Abstract
Given the limitations of available studies, the objective of this study was to explore the role played by current and remitted major depression in the occurrence of comorbid insomnia disorder for apneic patients. Data from 1488 apneic patients were extracted from the medical reports of polysomnographic recordings available in the database of the Sleep Laboratory. The presence of comorbid insomnia disorder in these apneic patients was defined based on the diagnostic criteria of the American Academy of Sleep Medicine Work Group. The risk of comorbid insomnia disorder associated with current or remitted major depression in apneic patients was investigated using multivariate logistic regression models. After adjustment for the main confounding factors, multivariate logistic regression analyses revealed that remitted and current major depression were significantly associated with the occurrence of comorbid insomnia disorder in apneic patients. The findings of this study seem to indicate that comorbid insomnia disorder could be a residual symptom and a marker of major depression in apneic patients, which justifies the establishment of an adequate treatment for major depressive episodes and their potential residual symptoms to allow the better management of comorbid insomnia disorder and the better prevention of its potential negative consequences in this particular subpopulation.
1. Introduction
In the literature, there are arguments in favor of a particular relationship between obstructive sleep apnea syndrome (OSAS) and insomnia disorder. Indeed, insomnia disorder is a frequent comorbidity (43.0%) in apneic patients, whereas the prevalence of OSAS is estimated at 34.5% among insomniac patients [1,2]. Furthermore, in apneic patients, the occurrence of comorbid insomnia disorder seems to favor the development of cardiovascular diseases since apneic patients with comorbid insomnia disorder have a higher risk of cardiovascular disease than those without comorbid insomnia [3,4,5]. In addition, the presence of comorbid insomnia disorder appears to be associated with a negative impact on mental health, life quality and professional performance in apneic patients [6]. Concerning the therapeutic aspect, it has been shown that apneic patients with comorbid insomnia disorder are less adherent and compliant to OSA treatments than those without comorbid insomnia disorder [7,8]. Thus, given these data, it seems essential to identify the potential factors favoring the occurrence of comorbid insomnia disorder in apneic patients in order to allow the better management of this sleep disorder in this particular subpopulation.
Based on the available studies, some evidence seems to indicate the existence of a bidirectional relationship between major depression and insomnia disorder. Indeed, insomnia complaints are very frequent among major depressed patients and the prevalence of depressive symptoms is high among insomniac patients [9,10]. In addition, insomnia disorder is associated with an increased risk of developing a major depressive episode, whereas major depression may lead to the occurrence of insomnia complaints [11,12]. Furthermore, in major depressed patients in remission, insomnia complaints are a frequent residual symptom that may contribute to the occurrence of depressive relapse [13,14]. However, despite the high prevalence of major depression in apneic patients [15], few studies have currently investigated the role played by this psychiatric disorder in the occurrence of comorbid insomnia disorder for this particular subpopulation [16,17,18,19,20]. In addition, most of these studies have mainly investigated the impact of depressive symptoms, measured by a self-questionnaire or a self-reported diagnosis of depression, on the occurrence of insomnia complaints in apneic patients, which may limit the interpretation of their results [16,17,18,19,20]. In this context, it could therefore be interesting to study the risk of comorbid insomnia disorder associated with remitted and current major depression (diagnosed during a systematic psychiatric interview) in apneic patients to allow the better identification of individuals at risk of insomnia complaints in this particular subpopulation.
Given these different previous data available in the literature, the hypothesis of this study was that remitted and current major depression are associated with an increased risk of comorbid insomnia disorder in apneic patients, which could indicate that this sleep disorder is a residual symptom and a marker of major depression in this particular subpopulation. To confirm this hypothesis, the risk of comorbid insomnia disorder associated with remitted and current major depression was investigated in a large sample of apneic patients. The goal of this approach is to allow health professionals to obtain reliable data regarding the risk of comorbid insomnia disorder associated with remitted and current major depression in apneic patients in order to allow the better prevention of the multiple negative consequences associated with the occurrence of this sleep disorder in this particular subpopulation.
2. Results
2.1. Polysomnographic Data (Table 1)

Table 1.
Polysomnographic data (n = 1488).
Apneic patients with comorbid insomnia disorder had a longer sleep latency than those without comorbid insomnia disorder. In addition, the sleep efficiency, sleep period time, micro-arousal index, apnea–hypopnea index, oxygen desaturation index and total time under 90% of SaO2 were lower in apneic patients with comorbid insomnia disorder than in those without comorbid insomnia disorder. The two groups of apneic patients did not differ significantly for other polysomnographic parameters.
2.2. Demographic Data (Table 2)

Table 2.
Univariate analyses (n = 1488).
Insomnia disorder was a frequent comorbidity (40.9%) in our sample of apneic patients. Sex, age, body mass index, antidepressant therapy, benzodiazepine receptor agonists, cardiometabolic comorbidities, OSAS severity, sleep duration, excessive daytime sleepiness and the major depression status were significantly associated with the occurrence of comorbid insomnia disorder in apneic patients. Furthermore, compared to those without comorbid insomnia disorder, apneic patients with comorbid insomnia disorder were younger in age and had higher scores on the Beck Depression Inventory/Insomnia Severity Index/Epworth Sleepiness Scale. The two groups of apneic patients did not differ significantly for other demographic parameters. Finally, the prevalence of remitted and current major depression was 16.6% and 23.9%, respectively, in our sample of apneic patients.
2.3. Multivariate Analyses (Table 3)

Table 3.
Multivariate analyses (n = 1488).
After adjustment via the hierarchical introduction of the main confounding factors identified during the univariate analyses, the multivariate logistic regression analyses revealed that remitted and current major depression were significantly associated with the occurrence of comorbid insomnia disorder in apneic patients.
3. Discussion
Given the high prevalence of comorbid insomnia disorder (40.9%) in our sample of apneic patients, the results of our study seem to confirm that this sleep disorder is a frequent comorbidity in this particular subpopulation [3]. However, this prevalence is higher than that in the studies by Stelzer et al. (2021) (29.0%) and Cho et al. (2018) (29.2%), which could be explained by the recruitment of more severe apneic patients in these studies than in our study [17,21]. Indeed, in the literature, it has been shown that insomnia complaints tend to decrease with OSAS severity [22,23], which may have led to an underestimation of the prevalence of comorbid insomnia disorder in these two studies. Furthermore, the prevalence of comorbid insomnia disorder highlighted in our study is lower than that of the studies by Wallace et al. (2019) (74%) and Hagen et al. (2009) (61%), which could be explained by the fact that unlike our study where the comorbid insomnia disorder was diagnosed based on the American Academy of Sleep Medicine Work Group diagnostic criteria, insomnia complaints were investigated in these two studies using self-questionnaires [18,20]. However, it has been shown that the use of self-questionnaires for the diagnosis of insomnia disorder may promote the overdiagnosis of this sleep disorder [24], which could provide a better understanding of this higher prevalence of insomnia complaints in these two latter studies. Finally, the prevalence of comorbid insomnia disorder demonstrated in our study is similar to that of the meta-analysis by Zhang et al. (2019) (38.0%), which seems to confirm that despite the methodological differences between the available studies, apneic patients are a subpopulation at high risk of comorbid insomnia disorder [25]. Thus, in this context, it seems essential to systematically search for the presence of comorbid insomnia disorder in apneic patients given the potential negative consequences associated with this sleep disorder in this particular subpopulation.
Similar to the available literature [3,15,26], we demonstrated that remitted and current major depression are frequent comorbidities in apneic patients since their prevalence was, respectively, 16.6% and 23.9% in our sample, which confirms that the occurrence of this psychiatric disorder is a significant problem in this particular subpopulation. In addition, we have shown that remitted and current major depression are significantly associated with the occurrence of comorbid insomnia disorder in apneic patients. However, this high prevalence of remitted and current major depression and their potential implication in the occurrence of comorbid insomnia disorder for apneic patients may potentially be explained by several pathophysiological elements. First, excessive sleep fragmentation and intermittent hypoxia related to OSAS may induce biological alterations (modification of cerebral monoaminergic neurotransmission, activation of pro-inflammatory mechanisms and alteration of some cerebral structures [hippocampus and frontal lobes]) and promote the occurrence of complaints of excessive daytime sleepiness in apneic patients [27,28,29]. However, there are numerous arguments in favor of the central involvement of these biological alterations and complaints of excessive daytime sleepiness in the pathophysiology of major depression, which could provide a better understanding of the high prevalence of this psychiatric disorder highlighted in our sample of apneic patients [27,28,29]. Second, in patients with current major depression, one of the theories currently proposed to explain the frequent occurrence of insomnia disorder is the phenomenon of hyperarousal that may be divided into three highly interrelated categories: somatic, cortical and cognitive hyperarousal [30,31,32,33]. The presence of hyperarousal in patients with current major depression is characterized by the existence of a state of hypervigilance present throughout the 24-hour cycle, favoring the occurrence of complaints of insomnia (difficulty falling asleep, nocturnal awakenings and early morning awakenings) [30,31,32,33]. The occurrence of hyperarousal related to current major depression could be one of the main pathophysiological mechanisms related to the increased risk of comorbid insomnia disorder associated with this psychiatric disorder in the apneic patients in our sample. Third, in patients with remitted major depression, the phenomenon of hyperarousal may persist despite the remission of the main depressive symptoms [34,35,36]. However, this persistence of the phenomenon of hyperarousal in patients with remitted major depression may be manifested by the existence of a residual insomnia disorder favoring the occurrence of depressive relapses [34,35,36], which could help to better understand the increased risk of comorbid insomnia disorder associated with remitted major depression in our sample of apneic patients. Thus, these different elements seem to indicate that comorbid insomnia disorder could be a residual symptom and a marker of major depression in apneic patients, which seems to justify systematic screening for this psychiatric disorder in patients with OSAS and comorbid insomnia disorder.
The demonstration of this increased risk of comorbid insomnia disorder associated with current or remitted major depression in apneic patients could open up new therapeutic perspectives for the management of this sleep disorder in this particular subpopulation. Indeed, given that insomnia complaints are one of the symptoms frequently present during major depressive episodes [37,38], it is important to start an appropriate antidepressant treatment in apneic patients with current major depression in order to target the complete remission of the affective, cognitive and neurovegetative symptoms of this psychiatric disorder [39,40]. However, even in the case of clinical remission, the optimization of this antidepressant treatment may be necessary in some apneic patients with remitted major depression to avoid the persistence of residual insomnia complaints [39,40]. Regarding psychotherapeutic treatments that could be used alone or in combination with antidepressant treatment in apneic patients with current or remitted major depression, cognitive–behavioral therapy for insomnia seems to be a promising option given its positive results for both depressive symptoms and insomnia complaints [41]. Indeed, it has been demonstrated that cognitive behavioral therapy for insomnia may be used to enhance the effectiveness of antidepressant treatments in patients with current major depression and treat residual insomnia complaints in patients with remitted major depression [13,42]. Furthermore, alongside this specific management of major depression in apneic patients, it is essential to adequately treat OSAS in order to reinforce the improvement of depressive symptoms and to avoid the persistence of pathophysiological mechanisms related to obstructive respiratory events that may promote the maintenance of insomnia complaints [43,44]. Thus, in patients with OSAS and major depression, the establishment of an adequate combined treatment of these two pathologies could allow the better management of comorbid insomnia disorder and the better prevention of its potential negative consequences.
Finally, although there is evidence showing that more frequent alcohol consumption and smoking are associated with insomniac patients in the literature [45], there were no significant differences in these consumptions between apneic patients with and without insomnia disorder in this study. This difference from the literature could be explained by the fact that all subjects included in this study were apneic patients with sleep complaints justifying polysomnographic recording regardless of their insomnia complaints. This recruitment, limited only to apneic patients, may potentially have masked the impact of insomnia disorder on alcohol consumption and smoking in this study, since apneic patients are already a subpopulation at a higher risk of alcohol consumption and smoking following their sleep complaints [46,47].
Limitations and Strengths
Given that the collection of data used was carried out retrospectively without direct verification from the apneic patients included in this study, the performance of additional prospective studies is essential to confirm our findings. Additionally, since only patients with OSAS were recruited for this study, our results cannot be extrapolated to patients with other sleep-related breathing disorders. Furthermore, given that we focused on the potential role played by major depression in the occurrence of comorbid insomnia disorder among apneic patients, the findings of this study cannot be generalized to other psychiatric disorders. Moreover, only apneic patients who have agreed to stay at the Sleep Laboratory for a polysomnography recording are present in the database of the Brussels University Hospital, which may be a limitation regarding the generalization of our results. Finally, despite its limitations, our study is one of the first to investigate the impact of remitted and current major depression (diagnosed during a systematic psychiatric interview) in a large sample of apneic patients, which adds real value compared to the available literature.
4. Materials and Methods
4.1. Population
Data from 1488 apneic patients who stayed at the Sleep Laboratory between 1 January 2002 and 31 December 2020 were extracted from the medical reports of polysomnographic recordings available in the database of the Brussels University Hospital (Figure 1). The criteria applied for the selection of these apneic patients are detailed in Table 4 [48]. Furthermore, we decided to focus only on apneic patients for this study, given the potential negative consequences associated with the occurrence of comorbid insomnia disorder in this particular subpopulation [3,4,5,6,7,8]. Finally, the description of the outpatient care journey for these apneic patients, from the specialized consultation for sleep medicine to their admission to the Sleep Laboratory, is detailed in the Supplementary Data—Annex 1 [26].
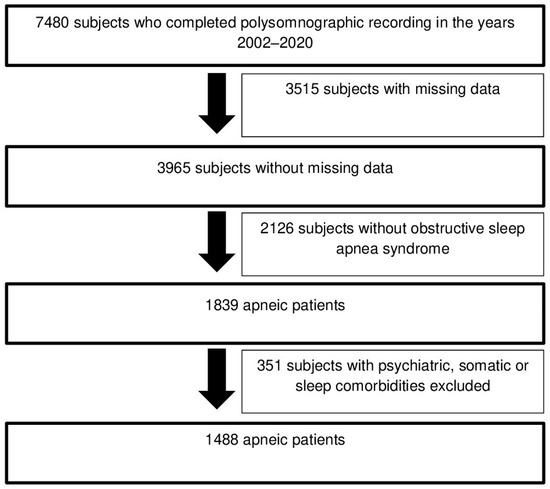
Figure 1.
Selection diagram of apneic patients included in this study.

Table 4.
Selection criteria.
4.2. Method
4.2.1. Medical and Psychiatric Assessment
During their hospitalization for polysomnographic recording, all these apneic patients benefited from a standardized somatic check-up specific to the Sleep Laboratory of the Brussels University Hospital (review of medical records, clinical interview, physical examination and complementary tests [blood test, electrocardiogram, daytime electroencephalogram and urine analyzes]) in order to systematically diagnose their potential medical comorbidities.
Subsequently, a systematic psychiatric interview based on the diagnostic criteria of DSM-IV-TR (before 2013) and DSM 5 (after 2013) was carried out by a psychiatrist assigned to the Sleep Laboratory for all these apneic patients to identify their potential past or current psychiatric comorbidities [37,38]. Thus, following this systematic psychiatric interview, the status of potential major depressive episodes (remitted or current) was determined according to the following criteria:
- The absence of significant symptoms or signs of major depression during a period of at least 2 months before hospitalization for polysomnographic recording was used to define remitted major depressive episodes [37,38].
- The presence of significant symptoms or signs of major depression during a period of at least 2 weeks (DSM 5) or at least 4 weeks (DSM-IV-TR) before hospitalization for polysomnographic recording was used to define current major depressive episodes [37,38].
Finally, after these somatic and psychiatric assessments, all these apneic patients completed a series of questionnaires to determine the severity of their self-reported complaints of depression, insomnia and daytime sleepiness.
- The Beck Depression Inventory (reduced to 13 items) was used to investigate the presence of depressive symptoms. The 13 items of this scale may be scored from 0 to 3, which means that the total score may vary from 0 to 39. A final score of 0–4 indicates an absence of depressive symptoms, 5–7 indicates mild depressive symptoms, 8–15 indicates moderate depressive symptoms, and ≥16 indicates severe depressive symptoms [49]. The internal consistency reliability measure showed a Cronbach α coefficient of 0.90 for the French version of The Beck Depression Inventory (reduced to 13 items) [50].
- The Insomnia Severity Index was used to investigate the severity of insomnia complaints. The 7 items of this index may be scored from 0 to 4, which means that the total score may vary from 0 to 28. A final score of 0–7 indicates an absence of insomnia complaints, 8–14 indicates subclinical insomnia complaints, 15–21 indicates moderate insomnia complaints, and 22–28 indicates severe insomnia complaints [51]. The internal consistency reliability measure showed a Cronbach α coefficient of 0.92 for the French version of the Insomnia Severity Index [52].
- The Epworth Sleepiness Scale was used to investigate daytime sleepiness. The 8 items of this scale assessing sleepiness in different daytime situations may be scored from 0 to 3, which means that the total score may vary from 0 to 24. A final score greater than 10 indicates excessive daytime sleepiness [53]. The internal consistency reliability measure showed a Cronbach α coefficient of 0.88 for the French version of the Epworth Sleepiness Scale [54].
4.2.2. Sleep Evaluation and Study
In all these apneic patients, a systematic interview investigating their sleep habits and their sleep-related complaints was conducted by a psychiatrist specializing in sleep medicine during their hospitalization at the Sleep Laboratory in order to highlight the presence of potential signs suggestive of the main sleep disorders.
Subsequently, to complete this interview focused on sleep, a polysomnographic recording meeting the criteria of the American Academy of Sleep Medicine was carried out in all these apneic patients [55]. The polysomnography instruments applied were as follows: two electro-oculogram channels, three electroencephalogram channels, one submental electromyogram channel, an electrocardiogram, a pressure cannula to detect the oro-nasal airflow, a finger pulse oximeter, a microphone to record breathing sounds and snoring, plethysmographic inductive belts to measure thoracic and abdominal breathing, and anterior tibialis electrodes. Furthermore, the conditions of hospitalization at the Sleep Laboratory for all apneic patients were as follows: (1) the patients went to bed between 22:00–24:00 and got up between 6:00–8:00 (following their usual schedule); (2) during bedtime hours, the subjects were recumbent and the lights were turned off; and (3) daytime naps were not permitted. Finally, a technical report of these polysomnographic recordings was produced by specialized technicians after visual scoring based on the criteria of the American Academy of Sleep Medicine in order to allow their clinical interpretation by physicians specializing in sleep medicine [56,57,58].
Obstructive apneas were scored if the decrease in air flow was ≥90% for at least 10 s whereas obstructive hypopneas were scored if the decrease in airflow was ≥30% for at least 10 s, with a 3% decrease in oxygen saturation or micro-arousal [57]. The obstructive apnea–hypopnea index corresponds to the total number of obstructive apneas and hypopneas divided by the period of sleep in hours [57].
Periodic limb movements were scored based on the following strict criteria: (F1) duration between 0.5 to 10 s; (2) interval between 5 and 90 s from leg movement onset; and 3) movements that were part of a series of ≥4 consecutive movements meeting these criteria [58]. The periodic limb movement index corresponds to the total number of periodic limb movements divided by the period of sleep in hours [58].
The completion of this interview focused on sleep and this polysomnographic recording therefore made it possible to confirm the OSAS diagnosis, to determine the OSAS severity (mild [apnea–hypopnea index ≥ 5/h and <15/h], moderate [apnea–hypopnea index ≥ 15/hour and <30/hour], severe [apnea–hypopnea index ≥ 30/hour]) and to systematically screen for all potential comorbid sleep disorders (insomnia disorder [American Academy of Sleep Medicine Work Group diagnostic criteria], moderate to severe periodic limb movement syndrome [periodic limb movement index ≥ 15/hour], restless legs syndrome [International Restless Legs Syndrome Study Group diagnostic criteria] and short sleep duration [<6 h]) in the apneic patients recruited for this study [59,60,61,62,63].
4.3. Statistical Analyses
In order to carry out statistical analyses using Stata 14 software, the 1488 apneic patients were categorized into a subgroup without comorbid insomnia disorder and a subgroup with comorbid insomnia disorder. The presence of comorbid insomnia disorder in these apneic patients was defined based on the diagnostic criteria of the American Academy of Sleep Medicine Work Group [60].
Since the majority of continuous data were distributed asymmetrically (histograms, boxplots and quantile–quantile plots to check the data distribution, and Levene’s test to check the equality of variances), medians with their P25-P75 were used for descriptive analyses and Wilcoxon tests were used for comparison tests. For categorical data, descriptive analyses were carried out using percentages and comparison analyses were carried out using Chi2 tests.
The risk of comorbid insomnia disorder (dependent variable) associated with a major depression status (categorized: no, remitted, current) and potential confounding factors (independent variables) was investigated using univariate logistic regression models. After a review of the literature on the risk factors for comorbid insomnia disorder in apneic patients [1,21,64,65,66], the potential confounding factors included in this study were body mass index (categorized: <25 kg/m2, ≥25 kg/m2), age (categorized: <50 years, ≥50 years), cardiometabolic comorbidities (categorized: 0, 1–2, ≥3), OSAS severity (categorized: mild, moderate, severe), sleep movement disorders (categorized: no, moderate to severe periodic limb movement syndrome, restless legs syndrome alone or combined with periodic limb movements), sleep duration (categorized: <6 h, ≥6 h), and the following binary variables: sex, antidepressant therapy, benzodiazepine receptor agonists, alcohol consumption, smoking and excessive daytime sleepiness. Subsequently, following a hierarchical introduction of the significant confounding factors identified during the univariate analyses, this risk of comorbid insomnia disorder associated with major depression status was adjusted using multivariate logistic regression models.
For the final multivariate logistic regression model, the adequacy was verified by the Hosmer and Lemeshow test, whereas the specificity was verified by the Link test. Additionally, the Wald test and the Nagelkerke R-square were used as additional fit criteria.
Following the conditions of use of multivariate logistic regression analyses (number of subjects per predictor > 10) [67,68], each of the two groups of apneic patients for this study had to contain at least 130 subjects (10 subjects * 13 potential predictors) to ensure the validity of the analyses performed, which was largely achieved in this study.
The results were considered significant when the p-value was <0.05.
5. Conclusions
Comorbid insomnia disorder was present in 40.9% of apneic patients from our sample, which seems to confirm that this sleep disorder is a frequent comorbidity in this particular subpopulation. In addition, we demonstrated that current and remitted major depression were significantly associated with the occurrence of comorbid insomnia disorder in apneic patients, which seems to indicate that this sleep disorder could be a residual symptom and a marker of major depression in this specific subgroup of patients. Moreover, given the results of this study, the establishment of an adequate treatment for major depressive episodes and their potential residual symptoms seems to be essential for apneic patients to allow the better management of comorbid insomnia disorder and the better prevention of its potential negative consequences in this particular subpopulation. Finally, given the retrospective design of our study, it seems necessary to carry out additional prospective studies to confirm this potential role of major depression in the occurrence of comorbid insomnia disorder in apneic patients thanks to a better level of scientific evidence.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/clockssleep6030026/s1, Annex 1: Description of the outpatient care journey for apneic patients from the consultation specialized in sleep medicine to their admission to the Sleep Laboratory.
Author Contributions
Conceptualization: M.H. and C.P.; Methodology: M.H., B.W., M.C., J.-P.L. and C.P.; Formal Analysis: M.H., M.C. and C.P.; Investigation: M.H. and C.P.; Software: B.W. and J.-P.L.; Data Curation: M.H., B.W., M.C., J.-P.L. and C.P.; Writing–Original Draft Preparation: M.H., B.W., M.C., J.-P.L. and C.P.; Supervision: C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any funding.
Institutional Review Board Statement
This study was approved by the Hospital and Medical School Ethics Committee of the Erasme Hospital (Brussels University Hospital) (reference: P2023/603-approval date: 21 February 2024) in compliance with the recommendations of the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author (the data are not publicly available due to privacy restrictions).
Acknowledgments
I thank Christelle Bouchart for their support, as well as the sleep laboratory team from the Brussels University Hospital for their technical support.
Conflicts of Interest
The authors of this study have no conflicts of interest to report.
References
- Vozoris, N.T. Sleep apnea-plus: Prevalence, risk factors, and association with cardiovascular diseases using United States population-level data. Sleep Med. 2012, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of excessive daytime sleepiness in insomnia sufferers: A study with 1311 individuals. J. Psychosom. Res. 2017, 103, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Wacquier, B.; Lanquart, J.P.; Loas, G. Risk of Cardiovascular Disease in Apnoeic Individuals: Role of Comorbid Insomnia Disorder. Life 2022, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Mungo, A.; Loas, G. Cardiovascular risk associated with co-morbid insomnia and sleep apnoea (COMISA) in type 2 diabetics. Sleep Sci. 2022, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Draelants, L.; Point, C.; Wacquier, B.; Lanquart, J.P.; Loas, G.; Hein, M. 10-Year Risk for Cardiovascular Disease Associated with COMISA (Co-Morbid Insomnia and Sleep Apnea) in Hypertensive Subjects. Life 2023, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.; Crawford, M.R.; Wallace, D.M. Sleep Apnea and Insomnia: Emerging Evidence for Effective Clinical Management. Chest 2021, 159, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.M.; Sawyer, A.M.; Shafazand, S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018, 22, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pieh, C.; Bach, M.; Popp, R.; Jara, C.; Crönlein, T.; Hajak, G.; Geisler, P. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013, 17, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of excessive daytime sleepiness in major depression: A study with 703 individuals referred for polysomnography. J. Affect. Disord. 2019, 243, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dosogne, M.; Wacquier, B.; Al Faker, M.; Willame, H.; Point, C.; Loas, G.; Hein, M. Risk of current suicidal ideations associated with lifelong anhedonia and recent change of anhedonia in individuals with insomnia: A cross-sectional study. J. Psychiatr. Res. 2022, 150, 338–345. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Gan, Y.; Qu, X.; Lu, Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016, 16, 375. [Google Scholar] [CrossRef]
- Bao, Y.P.; Han, Y.; Ma, J.; Wang, R.J.; Shi, L.; Wang, T.Y.; He, J.; Yue, J.L.; Shi, J.; Tang, X.D.; et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: Meta-analysis and systematic review. Neurosci. Biobehav. Rev. 2017, 75, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Kwaśny, A.; Włodarczyk, A.; Dywel, A.; Szarmach, J.; Strandberg, O.; Cubała, W.J. Residual insomnia in major depressive disorder: A systematic review. Front. Psychiatry 2023, 14, 1190415. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Suzuki, T.; Yoshimura, K.; Mimura, M.; Uchida, H. Predicting relapse with individual residual symptoms in major depressive disorder: A reanalysis of the STAR*D data. Psychopharmacology 2017, 234, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Tolson, J.; Bartlett, D.; Berlowitz, D.J.; Varma, P.; Barnes, M. Clinical depression in untreated obstructive sleep apnea: Examining predictors and a meta-analysis of prevalence rates. Sleep Med. 2019, 62, 22–28. [Google Scholar] [CrossRef]
- Sweetman, A.; Melaku, Y.A.; Lack, L.; Reynolds, A.; Gill, T.K.; Adams, R.; Appleton, S. Prevalence and associations of co-morbid insomnia and sleep apnoea in an Australian population-based sample. Sleep Med. 2021, 82, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, F.G.; Garcia, E.; Schorr, F.; Barea, L.M.; Barros, H.T. Prevalence of chronic insomnia in patients with obstructive sleep apnea. Braz. J. Psychiatry 2021, 43, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.M.; Wohlgemuth, W.K. Predictors of Insomnia Severity Index Profiles in United States Veterans with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.J.; Appleton, S.L.; Vakulin, A.; McEvoy, R.D.; Wittert, G.A.; Martin, S.A.; Catcheside, P.G.; Antic, N.A.; Lack, L.; Adams, R.J. Co-morbid OSA and insomnia increases depression prevalence and severity in men. Respirology 2017, 22, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Patel, A.; McCall, W.V. Prevalence of insomnia symptoms in sleep laboratory patients with and without sleep apnea. Psychiatry Res. 2009, 170, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Kim, K.T.; Moon, H.J.; Korostyshevskiy, V.R.; Motamedi, G.K.; Yang, K.I. Comorbid Insomnia with Obstructive Sleep Apnea: Clinical Characteristics and Risk Factors. J. Clin. Sleep Med. 2018, 14, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, B.L.; Hagen, K.; Engstrøm, M.; Stjern, M.; Gravdahl, G.B.; Sand, T. The relationship between obstructive sleep apnea and insomnia: A population-based cross-sectional polysomnographic study. Sleep Med. 2019, 54, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of moderate to severe obstructive sleep apnea syndrome in insomnia sufferers: A study on 1311 subjects. Respir. Res. 2017, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Chang, L.Y.; Hsieh, Y.J.; Tsai, P.S. A meta-analysis of diagnostic accuracy of three screening tools for insomnia. J. Psychosom. Res. 2016, 87, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Lei, F.; Zhou, J.; Zhang, J.; Wing, Y.K.; Sanford, L.D.; Tang, X. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Al Faker, M.; Wacquier, B.; Willame, H.; Point, C.; Dosogne, M.; Loas, G.; Hein, M. The association between type 2 diabetes and major depression in apnoeic individuals. Sleep Biol. Rhythm. 2022, 20, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.M.; O’Hara, R. Depression and Obstructive Sleep Apnea (OSA). Ann. Gen. Psychiatry 2005, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Glozier, N.; Ratnavadivel, R.; Grunstein, R.R. Obstructive sleep apnea and depression. Sleep Med. Rev. 2009, 13, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innov. Clin. Neurosci. 2011, 8, 17–25. [Google Scholar] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Contribution to the study of physiology and pathophysiology of sleep in healthy individual and patients suffering from major depression and primary insomnia. Rev. Med. Brux. 2020, 41, 137–142. [Google Scholar] [CrossRef]
- Hein, M.; Senterre, C.; Lanquart, J.P.; Montana, X.; Loas, G.; Linkowski, P.; Hubain, P. Hyperarousal during sleep in untreated primary insomnia sufferers: A polysomnographic study. Psychiatry Res. 2017, 253, 71–78. [Google Scholar] [CrossRef]
- Hein, M.; Senterre, C.; Lanquart, J.P.; Montana, X.; Loas, G.; Linkowski, P.; Hubain, P. Hyperarousal during sleep in untreated, major depressed subjects with prodromal insomnia: A polysomnographic study. Psychiatry Res. 2017, 258, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Similar polysomnographic pattern in primary insomnia and major depression with objective insomnia: A sign of common pathophysiology? BMC Psychiatry 2017, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Dombrovski, A.Y.; Cyranowski, J.M.; Mulsant, B.H.; Houck, P.R.; Buysse, D.J.; Andreescu, C.; Thase, M.E.; Mallinger, A.G.; Frank, E. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress. Anxiety 2008, 25, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Meaklim, H.; Saunders, W.J.; Byrne, M.L.; Junge, M.F.; Varma, P.; Finck, W.A.; Jackson, M.L. Insomnia is a key risk factor for persistent anxiety and depressive symptoms: A 12-month longitudinal cohort study during the COVID-19 pandemic. J. Affect. Disord. 2023, 322, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Manber, R.; Chambers, A.S. Insomnia and depression: A multifaceted interplay. Curr. Psychiatry Rep. 2009, 11, 437–442. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; text rev; American Psychiatric Publishing: Arlington, VA, USA, 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Heck, T.; Zolezzi, M. Obstructive sleep apnea: Management considerations in psychiatric patients. Neuropsychiatr. Dis. Treat. 2015, 11, 2691–2698. [Google Scholar] [PubMed]
- Benca, R.M.; Krystal, A.; Chepke, C.; Doghramji, K. Recognition and Management of Obstructive Sleep Apnea in Psychiatric Practice. J. Clin. Psychiatry 2023, 84, 22r14521. [Google Scholar] [CrossRef]
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Züst, M.A.; Fehér, K.D.; Su, T.; Straten, A.V.; Berger, T.; Baglioni, C.; et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef] [PubMed]
- Manber, R.; Buysse, D.J.; Edinger, J.; Krystal, A.; Luther, J.F.; Wisniewski, S.R.; Trockel, M.; Kraemer, H.C.; Thase, M.E. Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined with Antidepressant Pharmacotherapy in Patients with Comorbid Depression and Insomnia: A Randomized Controlled Trial. J. Clin. Psychiatry 2016, 77, e1316–e1323. [Google Scholar] [CrossRef]
- Fu, W.; Li, L.; Zhang, S.; Liu, S.; Liu, W. Effects of CPAP and Mandibular Advancement Devices on depressive symptoms in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. Sleep Breath. 2023, 27, 2123–2137. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.; Lack, L.; McEvoy, R.D.; Smith, S.; Eckert, D.J.; Osman, A.; Carberry, J.C.; Wallace, D.; Nguyen, P.D.; Catcheside, P. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med. Rev. 2021, 60, 101519. [Google Scholar] [CrossRef] [PubMed]
- Skarupke, C.; Schlack, R.; Lange, K.; Goerke, M.; Dueck, A.; Thome, J.; Szagun, B.; Cohrs, S. Insomnia complaints and substance use in German adolescents: Did we underestimate the role of coffee consumption? Results of the KiGGS study. J. Neural Transm. 2017, 124 (Suppl. S1), 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of moderate to severe obstructive sleep apnea syndrome in major depression: A observational and retrospective study on 703 subjects. BMC Pulm. Med. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Dixon-Williams, S.; Thornton, J.D. Where there is smoke…there is sleep apnea: Exploring the relationship between smoking and sleep apnea. Chest 2014, 146, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Bourque, P.; Beaudette, D. Psychometric study of the Beck Depression Inventory on a sample of French-speaking university students. Can. J. Behav. Sci.-Rev. Can. Sci. Comport. 1982, 14, 211–218. [Google Scholar]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993. [Google Scholar]
- Gagnon, C.; Bélanger, L.; Ivers, H.; Morin, C.M. Validation of the Insomnia Severity Index in Primary Care. J. Am. Board Fam. Med. 2013, 26, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, M.; Jobin, V.; Mayer, P.; Amyot, R.; Perraton-Brillon, M.; Bellemare, F. The Epworth Sleepiness Scale: Self-Administration Versus Administration by the Physician, and Validation of a French Version. Can. Respir. J. 2010, 17, e27–e34. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Koo, B.B.; Picchietti, D.L.; Fulda, S. Periodic leg movements during sleep: Phenotype, neurophysiology, and clinical significance. Sleep Med. 2017, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fleetham, J.; Ayas, N.; Bradley, D.; Ferguson, K.; Fitzpatrick, M.; George, C.; Hanly, P.; Hill, F.; Kimoff, J.; Kryger, M.; et al. Canadian Thoracic Society guidelines: Diagnosis and treatment of sleep disordered breathing in adults. Can. Respir. J. 2006, 13, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Jamieson, A.O.; McCall, W.V.; Morin, C.M.; Stepanski, E.J. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef] [PubMed]
- Haba-Rubio, J.; Marti-Soler, H.; Tobback, N.; Andries, D.; Marques-Vidal, P.; Vollenweider, P.; Preisig, M.; Heinzer, R. Clinical significance of periodic limb movements during sleep: The HypnoLaus study. Sleep Med. 2018, 41, 45–50. [Google Scholar] [CrossRef]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B.; et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–History, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Insomnia with short sleep duration as risk factor for type 2 diabetes: A systematic review of the literature. Rev. Med. Brux. 2020, 41, 98–104. [Google Scholar] [CrossRef]
- Krell, S.B.; Kapur, V.K. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, C.; Liao, Y.; Dai, Q.; Cao, S. Smoking and incidence of insomnia: A systematic review and meta-analysis of cohort studies. Public Health 2021, 198, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Buysse, D.J.; Strollo, P.J., Jr. Comorbid insomnia and obstructive sleep apnea: Challenges for clinical practice and research. J. Clin. Sleep Med. 2010, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).