Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles
Abstract
1. Introduction
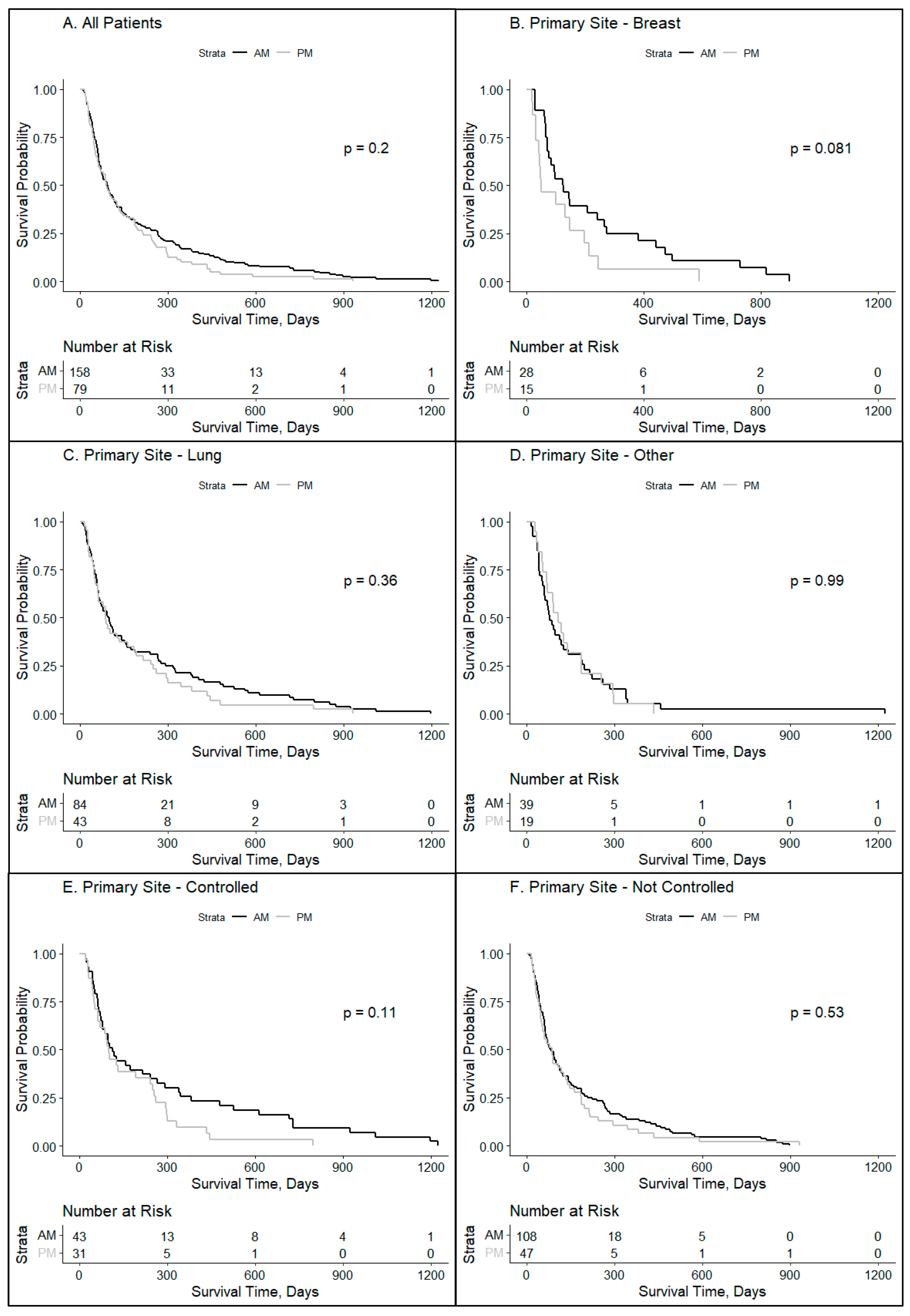
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Wang, C.; Barnoud, C.; Cenerenti, M.; Sun, M.; Caffa, I.; Kizil, B.; Bill, R.; Liu, Y.; Pick, R.; Garnier, L.; et al. Dendritic cells direct circadian anti-tumour immune responses. Nature 2023, 614, 136–143. [Google Scholar] [CrossRef]
- Kim, D.W.; Byun, J.M.; Lee, J.O.; Kim, J.K.; Koh, Y. Chemotherapy delivery time affects treatment outcomes of female patients with diffuse large B cell lymphoma. JCI Insight 2023, 8, 164767. [Google Scholar] [CrossRef]
- Haus, E. Chronobiology of the mammalian response to ionizing radiation. Potential applications in oncology. Chronobiol. Int. 2002, 19, 77–100. [Google Scholar] [CrossRef]
- Bermúdez-Guzmán, L.; Blanco-Saborío, A.; Ramírez-Zamora, J.; Lovo, E. The Time for Chronotherapy in Radiation Oncology. Front. Oncol. 2021, 11, 687672. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Lombardo, J.; Matlack, L.; Smith, A.; Hines, K.; Shi, W.; Simone, N.L. Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology. Int. J. Mol. Sci. 2022, 23, 1331. [Google Scholar] [CrossRef]
- Chan, S.; Rowbottom, L.; McDonald, R.; Zhang, L.; Bjarnason, G.A.; Tsao, M.; Chow, E. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann. Palliat. Med. 2016, 5, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Ferraro, D.J.; Yaddanapudi, S.; Drzymala, R.E.; Lee, A.Y.; Silver, S.A.; Dyk, P.; DeWees, T.; Simpson, J.R.; Rich, K.M.; et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer 2013, 119, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.A.; Ray, D.K.; Schlesinger, D.J.; Steiner, L.; Sheehan, J.P.; O’Quigley, J.M.; Rich, T. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: Is there a difference in outcome between morning and afternoon treatment? Cancer 2011, 117, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kabolizadeh, P.; Wegner, R.; Bernard, M.; Heron, D.; Mintz, A.; Burton, S. The effect of treatment time on outcome in non-small cell lung cancer brain metastases treated with stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, S301. [Google Scholar] [CrossRef]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saenz, A.; Sánchez de Miguel, A.; Espinosa, A.; Valentin, A.; Aragonés, N.; Llorca, J. Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study). Environ. Health Perspect. 2018, 126, 047011. [Google Scholar] [CrossRef] [PubMed]
- deHaro, D.; Kines, K.J.; Sokolowski, M.; Dauchy, R.T.; Streva, V.A.; Hill, S.M.; Hanifin, J.P.; Brainard, G.C.; Blask, D.E.; Belancio, V.P. Regulation of L1 expression and retrotransposition by melatonin and its receptor: Implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014, 42, 7694–7707. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; He, C.; Zhao, W.; Hu, N.; Long, D. Circadian clock and cell cycle: Cancer and chronotherapy. Acta Histochem. 2021, 123, 151816. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Przybek, M.; Muurlink, O.; Pepłonska, B. Circadian gene variants and breast cancer. Cancer Lett. 2017, 390, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.D.; Bussard, K.M. The Clock Is Ticking: Countdown to Metastases. PLoS Genet. 2016, 12, e1006299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Chang-Claude, J.; Critchley, A.M.; Kyriacou, C.; Lavers, S.; Rattay, T.; Webb, A.; Azria, D.; Brookes, A.; Burr, T.; et al. Genetic Variants Predict Optimal Timing of Radiotherapy to Reduce Side-effects in Breast Cancer Patients. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, H.; Ji, M.; Wang, Z.; Wu, W. Low circadian clock genes expression in cancers: A meta-analysis of its association with clinicopathological features and prognosis. PLoS ONE 2020, 15, e0233508. [Google Scholar] [CrossRef]
- Chan, S.; Zhang, L.; Rowbottom, L.; McDonald, R.; Bjarnason, G.A.; Tsao, M.; Barnes, E.; Danjoux, C.; Popovic, M.; Lam, H.; et al. Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann. Palliat. Med. 2017, 6, 14–25. [Google Scholar] [CrossRef]
- Steele, T.A.; St Louis, E.K.; Videnovic, A.; Auger, R.R. Circadian Rhythm Sleep-Wake Disorders: A Contemporary Review of Neurobiology, Treatment, and Dysregulation in Neurodegenerative Disease. Neurotherapeutics 2021, 18, 53–74. [Google Scholar] [CrossRef]
- Hamilton, T. Influence of environmental light and melatonin upon mammary tumour inductino. Br. J. Surg. 1969, 56, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Choo, K.B.; Hou, M.F.; Yeh, K.T.; Kuo, S.J.; Chang, J.G. Deregualted expression of the PER1, PER2, and PER3 genes in breast cancers. Carcinogenesis 2005, 26, 1241–1246. [Google Scholar] [CrossRef]
- Innominato, P.F.; Roche, V.P.; Palesh, O.G.; Ulusakarya, A.; Spiegel, D.; Levi, F.A. The circadian timing system in clinical oncology. Ann. Med. 2014, 46, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological impications. Fertil. Sterlity 2008, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Marzouk, M.A.; Adhikari, S.; Wright, T.D.; Miller, B.P.; Matossian, M.D.; Elliott, S.; Wright, M.; Alzoubi, M.; Collins-Burow, B.M.; et al. Pharmacological, Mechanistic, and Pharmacokinetic Assessment of Novel Melatonin-Tamoxifen Drug Conjugates as Breast Cancer Drugs. Mol. Pharmacol. 2019, 96, 272–296. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Palesh, O.; Kriegsfeld, L.J. The Pathophysiologic Role of Disrupted Circadian and Neuroendocrine Rhythms in Breast Carcinogenesis. Endocr. Rev. 2016, 37, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Hoogstraat, M.; Stelloo, S.; Eickhoff, N.; Schuurman, K.; de Barros, H.; Alkemade, M.; Bekers, E.M.; Severson, T.M.; Sanders, J.; et al. Drug-Induced Epigenomic Plasticity Reprograms Circadian Rhythm Regulation to Drive Prostate Cancer toward Androgen Independence. Cancer Discov. 2022, 12, 2074–2097. [Google Scholar] [CrossRef]
- Zhu, Y.; Brown, H.N.; Zhang, Y.; Stevens, R.G.; Zheng, T. Period3 structural variation: A circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 268–270. [Google Scholar] [CrossRef]
- Duffy, J.F.; Cain, S.W.; Chang, A.M.; Phillips, A.J.; Munch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.J.; Wright, K.P., Jr.; Czeisler, C.A. Sex difference in the near-24-hour insrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108, 15602–15608. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhytms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zitting, K.M.; Chinoy, E.D. Aging and Circadian rhythms. Sleep. Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; McWhirter, L.; Diniz Behn, C.; Bubar, K.M.; Kaar, J.L.; Pyle, L.; Rahat, H.; Garcia-Reyes, Y.; Carreau, A.M.; Wright, K.P.; et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3525–3534. [Google Scholar] [CrossRef]
- Dasari, S.S.; Archer, M.; Mohamed, N.E.; Tewari, A.K.; Figueiro, M.G.; Kypinaou, N. Circadian Rhythm Disruption as a contributor to racial disparities in prostate cancer. Cancers 2002, 14, 5116. [Google Scholar] [CrossRef]
- Smith, M.R.; Burgess, H.J.; Fogg, L.F.; Eastman, C.I. Racial differences in the human endogenous circadian period. PLoS ONE 2009, 4, e6014. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.M.; Kong, X.; Hut, R.; Suchecki, D.; Meerlo, P. The impact of stress and stress hormones on endogenous clocks and circadian rhythms. Front. Neuroendocr. 2023, 63, 100931. [Google Scholar] [CrossRef]
- Walker, W.H.; Borniger, J.C. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]


| Patients | ≥80% AM Treatment % (n = 158) | ≥80% PM Treatment % (n = 79) | All Patients |
|---|---|---|---|
| Deceased | 133 (84.18%) | 71 (89.87%) | 204 (86.08%) |
| Alive | 25 (15.82%) | 8 (10.13%) | 33 (13.92%) |
| Total | 158 | 79 | 237 |
| Sex | ≥80% AM | ≥80% PM | All ≥ 80% |
| Female | 100 (63.29%) | 47 (59.49%) | 147 (62.03%) |
| Male | 58 (36.71%) | 32 (40.51%) | 90 (37.97%) |
| Age at Dx | ≥80% AM | ≥80% PM | All ≥ 80% |
| <65 Years | 76 (48.10%) | 41 (51.90%) | 117 (49.37%) |
| ≥65 Years | 82 (51.90%) | 38 (48.10%) | 120 (50.63%) |
| Primary Site | ≥80% AM | ≥80% PM | All ≥ 80% |
| Breast | 28 (17.72%) | 15 (18.99%) | 43 (18.14%) |
| Lung | 84 (53.16%) | 43 (54.43%) | 127 (53.59%) |
| Other | 39 (24.68%) | 19 (24.05%) | 58 (24.47%) |
| Unknown | 7 (4.43%) | 2 (2.53%) | 9 (3.80%) |
| Primary Controlled | ≥80% AM | ≥80% PM | All ≥ 80% |
| Yes | 43 (27.22%) | 31 (39.24%) | 74 (31.22%) |
| No | 108 (68.35%) | 47 (59.49%) | 155 (65.40%) |
| N/A | 7 (4.43%) | 1 (1.27%) | 8 (3.38%) |
| KPS Index | ≥80% AM | ≥80% PM | All ≥ 80% |
| ≥70 | 97 (61.39%) | 47 (59.49%) | 144 (60.76%) |
| <70 | 33 (20.89%) | 16 (20.25%) | 49 (20.68%) |
| N/A | 28 (17.72%) | 16 (20.25%) | 44 (18.57%) |
| RPA Group | ≥80% AM | ≥80% PM | All ≥ 80% |
| Class 1 | 17 (10.76%) | 16 (20.25%) | 33 (13.92%) |
| Class 2 | 80 (50.63%) | 31 (39.24%) | 111 (46.84%) |
| Class 3 | 32 (20.25%) | 17 (21.52%) | 49 (20.68%) |
| N/A | 29 (18.35%) | 15 (18.99%) | 44 (18.57%) |
| Race/Ethnicity | ≥80% AM | ≥80% PM | All ≥ 80% |
| Asian | 4 (2.53%) | 1 (1.27%) | 5 (2.11%) |
| Black | 24 (15.19%) | 15 (18.99%) | 39 (16.46%) |
| Hispanic | 4 (2.53%) | 1 (1.27%) | 5 (2.11%) |
| White | 111 (70.25%) | 53 (67.09%) | 164 (69.25%) |
| N/A | 15 (9.49%) | 9 (11.39%) | 24 (10.13%) |
| BMI | ≥80% AM | ≥80% PM | All ≥ 80% |
| <25 | 60 (37.97%) | 31 (39.24%) | 91 (38.40%) |
| 25–30 | 48 (30.38%) | 21 (26.58%) | 69 (29.11%) |
| >30 | 38 (24.05%) | 22 (27.85%) | 60 (25.32%) |
| N/A | 12 (7.59%) | 5 (6.33%) | 17 (7.17%) |
| Patient Zip Code Median Income | ≥80% AM | ≥80% PM | All ≥ 80% |
| <$57,550 K | 52 (32.91%) | 27 (34.18%) | 79 (33.33%) |
| >$57,500 K | 106 (67.09%) | 52 (65.82%) | 158 (66.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, N.G.; Burke, S.E.; Cappelli, L.; Matlack, L.E.; Smith, A.P.; Francois, N.; Lombardo, J.F.; Shah, Y.B.; Wen, K.-Y.; Shafi, A.A.; et al. Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles. Clocks & Sleep 2024, 6, 200-210. https://doi.org/10.3390/clockssleep6010014
Nelson NG, Burke SE, Cappelli L, Matlack LE, Smith AP, Francois N, Lombardo JF, Shah YB, Wen K-Y, Shafi AA, et al. Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles. Clocks & Sleep. 2024; 6(1):200-210. https://doi.org/10.3390/clockssleep6010014
Chicago/Turabian StyleNelson, Nicolas G., Sara E. Burke, Louis Cappelli, Lauren E. Matlack, Alexandria P. Smith, Noelle Francois, Joseph F. Lombardo, Yash B. Shah, Kuang-Yi Wen, Ayesha A. Shafi, and et al. 2024. "Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles" Clocks & Sleep 6, no. 1: 200-210. https://doi.org/10.3390/clockssleep6010014
APA StyleNelson, N. G., Burke, S. E., Cappelli, L., Matlack, L. E., Smith, A. P., Francois, N., Lombardo, J. F., Shah, Y. B., Wen, K.-Y., Shafi, A. A., & Simone, N. L. (2024). Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles. Clocks & Sleep, 6(1), 200-210. https://doi.org/10.3390/clockssleep6010014






