Polygraphic Results in High-Risk Infants Aged Under 3 Months
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods
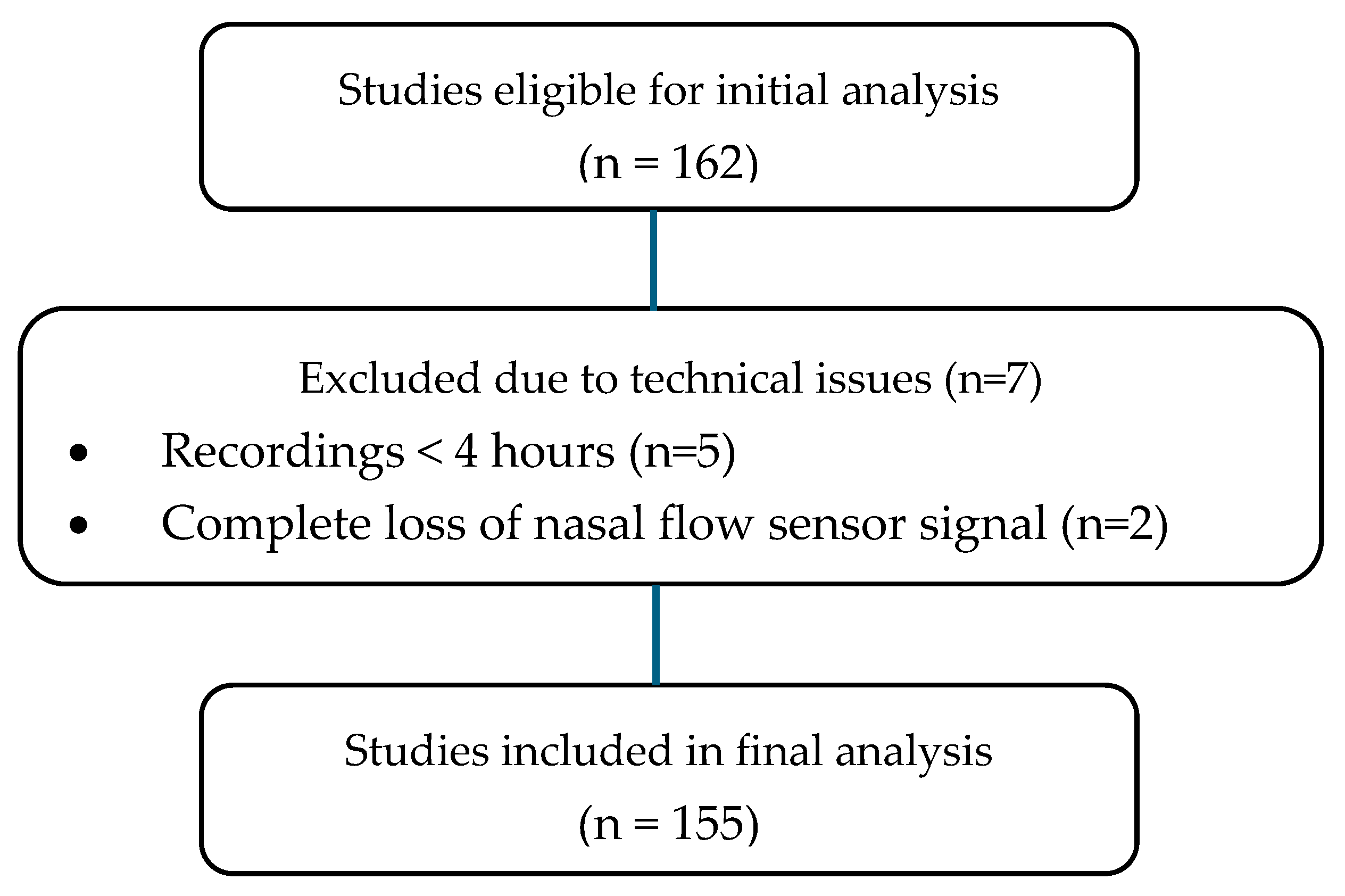
4.1. Study Design and Participants
- Infants with resolved respiratory conditions but a history of apparent life-threatening events (ALTE), later redefined as brief resolved unexplained events (BRUE), for whom PG was used as part of extended evaluation.
- Former preterm infants or neonates with episodes of oxygen desaturation—either nocturnal or during feeding—combined with abnormal overnight oximetry, in whom PG was indicated to evaluate apnea of prematurity.
- Infants with clinical suspicion of central or obstructive events due to underlying conditions such as laryngomalacia, craniofacial malformations, or neuromuscular hypotonia, whether hospitalized or referred from outpatient follow-up.
4.2. Sleep Studies
4.3. Polysomnography Acceptability and Exclusion Criteria
- Total sleep time of at least 4 h;
- Less than 20% of the recording time affected by major artifacts or sensor disconnections across critical channels.
- PGs not meeting the above acceptability thresholds;
- Studies conducted under supplemental oxygen or ventilatory support;
- Presence of congenital heart disease;
- Repeated PGs (only the first baseline study was analyzed).
4.4. Variables and Definitions
- Apnea–hypopnea index (AHI): total number of apneas and hypopneas per hour of valid recording time.
- Central apnea: absence or ≥90% reduction in airflow, without respiratory effort, lasting longer than the duration of two respiratory cycles.
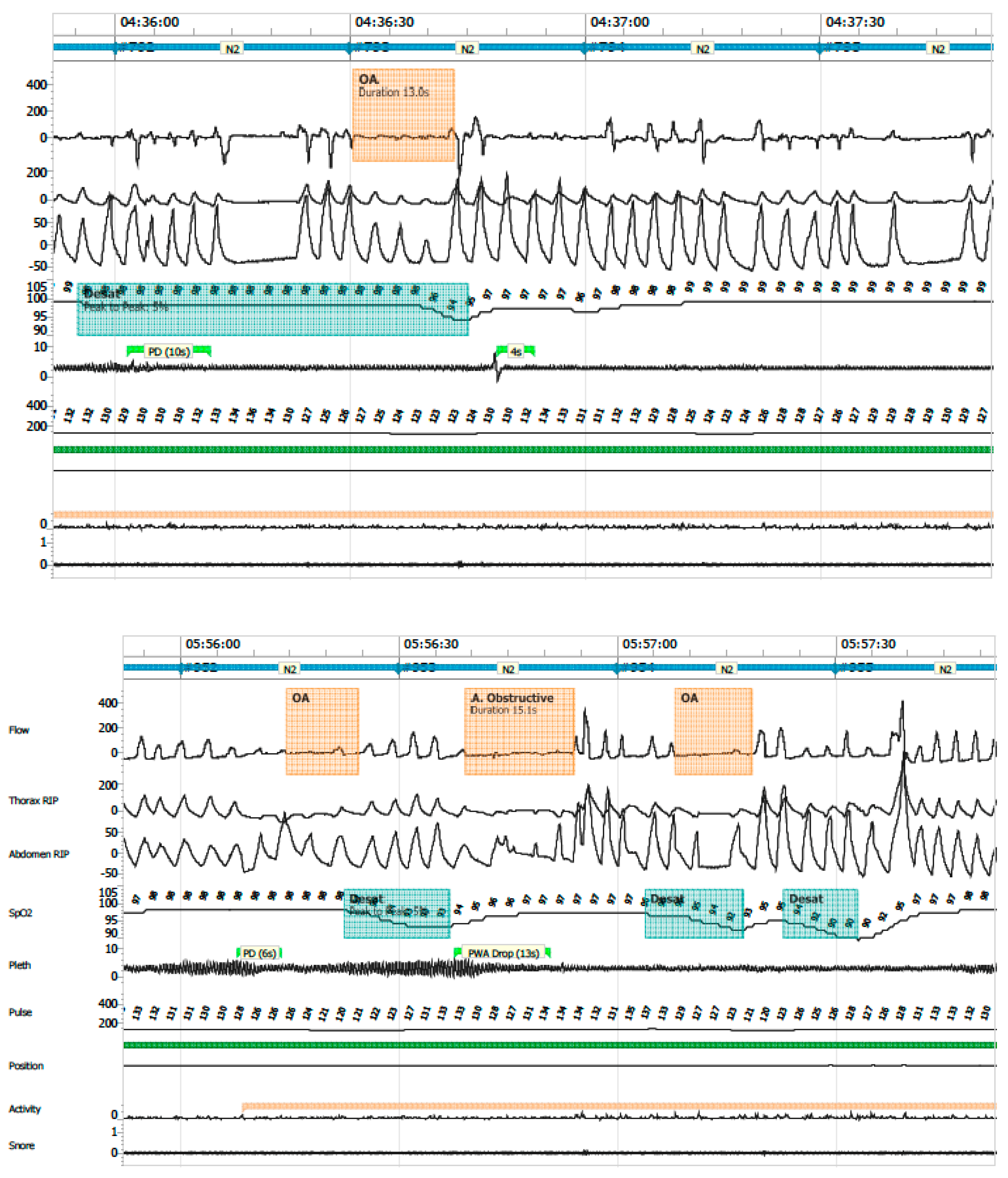
- Obstructive apnea: absence or ≥90% reduction in airflow with persistent respiratory effort, lasting longer than two respiratory cycles (Figure 6).
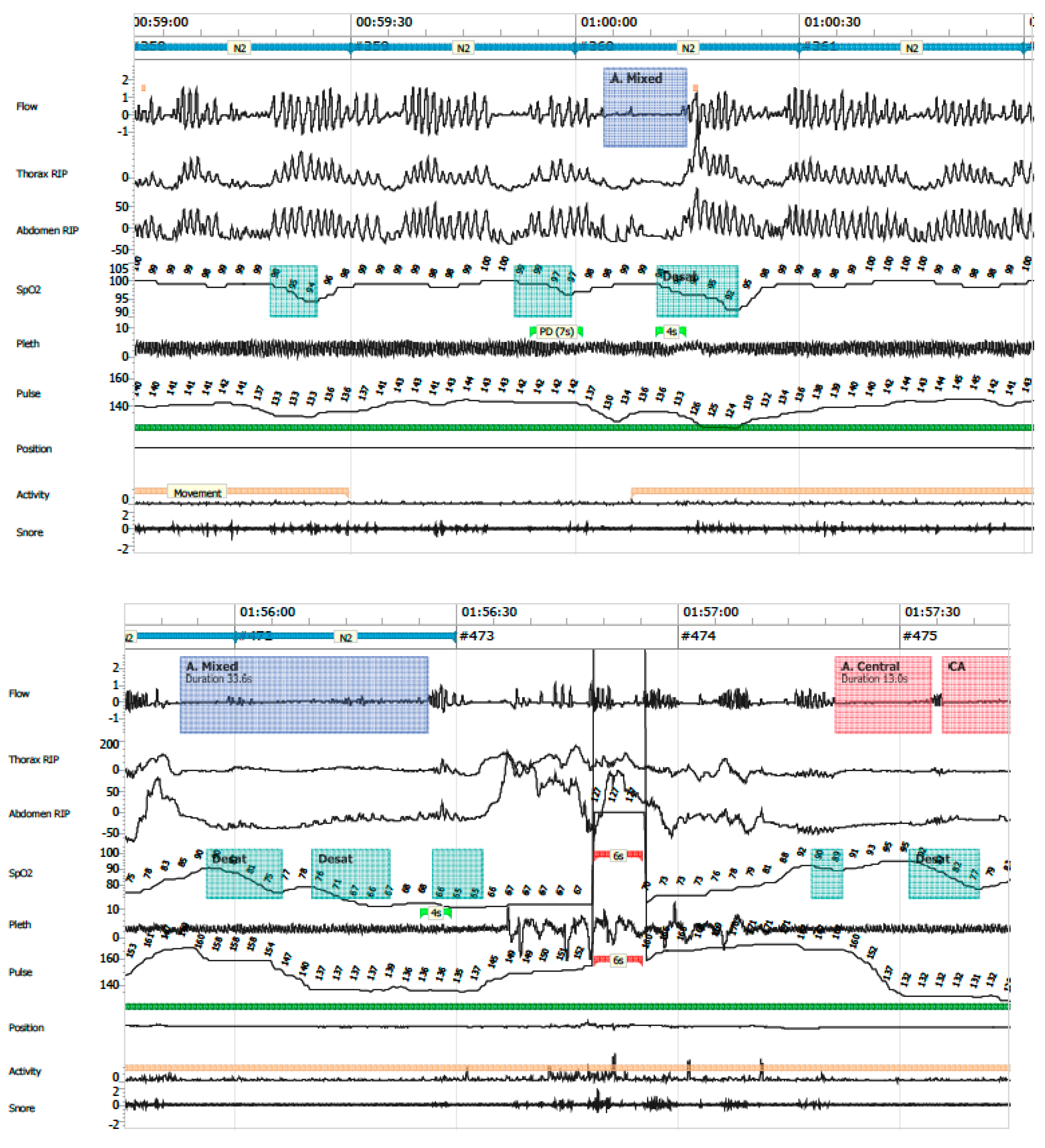
- Mixed apneas: an apnea event with a central component (absence of respiratory effort) and transitions into an obstructive component (airflow limitation with effort) or vice versa (Figure 7).
- Hypopnea: ≥30% reduction in nasal airflow from baseline, lasting more than the duration of two respiratory cycles, associated with a ≥3% drop in oxygen saturation.
- Oxygen desaturation index (ODI ≤ 80): number of desaturation events to ≤80% SpO2 per hour.
- Central apnea index (CAI): number of central apneas per hour of recording; clinical relevance was assigned to events associated with ≤80% desaturation.
- Mean SpO2 and time spent with SpO2 ≤ 90%
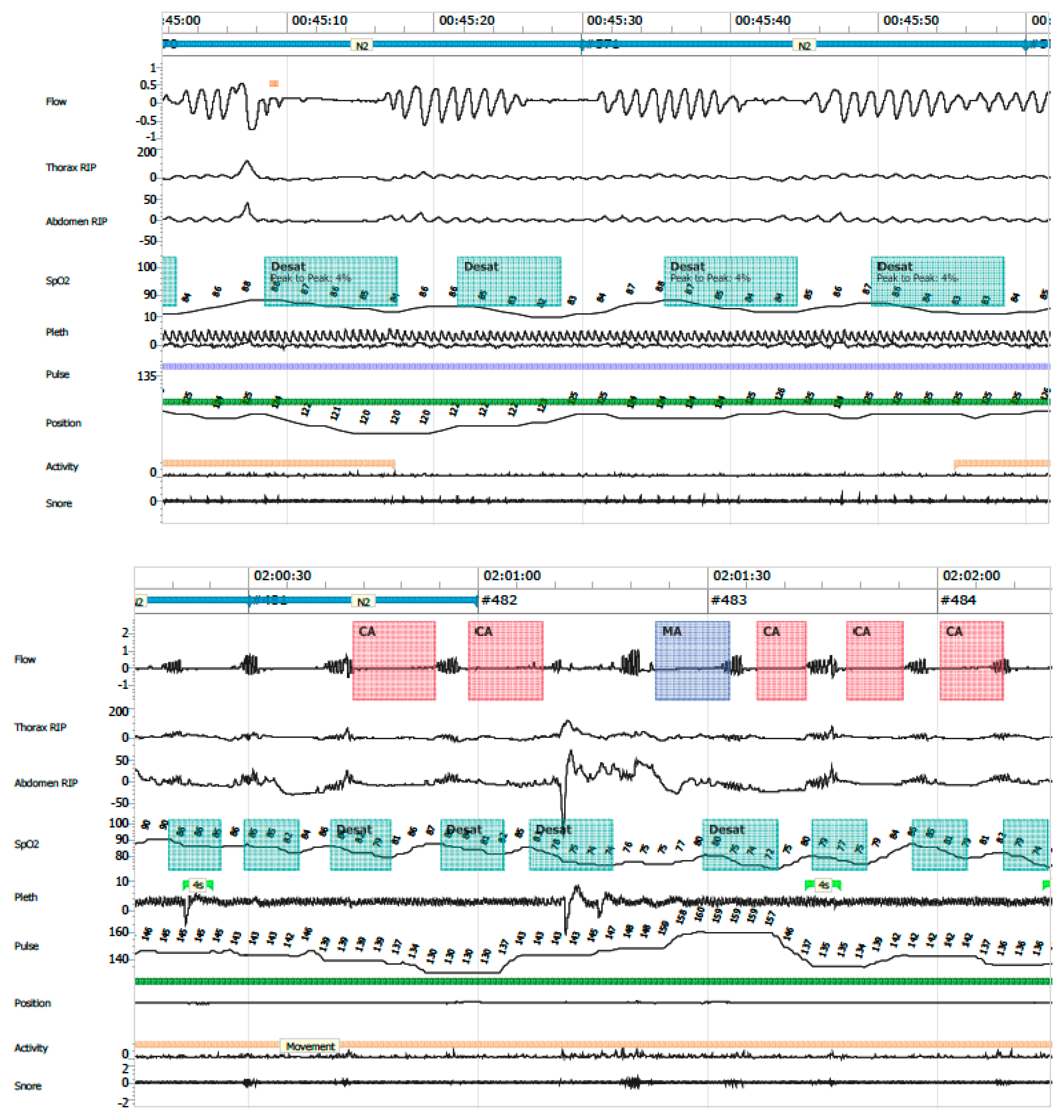
- Periodic breathing: defined as cyclic respiratory pattern with ≥3 successive central pauses and ≥3% desaturation (Figure 8).
- AHI ≥ 5 events/hour;
- ODI ≤ 80 ≥ 1 event/hour;
- CAI ≥ 1 event/hour associated with SpO2 ≤ 80%;
- Mean SpO2 < 93%;
- Percentage of time with SpO2 ≤ 90% exceeding 5% of the total recording time;
- Increased periodic breathing defined as >20% of total recording time in infants younger than 37 weeks corrected gestational age, or >10% in infants older than 37 weeks corrected age.
4.5. Ethical Considerations
4.6. Statistical Analysis
5. Conclusions
Further Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Abara, S.; Campos, C.; Holmgren, N.L.; Montes, S.; Sepúlveda, H.; Zenteno, D. Consenso sobre el manejo de eventos de aparente amenaza a la vida del lactante (ALTE). Comisión de Sueño, Sociedad Chilena de Neumología Pediátrica 2013. Rev. Chil. Pediatr. 2014, 85, 378–387. [Google Scholar] [CrossRef]
- Tieder, J.S.; Bonkowsky, J.L.; Etzel, R.A.; Franklin, W.H.; Gremse, D.A.; Herman, B.; Katz, E.S.; Krilov, L.R.; Merritt, J.L.; Norlin, C.; et al. Brief Resolved Unexplained Events (Formerly Apparent Life-Threatening Events) and Evaluation of Lower-Risk Infants. Pediatrics 2016, 137, e20160590. [Google Scholar]
- Brockmann, P.E.; Poets, A.; Poets, C.F. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3 months. Sleep Med. 2013, 14, 1323–1327. [Google Scholar] [CrossRef]
- Zenteno, D.; Rodríguez-Núñez, I.; Molina, I.; Peña, R.; Rivas, C.; Tapia, J.; Brockmann, P. Poligrafía en menores de 3 meses hospitalizados. Rev. Chil. Pediatr. 2017, 88, 230–235. [Google Scholar] [CrossRef][Green Version]
- Michelet, M.; Blanchon, S.; Guinand, S.; Ruchonnet-Métrailler, I.; Mornand, A.; Van, H.C.; Barazzone-Argiroffo, C.; Corbelli, R. Successful home respiratory polygraphy to investigate sleep-disordered breathing in children. Sleep Med. 2020, 68, 146–152. [Google Scholar] [CrossRef]
- Zenteno, D.; Verbal, D.; Navarro, X.; Torres, G.; Rivas, C.; Rodríguez-Núñez, I.; Elso, M.-J.; Tapia, J. Pediatric polygraphy: A 6-year experience. Rev. Chil. Pediatr. 2019, 90, 309–315. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Abara, S.; Mesa, T.; Zenteno, D.; Bertrán, K.; Astudillo, C.; Farbinger, F.; García, M.; Hernández, A.; Sierra, G. Recomendación de Experto: Apneas Respiratorias y Parámetros de Sueño en el Recién Nacido y Lactantes Menores de 3 meses. Rev. Chil. Psiquiatr. Neurol. Infanc. Adolesc. 2020, 31, 81–97. [Google Scholar]
- Erickson, G.; Dobson, N.R.; Hunt, C.E. Immature control of breathing and apnea of prematurity: The known and unknown. J. Perinatol. 2021, 41, 2111–2123. [Google Scholar] [CrossRef]
- Verkest, V.; Verhulst, S.; Van Hoorenbeeck, K.; Vanderveken, O.; Saldien, V.; Boudewyns, A. Prevalence of obstructive sleep apnea in children with laryngomalacia and value of polysomnography in treatment decisions. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110255. [Google Scholar] [CrossRef]
- Mittal, M.K.; Tieder, J.S.; Westphal, K.; Sullivan, E.; Hall, M.; Bochner, R.; Cohen, A.; Colgan, J.Y.; Delaney, A.C.; DeLaroche, A.M.; et al. Diagnostic testing for evaluation of brief resolved unexplained events. Acad. Emerg. Med. 2023, 30, 662–670. [Google Scholar] [CrossRef]
- Brockmann, P.E.; Perez, J.L.; Moya, A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1960–1964. [Google Scholar] [CrossRef]
- Chiner, E.; Cánovas, C.; Molina, V.; Sancho-Chust, J.N.; Vañes, S.; Pastor, E.; Martinez-Garcia, M.A. Home Respiratory Polygraphy is Useful in the Diagnosis of Childhood Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2020, 9, 2067. [Google Scholar] [CrossRef]
- Arms, J.L.; Ortega, H.; Reid, S. Chronological and clinical characteristics of apnea associated with respiratory syncytial virus infection: A retrospective case series. Clin. Pediatr. 2008, 47, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Brulé, C.; Lauzier, B.; Brossier, D.; Porcheret, F. Brief resolved unexplained event: Severity-associated factors at admission in the pediatric emergency ward. Arch. Pediatr. 2023, 30, 389–395. [Google Scholar] [CrossRef]
- Pongchangyoo, J.; Hantragool, S.; Rojvachiranonda, N.; Niyomkarn, W. Sleep-disordered Breathing in Children With Craniofacial Anomalies. J. Craniofacial Surg. 2023, 34, 1962–1965. [Google Scholar] [CrossRef]
- Zenteno, D.; Cancino-Mella, M.; Torres-Puebla, G.; Barrientos, G.; Islas, C.; Tapia, J.; Elso, M.J.; Brockmann, P. Studies of sleep and therapeutic actions in children and adolescents with craniofacial anomalies. Andes Pediatr. 2023, 94, 37–44. [Google Scholar] [CrossRef]
- Cielo, C.M.; Tapia, I.E. What’s New in Pediatric Obstructive Sleep Apnea? Sleep Med. Clin. 2023, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J.; Zenteno, D.; Fuentes, C.; Brockmann, P. Sudden unexpected death in infancy: Update and preventive measures. Andes Pediatr. 2021, 92, 609–616. [Google Scholar]
- Al-Saleh, S.; Khayat, A.; Bin-Hassan, S. Polysomnographic findings in infants with Pierre Robin sequence. Ann. Thorac. Med. 2017, 12, 25–29. [Google Scholar] [CrossRef]
- Poets, C.F.; Wiechers, C.; Koos, B.; Muzaffar, A.R.; Gozal, D. Pierre Robin and breathing: What to do and when? Pediatr. Pulmonol. 2022, 57, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Zenteno, D.; Bancalari, A.; Navarro, X.; Díaz, V.; Rodríguez-Núñez, I.; Brockmann, P. Diagnosis of respiratory sleep disorders in newborns with suspected apneas: Comparison between nocturnal saturometry and polygraphy. Rev. Chil. Pediatr. 2017, 88, 759–764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]

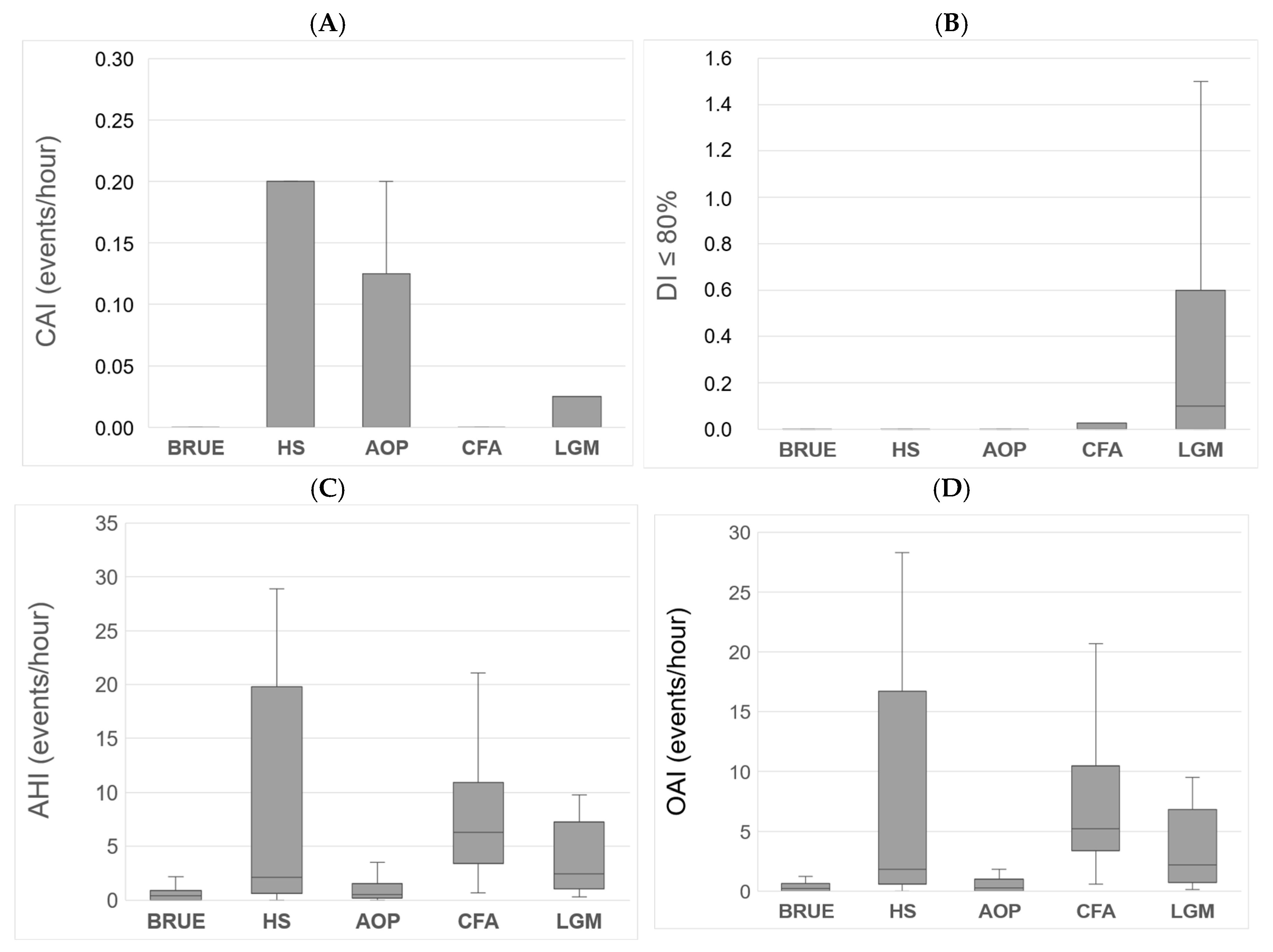
| Polygraphic Variables | Median | IQR | Min–Max Range |
|---|---|---|---|
| Total duration (h) | 9.6 | 8.8–10.3 | 5.2–14.0 |
| Validated duration (h) | 6.7 | 5.9–7.9 | 4.0–10.5 |
| AHI | 0.5 | 0.1–1.7 | 0.0–51.1 |
| MOAHI | 0.3 | 0.0–1.0 | 0.0–49.1 |
| CAI | 0.0 | 0.0–0.0 | 0.0–17.0 |
| OAI | 0.35 | 0.0–0.9 | 0.0–49.1 |
| DI < 80% | 0.0 | 0.0–0.0 | 0.0–1.5 |
| SpO2 (%) | 97 | 96.0–98.0 | 90.0–99.0 |
| Minimum SpO2 (%) | 85 | 79.5–87.5 | 40.0–95.0 |
| Periodic respiration (min) | 3.9 | 0.2–12.7 | 0.0–179.9 |
| Periodic respiration (%) | 1.0 | 0.1–3.1 | 0.0–41.1 |
| Alteration | % (n) |
|---|---|
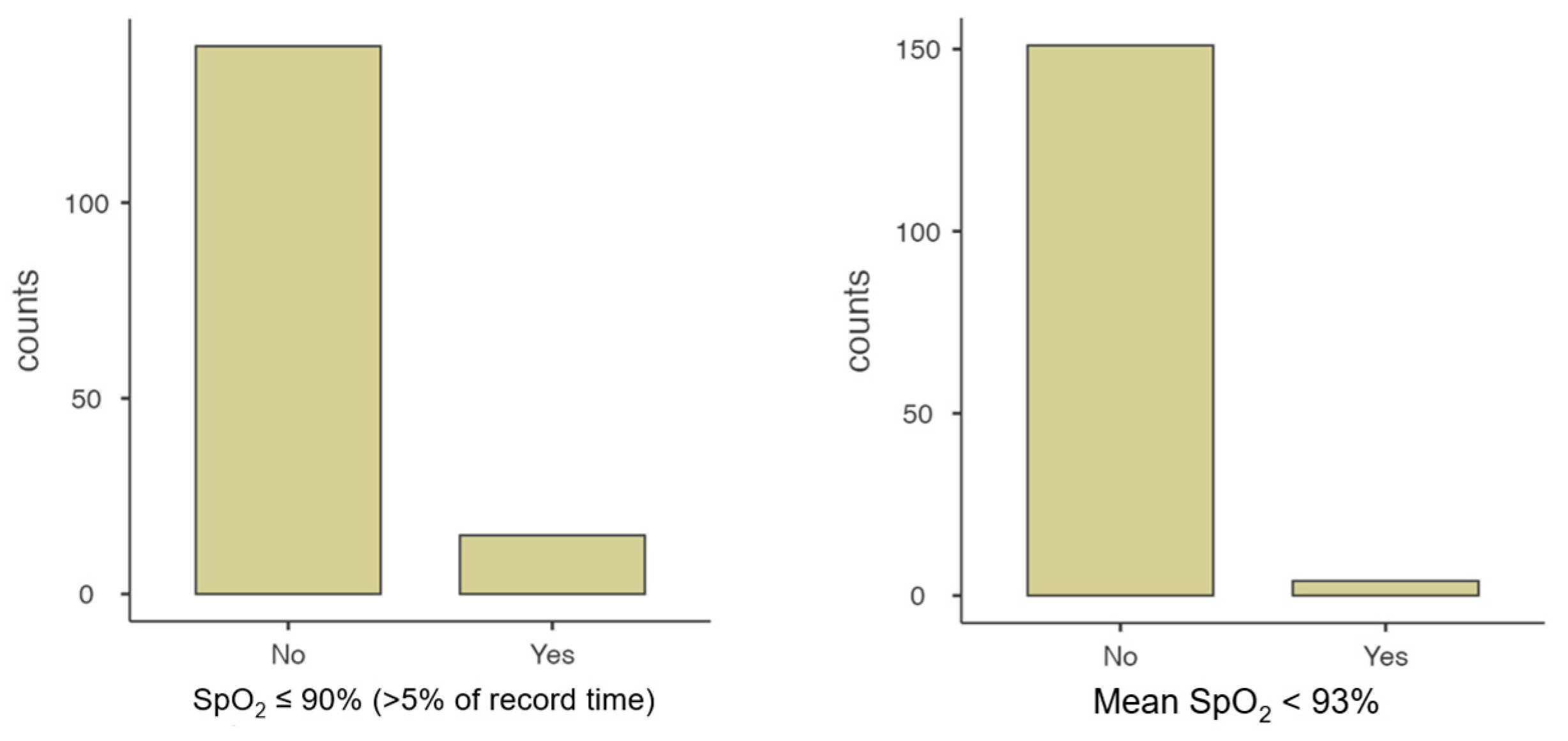
| AHI ≥ 5/h | 50.00 [10] |
| SpO2 ≤ 90%, >5% of record | 39.47 [11] |
| CAI ≥ 1/h with SpO2 ≤ 80% | 23.68 [9] |
| Average SpO2 < 93 | 10.53 [4] |
| DI ≤ 80% ≥ 1/h | 5.26 [2] |
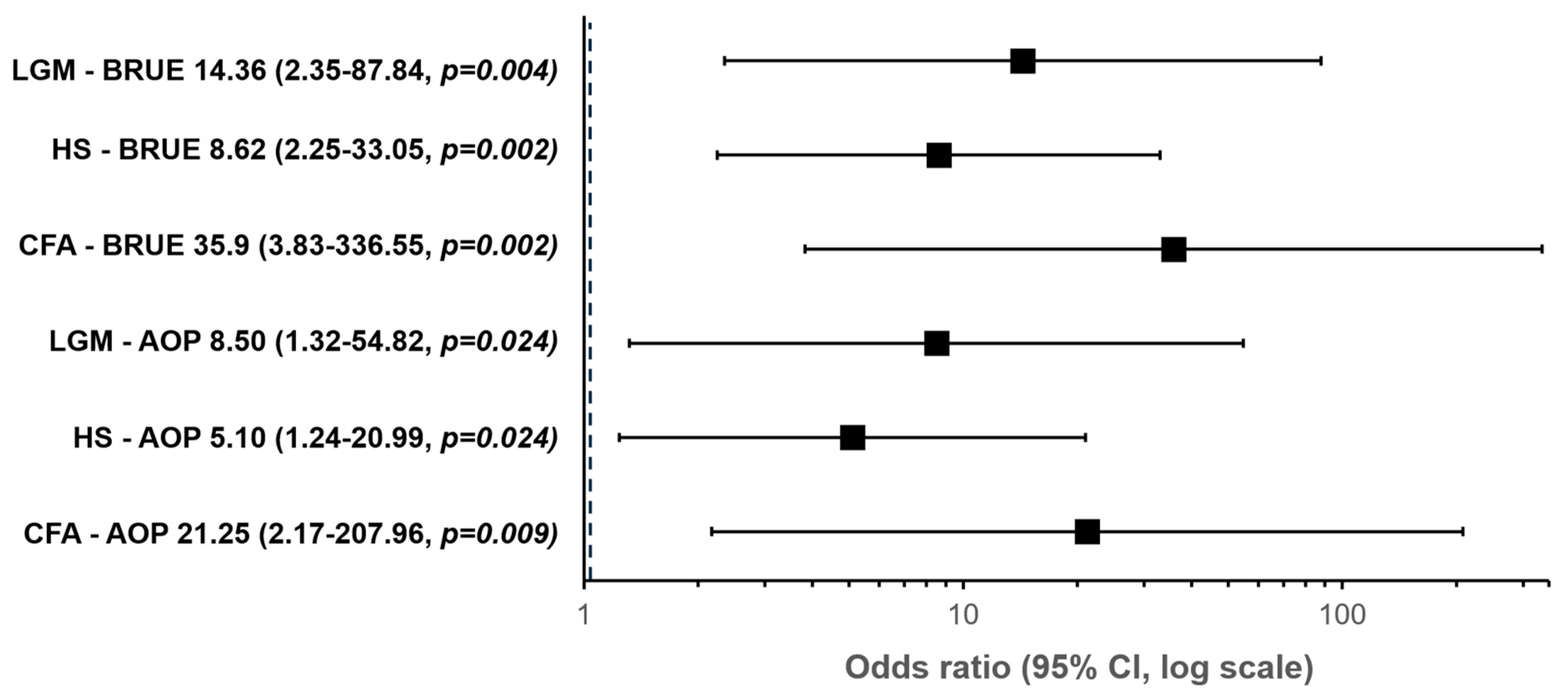
| Predictor | Z | p Value | OR | 95% Confidence Interval |
|---|---|---|---|---|
| Diagnosis | ||||
| CFA—AOP | 3.056 | 0.009 | 21.250 | 2.171–207.960 |
| HS—AOP | 1.629 | 0.024 | 5.100 | 1.239–20.990 |
| LGM—AOP | 2.140 | 0.024 | 8.500 | 1.318–54.817 |
| CFA—BRUE | 3.581 | 0.002 | 35.909 | 3.831–336.553 |
| HS—BRUE | 2.154 | 0.002 | 8.618 | 2.248–33.046 |
| LGM—BRUE | 2.665 | 0.004 | 14.364 | 2.349–87.837 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenteno, D.; Torres-Puebla, G.; Sánchez, C.; Gutiérrez, R.; Elso, M.J.; Brockmann, P.E. Polygraphic Results in High-Risk Infants Aged Under 3 Months. Clocks & Sleep 2025, 7, 42. https://doi.org/10.3390/clockssleep7030042
Zenteno D, Torres-Puebla G, Sánchez C, Gutiérrez R, Elso MJ, Brockmann PE. Polygraphic Results in High-Risk Infants Aged Under 3 Months. Clocks & Sleep. 2025; 7(3):42. https://doi.org/10.3390/clockssleep7030042
Chicago/Turabian StyleZenteno, Daniel, Gerardo Torres-Puebla, Camila Sánchez, Rocío Gutiérrez, María José Elso, and Pablo E. Brockmann. 2025. "Polygraphic Results in High-Risk Infants Aged Under 3 Months" Clocks & Sleep 7, no. 3: 42. https://doi.org/10.3390/clockssleep7030042
APA StyleZenteno, D., Torres-Puebla, G., Sánchez, C., Gutiérrez, R., Elso, M. J., & Brockmann, P. E. (2025). Polygraphic Results in High-Risk Infants Aged Under 3 Months. Clocks & Sleep, 7(3), 42. https://doi.org/10.3390/clockssleep7030042






