Time of Day and Sleep Deprivation Effects on Risky Decision Making
Abstract
1. Introduction
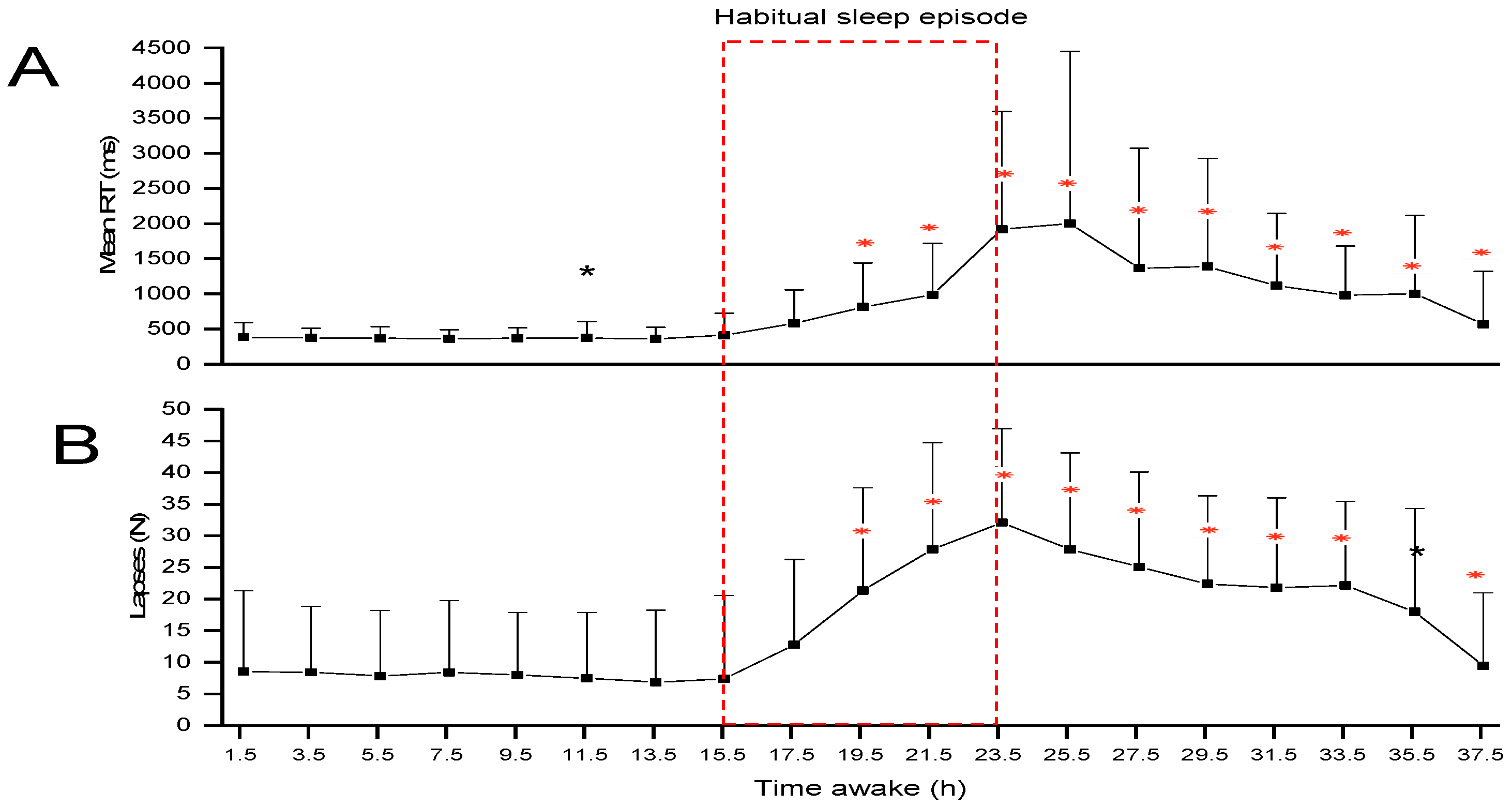
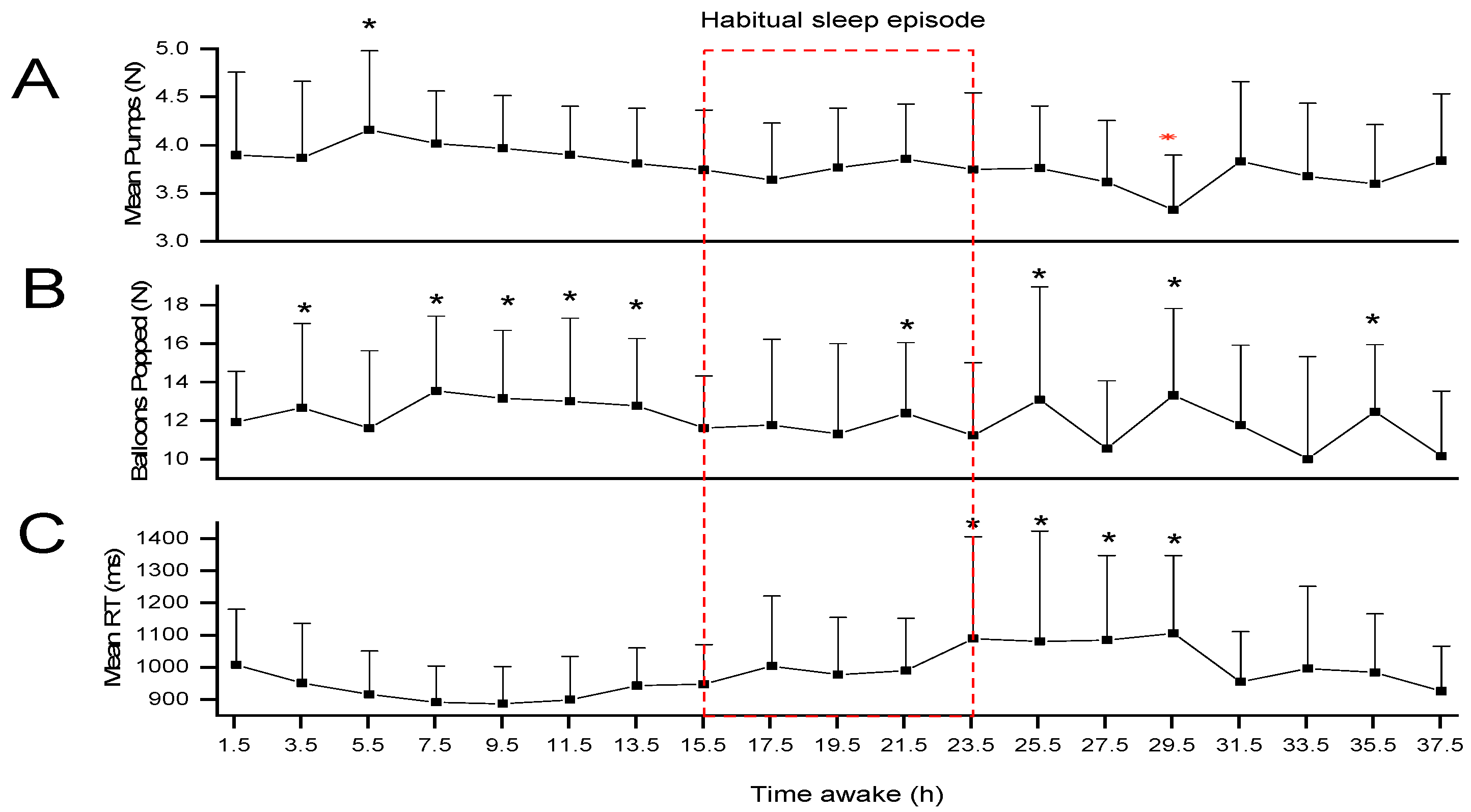
2. Results
Additional Analysis
3. Discussion
4. Materials and Methods
4.1. Participant Recruitment and Eligibility Criteria
4.2. Study Protocol
4.3. Test Battery
4.3.1. Psychomotor Vigilance Task (PVT)
4.3.2. Balloon Analogue Risk Task (BART)
4.3.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, M.G. Circadian regulation of synaptic plasticity. Biology 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G. Clocking in: A circadian model of synaptic plasticity. Curr. Opin. Physiol. 2020, 15, 96–103. [Google Scholar] [CrossRef]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar] [PubMed]
- Choshen-Hillel, S.; Ishqer, A.; Mahameed, F.; Reiter, J.; Gozal, D.; Gileles-Hillel, A.; Berger, I. Acute and chronic sleep deprivation in residents: Cognition and stress biomarkers. Med. Educ. 2021, 55, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Groeger, J.A.; Viola, A.U.; Lo, J.C.; von Schantz, M.; Archer, S.N.; Dijk, D.J. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep 2008, 31, 1159–1167. [Google Scholar] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.N.; Van Dongen, H.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992, 1, 112–117. [Google Scholar] [CrossRef]
- Stojanoski, B.; Benoit, A.; Van Den Berg, N.; Ray, L.B.; Owen, A.M.; Shahidi Zandi, A.; Quddus, A.; Comeau, F.J.E.; Fogel, S.M. Sustained vigilance is negatively affected by mild and acute sleep loss reflected by reduced capacity for decision making, motor preparation, and execution. Sleep 2019, 42, zsy200. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Morris, C.J.; Scheer, F.A. Daily circadian misalignment impairs human cognitive performance task-dependently. Sci. Rep. 2018, 8, 3041. [Google Scholar] [CrossRef] [PubMed]
- Blatter, K.; Cajochen, C. Circadian rhythms in cognitive performance: Methodological constraints, protocols, theoretical underpinnings. Physiol. Behav. 2007, 90, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Collette, F.; Cajochen, C.; Peigneux, P. A time to think: Circadian rhythms in human cognition. Cogn. Neuropsychol. 2007, 24, 755–789. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Y.; Horne, J.A. The impact of sleep deprivation on decision making: A review. Exp. Psychol. Appl. 2000, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Harrison, Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med. Rev. 2001, 5, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Y.; Horne, J. Sleep loss impairs short and novel language tasks having a prefrontal focus. J. Sleep Res. 1998, 7, 95–100. [Google Scholar] [CrossRef] [PubMed]
- McMahon, W.R.; Ftouni, S.; Diep, C.; Collet, J.; Lockley, S.W.; Rajaratnam, S.M.W.; Maruff, P.; Drummond, S.P.A.; Anderson, C. The impact of the wake maintenance zone on attentional capacity, physiological drowsiness, and subjective task demands during sleep deprivation. J. Sleep Res. 2021, 30, e13312. [Google Scholar] [CrossRef] [PubMed]
- May, C.P.; Hasher, L. Synchrony effects in inhibitory control over thought and action. J. Exp. Psychol. Hum. Percept. Perform. 1998, 24, 363. [Google Scholar] [CrossRef]
- Wimmer, R.; Hoffman, R.P.; Bonato, R.A.; Moffitt, A.R. The effects of sleep deprivation on divergent thinking and attention processes. J. Sleep Res. 1992, 1, 223–230. [Google Scholar] [CrossRef]
- Correa, A.; Alguacil, S.; Ciria, L.F.; Jiménez, A.; Ruz, M. Circadian rhythms and decision-making: A review and new evidence from electroencephalography. Chronobiol. Int. 2020, 37, 520–541. [Google Scholar] [CrossRef]
- Byrne, J.E.; Murray, G. Diurnal rhythms in psychological reward functioning in healthy young men: ‘Wanting’, liking, and learning. Chronobiol. Int. 2017, 34, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mai, Z.; Yang, J.; Zhang, B.; Ma, N. Ideal time of day for risky decision making: Evidence from the Balloon Analogue Risk Task. Nat. Sci. Sleep. 2020, 12, 477–486. [Google Scholar] [CrossRef]
- Correa, A.; Ruiz-Herrera, N.; Ruz, M.; Tonetti, L.; Martoni, M.; Fabbri, M.; Natale, V. Economic decision-making in morning/evening-type people as a function of time of day. Chronobiol. Int. 2017, 34, 139–147. [Google Scholar] [CrossRef]
- Ingram, K.K.; Ay, A.; Kwon, S.B.; Woods, K.; Escobar, S.; Gordon, M.; Smith, I.H.; Bearden, N.; Filipowicz, A.; Jain, K. Molecular insights into chronotype and time-of-day effects on decision-making. Sci. Rep. 2016, 6, 29392. [Google Scholar] [CrossRef] [PubMed]
- Maric, A.; Montvai, E.; Werth, E.; Storz, M.; Leemann, J.; Weissengruber, S.; Ruff, C.C.; Huber, R.; Poryazova, R.; Baumann, C.R. Insufficient sleep: Enhanced risk-seeking relates to low local sleep intensity. Ann. Neurol. 2017, 82, 409–418. [Google Scholar] [CrossRef]
- Killgore, W.D.; Balkin, T.J.; Wesensten, N.J. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 2006, 15, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Chuah, Y.L.; Huettel, S.A.; Chee, M.W. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 2007, 30, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Huettel, S.A.; Chuah, L.Y.; Payne, J.W.; Chee, M.W. Sleep deprivation biases the neural mechanisms underlying economic preferences. J. Neurosci. 2011, 31, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- Linde, L.; Edland, A.; Bergström, M. Auditory attention and multiattribute decision-making during a 33h sleep-deprivation period: Mean performance and between-subject dispersions. Ergonomics 1999, 42, 696–713. [Google Scholar] [CrossRef]
- Dorrian, J.; Rogers, N.L.; Dinges, D.F. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In Sleep Deprivation; CRC Press: Boca Raton, FL, USA, 2004; pp. 39–70. [Google Scholar]
- Graw, P.; Kräuchi, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol. Behav. 2004, 80, 695–701. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Corr, P.J. (Ed.) Reinforcement Sensitivity Theory (RST): Introduction. In The Reinforcement Sensitivity Theory of Personality; Cambridge University Press: New York, NY, USA, 2008; pp. 1–43. [Google Scholar]
- Fantino, E.; Gaitan, S.; Kennelly, A.; Stolarz-Fantino, S. How reinforcer type affects choice in economic games. Behav. Process. 2007, 75, 107–114. [Google Scholar] [CrossRef]
- Xu, S.; Pan, Y.; Wang, Y.; Spaeth, A.M.; Qu, Z.; Rao, H. Real and hypothetical monetary rewards modulate risk taking in the brain. Sci. Rep. 2016, 6, 29520. [Google Scholar] [CrossRef]
- Xu, S.; Pan, Y.; Qu, Z.; Fang, Z.; Yang, Z.; Yang, F.; Wang, F.; Rao, H. Differential effects of real versus hypothetical monetary reward magnitude on risk-taking behavior and brain activity. Sci. Rep. 2018, 8, 3712. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.N.; Dahlstrom, W.G.; Graham, J.R.; Tellegen, A.; Kaemmer, B. The Minnesota Multiphasic Personality Inventory-2 (MMPI-2): Manual for Administration and Scoring; University of Minnesota Press: Minneapolis, MN, USA, 1989. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Derogatis, L.R.; Lipman, R.S.; Rickels, K.; Uhlenhuth, E.H.; Covi, L. The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behav. Sci. 1974, 19, 1–15. [Google Scholar] [CrossRef]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Amira, S.A.; Bressler, B.L.; Lee, J.H.; Czeisler, C.A.; Duffy, J.F. Psychological screening for exceptional environments: Laboratory circadian rhythm and sleep research. Clocks Sleep 2020, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Dijk, D.J. Getting through to circadian oscillators: Why use constant routines? J. Biol. Rhythm. 2002, 17, 4–13. [Google Scholar] [CrossRef]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh. Res. Meth. Instr. Comp. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Silva, E.J.; Wang, W.; Ronda, J.M.; Wyatt, J.K.; Duffy, J.F. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep 2010, 33, 481–490. [Google Scholar] [CrossRef]
- Duffy, J.F.; Willson, H.J.; Wang, W.; Czeisler, C.A. Healthy older adults better tolerate sleep deprivation than young adults. J. Am. Geriatr. Soc. 2009, 57, 1245–1251. [Google Scholar] [CrossRef]
- Wyatt, J.K.; Ritz-De Cecco, A.; Czeisler, C.A.; Dijk, D.J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, 1152–1163. [Google Scholar] [CrossRef]
- Lejuez, C.W.; Aklin, W.M.; Zvolensky, M.J.; Pedulla, C.M. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2002, 26, 475–479. [Google Scholar] [CrossRef]
- Yun, C.H.; Kim, H.; Lee, S.K.; Suh, S.; Lee, S.H.; Park, S.H.; Thomas, R.J.; Au, R.; Shin, C. Daytime sleepiness associated with poor sustained attention in middle and late adulthood. Sleep Med. 2015, 16, 143–151. [Google Scholar] [CrossRef]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A.I. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Herrera, N.; Friedman, M.; St. Hilaire, M.A.; Arrona-Palacios, A.; Czeisler, C.A.; Duffy, J.F. Time of Day and Sleep Deprivation Effects on Risky Decision Making. Clocks & Sleep 2024, 6, 281-290. https://doi.org/10.3390/clockssleep6020020
Ruiz-Herrera N, Friedman M, St. Hilaire MA, Arrona-Palacios A, Czeisler CA, Duffy JF. Time of Day and Sleep Deprivation Effects on Risky Decision Making. Clocks & Sleep. 2024; 6(2):281-290. https://doi.org/10.3390/clockssleep6020020
Chicago/Turabian StyleRuiz-Herrera, Noelia, Mia Friedman, Melissa A. St. Hilaire, Arturo Arrona-Palacios, Charles A. Czeisler, and Jeanne F. Duffy. 2024. "Time of Day and Sleep Deprivation Effects on Risky Decision Making" Clocks & Sleep 6, no. 2: 281-290. https://doi.org/10.3390/clockssleep6020020
APA StyleRuiz-Herrera, N., Friedman, M., St. Hilaire, M. A., Arrona-Palacios, A., Czeisler, C. A., & Duffy, J. F. (2024). Time of Day and Sleep Deprivation Effects on Risky Decision Making. Clocks & Sleep, 6(2), 281-290. https://doi.org/10.3390/clockssleep6020020






