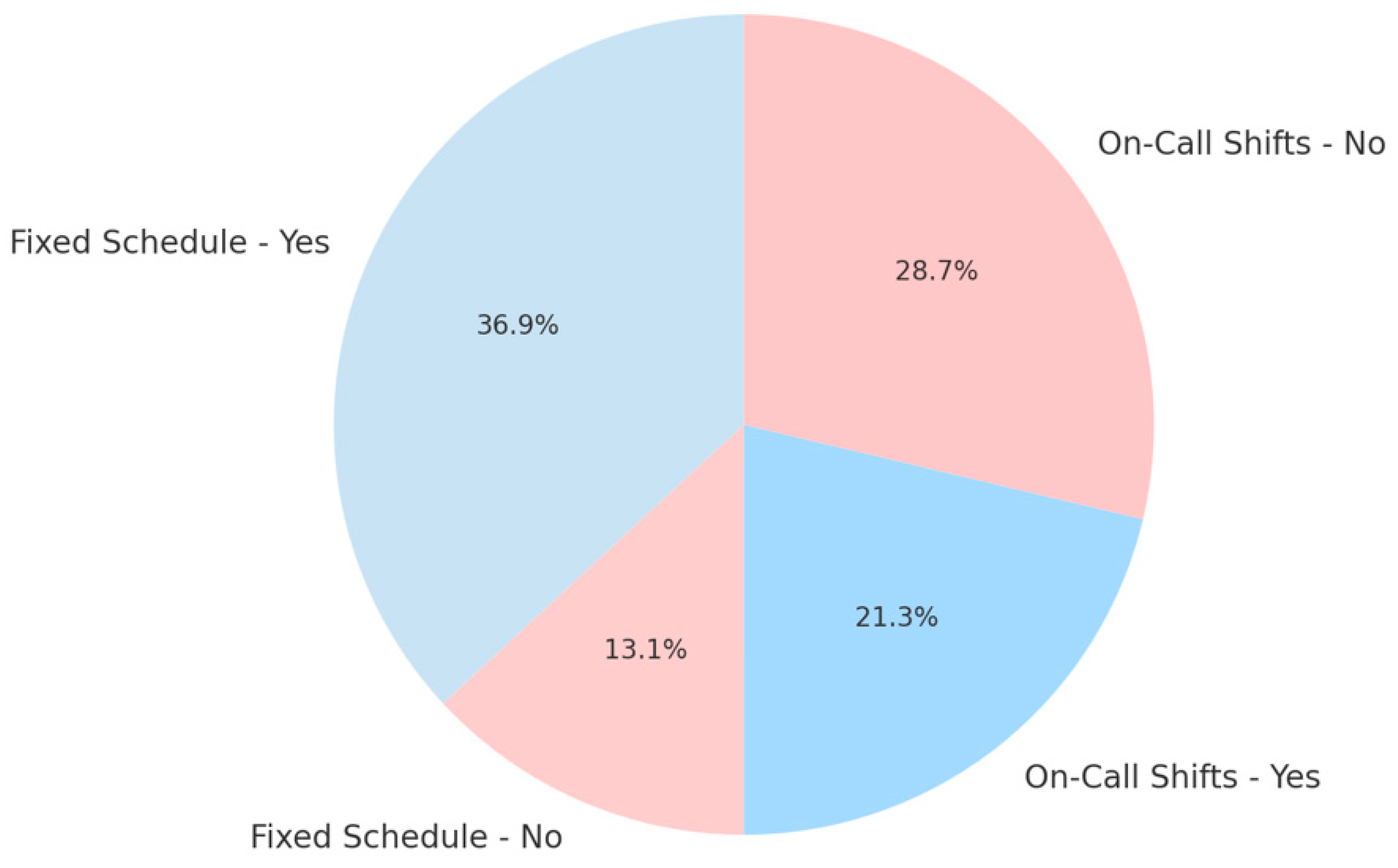
| On-call Shifts (Irregular schedule) | 0.26 (moderate positive) | Irregular shift work contributes notably to burnout, likely due to disruptions in circadian rhythm and melatonin secretion. |
| Fixed Schedule (Regular schedule) | −0.15 (negative correlation) | Regular schedules show a protective effect, suggesting that circadian stability may mitigate burnout. |
| Experience Years | −0.17 (negative correlation) | Greater experience slightly reduces burnout risk, feasibly through improved coping mechanisms. |
| BMI | −0.02 (no significant correlation) | BMI does not significantly correlate with burnout in this analysis, indicating limited immediate metabolic impact within this context. |
| Gender | −0.03 (no significant correlation) | Gender showed negligible correlation with burnout severity, suggesting burnout prevalence is similar across genders in this context. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungurianu, A.; Marina, V. The Biological Clock Influenced by Burnout, Hormonal Dysregulation and Circadian Misalignment: A Systematic Review. Clocks & Sleep 2025, 7, 63. https://doi.org/10.3390/clockssleep7040063
Ungurianu A, Marina V. The Biological Clock Influenced by Burnout, Hormonal Dysregulation and Circadian Misalignment: A Systematic Review. Clocks & Sleep. 2025; 7(4):63. https://doi.org/10.3390/clockssleep7040063
Chicago/Turabian StyleUngurianu, Alexandru, and Virginia Marina. 2025. "The Biological Clock Influenced by Burnout, Hormonal Dysregulation and Circadian Misalignment: A Systematic Review" Clocks & Sleep 7, no. 4: 63. https://doi.org/10.3390/clockssleep7040063
APA StyleUngurianu, A., & Marina, V. (2025). The Biological Clock Influenced by Burnout, Hormonal Dysregulation and Circadian Misalignment: A Systematic Review. Clocks & Sleep, 7(4), 63. https://doi.org/10.3390/clockssleep7040063






