The Sleep–Wake Cycle Pattern of a Blind Trail Ultramarathon Runner and His Guide: The World’s First Case
Abstract
1. Introduction
2. Results
2.1. Subjective Sleep Outcomes
2.2. Objective Sleep Outcomes
2.3. Objective Circadian Rhythms Outcomes
3. Discussion
4. Materials and Methods
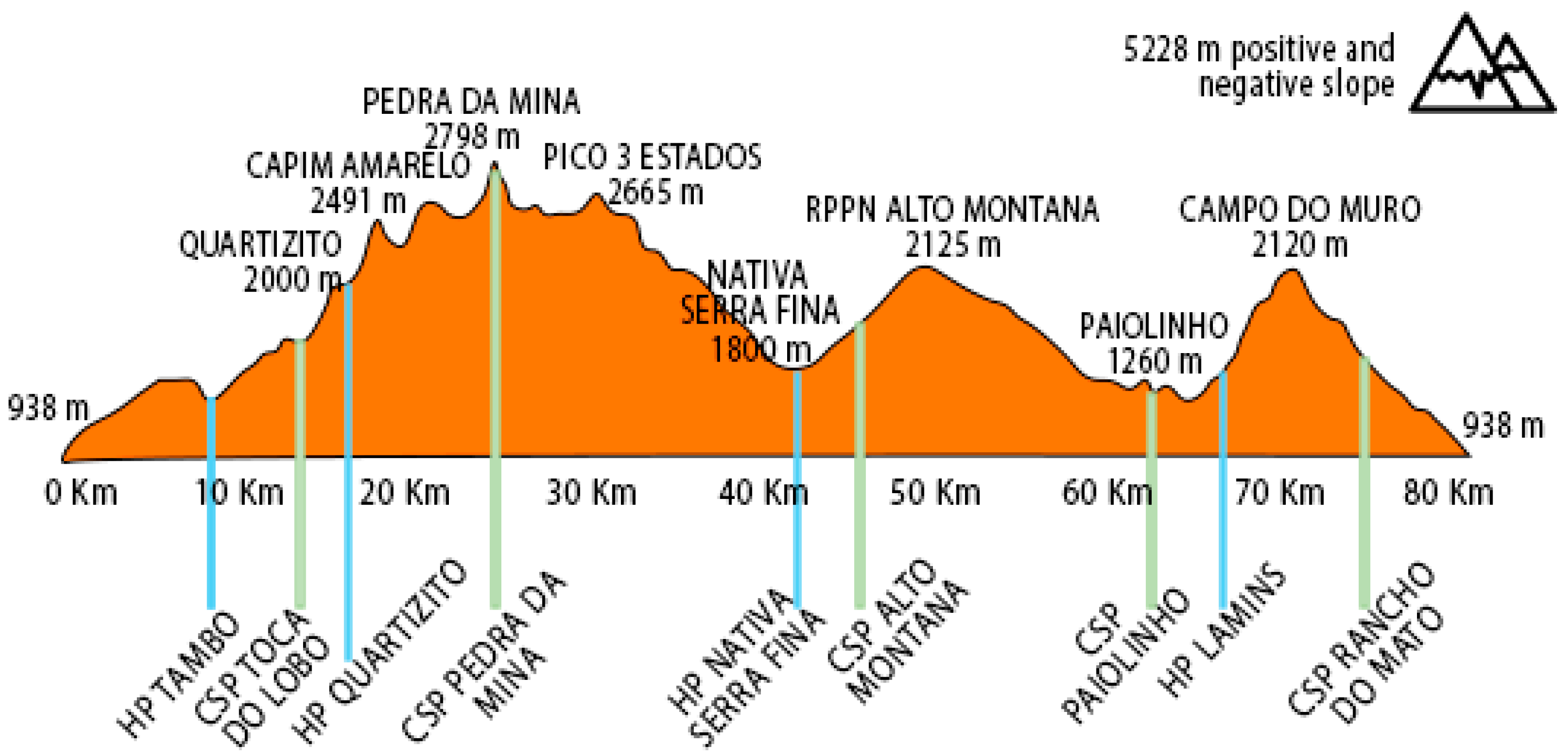
4.1. The Case
4.2. Assessment Instruments
4.3. Statistical Analyses
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valentin, S.; Pham, L.A.; Macrae, E. Enablers and barriers in ultra-running: A comparison of male and female ultra-runners. Sport Soc. 2021, 25, 2193–2212. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Knechtle, B.; Tarnopolsky, M.; Hoffman, M.D. Nutrition for Ultramarathon Running: Trail, Track, and Road. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and pathophysiological responses to ultramarathon running in non-elite runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef]
- dos Santos, N.E.; Daniel, N.V.S.; Franco, B.; Bastos, A.M.; Belli, T.; Esteves, A.M. Sleep and nutritional profile of endurance and ultra-endurance running athletes. Sleep Sci. 2022, 15, 441–447. [Google Scholar] [CrossRef]
- Belinchón-Demiguel, P.; Ruisoto, P.; Knechtle, B.; Nikolaidis, P.T.; Herrera-Tapias, B.; Clemente-Suárez, V.J. Predictors of Athlete’s Performance in Ultra-Endurance Mountain Races. Int. J. Environ. Res. Public Health 2021, 18, 956. [Google Scholar] [CrossRef]
- Lecina, M.; Castellar-Otín, C.; López-Laval, I.; Páez, L.C.; Pradas, F. Acute Kidney Injury and Hyponatremia in Ultra-Trail Racing: A Systematic Review. Medicina 2022, 58, 569. [Google Scholar] [CrossRef]
- Martin, T.; Arnal, P.J.; Hoffman, M.D.; Millet, G.Y. Sleep habits and strategies of ultramarathon runners. PLoS ONE 2018, 13, e0194705. [Google Scholar] [CrossRef]
- Driller, M.W.; Dunican, I.C.; Omond, S.E.T.; Boukhris, O.; Stevenson, S.; Lambing, K.; Bender, A.M. Pyjamas, Polysomnography and Professional Athletes: The Role of Sleep Tracking Technology in Sport. Sports 2023, 11, 14. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Weiss, K.; Knechtle, B.; Trakada, G. Sleep in marathon and ultramarathon runners: A brief narrative review. Front. Neurol. 2023, 14, 1217788. [Google Scholar] [CrossRef]
- Warman, G.R.; Pawley, M.D.M.; Bolton, C.; Cheeseman, J.F.; Fernando, A.T.; Arendt, J.; Wirz-Justice, A. Circadian-related sleep disorders and sleep medication use in the New Zealand blind population: An observational prevalence survey. PLoS ONE 2011, 6, e22073. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.; Dauvilliers, Y.; Quera-Salva, M.A. Circadian Rhythm Disturbances in the Blind. Curr. Neurol. Neurosci. Rep. 2018, 18, 65. [Google Scholar] [CrossRef]
- Tamura, N.; Sasai-Sakuma, T.; Morita, Y.; Okawa, M.; Inoue, S.; Inoue, Y. A Nationwide Cross-Sectional Survey of Sleep-Related Problems in Japanese Visually Impaired Patients: Prevalence and Association with Health-Related Quality of Life. J. Clin. Sleep Med. 2016, 12, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Atan, Y.S.; Subaşı, M.; Özdemir, P.G.; Batur, M. The Effect of Blindness on Biological Rhythms and the Consequences of Circadian Rhythm Disorder. Turk. J. Ophthalmol. 2023, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Monma, T.; Kohda, Y.; Yamane, M.; Mitsui, T.; Ando, K.; Takeda, F. Prevalence and risk factors of sleep disorders in visually impaired athletes. Sleep Med. 2020, 79, 175–182. [Google Scholar] [CrossRef]
- Salva, M.A.Q.; Hartley, S.; Léger, D.; Dauvilliers, Y.A. Non-24-Hour Sleep-Wake Rhythm Disorder in the Totally Blind: Diagnosis and Management. Front. Neurol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Swinbourne, R.; Gill, N.; Vaile, J.; Smart, D. Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes. Eur. J. Sport Sci. 2015, 16, 850–858. [Google Scholar] [CrossRef]
- Knufinke, M.; Nieuwenhuys, A.; Geurts, S.A.E.; Coenen, A.M.L.; Kompier, M.A.J. Self-reported sleep quantity, quality and sleep hygiene in elite athletes. J. Sleep Res. 2017, 27, 78–85. [Google Scholar] [CrossRef]
- Brager, A.J.; Demiral, S.; Choynowski, J.; Kim, J.; Campbell, B.; Capaldi, V.F.; Simonelli, G.; Hammer, S. Earlier shift in race pacing can predict future performance during a single-effort ultramarathon under sleep deprivation. Sleep Sci. 2020, 13, 25–31. [Google Scholar] [CrossRef]
- Bianchi, D.; Miller, D.J.; Lastella, M. Sleep-Wake Behaviour of 200-Mile Ultra-Marathon Competitors: A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 3006. [Google Scholar] [CrossRef]
- Kishi, A.; Millet, G.Y.; Desplan, M.; Lemarchand, B.; Bouscaren, N. Sleep and Ultramarathon: Exploring Patterns, Strategies, and Repercussions of 1,154 Mountain Ultramarathons Finishers. Sports Med. Open 2024, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, E.A.; Diamond, A.B. The Value of Sleep on Athletic Performance, Injury, and Recovery in the Young Athlete. Pediatr. Ann. 2017, 46, e106–e111. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2020, 55, 356–368. [Google Scholar] [CrossRef]
- Roberts, I.E.; Murphy, C.J.; Goosey-Tolfrey, V.L. Sleep disruption considerations for Paralympic athletes competing at Tokyo 2020. J. Sports Med. Phys. Fit. 2021, 61, 1159–1172. [Google Scholar] [CrossRef]
- Grade, I.; Andrade, H.; Guerreiro, R.; Stieler, E.; da Silva, F.R.; da Silva, H.G.; Vital, R.; Resende, R.A.; Gonçalves, D.A.; Andrade, A.G.; et al. The Sleep Parameters of Paralympic Athletes: Characteristics and Assessment Instruments. J. Sport Rehabilit. 2023, 32, 203–214. [Google Scholar] [CrossRef]
- Rosa, J.P.P.; Silva, A.; de Lira, C.A.B.; de Mello, M.T. The Potential Impact of Heat on Athletes’ Sleep at the Paris 2024 Olympics and Paralympics Games. Sleep Sci. 2024. [Google Scholar] [CrossRef]
- Benchetrit, S.; Badariotti, J.I.; Corbett, J.; Costello, J.T. The effects of sleep deprivation and extreme exertion on cognitive performance at the world-record breaking Suffolk Back Yard Ultra-marathon. PLoS ONE 2024, 19, e0299475. [Google Scholar] [CrossRef]
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance: Impacts on Physical Performance, Mental Performance, Injury Risk and Recovery, and Mental Health. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef]
- Huang, M.K.; Chang, K.S.; Kao, W.F.; Li, L.H.; How, C.K.; Wang, S.H.; Lin, Y.K.; Hwang, Y.S.; Chien, D.K.; Chiu, Y.H. Visual hallucinations in 246-km mountain ultra-marathoners: An observational study. Chin. J. Physiol. 2021, 64, 225–231. [Google Scholar] [CrossRef]
- Stuldreher, I.V.; Maasland, E.; Bottenheft, C.; van Erp, J.B.F.; Brouwer, A.M. Physiological synchrony in electrodermal activity predicts decreased vigilant attention induced by sleep deprivation. Front. Neuroergon. 2023, 4, 1199347. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.; Thomas, G.; Wadley, A.J. Sleep Deprivation: Cytokine and Neuroendocrine Effects on Perception of Effort. Med. Sci. Sports Exerc. 2020, 52, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Odle-Dusseau, H.N.; Bradley, J.L.; Pilcher, J.J. Subjective perceptions of the effects of sustained performance under sleep-deprivation conditions. Chrono. Int. 2010, 27, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Souissi, W.; Hammouda, O.; Ayachi, M.; Ammar, A.; Khcharem, A.; de Marco, G.; Souissi, M.; Driss, T. Partial sleep deprivation affects endurance performance and psychophysiological responses during 12-minute self-paced running exercise. Physiol. Behav. 2020, 227, 113165. [Google Scholar] [CrossRef]
- Daniel, N.V.S.; Barreira, J.; Bastos, A.M.; dos Santos, N.E.; Franco, B.; Esteves, A.M.; Belli, T. Ultramarathon runners and support crew: The influence of pre-race sleep and training profiles on performance in a 217-km mountain race. Sleep Med. 2024, 120, 85–89. [Google Scholar] [CrossRef]
- Gong, M.; Sun, M.; Sun, Y.; Jin, L.; Li, S. Effects of Acute Sleep Deprivation on Sporting Performance in Athletes: A Comprehensive Systematic Review and Meta-Analysis. Nat. Sci. Sleep 2024, 16, 935–948. [Google Scholar] [CrossRef]
- Anderson, T.; Adams, W.M.; Burns, G.T.; Post, E.G.; Baumann, S.; Clark, E.; Cogan, k.; Finnoff, J.T. Addressing Circadian Disruptions in Visually Impaired Paralympic Athletes. Int. J. Sports Physiol. Perform. 2024, 19, 212–218. [Google Scholar] [CrossRef]
- O’Donnell, S.; Beaven, C.M.; Driller, M.W. From pillow to podium: A review on understanding sleep for elite athletes. Nat. Sci. Sleep 2018, 10, 243–253. [Google Scholar] [CrossRef]
- Lockley, S.W.; Arendt, J.; Skene, D.J. Visual impairment and circadian rhythm disorders. Dialog. Clin. Neurosci. 2007, 9, 301–314. [Google Scholar] [CrossRef]
- Cunha, L.A.; Costa, J.A.; Marques, E.A.; Brito, J.; Lastella, M.; Figueiredo, P. The Impact of Sleep Interventions on Athletic Performance: A Systematic Review. Sports Med. Open 2023, 9, 58. [Google Scholar] [CrossRef]
- Silva, A.; Pinheiro, L.S.P.; Silva, S.; Andrade, H.; Pereira, A.G.; da Silva, F.R.; Guerreiro, R.; Barreto, B.; Resende, R.; de Mello, M.T. Sleep in Paralympic athletes and its relationship with injuries and illnesses. Phys. Ther. Sport 2022, 56, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. Available online: https://pubmed.ncbi.nlm.nih.gov/1027738/ (accessed on 7 January 2025). [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bertolazi, A.N.; Fagondes, S.C.; Hoff, L.S.; Pedro, V.D.; Barreto, S.S.M.; Johns, M.W. Validação da escala de sonolência de Epworth em português para uso no Brasil. J. Bras. Pneumol. 2009, 35, 877–883. [Google Scholar] [CrossRef]
- Laura de Siqueira, C. Adaptação e Validação do Índice de Gravidade de Insânia (IGI): Caracterização Populacional, Valores Normativos e Aspectos Associados. Universidade Federal de São Paulo (UNIFESP). 2011. Available online: http://repositorio.unifesp.br/handle/11600/23193 (accessed on 7 January 2025).
- Bertolazi, A.N.; Fagondes, S.C.; Hoff, L.S.; Dartora, E.G.; da Silva Miozzo, I.C.; de Barba, M.E.F.; Barreto, S.S. Validation of the Brazilian Portuguese Version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011, 12, 70–75. [Google Scholar] [CrossRef]
- Claudino, J.G.; Gabbett, T.J.; de Sá Souza, H.; Simim, M.; Fowler, P.; Borba, D.D.A.; Melo, M.; Bottino, A.; Loturco, I.; D’Almeida, V.; et al. Which parameters to use for sleep quality monitoring in team sport athletes? A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2019, 5, e000475. [Google Scholar] [CrossRef]
- Gonçalves, B.S.; Cavalcanti, P.R.; Tavares, G.R.; Campos, T.F.; Araujo, J.F. Nonparametric methods in actigraphy: An update. Sleep Sci. 2014, 7, 158–164. [Google Scholar] [CrossRef]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef]
- Simim, M.A.d.M.; Souza, H.d.S.; Filho, C.A.C.; Gianoni, R.L.d.S.; Bezerra, R.R.; Affonso, H.d.O.; Amadio, A.C.; D’Almeida, V.; Serrao, J.C.; Claudino, J.G. Sleep quality monitoring in individual sports athletes: Parameters and definitions by systematic review. Sleep Sci. 2020, 13, 267–285. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work. Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef]
- Greene, W. Econometric Analysis, 8th ed.; Pearson Education Limited: London, UK, 2018. [Google Scholar]
- Jebb, A.T.; Tay, L.; Wang, W.; Huang, Q. Time series analysis for psychological research: Examining and forecasting change. Front. Psychol. 2015, 6, 727. [Google Scholar] [CrossRef]
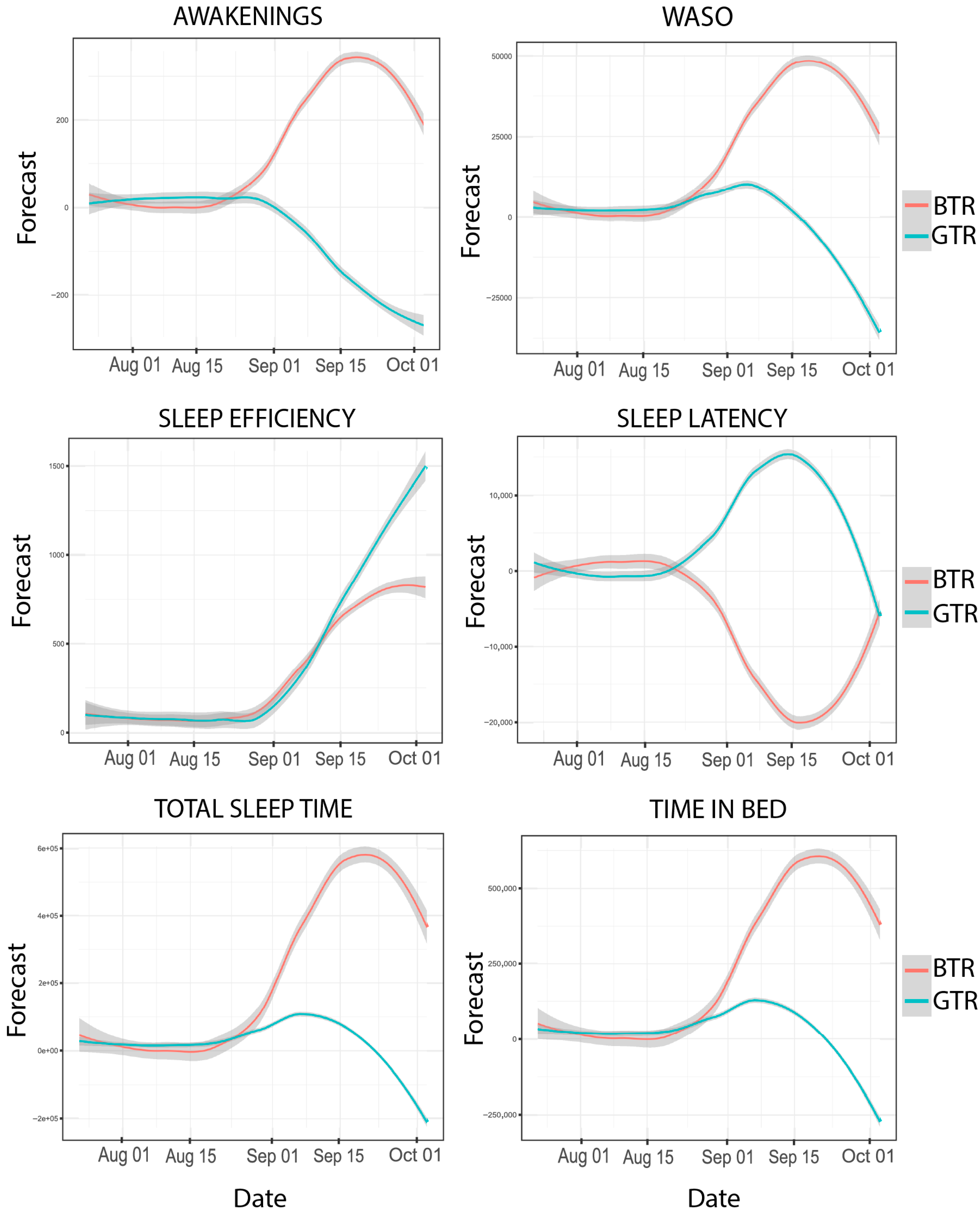
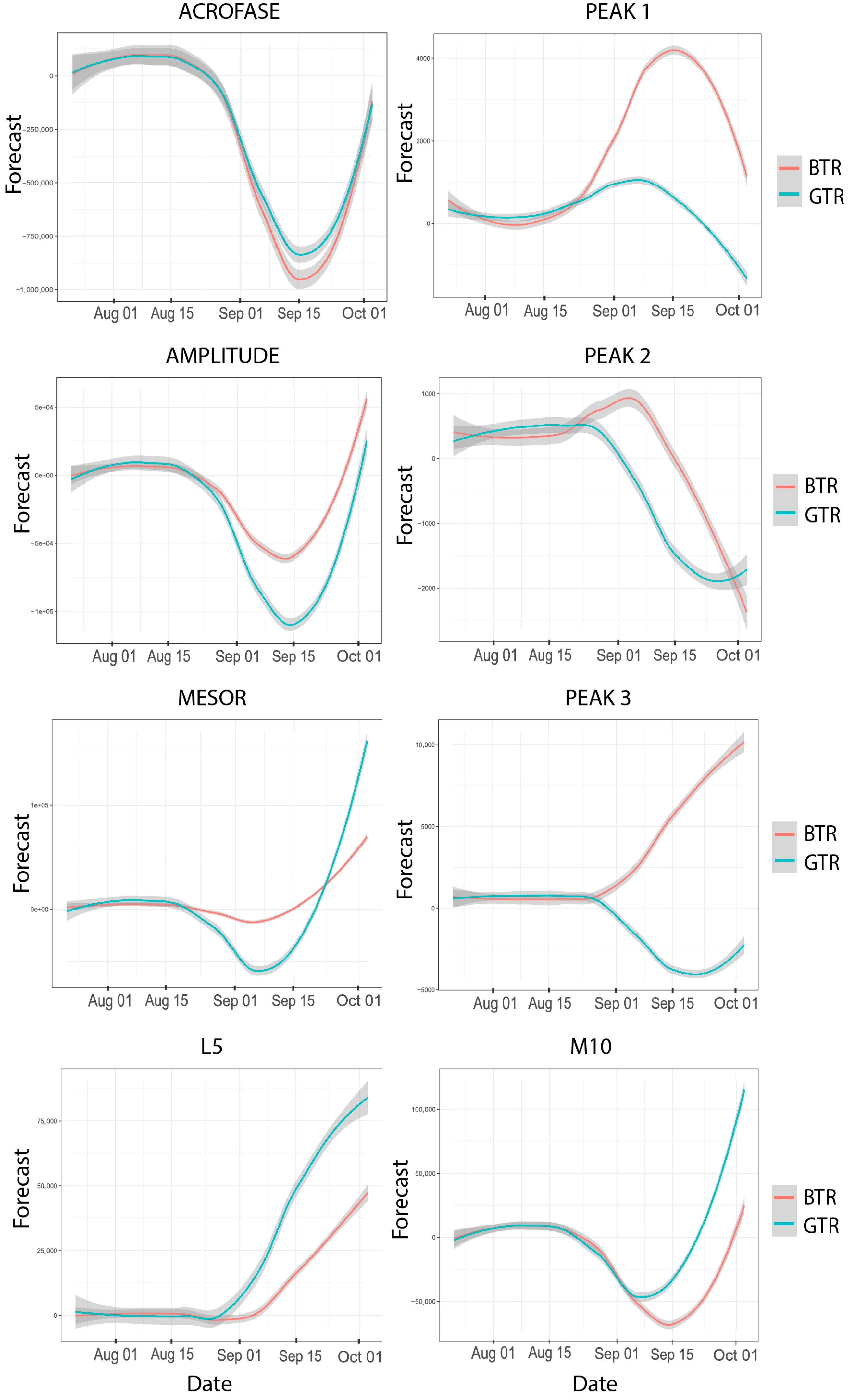
| Variable | BTR | GTR | ||||||
|---|---|---|---|---|---|---|---|---|
| BIC | RMSE | Ljung-Box Q | p | BIC | RMSE | Ljung-Box Q | p | |
| Bedtime (s) | 20.89 | 32,501.63 | 52.15 | <0.01 | 20.98 | 34,160.47 | 30.67 | 0.03 |
| To wake up (s) | 17.73 | 6733.56 | 14.93 | 0.66 | 17.09 | 4862.9 | 30.46 | 0.03 |
| Time in bed (s) | 17.39 | 5655.01 | 29.70 | 0.04 | 18.10 | 8072.47 | 20.06 | 0.33 |
| TST (s) | 17.07 | 4815.80 | 23.30 | 0.17 | 17.90 | 7308.73 | 23.83 | 0.16 |
| Sleep Latency (s) | 10.77 | 206.50 | 63.38 | <0.01 | 10.54 | 184.79 | 47.87 | <0.01 |
| Sleep efficiency (%) | 2.60 | 3.48 | 34.92 | 0.01 | 3.86 | 6.52 | 29.78 | 0.04 |
| WASO (s) | 14.50 | 1339.24 | 22.88 | 0.19 | 14.75 | 1511.29 | 24.58 | 0.13 |
| Awakenings (nº) | 3.26 | 4.84 | 28.36 | 0.05 | 4.74 | 10.10 | 41.04 | 0.002 |
| Variable | BTR | GTR | ||||||
|---|---|---|---|---|---|---|---|---|
| BIC | RMSE | Ljung-Box Q | p | BIC | RMSE | Ljung-Box Q | p | |
| M10 | 16.11 | 2998.08 | 216.84 | 0.53 | 16.75 | 4124.71 | 24.64 | 0.13 |
| L5 | 14.08 | 1086.12 | 10.95 | 0.89 | 14.41 | 1275.71 | 7.97 | 0.97 |
| Mesor (0.0.2) | 15.49 | 2079.98 | 11.18 | 0.84 | 15.89 | 2674.11 | 10.12 | 0.92 |
| Amplitude | 15.74 | 2489.47 | 23.18 | 0.18 | 16.34 | 3353.68 | 20.22 | 0.32 |
| Acrophase (hours) | 0.22 | 1.06 | 18.13 | 0.44 | 0.31 | 1.11 | 38.53 | 0.003 |
| Acrophase (radian) | 19.28 | 14,575.13 | 18.13 | 0.44 | 19.36 | 15,203.32 | 38.53 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilherme, L.Q.; Matos, J.P.; Kravchychyn, A.C.P.; De Mello, M.T.; dos Santos Amorim, P.R.; de Sá Souza, H. The Sleep–Wake Cycle Pattern of a Blind Trail Ultramarathon Runner and His Guide: The World’s First Case. Clocks & Sleep 2025, 7, 20. https://doi.org/10.3390/clockssleep7020020
Guilherme LQ, Matos JP, Kravchychyn ACP, De Mello MT, dos Santos Amorim PR, de Sá Souza H. The Sleep–Wake Cycle Pattern of a Blind Trail Ultramarathon Runner and His Guide: The World’s First Case. Clocks & Sleep. 2025; 7(2):20. https://doi.org/10.3390/clockssleep7020020
Chicago/Turabian StyleGuilherme, Larissa Quintão, Julia Pagotto Matos, Ana Claudia Pelissari Kravchychyn, Marco Tulio De Mello, Paulo Roberto dos Santos Amorim, and Helton de Sá Souza. 2025. "The Sleep–Wake Cycle Pattern of a Blind Trail Ultramarathon Runner and His Guide: The World’s First Case" Clocks & Sleep 7, no. 2: 20. https://doi.org/10.3390/clockssleep7020020
APA StyleGuilherme, L. Q., Matos, J. P., Kravchychyn, A. C. P., De Mello, M. T., dos Santos Amorim, P. R., & de Sá Souza, H. (2025). The Sleep–Wake Cycle Pattern of a Blind Trail Ultramarathon Runner and His Guide: The World’s First Case. Clocks & Sleep, 7(2), 20. https://doi.org/10.3390/clockssleep7020020









