A Longitudinal Examination between Chronotype and Insomnia in Youths: A Cross-Lagged Panel Analysis
Abstract
1. Introduction
2. Results
2.1. Descriptive and Correlation Analyses
2.2. Prevalence and Incidence of Insomnia
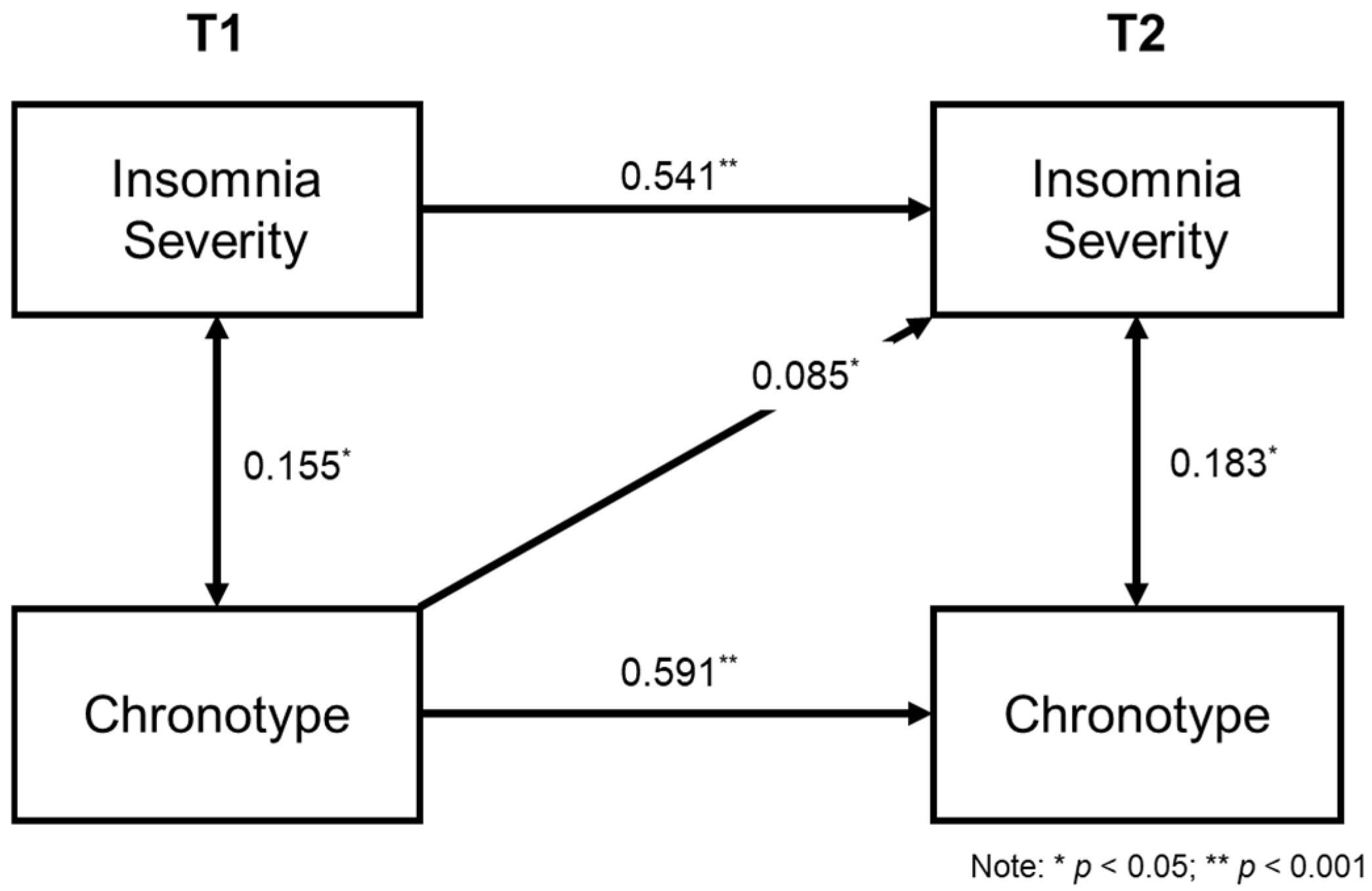
2.3. Cross-Lagged Model of Insomnia and Chronotype
3. Discussion
3.1. Implications
3.2. Limitations
4. Materials and Methods
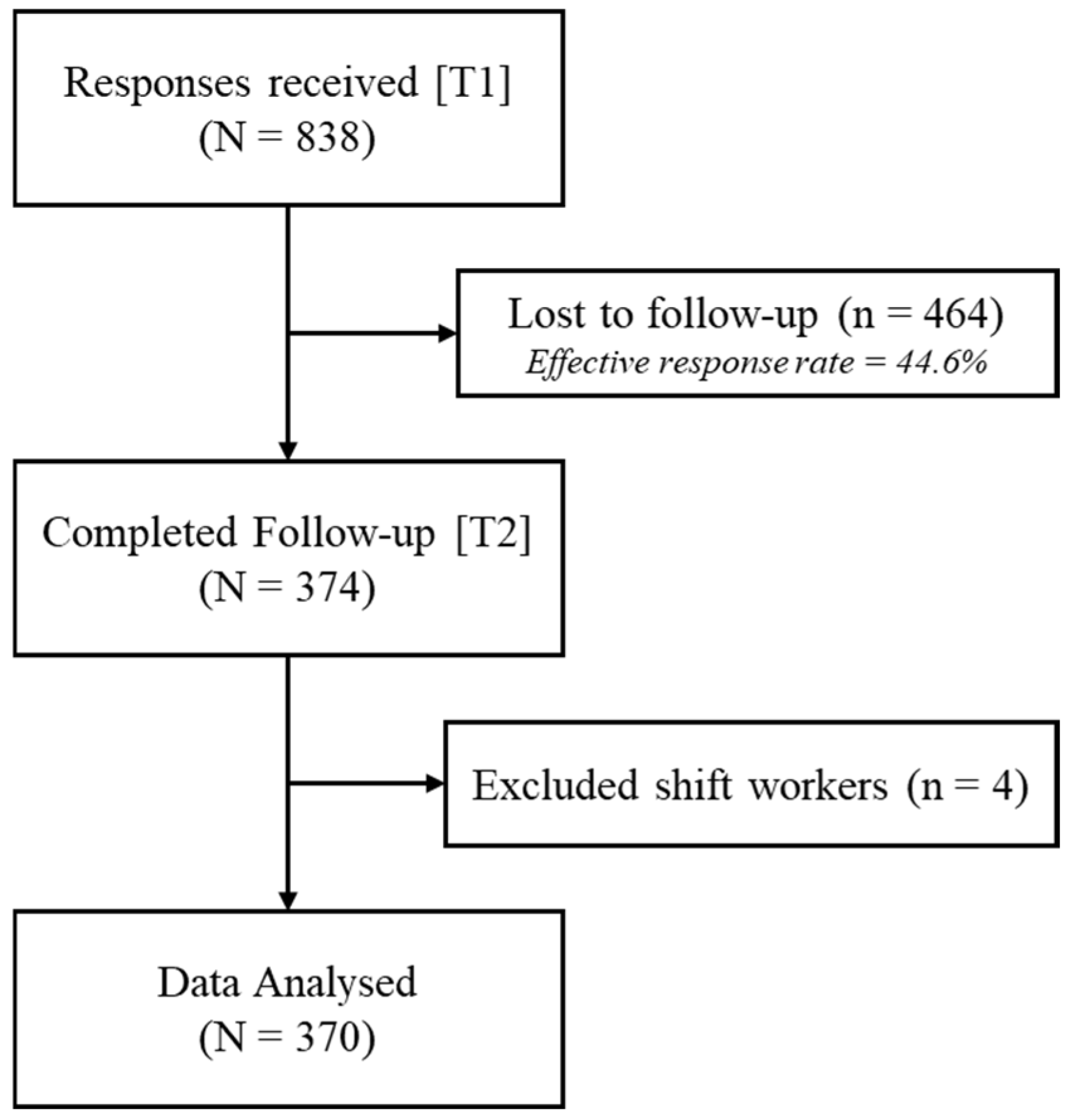
4.1. Participants
4.2. Procedure
4.3. Measurements
4.3.1. Measure of Insomnia Symptoms
4.3.2. Measure of Chronotype
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Chan, N.Y.; Lam, S.P.; Li, S.X.; Liu, Y.; Chan, J.W.; Kong, A.P.; Ma, R.C.; Chan, K.C.; Li, A.M.; et al. Emergence of sex differences in insomnia symptoms in adolescents: A large-scale school-based study. Sleep 2016, 39, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Randler, C.; Fassl, C.; Kalb, N. From lark to owl: Developmental changes in morningness-eveningness from new-borns to early adulthood. Sci. Rep. 2017, 7, 45874. [Google Scholar] [CrossRef]
- Cheung, F.T.W.; Li, X.; Hui, T.K.; Chan, N.Y.; Chan, J.W.; Wing, Y.K.; Li, S.X. Circadian preference and mental health outcomes in youth: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 72, 101851. [Google Scholar] [CrossRef]
- Phiri, D.; Amelia, V.L.; Muslih, M.; Dlamini, L.P.; Chung, M.-H.; Chang, P.-C. Prevalence of sleep disturbance among adolescents with substance use: A systematic review and meta-analysis. Child Adolesc. Psychiatry Ment. Health 2023, 17, 100. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- Zhang, B.; Wing, Y.-K. Sex differences in insomnia: A meta-analysis. Sleep 2006, 29, 85–93. [Google Scholar] [CrossRef]
- Scott, J.; Kallestad, H.; Vedaa, O.; Sivertsen, B.; Etain, B. Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: A systematic review and meta-analysis. Sleep. Med. Rev. 2021, 57, 101429. [Google Scholar] [CrossRef]
- Li, S.X.; Chan, N.Y.; Yu, M.W.; Lam, S.P.; Zhang, J.; Yan Chan, J.W.; Li, A.M.; Wing, Y.K. Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med. 2018, 47, 93–99. [Google Scholar] [CrossRef]
- Chen, S.-J.; Zhang, J.-H.; Li, S.X.; Tsang, C.C.; Chan, K.C.C.; Au, C.T.; Li, A.M.; Kong, A.P.S.; Wing, Y.K.; Chan, N.Y. The trajectories and associations of eveningness and insomnia with daytime sleepiness, depression and suicidal ideation in adolescents: A 3-year longitudinal study. J. Affect. Disord. 2021, 294, 533–542. [Google Scholar] [CrossRef]
- Gau, S.S.F.; Shang, C.Y.; Merikangas, K.R.; Chiu, Y.N.; Soong, W.T.; Cheng, A.T.A. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J. Biol. Rhythms 2007, 22, 268–274. [Google Scholar] [CrossRef]
- Lack, L.C.; Micic, G.; Lovato, N. Circadian aspects in the aetiology and pathophysiology of insomnia. J. Sleep Res. 2023, 32, e13976. [Google Scholar] [CrossRef]
- Tetsuo Harada, D.; Mami Morikuni, B.; Sato Yoshii, B.; Yasuhiro Yamashita, B.; Hitomi Takeuchi, M. Usage of mobile phone in the evening or at night makes Japanese students evening-typed and night sleep uncomfortable. Sleep Hypn. 2002, 4, 149–153. [Google Scholar]
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. 2014, 15, 934–941. [Google Scholar] [CrossRef]
- Yim, S.H.; Yang, K.I.; Kim, J.H.; Hwangbo, Y.; Kim, D.; Hong, S.B. Association between eveningness preference, socio-behavioral factors, and insomnia symptoms in Korean adolescents. Sleep Med. 2021, 82, 144–150. [Google Scholar] [CrossRef]
- Lin, C.Y.; Imani, V.; Griffiths, M.D.; Broström, A.; Nygårdh, A.; Demetrovics, Z.; Pakpour, A.H. Temporal associations between morningness/eveningness, problematic social media use, psychological distress and daytime sleepiness: Mediated roles of sleep quality and insomnia among young adults. J. Sleep Res. 2020, 30, e13076. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Ru, T.; Niu, J.; He, M.; Zhou, G. How does the COVID-19 affect mental health and sleep among Chinese adolescents: A longitudinal follow-up study. Sleep Med. 2021, 85, 246–258. [Google Scholar] [CrossRef]
- Duncan, O.D. Some linear models for two-wave, two-variable panel analysis. Psychol. Bull. 1969, 72, 177. [Google Scholar] [CrossRef]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef]
- Zeeuw, J.D.; Wisniewski, S.; Papakonstantinou, A.; Bes, F.; Wahnschaffe, A.; Zaleska, M.; Kunz, D.; Münch, M. The alerting effect of the wake maintenance zone during 40 hours of sleep deprivation. Sci. Rep. 2018, 8, 11012. [Google Scholar] [CrossRef]
- Morris, M.; Lack, L.; Dawson, D. Sleep-onset insomniacs have delayed temperature rhythms. Sleep 1990, 13, 1–14. [Google Scholar] [CrossRef]
- Wright, H.; Lack, L.; Bootzin, R. Relationship between dim light melatonin onset and the timing of sleep in sleep onset insomniacs. Sleep Biol. Rhythms 2006, 4, 78–80. [Google Scholar] [CrossRef]
- Hill, V.M.; Rebar, A.L.; Ferguson, S.A.; Shriane, A.E.; Vincent, G.E. Go to bed! A systematic review and meta-analysis of bedtime procrastination correlates and sleep outcomes. Sleep Med. Rev. 2022, 66, 101697. [Google Scholar] [CrossRef]
- Richardson, C.; Micic, G.; Cain, N.; Bartel, K.; Maddock, B.; Gradisar, M. Cognitive “insomnia” processes in delayed sleep–wake phase disorder: Do they exist and are they responsive to chronobiological treatment? J. Consult. Clin. Psychol. 2019, 87, 16. [Google Scholar] [CrossRef]
- Suh, S.; Nowakowski, S.; Bernert, R.A.; Ong, J.C.; Siebern, A.T.; Dowdle, C.L.; Manber, R. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012, 13, 469–475. [Google Scholar] [CrossRef]
- Harvey, A.G. A transdiagnostic intervention for youth sleep and circadian problems. Cogn. Behav. Pract. 2016, 23, 341–355. [Google Scholar] [CrossRef]
- Harvey, A.G.; Hein, K.; Dolsen, M.R.; Dong, L.; Rabe-Hesketh, S.; Gumport, N.B.; Kanady, J.; Wyatt, J.K.; Hinshaw, S.P.; Silk, J.S. Modifying the impact of eveningness chronotype (“Night-Owls”) in youth: A randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 742–754. [Google Scholar] [CrossRef]
- Van Maanen, A.; Meijer, A.M.; Van Der Heijden, K.B.; Oort, F.J. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 29, 52–62. [Google Scholar] [CrossRef]
- Gradisar, M.; Dohnt, H.; Gardner, G.; Paine, S.; Starkey, K.; Menne, A.; Slater, A.; Wright, H.; Hudson, J.L.; Weaver, E.; et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep 2011, 34, 1671–1680. [Google Scholar] [CrossRef]
- Danielsson, K.; Jansson-Fröjmark, M.; Broman, J.-E.; Markström, A. Cognitive behavioral therapy as an adjunct treatment to light therapy for delayed sleep phase disorder in young adults: A randomized controlled feasibility study. Behav. Sleep Med. 2016, 14, 212–232. [Google Scholar] [CrossRef]
- Morin, C.M.; Bjorvatn, B.; Chung, F.; Holzinger, B.; Partinen, M.; Penzel, T.; Ivers, H.; Wing, Y.K.; Chan, N.Y.; Merikanto, I.; et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: An international collaborative study. Sleep Med. 2021, 87, 38–45. [Google Scholar] [CrossRef]
- Genta, F.D.; Neto, G.B.R.; Sunfeld, J.P.V.; Porto, J.F.; Xavier, A.D.; Moreno, C.R.C.; Lorenzi-Filho, G.; Genta, P.R. COVID-19 pandemic impact on sleep habits, chronotype, and health-related quality of life among high school students: A longitudinal study. J. Clin. Sleep Med. 2021, 17, 1371–1377. [Google Scholar] [CrossRef]
- Randler, C.; Freyth-Weber, K.; Rahafar, A.; Florez Jurado, A.; Kriegs, J.O. Morningness-eveningness in a large sample of German adolescents and adults. Heliyon 2016, 2, e00200. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Vigod, S.; Brittain, H.; Vaillancourt, T.; Szatmari, P.; Pullenayegum, E. Application of transactional (cross-lagged panel) models in mental health research: An introduction and review of methodological considerations. J. Can. Acad. Child Adolesc. Psychiatry 2022, 31, 124. [Google Scholar]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Chung, K.-F.; Kan, K.K.-K.; Yeung, W.-F. Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med. 2011, 12, 463–470. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef]
- Cheung, F.T.W.; Ho, A.W.Y.; Chan, J.W.Y.; Li, X.; Chan, N.Y.; Zhang, J.; Ho, C.S.; Wing, Y.K.; Li, S.X. Validation of the Chinese version of the Munich Chronotype Questionnaire (MCTQHK) in Hong Kong Chinese youths. Chronobiol. Int. 2022, 39, 678–689. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
| T1 | T2 | p | |
|---|---|---|---|
| Demographic | |||
| Sex, (n, % Female) | 269 (72.7%) | - | |
| Age | 21.08 (2.00) | 22.10 (2.00) | <0.001 |
| Derived from MCTQ | |||
| Sleep Time (hh:mm) | |||
| Free days | 02:04 (01:38) | 02:08 (01:37) | 0.310 |
| Workdays | 01:21 (01:16) | 01:23 (01:27) | 0.510 |
| Wake Time (hh:mm) | |||
| Free days | 10:26 (01:59) | 10:39 (01:48) | 0.013 |
| Workdays | 08:10 (01:27) | 08:40 (01:39) | <0.001 |
| Sleep Duration (hours) | |||
| Free days | 8.09 (1.88) | 8.03 (1.35) | 0.540 |
| Workdays | 6.70 (1.80) | 7.03 (1.44) | 0.002 |
| Chronotype (hh:mm) | 05:38 (01:39) | 05:48 (01:31) | 0.016 |
| Derived from ISI | |||
| Insomnia symptoms | 8.09 (4.72) | 7.68 (4.82) | 0.080 |
| Age | T1 ISI | T1 MSFsc | T2 ISI | T2 MSFsc | |
|---|---|---|---|---|---|
| T1 ISI | 0.101 | - | |||
| T1 MSFsc | −0.093 | 0.143 ** | - | ||
| T2 ISI | 0.102 * | 0.561 ** | 0.156 ** | - | |
| T2 MSFsc | −0.262 ** | 0.077 | 0.611 ** | 0.193 ** | - |
| Model Fit | Model Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Competing Models | df | CFI | AIC | RMSEA | SRMR | p-Value | ||
| [Model 1] Autoregressive model | 12.86 | 4 | 0.975 | 8890.45 | 0.077 | 0.059 | ||
| [Model 2] Cross-Sectional model | 3.90 | 3 | 0.997 | 8883.48 | 0.028 | 0.022 | ||
| Comparison: M2 vs. M1 | 8.97 | 0.003 | ||||||
| [Model 3a] Causal model (Insomnia to Chronotype) | 3.74 | 2 | 0.995 | 8885.33 | 0.049 | 0.021 | ||
| Comparison: M3a vs. M1 | 9.12 | 0.010 | ||||||
| Comparison: M3a vs. M2 | 0.15 | 0.69 | ||||||
| [Model 3b] Reversed causal model (Chronotype to Insomnia) | 0.13 | 2 | 1.000 | 8881.71 | 0.000 | 0.004 | ||
| Comparison: M3b vs. M1 | 12.73 | 0.002 | ||||||
| Comparison: M3b vs. M2 | 3.77 | 0.052 | ||||||
| [Model 4] Reciprocal model | 0.02 | 1 | 1.000 | 8883.60 | 0.000 | 0.001 | ||
| Comparison: M4 vs. M1 | 12.85 | 0.005 | ||||||
| Comparison: M4 vs. M2 | 3.88 | 0.14 | ||||||
| Comparison: M4 vs. M3a | 3.73 | 0.054 | ||||||
| Comparison: M4 vs. M3b | 0.12 | 0.73 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, F.T.W.; Sit, H.F.; Li, X.; Chan, J.W.Y.; Chan, N.Y.; Wing, Y.K.; Li, S.X. A Longitudinal Examination between Chronotype and Insomnia in Youths: A Cross-Lagged Panel Analysis. Clocks & Sleep 2024, 6, 557-567. https://doi.org/10.3390/clockssleep6040037
Cheung FTW, Sit HF, Li X, Chan JWY, Chan NY, Wing YK, Li SX. A Longitudinal Examination between Chronotype and Insomnia in Youths: A Cross-Lagged Panel Analysis. Clocks & Sleep. 2024; 6(4):557-567. https://doi.org/10.3390/clockssleep6040037
Chicago/Turabian StyleCheung, Forrest Tin Wai, Hao Fong Sit, Xiao Li, Joey Wing Yan Chan, Ngan Yin Chan, Yun Kwok Wing, and Shirley Xin Li. 2024. "A Longitudinal Examination between Chronotype and Insomnia in Youths: A Cross-Lagged Panel Analysis" Clocks & Sleep 6, no. 4: 557-567. https://doi.org/10.3390/clockssleep6040037
APA StyleCheung, F. T. W., Sit, H. F., Li, X., Chan, J. W. Y., Chan, N. Y., Wing, Y. K., & Li, S. X. (2024). A Longitudinal Examination between Chronotype and Insomnia in Youths: A Cross-Lagged Panel Analysis. Clocks & Sleep, 6(4), 557-567. https://doi.org/10.3390/clockssleep6040037






