Appropriate Lifelong Circadian Rhythms Are Established During Infancy: A Narrative Review
Abstract
1. Introduction
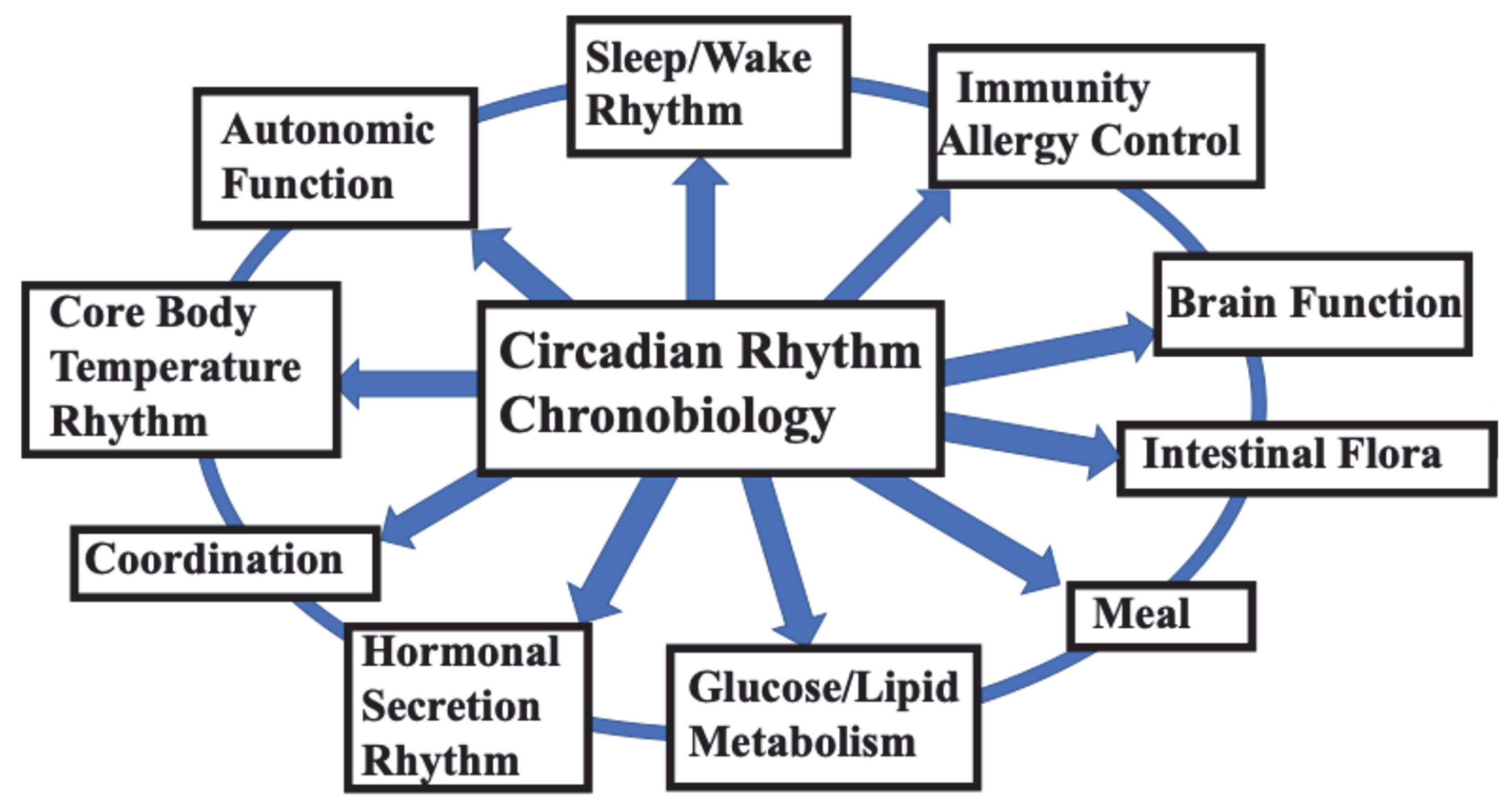
2. Two Circadian Clocks
3. The Process of Circadian Clock Development
3.1. Formation of Fetal Ultradian Rhythm
3.2. Formation of Circadian Rhythms During Fetal Development
3.3. From Ultradian to Circadian Rhythms
4. Factors Involved in the Formation of the Biological Clock
4.1. Fetal Period
4.1.1. Living Environment of the Mother During Pregnancy
4.1.2. Environmental Pollutants
4.1.3. DOHaD Theory
4.2. Newborns and Infants
4.2.1. Premature Infants/Newborns
4.2.2. Circadian Rhythms in Infancy
 : Self-awakening, ★: Bow defecation.
: Self-awakening, ★: Bow defecation.
 : Self-awakening, ★: Bow defecation.
: Self-awakening, ★: Bow defecation.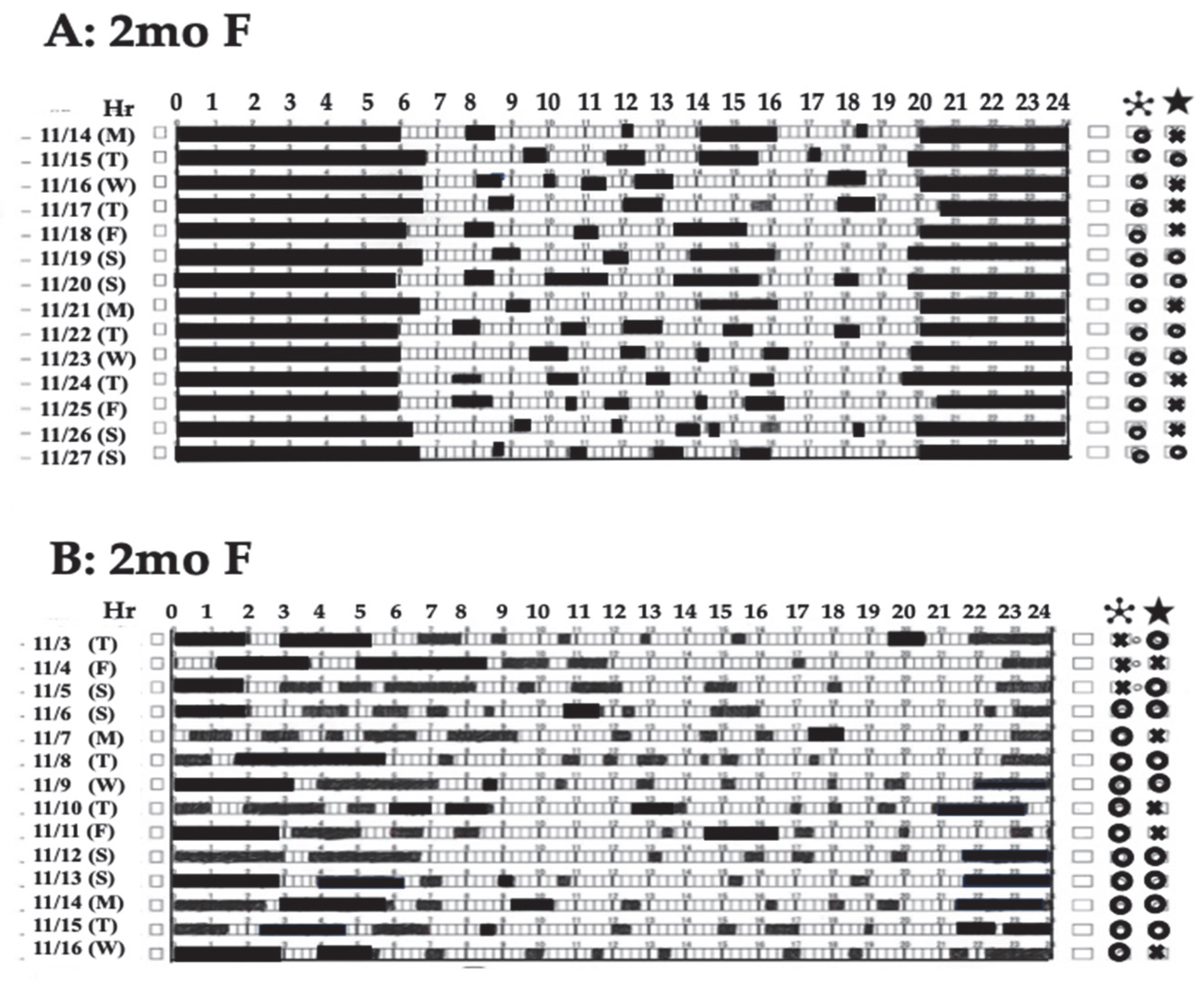
4.2.3. Breastfeeding/Nighttime Feeding
4.2.4. Stopping Nighttime Breastfeeding
4.2.5. Development of Circadian Clocks and Naps
4.2.6. The Biological Clock Adapts to the School/Social Life Schedule
5. Disruption of Biological Clocks
5.1. Disruption of the Circadian Rhythms
5.1.1. Jet Lag
5.1.2. Social Jet Lag (SJL)
5.2. Chronodisruption
5.3. Chronodisruption and DOHaD
6. Preventing and Dealing with Deviations in Daily Rhythms
6.1. Daily Life of Pregnant Mothers
- (1)
- Avoid staying up late (go to bed on the same day).
- (2)
- Ensure the amount of sleep and the time of day (ensure the amount of sleep required before waking up on weekdays).
- (3)
- Keep mealtimes consistent (avoid late-night snacks as much as possible).
- (4)
- Improve work style (avoid long hours and night shifts).
- (5)
- Avoid traveling abroad with large time differences.
- (6)
- Eliminate the effects of tobacco, alcohol, and other pollutants as much as possible.
6.2. Life After Birth
- (1)
- Establish a daily rhythm that allows you to ensure the basic nocturnal sleep duration (10–11 h: NBSD) according to the required wake-up time in the morning (usually before 7:00). For this purpose, a life rhythm in which the sleep onset time is set to before 7–9 p.m. in early infancy is recommended.
- (2)
- Nighttime feeding should be stopped in early infancy (2–6 months after birth) to foster sleep continuity in children.
- (3)
- Keep mealtimes consistent.
- (4)
- Correct the biological clock as soon as possible if it is out of sync.
6.3. Correcting the Child’s Life Rhythm
- (1)
- Family therapy.
- (2)
- Pharmacological therapy.
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCN | Suprachiasmatic nucleus |
| ASD | Autism spectrum disorder |
| NBSD | Nighttime Basic Sleep Duration |
| DOHaD | Developmental Origin Health and Disease |
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Kornhauser, J.M.; Mayo, K.E.; Takahashi, J.S. Light, immediate-early genes. Behav. Genet. 1996, 26, 221–240. [Google Scholar] [CrossRef]
- Inoue, S. Science of Sleep; Asakura-Shoten: Tokyo, Japan, 2006. (In Japanese) [Google Scholar]
- Telias, I.; Wilcox, M.E. Sleep and Circadian Rhythm in Critical Illness. Crit. Care 2019, 23, 82. [Google Scholar] [CrossRef]
- Borbély, A.J. The two-process model of sleep regulation: Beginnings and outlook. J. Sleep Res. 2022, 31, e13598. [Google Scholar] [CrossRef]
- Tomoda, A.; Miike, T.; Yonamine, K.; Adachi, K.; Shiraishi, S. Disturbed circadian core body temperature rhythm and sleep disturbance in school refusal children and adolescents. Biol. Psychiatry 1997, 41, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Lodemore, M.; Petersen, S.A.; Wailoo, M.P. Development of night time temperature rhythms over the first six months of life. Arch. Dis. Child. 1991, 66, 521–524. [Google Scholar] [CrossRef][Green Version]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy. metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Ding, G.; Gong, Y.; Eckel-Mahan, K.L.; Sun, Z. Central Circadian Clock Regulates Energy Metabolism. Adv. Exp. Med. Biol. 2018, 1090, 79–103. [Google Scholar] [PubMed]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef]
- Peters, B.; Vahlhaus, J.; Pivovarova-Ramich, O. Meal timing and its role in obesity and associated diseases. Front. Endocrinol. 2024, 15, 1359772. [Google Scholar] [CrossRef]
- Mavroudis, P.D.; Scheff, J.D.; Calvano, S.E.; Androulakis, I.P. Systems biology of circadian-immune interactions. J. Innate Immun. 2013, 5, 153–162. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Buijs, R.M.; Escobar, C.; Swaab, D.F. The circadian system and the balance of the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 173–191. [Google Scholar] [PubMed]
- Baschieri, F.; Cortelli, P. Circadian rhythms of cardiovascular autonomic function: Physiology and clinical implications in neurodegenerative diseases. Auton. Neurosci. 2019, 217, 91–101. [Google Scholar] [CrossRef]
- Bering, T.; Hertz, H.; Rath, M.F. Rhythmic Release of Corticosterone Induces Circadian Clock Gene Expression in the Cerebellum. Neuroendocrinology 2020, 110, 604–615. [Google Scholar] [CrossRef]
- Lotti, S.; Dinu, M.; Colombini, B.; Amedei, A.; Sofi, F. Circadian rhythms, gut microbiota, and diet: Possible implications for health. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1490–1500. [Google Scholar] [CrossRef]
- Vandewalle, G. Circadian, sleep-wake dependent or both? A preface to the special issue Circadian rhythm and sleep-wake dependent regulation of behavior and brain function. Biochem. Pharmacol. 2021, 191, 114535. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992, 1, 112–117. [Google Scholar] [CrossRef]
- von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. [Google Scholar] [CrossRef]
- Murray, A.; Tharmalingam, S.; Khurana, S.; Lalonde, C.; Nguyen, P.; Tai, T.C. Effect of Prenatal Glucocorticoid Exposure on Circadian Rhythm Gene Expression in the Brains of Adult Rat Offspring. Cells 2022, 11, 1613. [Google Scholar] [CrossRef]
- Koukkari, W.L.; Sothern, R.B. Introducing Biological Rhythms: A Primer on the Temporal Organization of Life, with Implications for Health, Society, Reproduction, and the Natural Environment; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Kumar, V. Biological Rhythms; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Refinetti, R. Integration of biological clocks and rhythms. Compr. Physiol. 2012, 2, 1213–1239. [Google Scholar] [CrossRef]
- Foster, R.G.; Roenneberg, T. Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 2008, 18, R784–R794. [Google Scholar] [CrossRef] [PubMed]
- Serón-Ferré, M.; Torres-Farfán, C.; Forcelledo, M.L.; Valenzuela, G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Hao, H. Developing circadian rhythmicity. Semin. Perinatol. 2000, 24, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Serón-Ferré, M.; Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Valenzuela, F.J.; Reynolds, H.E.; Llanos, A.J.; Rojas, A.; Valenzuela, G.J.; Torres-Farfan, C. Circadian rhythms in the fetus. Mol. Cell. Endocrinol. 2012, 349, 68–75. [Google Scholar] [CrossRef]
- Touchette, E.; Dionne, G.; Forget-Dubois, N.; Petit, D.; Pérusse, D.; Falissard, B.; Tremblay, R.E.; Boivin, M.; Montplaisir, J.Y. Genetic and environmental influences on daytime and nighttime sleep duration in early childhood. Pediatrics 2013, 131, e1874–e1880. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Carmona-Alcocer, V.; Rohr, K.E.; Joye, D.A.M.; Evans, J.A. Circuit development in the master clock network of mammals. Eur. J. Neurosci. 2020, 51, 82–108. [Google Scholar] [CrossRef]
- Schibler, U.; Gotic, I.; Saini, C.; Gos, P.; Curie, T.; Emmenegger, Y.; Sinturel, F.; Gosselin, P.; Gerber, A.; Fleury-Olela, F.; et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 223–232. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian clocks: Setting time by food. J. Neuroendocrinol. 2007, 19, 127–137. [Google Scholar] [CrossRef]
- Qian, J.; Scheer, F.A.J.L. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef]
- Takimoto, M.; Hamada, A.; Tomoda, A.; Ohdo, S.; Ohmura, T.; Sakato, H.; Kawatani, J.; Jodoi, T.; Nakagawa, H.; Terazono, H.; et al. Daily expression of clock genes in whole blood cells in healthy subjects and a patient with circadian rhythm sleep disorder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1273–R1279. [Google Scholar] [CrossRef]
- Cuninkova, L.; Brown, S.A. Peripheral circadian oscillators: Interesting. mechanisms and powerful tools. Ann. N. Y. Acad. Sci. 2008, 1129, 358–370. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, M.; Wang, X.; Jiang, S.H.; Hu, L.P.; Zhang, X.L.; Zhang, Z.G. Signalling entrains the peripheral circadian clock. Cell. Signal. 2020, 69, 109433. [Google Scholar] [CrossRef]
- Yildirim, E.; Curtis, R.; Hwangbo, D.S. Roles of peripheral clocks: Lessons from the fly. FEBS. Lett. 2022, 596, 263–293. [Google Scholar] [CrossRef]
- Wakayama, K.; Ogawa, T.; Goto, K.; Sonoda, H. Development of ultradian rhythm of EEG activities in premature babies. Early Hum. Dev. 1993, 32, 11–30. [Google Scholar] [CrossRef]
- Fukushima, K.; Morokuma, S.; Nakano, H. Fetal behavior: Ontogenesis and transition to neonate. In Donald School Textbook of Ultrasound in Obstetics and Gynecology, 2nd ed.; Kurjak, A., Arenas, J.B., Eds.; Jaypee Brothers Medical Publishers: New Delhi, India, 2008; pp. 641–655. [Google Scholar]
- Bueno, C.; Menna-Barretoet, L. Development of sleep/wake, activity and temperature rhythms in newborns maintained in a neonatal intensive care unit and the impact of feeding schedules. Infant Behav. Dev. 2016, 44, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Serón-Ferré, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. Part C Embryo Today 2007, 81, 204–214. [Google Scholar] [CrossRef]
- de Vries, J.I.; Visser, G.H.; Mulder, E.J.; Prechtl, H.F. Diurnal and other variations in fetal movement and heart rate patterns at 20–22 weeks. Early Hum. Dev. 1987, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A. Developing circadian rhythmicity. Basic and clinical aspects. Pediatr. Clin. N. Am. 1997, 44, 467–487. [Google Scholar] [CrossRef]
- Varcoe, T.J.; Gatford, K.L.; Kennaway, D.J. Maternal circadian rhythms and the programming of adult health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R231–R241. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Hillard, P.J.A.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, M.; Maas, Y.G.; Ariagno, R.L. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003, 7, 321–334. [Google Scholar] [CrossRef]
- Meier-Koll, A.; Hall, U.; Hellwig, U.; Kott, G.; Meier-Koll, V. A biological oscillator system and the development of sleep-waking behavior during early infancy. Chronobiologia 1978, 5, 425–440. [Google Scholar]
- Stephenson, R.; Lim, J.; Famina, S.; Caron, A.M.; Dowse, H.B. Sleep-wake behavior in the rat: Ultradian rhythms in a light-dark cycle and continuous bright light. J. Biol. Rhythm. 2012, 27, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Miike, T.; Toyoura, M.; Tonooka, S.; Konishi, Y.; Oniki, K.; Saruwatari, J.; Tajima, S.; Kinoshita, J.; Nakai, A.; Kikuchi, K. Neonatal Irritable Sleep-Wake Rhythm as a Predictor of Autism Spectrum Disorders. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100053. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Schwartz, W.J. Maternal coordination of the fetal biological clock in utero. Science 1983, 22, 969–971. [Google Scholar] [CrossRef]
- Yellon, S.M.; Longo, L.D. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep, fetus during the last trimester of pregnancy. Biol. Reprod. 1988, 39, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Micheli, K.; Komninos, I.; Bagkeris, E.; Roumeliotaki, T.; Koutis, A.; Kogevinas, M.; Chatzi, L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 2011, 22, 738–744. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Weaver, D.R.; Reppert, S.M. Periodic feeding of SCN-lesioned pregnant rats entrains the fetal. biological clock. Brain Res. Dev. Brain Res. 1989, 46, 291–296. [Google Scholar] [CrossRef]
- Serón-Ferré, M.; Torres, C.; Parraguez, V.H.; Vergara, M.; Valladares, L.; Forcelledo, M.L.; Constandil, L.; Valenzuela, G.J. Perinatal neuroendocrine regulation. Development of the circadian time-keeping system. Mol. Cell. Endocrinol. 2002, 186, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. Int. J. Mol. Sci. 2020, 21, 2232. [Google Scholar] [CrossRef]
- Bisanti, L.; Olsen, J.; Basso, O.; Thonneau, P.; Karmaus, W. Shift work and subfecundity: A European multicenter study. European Study Group on Infertility and Subfecundity. J. Occup. Environ. Med. 1996, 38, 352–358. [Google Scholar] [CrossRef]
- Aspholm, R.; Lindbohm, M.L.; Paakkulainen, H.; Taskinen, H.; Nurminen, T.; Tiitinen, A. Spontaneous abortions among Finnish flight attendants. J. Occup. Environ. Med. 1999, 41, 486–491. [Google Scholar] [CrossRef]
- Cone, J.E.; Vaughan, L.M.; Huete, A.; Samuels, S.J. Reproductive health outcomes among female flight attendants: An exploratory study. J. Occup. Environ. Med. 1998, 40, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, M.M. Shift work, jet lag, and female reproduction. Int. J. Endocrinol. 2010, 2010, 813764. [Google Scholar] [CrossRef]
- Loy, S.L.; Loo, R.S.L.; Godfrey, K.M.; Chong, Y.S.; Shek, L.P.C.; Tan, K.H.; Chong, M.F.F.; Chan, J.K.Y.; Yap, F. Chrononutrition during Pregnancy: A Review on Maternal Night-Time Eating. Nutrients 2020, 12, 2783. [Google Scholar] [CrossRef]
- Varcoe, T.J.; Boden, M.J.; Voultsios, A.; Salkeld, M.D.; Rattanatray, L.; Kennaway, D.J. Characterisation of the maternal response to chronic phase shifts during gestation in the rat: Implications for fetal metabolic programming. PLoS ONE 2013, 8, e53800. [Google Scholar] [CrossRef]
- Torres-Farfan, C.; Richter, H.G.; Germain, A.G.; Valenzuela, G.J.; Campino, C.; Rojas-García, P.; Forcelledo, M.L.; Torrealba, F.; Serón-Ferré, M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004, 554, 841–856. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Forcelledo, M.L.; Torres-Farfan, C.; Valenzuela, F.J.; Rojas, A.; Vergara, M.; Rojas-Garcia, P.P.; Recabarren, M.P.; Valenzuela, G.J. Impact of chronodisruption during primate pregnancy on the maternal and newborn temperature rhythms. PLoS ONE 2013, 8, e57710. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, T.; Hess, D.L.; Kaushal, K.M.; Valenzuela, G.J.; Yellon, S.M.; Ducsay, C.A. Circadian myometrial and endocrine rhythms in the pregnant rhesus macaque: Effects of constant light and timed melatonin infusion. Am. J. Obstet. Gynecol. 1991, 165, 1777–1784. [Google Scholar] [CrossRef]
- Houdek, P.; Polidarová, L.; Nováková, M.; Matějů, K.; Kubík, S.; Sumová, A. Melatonin administered during the fetal stage affects circadian clock in the suprachiasmatic nucleus but not in the liver. Dev. Neurobiol. 2015, 75, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.O.; Evsyukova, I.I.; Mironova, E.S.; Polyakova, V.O.; Kvetnoy, I.M.; Ruslan, A.; Nasyrov, R.A. Maternal Melatonin Deficiency Leads to Endocrine Pathologies in Children in Early Ontogenesis. Int. J. Mol. Sci. 2021, 22, 2058. [Google Scholar] [CrossRef]
- Vilches, N.; Spichiger, C.; Mendez, N.; Abarzua-Catalan, L.; Galdames, H.A.; Hazlerigg, D.G.; Hans, G.; Richter, H.G.; Torres-Farfan, C. Gestational chronodisruption impairs hippocampal expression of NMDA receptor subunits Grin1b/Grin3a and spatial memory in the adult offspring. PLoS ONE 2014, 9, e91313. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Huang, L.T.; Tain, Y.L. Perinatal Use of Melatonin for Offspring Health: Focus on Cardiovascular and Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 5681. [Google Scholar] [CrossRef]
- Méndez, N.; Corvalan, F.; Halabi, D.; Vasquez, A.; Vergara, K.; Noriega, H.; Ehrenfeld, P.; Sanhueza, K.; Seron-Ferre, M.; Valenzuela, G.J.; et al. Sex-Specific Metabolic Effects of Gestational Chronodisruption and Maternal Melatonin Supplementation in Rat Offspring. J. Pineal Res. 2024, 76, e70015. [Google Scholar] [CrossRef]
- Lunn, R.M.; Blask, D.E.; Andrew, N.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Janet E Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607–608, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.P.; Jedrychowski, W.; Rauh, V.; Whyatt, R.M. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ. Health Perspect. 1999, 107 (Suppl. S3), 451–460. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Dhok, A. Effects of Pollution on Pregnancy and Infants. Cureus 2023, 15, e33906. [Google Scholar] [CrossRef]
- Ghazi, T.; Naidoo, P.; Naidoo, R.N.; Chuturgoon, A.A. Prenatal Air Pollution Exposure and. Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells 2021, 10, 3025. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, G.P.; Stein, Z.A.; Susser, M.W. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976, 295, 349–353. [Google Scholar] [CrossRef]
- Varcoe, T.J.; Wight, N.; Voultsios, A.; Salkeld, M.D.; Kennaway, D.J. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS ONE 2011, 6, e18504. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, T.J. Timing is everything: Maternal circadian rhythms and the developmental origins of health and disease. J. Physiol. 2018, 596, 5493–5494. [Google Scholar] [CrossRef] [PubMed]
- Morag, I.; Ohlsson, A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst. Rev. 2013, 8, CD006982. [Google Scholar]
- Vásquez-Ruiz, S.; Maya-Barrios, J.A.; Torres-Narváez, P.; Vega-Martínez, B.R.; Rojas-Granados, A.; Escobar, C.; Angeles-Castellanos, M. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum. Dev. 2014, 90, 535–540. [Google Scholar] [CrossRef]
- Guyer, C.; Huber, R.; Fontijn, J.; Bucher, H.U.; Nicolai, H.; Werner, H.; Molinari, L.; Latal, B.; Jenni, O.G. Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics 2012, 130, e145–e151. [Google Scholar] [CrossRef]
- Guyer, C.; Huber, R.; Fontijn, J.; Bucher, H.U.; Nicolai, H.; Werner, H.; Molinari, L.; Latal, B.; Jenni, O.G. Very preterm infants show earlier emergence of 24-hour sleep-wake rhythms compared to term infants. Early Hum. Dev. 2015, 91, 37–42. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Mayes, L.; Jacobs, H.; Gross, I. Rest-activity patterns of premature infants are regulated by cycled lighting. Pediatrics 2004, 113, 833–839. [Google Scholar] [CrossRef]
- Kok, E.Y.; Kaur, S.; Shukri, N.H.M.; Razak, N.A.; Takahashi, M.; Teoh, S.C.; Tay, J.E.F.; Shibata, S. The role of light exposure in infant circadian rhythm establishment: A scoping review perspective. Eur. J. Pediatr. 2025, 184, 112. [Google Scholar] [CrossRef]
- Bruni, O.; Baumgartner, E.; Sette, S.; Ancona, M.; Caso, G.; Di Cosimo, M.E.; Mannini, A.; Ometto, M.; Pasquini, A.; Ulliana, A.; et al. Longitudinal study of sleep behavior in normal infants during the first year of life. J. Clin. Sleep Med. 2014, 10, 1119–1127. [Google Scholar] [CrossRef]
- Ardura, J.; Gutierrez, R.; Andres, J.; Agapito, T. Emergence and evolution of the circadian rhythm of melatonin in children. Horm. Res. 2003, 59, 66–72. [Google Scholar] [CrossRef]
- Rivkees, S.A. Developing circadian rhythmicity in infants. Pediatr. Endocrinol. Rev. 2003, 1, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, T.; Birch, L.L. Help me make it through the night: Behavioral entrainment of breast-fed infants’ sleep patterns. Pediatrics 1993, 91, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.T.; France, K.G.; Blampied, N.M. The consolidation of infants’ nocturnal sleep across the first year of life. Sleep Med. Rev. 2011, 15, 211–220. [Google Scholar] [CrossRef]
- Mohr, C.; Gross-Hemmi, M.H.; Meyer, A.H. Temporal Patterns of Infant Regulatory Behaviors in Relation to Maternal Mood and Soothing Strategies. Child Psychiatry Hum. Dev. 2019, 50, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Juda-Hanael, M.; Livne-Karp, E.; Kahn, M.; Tikotzky, L.; Anders, T.F.; Calkins, S.; Sivan, Y. Low parental tolerance for infant crying: An underlying factor in infant sleep problems? J. Sleep Res. 2016, 25, 501–507. [Google Scholar] [CrossRef]
- Paul, I.M.; Savage, J.S.; Anzman-Frasca, S.; Marini, M.E.; Mindell, J.A.; Birch, L.L. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics 2016, 138, e20160762. [Google Scholar] [CrossRef]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef]
- Kikuchi, S.; Nishihara, K.; Horiuchi, S.; Eto, H. The influence of feeding method on mother’s circadian rhythm and on the development of her infant’s circadian rest activity rhythm. Early Hum. Dev. 2020, 145, 105046. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Pianese, G.; Mureddu, P.; Fanos, V.; Bosco, A. From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression? Nutrients 2024, 16, 2285. [Google Scholar] [CrossRef] [PubMed]
- Akanalçı, C.; Bilici, S. Biological clock and circadian rhythm of breast milk composition. Chronobiol. Int. 2024, 41, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Italianer, M.F.; Naninck, E.F.G.; Roelants, J.A.; van der Horst, G.T.J.; Reiss, I.K.M.; Goudoever, J.B.V.; Joosten, K.F.M.; Chaves, I.; Vermeulen, M.J. Circadian Variation in Human Milk Composition, a Systematic Review. Nutrients 2020, 12, 2328. [Google Scholar] [CrossRef]
- Booker, L.A.; Wilson, D.; Spong, J.; Fitzgibbon, C.; Deacon-Crouch, M.; Lenz, K.E.; Skinner, T.C. Maternal Circadian Disruption from Shift Work and the Impact on the Concentration of Melatonin in Breast Milk. Breastfeed. Med. 2024, 19, 33–39. [Google Scholar] [CrossRef]
- Manková, D.; Švancarová, S.; Štenclová, E. Does the feeding method affect the quality of infant and maternal sleep? A systematic review. Infant Behav. Dev. 2023, 73, 101868. [Google Scholar] [CrossRef]
- Madar, A.A.; Kurniasari, A.; Marjerrison, N.; Mdala, I. Breastfeeding and Sleeping Patterns Among 6–12-Month-Old Infants in Norway. Matern. Child Health J. 2024, 28, 496–505. [Google Scholar] [CrossRef]
- Smith, J.P.; Forrester, R.I. Association between breastfeeding and new mothers’ sleep: A unique Australian time use study. Int. Breastfeed. J. 2021, 16, 7. [Google Scholar] [CrossRef]
- Vitzthum, V.J.; Thornburg, J.; Spielvogel, H. Impacts of nocturnal breastfeeding, photoperiod, and access to electricity on maternal sleep behaviors in a non-industrial rural Bolivian population. Sleep Health 2018, 4, 535–542. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Ganesh, R. Vitamin D deficiency in exclusively breast-fed infants. Indian J. Med. Res. 2008, 127, 250–255. [Google Scholar]
- Terashita, S.; Nakamura, T.; Igarashi, N. Longitudinal study on the effectiveness of vitamin D supplements in exclusively breast-fed infants. Clin. Pediatr. Endocrinol. 2017, 26, 215–222. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Pregnancy, Breastfeeding, and Vitamin D. Int. J. Mol. Sci. 2023, 24, 11881. [Google Scholar] [CrossRef]
- Benhamou, I. Sleep disorders of early childhood: A review. Isr. J. Psychiatry Relat. Sci. 2000, 37, 190–196. [Google Scholar] [PubMed]
- Sadeh, A.; Tikotzky, L.; Scher, A. Parenting and infant sleep. Sleep Med. Rev. 2010, 14, 89–96. [Google Scholar] [CrossRef]
- Hiscock, H.; Cook, F.; Bayer, J.; Le, H.N.D.; Mensah, F.; Cann, W.; Symon, B.; St James-Roberts, I. Preventing early infant sleep and crying problems and postnatal depression: A randomized trial. Pediatrics 2014, 133, e346–e354. [Google Scholar] [CrossRef]
- Miike, T.; Oniki, K.; Toyoura, M.; Tonooka, S.; Tajima, S.; Kinoshita, J.; Saruwatari, J.; Konishi, Y. Disruption of Circadian Sleep/Wake Rhythms in Infants May Herald Future Development of Autism Spectrum Disorder. Clocks Sleep 2024, 6, 170–182. [Google Scholar] [CrossRef]
- Dönmez, R.Ö.; Temel, A.B. Effect of soothing techniques on infants’ self- regulation behaviors (sleeping, crying, feeding): A randomized controlled study. Jpn. J. Nurs. Sci. 2019, 16, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.L.; Master, L.; Buxton, O.M.; Savage, J.S. Patterns of infant-only wake bouts and night feeds during early infancy: An exploratory study using actigraphy in mother-father-infant triads. Pediatr. Obes. 2020, 15, e12640. [Google Scholar] [CrossRef]
- Tordjman, S.; Davlantis, K.S.; Georgieff, N.; Geoffray, M.-M.; Speranza, M.; Anderson, G.M.; Xavier, J.; Botbol, M.; Oriol, C.; Bellissan, E.; et al. Autism as a disorder of biological and behavioral rhythms: Toward new therapeutic perspectives. Front. Pediatr. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Segawa, M.; Nomura, Y.; Kondo, Y.; Tanagitani, M.; Higurashi, M. The Development of Sleep-Wakefulness Rhythm in Normal Infants and Young Children. Tohoku J. Exp. Med. 1993, 171, 29–41. [Google Scholar] [CrossRef][Green Version]
- Staton, S.; Rankin, P.S.; Harding, M.; Smith, S.S.; Westwood, E.; LeBourgeois, M.K.; Thorpe, K.J. Many naps, one nap, none: A systematic review and meta-analysis of napping patterns in children 0–12 years. Sleep Med. Rev. 2020, 101247. [Google Scholar] [CrossRef]
- Thorpe, K.; Santon, S.; Sawyer, E.; Pattinson, C.; Haden, C.; Smith, S. Napping, development and health from 0 to 5 years: A systematic review. Arch. Dis. Child. 2015, 100, 615–622. [Google Scholar] [CrossRef]
- Weissbluth, M. Naps in children: 6 months-7 years. Sleep 1995, 18, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Murata, S.; Kagimura, T.; Omae, K.; Tanaka, A.; Takahashi, K.; Narusawa, M.; Konishi, Y.; Oniki, K.; Miike, T. Characteristics and Transition of Sleep–Wake Rhythm in Nursery School Children: The Importance of Nocturnal Sleep. Clocks Sleep 2024, 6, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Levandovski, R.; Dantas, G.; Fernandes, L.C.; Caumo, W.; Torres, I.; Roenneberg, T.; Hidalgo, M.P.; Allebrandt, K.V. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 2011, 28, 771–778. [Google Scholar] [CrossRef]
- Remi, J. Humans Entrain to Sunlight—Impact of Social Jet Lag on Disease and Implications for Critical Illness. Curr. Pharm. Des. 2015, 21, 3431–3437. [Google Scholar] [CrossRef]
- Miike, T. Insufficient Sleep Syndrome in Childhood. Children 2024, 12, 19. [Google Scholar] [CrossRef]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep duration from infancy to adolescence: Reference values and generationaltrends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Blair, P.S.; Humphreys, J.S.; Gringras, P.; Taheri, S.; Scott, N.; Emond, A.; Henderson, J.; Fleming, P.J. Childhood sleep duration and associated demographic characteristics in an English cohort Sleep. Sleep 2012, 35, 353–360. [Google Scholar] [CrossRef]
- Herxheimer, A. Jet lag. BMJ Clin. Evid. 2008, 2008, 2303. [Google Scholar]
- Sack, R.L. The pathophysiology of jet lag. Travel Med. Infect. Dis. 2009, 7, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J. Approaches to the Pharmacological Management of Jet Lag. Drugs 2018, 78, 1419–1431. [Google Scholar] [CrossRef]
- Reid, K.J.; Abbott, S.M. Jet Lag and Shift Work Disorder. Sleep Med. Clin. 2015, 10, 523–535. [Google Scholar] [CrossRef]
- Kolla, B.P.; Auger, R.R. Jet lag and shift work sleep disorders: How to help reset the internal clock. Clevel. Clin. J. Med. 2011, 78, 675–684. [Google Scholar] [CrossRef]
- Owens, J.A.; Weiss, M.R. Insufficient Sleep in Adolescents: Causes and Consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Serin, Y.; Tek, N.A. Effect of Circadian Rhythm on Metabolic Processes and the. Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, A.; Miike, Y.; Uezono, K.; Kawasaki, T. A school refusal with biological rhythm disturbance and melatonin therapy. Brain Dev. 1994, 16, 71–76. [Google Scholar] [CrossRef]
- Oldham, M.A.; Lee, H.B.; Desan, P.H. Circadian Rhythm Disruption in the Critically Ill: An Opportunity for Improving Outcomes. Crit. Care Med. 2016, 44, 207–217. [Google Scholar] [CrossRef]
- Tomoda, A.; Jhodoi, T.; Miike, T. Chronic fatigue syndrome and abnormal biological rhythms in school children. J. Chronic Fatigue Syndr. 2001, 60, 607–612. [Google Scholar] [CrossRef]
- Miike, T.; Tomoda, A.; Jhodoi, T.; Iwatani, N.; Mabe, H. Learning and memorization impairment in childhood chronic fatigue syndrome manifesting as school phobia in Japan. Brain Dev. 2004, 26, 442–447. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J.; Piekarski, C. Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften 2003, 90, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, S.; Ramos, A.M.; Sanz, A.B.; Sanchez-Niño, M.D.; Kanbay, M.; Ortiz, A. Chronodisruption: A Poorly Recognized Feature of CKD. Toxins 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Lombardi, G.; Weydahl, A.; Banfi, G. Biological rhythms, chronodisruption and chrono-enhancement: The role of physical activity as synchronizer in correcting steroids circadian rhythm in metabolic dysfunctions and cancer. Chronobiol. Int. 2018, 35, 1185–1197. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A.J.L. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the metabolic syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Méndez, N.; Corvalan, F.; Halabi, D.; Ehrenfeld, P.; Maldonado, R.; Vergara, K.; Seron-Ferre, M.; Torres-Farfan, C. From gestational chronodisruption to noncommunicable diseases: Pathophysiological mechanisms of programming of adult diseases, and the potential therapeutic role of melatonin. J. Pineal Res. 2023, 75, e12908. [Google Scholar] [CrossRef]
- Mendez, N.; Halabi, D.; Spichiger, C.; Salazar, E.R.; Vergara, K.; Alonso-Vasquez, P.; Carmona, P.; Sarmiento, J.M.; Richter, H.G.; Seron-Ferre, M.; et al. Gestational Chronodisruption Impairs Circadian Physiology in Rat Male Offspring, Increasing the Risk of Chronic Disease. Endocrinology 2016, 157, 4654–4668. [Google Scholar] [CrossRef]
- Halabi, D.; Richter, H.G.; Natalia Mendez, N.; Kähne, T.; Spichiger, C.; Salazar, E.; Fabiola Torres, F.; Vergara, K.; Seron-Ferre, M.; Torres-Farfan, C. Maternal Chronodisruption Throughout Pregnancy Impairs Glucose Homeostasis and Adipose Tissue Physiology in the Male Rat Offspring. Front. Endocrinol. 2021, 12, 678468. [Google Scholar] [CrossRef]
- Delorme, T.C.; Srivastava, L.K.; Cermakian, M. Altered circadian rhythms in a mouse model of neurodevelopmental disorders based on prenatal maternal immune activation. Brain Behav. Immun. 2021, 93, 119–131. [Google Scholar] [CrossRef]
- Bustamante-Valdez, D.J.; Fuentes-Cano, M.A.; Gonzalez-Ruano, J.S.; Martinez-Canabal, A.; Cardenas-Vazquez, R.; Duran, P. Intrauterine and early-life malnutrition in rats disrupts the circadian rhythm programming of energy metabolites through adulthood. PLoS ONE 2024, 19, e0299554. [Google Scholar] [CrossRef] [PubMed]
- Kok, E.Y.; Kaur, S.; MohdShukri, N.H.; Razak, N.A.; Takahashi, M. Maternal dietary and environmental factors associated with infant circadian rhythm, growth, and temperament: Research protocol for a prospective cohort study. Nutr. Health 2024, 30, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Hoyniak, C.P.; Whalen, D.J.; Luby, J.L.; Barch, D.M.; Miller, J.P.; Zhao, P.; Triplett, R.L.; Ju, Y.E.; Smyser, C.D.; Warner, B.; et al. Sleep and circadian rhythms during pregnancy, social disadvantage, and alterations in brain development in neonates. Dev. Sci. 2024, 27, e13456. [Google Scholar] [CrossRef] [PubMed]
- Lassi, M.; Tomar, A.; Comas-Armangué, G.; Vogtmann, R.; Dijkstra, D.J.; Corujo, D.; Gerlini, R.; Darr, J.; Scheid, F.; Rozman, J.; et al. Disruption of paternal circadian rhythm affects metabolic health in male offspring via nongerm cell factors. Sci. Adv. 2021, 7, eabg6424. [Google Scholar] [CrossRef]
- Miike, T.; Toyoura, M.; Oniki, K.; Tonooka, S.; Tajima, S. Prophylactic Treatment of ASD Based on Sleep-Wake Circadian Rhythm Formation in Infancy to Early Childhood. In Neurobiology of Autism Spectrum Disorders; El Idrissi, A., McCloskey, D., Eds.; Springer: Cham, Switzerland, 2023; pp. 183–207. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miike, T. Appropriate Lifelong Circadian Rhythms Are Established During Infancy: A Narrative Review. Clocks & Sleep 2025, 7, 41. https://doi.org/10.3390/clockssleep7030041
Miike T. Appropriate Lifelong Circadian Rhythms Are Established During Infancy: A Narrative Review. Clocks & Sleep. 2025; 7(3):41. https://doi.org/10.3390/clockssleep7030041
Chicago/Turabian StyleMiike, Teruhisa. 2025. "Appropriate Lifelong Circadian Rhythms Are Established During Infancy: A Narrative Review" Clocks & Sleep 7, no. 3: 41. https://doi.org/10.3390/clockssleep7030041
APA StyleMiike, T. (2025). Appropriate Lifelong Circadian Rhythms Are Established During Infancy: A Narrative Review. Clocks & Sleep, 7(3), 41. https://doi.org/10.3390/clockssleep7030041