Associations between Rest–Activity Rhythms and Liver Function Tests: The US National Health and Nutrition Examination Survey, 2011–2014
Abstract
:1. Introduction
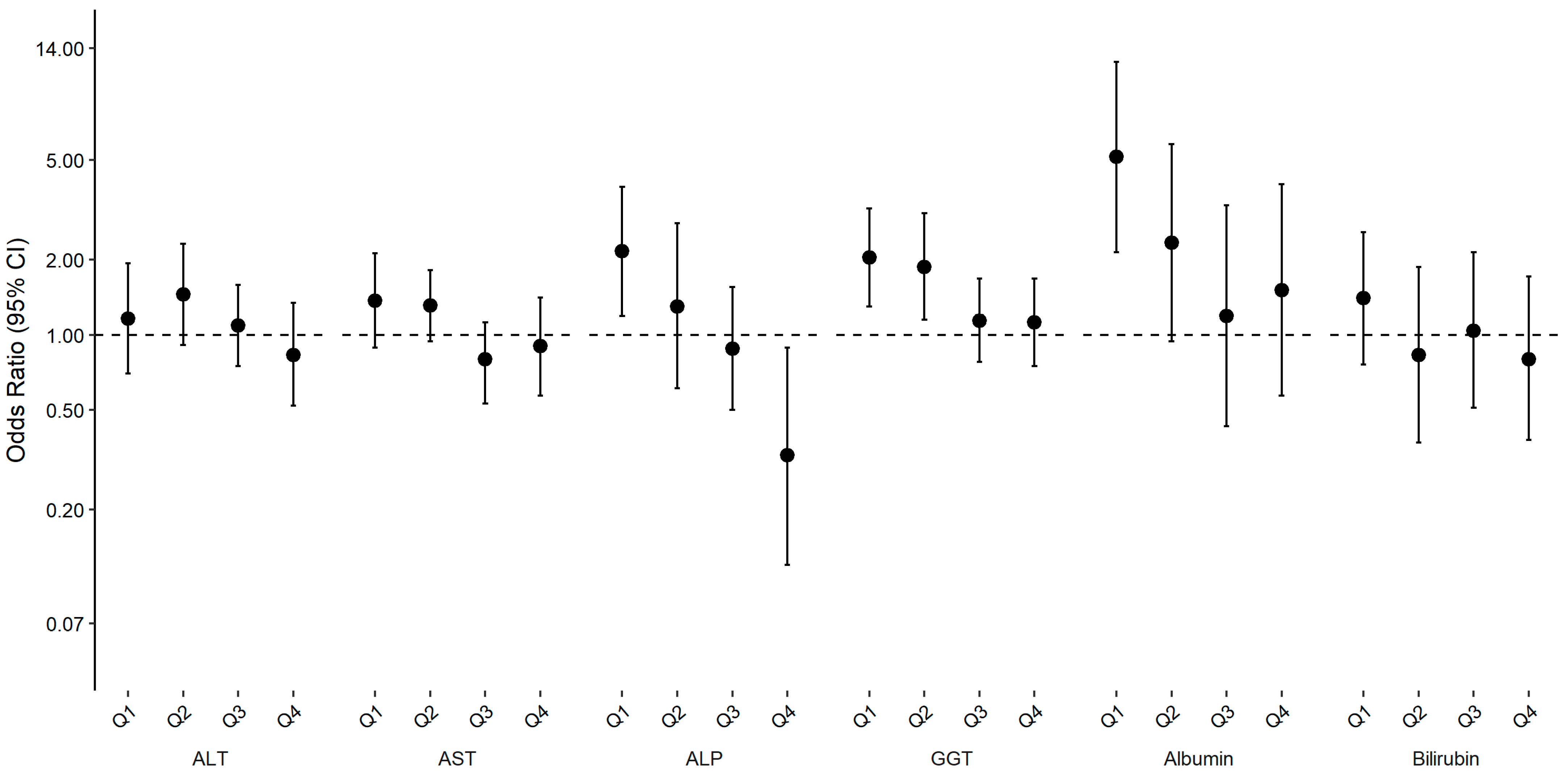
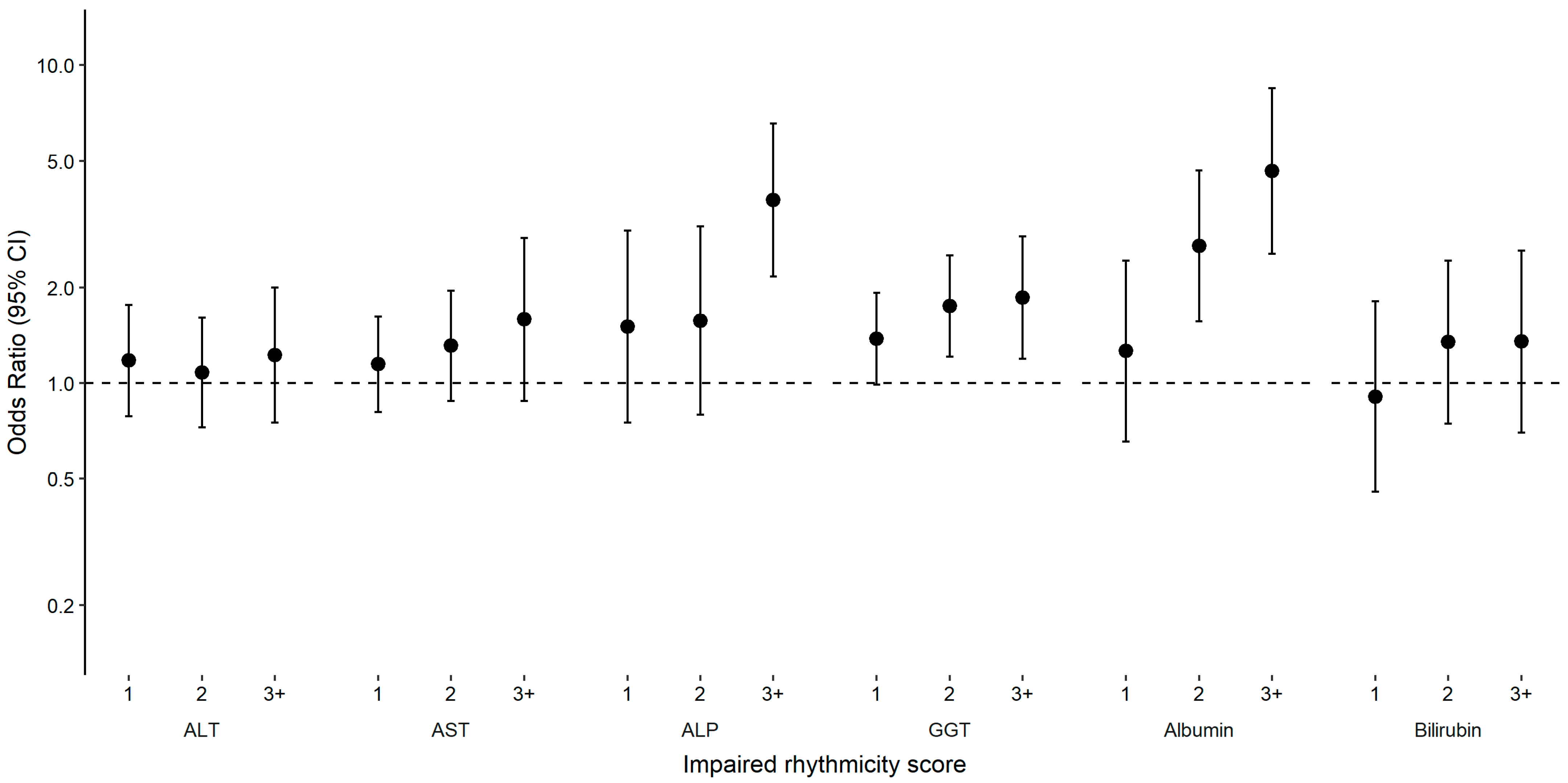
2. Results
3. Discussion
4. Methods
4.1. Study Population
4.2. Measurement of Rest–Activity Rhythms
4.3. Measurement of Liver Enzymes, Albumin and Bilirubin
4.4. Covariates
4.5. Analytic Sample
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Esquirol, Y.; Bongard, V.; Mabile, L.; Jonnier, B.; Soulat, J.; Perret, B. Shift work and metabolic syndrome: Respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol. Int. 2009, 26, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of Shift-Work, Painting, and Fire-Fighting; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Paudel, M.L.; Taylor, B.C.; Ancoli-Israel, S.; Stone, K.L.; Tranah, G.; Redline, S.; Barrett-Connor, E.; Stefanick, M.L.; Ensrud, K.E. Rest/Activity Rhythms and Cardiovascular Disease in Older Men. Chronobiol. Int. 2011, 28, 258–266. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Voigt, R.M.; Burgess, H.J.; Swanson, G.R.; Keshavarzian, A. Circadian rhythms, alcohol and gut interactions. Alcohol 2015, 49, 389–398. [Google Scholar] [CrossRef]
- Liu, J.; Au Yeung, S.L.; Lin, S.L.; Leung, G.M.; Schooling, C.M. Liver Enzymes and Risk of Ischemic Heart Disease and Type 2 Diabetes Mellitus: A Mendelian Randomization Study. Sci. Rep. 2016, 6, 38813. [Google Scholar] [CrossRef]
- Ghouri, N.; Preiss, D.; Sattar, N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: A narrative review and clinical perspective of prospective data. Hepatology 2010, 52, 1156–1161. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Wu, S.; Li, W.; Sun, M.; Feng, W.; Ding, D.; Yeung-Shan Wong, S.; Zhu, P.; Evans, G.J.; et al. Night shift work and abnormal liver function: Is non-alcohol fatty liver a necessary mediator? Occup. Environ. Med. 2019, 76, 83–89. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef]
- Xiao, Q.; Qian, J.; Evans, D.S.; Redline, S.; Lane, N.E.; Ancoli-Israel, S.; Scheer, F.A.J.L.; Stone, K.; Osteoporotic Fractures in Men (MrOS) Study Group. Cross-sectional and Prospective Associations of Rest-Activity Rhythms with Metabolic Markers and Type 2 Diabetes in Older Men. Diabetes Care 2020, 43, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Matthews, C.E.; Playdon, M.; Bauer, C. The association between rest-activity rhythms and glycemic markers: The US National Health and Nutrition Examination Survey, 2011–2014. Sleep 2022, 45, zsab291. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Resetting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; Van Someren, E.J.W.; Vogels, R.L.C.; Van Harten, B.; Scherder, E.J.A. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J. Sleep Res. 2009, 18, 129–135. [Google Scholar] [CrossRef]
- Xiao, Q.; Sampson, J.N.; LaCroix, A.Z.; Shadyab, A.H.; Zeitzer, J.M.; Ancoli-Israel, S.; Yaffe, K.; Stone, K.; Osteoporotic Fractures in Men (MrOS) Study Group. Nonparametric Parameters of 24-Hour Rest–Activity Rhythms and Long-Term Cognitive Decline and Incident Cognitive Impairment in Older Men. J. Gerontol. Ser. A 2022, 77, 250–258. [Google Scholar] [CrossRef]
- Mormont, M.-C.; Waterhouse, J.; Bleuzen, P.; Giacchetti, S.; Jami, A.; Bogdan, A.; Lellouch, J.; Misset, J.-L.; Touitou, Y.; Lévi, F. Marked 24-h Rest/Activity Rhythms Are Associated with Better Quality of Life, Better Response, and Longer Survival in Patients with Metastatic Colorectal Cancer and Good Performance Status. Clin. Cancer Res. 2000, 6, 3038–3045. [Google Scholar]
- Rivera-Coll, A.; Fuentes-Arderiu, X.; Díez-Noguera, A. Circadian Rhythms of Serum Concentrations of 12 Enzymes of Clinical Interest. Chronobiol. Int. 1993, 10, 190–200. [Google Scholar] [CrossRef]
- Minville, C.; Hilleret, M.-N.; Tamisier, R.; Aron-Wisnewsky, J.; Clement, K.; Trocme, C.; Borel, J.-C.; Lévy, P.; Zarski, J.-P.; Pépin, J.-L. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest 2014, 145, 525–533. [Google Scholar] [CrossRef]
- Mishra, P.; Nugent, C.; Afendy, A.; Bai, C.; Bhatia, P.; Afendy, M.; Fang, Y.; Elariny, H.; Goodman, Z.; Younossi, Z.M. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. Off. J. Int. Assoc. Study Liver 2008, 28, 1080–1086. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.-L.; Chen, R.; Wang, Y.; Xiong, K.-P.; Huang, J.-Y.; Han, F.; Liu, C.-F. Elevated Serum Liver Enzymes in Patients with Obstructive Sleep Apnea-hypopnea Syndrome. Chin. Med. J. 2015, 128, 2983–2987. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Obstructive Sleep Apnea Is Associated with Fatty Liver and Abnormal Liver Enzymes: A Meta-analysis. Obes. Surg. 2013, 23, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.-F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.A.; Reddy, A.B.; Maywood, E.S.; Clayton, J.D.; King, V.M.; Smith, A.G.; Gant, T.W.; Hastings, M.H.; Kyriacou, C.P. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Curr. Biol. 2002, 12, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Shigiyama, F.; Kumashiro, N.; Tsuneoka, Y.; Igarashi, H.; Yoshikawa, F.; Kakehi, S.; Funato, H.; Hirose, T. Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. Am. J. Physiol. Metab. 2018, 315, E848–E858. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kar, S.K. REM sleep deprivation of rats induces acute phase response in liver. Biochem. Biophys. Res. Commun. 2011, 410, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tong, X.; Nelson, B.B.; Jin, E.; Sit, J.; Charney, N.; Yang, M.; Omary, M.B.; Yin, L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatology 2018, 68, 883–896. [Google Scholar] [CrossRef]
- Greco, C.M.; Koronowski, K.B.; Smith, J.G.; Shi, J.; Kunderfranco, P.; Carriero, R.; Chen, S.; Samad, M.; Welz, P.-S.; Zinna, V.M.; et al. Integration of feeding behavior by the liver circadian clock reveals network dependency of metabolic rhythms. Sci. Adv. 2021, 7, eabi7828. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021, 236, 2393–2412. [Google Scholar] [CrossRef]
- Heckler, B.; Lee, M.; Stone, K.; Bauer, C.; Xiao, Q. Cross-sectional and Prospective Associations of Rest-Activity Rhythms with Body Mass Index in Older Men: A Novel Analysis Using Harmonic Hidden Markov Models. J. Biol. Rhythms 2023, 38, 87–97. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, J.; Li, X.; Fan, R.; Arsenault, B.; Gill, D.; Giovannucci, E.L.; Zheng, J.-S.; Larsson, S.C. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur. J. Epidemiol. 2022, 37, 723–733. [Google Scholar] [CrossRef]
- Limdi, J.K.; Hyde, G.M. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2003, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. 2 2014, 2, 1–33. [Google Scholar]
- National Center for Health Statistics. NCHS Research Ethics Review Board (ERB) Approval. 2017. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm#:~:text=NCHS%20Research%20Ethics%20Review%20Board%20%28ERB%29%20Approval%2A%20%2A,2017%20Content%20source%3A%20National%20Center%20for%20Health%20Statistics (accessed on 12 June 2023).
- Centers for Disease Control and Prevention (Organization/Institution). National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies Physical Activity Monitor-Day (PAXDAY_G). 2020. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PAXDAY_G.htm (accessed on 12 June 2023).
- Centers for Disease Control and Prevention (Organization/Institution). Health and Nutrition Examination Survey (NHANES) Physical Activity Monitor (PAM) Manual. 2011. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/Physical_Activity_Monitor_Manual.pdf (accessed on 12 June 2023).
- Marler, M.R.; Gehrman, P.; Martin, J.L.; Ancoli-Israel, S. The sigmoidally transformed cosine curve: A mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat. Med. 2006, 25, 3893–3904. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.R.; Walia, A. Liver function tests and their interpretation. Indian J. Pediatr. 2007, 74, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (Organization/Institution). National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies. 2013. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/BIOPRO_G.htm (accessed on 12 June 2023).
- Centers for Disease Control and Prevention (Organization/Institution). NHANES 2011–2012 Laboratory Methods. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?Cycle=2011-2012 (accessed on 12 June 2023).
- Glymour, M.M.; Weuve, J.; Chen, J.T. Methodological challenges in causal research on racial and ethnic patterns of cognitive trajectories: Measurement, selection, and bias. Neuropsychol. Rev. 2008, 18, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (Organization/Institution). National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies Demographic Variables & Sample Weights. 2015. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DEMO_G.htm#DMDEDUC2 (accessed on 12 June 2023).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
| F Statistic a | p Value b | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Age, median (IQR) | 48 (33, 60) | 50 (34, 60) | 47 (34, 59) | 48 (36, 62) | 50 (37, 60) | 0.11 |
| Women, % | 44 | 51 | 49 | 54 | 57 | <0.001 |
| Race, % | <0.001 | |||||
| Non-Hispanic white | 60 | 63 | 67 | 70 | 74 | |
| Non-Hispanic black | 18 | 14 | 11 | 9 | 4 | |
| Hispanic | 14 | 14 | 15 | 15 | 16 | |
| Others | 8 | 10 | 8 | 6 | 7 | |
| Education, % | <0.001 | |||||
| Less than high school | 15 | 16 | 14 | 15 | 16 | |
| High school graduate | 21 | 20 | 22 | 20 | 22 | |
| Some college | 40 | 36 | 31 | 29 | 27 | |
| College graduate or above | 24 | 27 | 33 | 36 | 35 | |
| Household Income, % | <0.001 | |||||
| <$20 k | 22 | 20 | 14 | 12 | 11 | |
| $20–$44.9 k | 31 | 27 | 25 | 23 | 24 | |
| $45–$74.9 k | 17 | 21 | 21 | 19 | 23 | |
| >$75 k | 30 | 32 | 41 | 45 | 41 | |
| Married, % | 42 | 51 | 59 | 61 | 68 | <0.001 |
| Smoking, % | <0.001 | |||||
| Never | 47 | 53 | 58 | 60 | 61 | |
| Former | 21 | 24 | 22 | 28 | 25 | |
| Current | 32 | 23 | 20 | 13 | 14 | |
| BMI, % | <0.001 | |||||
| <25 | 22 | 25 | 25 | 28 | 36 | |
| 25–30 | 29 | 31 | 34 | 37 | 35 | |
| ≥30 | 48 | 45 | 41 | 36 | 29 | |
| Diabetes, % | 21 | 18 | 14 | 12 | 9 | <0.001 |
| Alcohol, % | 0.3 | |||||
| Never/Former | 30 | 28 | 25 | 25 | 23 | |
| Light | 52 | 55 | 54 | 53 | 55 | |
| Moderate | 9.4 | 8.9 | 10 | 11 | 11 | |
| Heavy | 9.2 | 8 | 11 | 11 | 11 | |
| Hepatitis B core antibody or surface antigen positive, % | 5.5 | 5.7 | 5.0 | 4.6 | 3.7 | 0.12 |
| Hepatitis C antibody (confirmed) or RNA positive, % | 4.0 | 1.9 | 1.6 | 0.8 | 0.8 | <0.001 |
| Hepatitis E IgG or IgM positive, % | 7.7 | 6.2 | 6.6 | 6.4 | 8.6 | 0.40 |
| Total activity count, median (IQR) c | 9253 (7279, 11,709) | 10,105 (8455, 11,992) | 10,995 (9477, 12,664) | 11,567 (9961, 13,384) | 12,996 (11,262, 14,724) | <0.001 |
| Sleep duration (minutes), median (IQR) | 411 (357, 492) | 415 (365, 477) | 406 (364, 454) | 408 (364, 449) | 402 (362, 441) | <0.001 |
| Shift workers (proxied by L5MD), % | 16 | 4.3 | 2.1 | 2.4 | 2 | <0.001 |
| Abnormal Levels of Liver Enzymes a | F Statistic | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| F statistic, median (IQR) | 181 (128–225) | 325 (295–354) | 453 (420–481) | 599 (560–650) | 851 (764–997) | |
| ALT | ||||||
| N (%) b | 148 (11) | 146 (13) | 138 (11) | 102 (8) | 124 (9.8) | |
| OR (95% CI) | ||||||
| Model 1 | 1.21 (0.83, 1.75) | 1.37 (0.95, 1.98) | 1.1 (0.81, 1.49) | 0.8 (0.55, 1.18) | ref. | 0.02 |
| Model 2 | 1.16 (0.7, 1.93) | 1.45 (0.91, 2.31) | 1.09 (0.75, 1.58) | 0.83 (0.52, 1.34) | ref. | 0.08 |
| Model 3 | 1.06 (0.62, 1.81) | 1.36 (0.84, 2.2) | 1.03 (0.7, 1.52) | 0.8 (0.5, 1.29) | ref. | 0.20 |
| Model 4 | 1.02 (0.57, 1.82) | 1.32 (0.82, 2.12) | 1 (0.67, 1.48) | 0.79 (0.48, 1.31) | ref. | 0.26 |
| AST | ||||||
| N (%) b | 184 (14) | 157 (11) | 115 (8.2) | 103 (8.4) | 104 (9) | |
| OR (95% CI) | ||||||
| Model 1 | 1.55 (1.18, 2.03) | 1.27 (0.99, 1.63) | 0.86 (0.63, 1.19) | 0.91 (0.66, 1.26) | ref. | <0.001 |
| Model 2 | 1.37 (0.89, 2.12) | 1.31 (0.94, 1.81) | 0.8 (0.53, 1.23) | 0.9 (0.57, 1.41) | ref. | 0.04 |
| Model 3 | 1.26 (0.8, 1.99) | 1.24 (0.88, 1.75) | 0.77 (0.5, 1.18) | 0.87 (0.56, 1.37) | ref. | 0.10 |
| Model 4 | 1.32 (0.82, 2.14) | 1.26 (0.89, 1.8) | 0.79 (0.5, 1.23) | 0.88 (0.55, 1.42) | ref. | 0.07 |
| ALP | ||||||
| N (%) b | 78 (5.8) | 45 (3.5) | 31 (2.1) | 21 (0.9) | 32 (2.4) | |
| OR (95% CI) | ||||||
| Model 1 | 2.82 (1.7, 4.69) | 1.52 (0.77, 3.01) | 0.98 (0.62, 1.54) | 0.37 (0.18, 0.78) | ref. | <0.001 |
| Model 2 | 2.16 (1.19, 3.9) | 1.3 (0.61, 2.79) | 0.88 (0.5, 1.55) | 0.33 (0.12, 0.89) | ref. | 0.002 |
| Model 3 | 1.87 (1.02, 3.4) | 1.17 (0.54, 2.55) | 0.81 (0.46, 1.44) | 0.3 (0.11, 0.83) | ref. | 0.004 |
| Model 4 | 2.12 (1.09, 4.15) | 1.21 (0.54, 2.69) | 0.83 (0.45, 1.53) | 0.26 (0.09, 0.76) | ref. | 0.004 |
| GGT | ||||||
| N (%) b | 178 (13) | 148 (12) | 130 (7.9) | 106 (7.6) | 88 (7.1) | |
| OR (95% CI) | ||||||
| Model 1 | 2.24 (1.63, 3.07) | 1.9 (1.29, 2.79) | 1.22 (0.9, 1.66) | 1.11 (0.8, 1.54) | ref. | <0.001 |
| Model 2 | 2.04 (1.3, 3.2) | 1.87 (1.15, 3.06) | 1.14 (0.78, 1.68) | 1.12 (0.75, 1.68) | ref. | 0.003 |
| Model 3 | 1.85 (1.18, 2.91) | 1.75 (1.05, 2.89) | 1.08 (0.73, 1.6) | 1.07 (0.73, 1.59) | ref. | 0.01 |
| Model 4 | 1.75 (1.05, 2.93) | 1.66 (0.97, 2.84) | 1.02 (0.68, 1.55) | 1.05 (0.69, 1.61) | ref. | 0.02 |
| Albumin | ||||||
| N (%) b | 89 (6.1) | 40 (2.8) | 29 (1.5) | 28 (2.1) | 11 (1) | |
| OR (95% CI) | ||||||
| Model 1 | 7.25 (3.4, 15.48) | 2.95 (1.34, 6.49) | 1.56 (0.66, 3.73) | 2.13 (0.94, 4.84) | ref. | <0.001 |
| Model 2 | 5.15 (2.14, 12.38) | 2.33 (0.94, 5.78) | 1.19 (0.43, 3.3) | 1.51 (0.57, 4.01) | ref. | 0.001 |
| Model 3 | 4.64 (1.87, 11.53) | 2.12 (0.82, 5.49) | 1.1 (0.37, 3.28) | 1.45 (0.54, 3.87) | ref. | 0.002 |
| Model 4 | 4.28 (1.65, 11.1) | 1.85 (0.67, 5.15) | 1.01 (0.33, 3.13) | 1.35 (0.47, 3.88) | ref. | 0.003 |
| Bilirubin | ||||||
| N (%) b | 47 (4) | 26 (2.3) | 32 (3) | 34 (2.5) | 30 (2.5) | |
| OR (95% CI) | ||||||
| Model 1 | 1.37 (0.85, 2.22) | 0.84 (0.42, 1.7) | 1.05 (0.57, 1.95) | 0.92 (0.48, 1.77) | ref. | 0.34 |
| Model 2 | 1.4 (0.76, 2.58) | 0.83 (0.37, 1.87) | 1.04 (0.51, 2.14) | 0.8 (0.38, 1.71) | ref. | 0.34 |
| Model 3 | 1.38 (0.73, 2.61) | 0.82 (0.36, 1.85) | 1.03 (0.49, 2.17) | 0.8 (0.37, 1.74) | ref. | 0.36 |
| Model 4 | 1.51 (0.77, 2.96) | 0.88 (0.39, 2.02) | 1.12 (0.5, 2.49) | 0.85 (0.37, 1.96) | ref. | 0.24 |
| Abnormal Levels of Liver Enzymes a | Amplitude | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Amplitude, median (IQR) | 4.6 (3.7–5.2) | 6.7 (6.3–7.2) | 8.5 (8.1–9.0) | 10.8 (10.1–11.6) | 14.7 (13.4–16.9) | |
| ALT | ||||||
| N (%) b | 131 (11) | 153 (12) | 123 (9.7) | 121 (8.8) | 130 (11) | |
| OR (95% CI) | ||||||
| Model 1 | 1.14 (0.81, 1.60) | 1.24 (0.85, 1.82) | 0.91 (0.63, 1.32) | 0.79 (0.49, 1.29) | ref. | 0.07 |
| Model 2 | 1.30 (0.84, 2.01) | 1.47 (0.97, 2.23) | 1.09 (0.70, 1.69) | 0.93 (0.51, 1.69) | ref. | 0.02 |
| Model 3 | 1.17 (0.73, 1.87) | 1.38 (0.88, 2.16) | 1.06 (0.67, 1.68) | 0.92 (0.49, 1.72) | ref. | 0.08 |
| Model 4 | 1.18 (0.75, 1.86) | 1.38 (0.88, 2.14) | 1.06 (0.66, 1.69) | 0.9 (0.48, 1.7) | ref. | 0.06 |
| AST | ||||||
| N (%) b | 166 (13) | 142 (10) | 124 (9.4) | 110 (7.4) | 121 (11) | |
| OR (95% CI) | ||||||
| Model 1 | 1.21 (0.79, 1.84) | 0.95 (0.67, 1.37) | 0.90 (0.62, 1.30) | 0.69 (0.44, 1.09) | ref. | 0.20 |
| Model 2 | 1.34 (0.78, 2.31) | 1.09 (0.70, 1.70) | 1.07 (0.65, 1.74) | 0.78 (0.41, 1.46) | ref. | 0.13 |
| Model 3 | 1.23 (0.69, 2.18) | 1.04 (0.64, 1.67) | 1.05 (0.63, 1.74) | 0.77 (0.4, 1.49) | ref. | 0.24 |
| Model 4 | 1.31 (0.73, 2.37) | 1.07 (0.66, 1.71) | 1.06 (0.63, 1.79) | 0.77 (0.4, 1.51) | ref. | 0.16 |
| ALP | ||||||
| N (%) b | 75 (5.4) | 39 (2.7) | 28 (1.3) | 35 (3.2) | 30 (1.9) | |
| OR (95% CI) | ||||||
| Model 1 | 2.47 (1.49, 4.10) | 1.22 (0.69, 2.16) | 0.59 (0.28, 1.24) | 1.51 (0.85, 2.68) | ref. | 0.004 |
| Model 2 | 2.66 (1.24, 5.69) | 1.54 (0.71, 3.34) | 0.84 (0.32, 2.21) | 2.21 (0.96, 5.07) | ref. | 0.03 |
| Model 3 | 2.35 (1.08, 5.12) | 1.39 (0.62, 3.12) | 0.78 (0.28, 2.13) | 2.18 (0.93, 5.08) | ref. | 0.07 |
| Model 4 | 2.46 (1.09, 5.58) | 1.48 (0.65, 3.37) | 0.75 (0.27, 2.09) | 2.13 (0.88, 5.18) | ref. | 0.05 |
| GGT | ||||||
| N (%) b | 179 (13) | 144 (10) | 106 (8.3) | 110 (8.1) | 111 (7.7) | |
| OR (95% CI) | ||||||
| Model 1 | 1.67 (1.21, 2.31) | 1.29 (0.96, 1.73) | 0.97 (0.66, 1.42) | 0.95 (0.63, 1.43) | ref. | <0.001 |
| Model 2 | 1.82 (1.20, 2.76) | 1.50 (1.04, 2.15) | 1.23 (0.77, 1.96) | 1.18 (0.73, 1.89) | ref. | 0.004 |
| Model 3 | 1.65 (1.08, 2.53) | 1.4 (0.95, 2.07) | 1.19 (0.73, 1.94) | 1.17 (0.71, 1.92) | ref. | 0.02 |
| Model 4 | 1.65 (1.05, 2.58) | 1.37 (0.92, 2.04) | 1.18 (0.72, 1.95) | 1.14 (0.68, 1.92) | ref. | 0.02 |
| Albumin | ||||||
| N (%) b | 88 (6) | 35 (2.3) | 27 (1.4) | 21 (1.8) | 26 (1.7) | |
| OR (95% CI) | ||||||
| Model 1 | 3.88 (1.96, 7.7) | 1.35 (0.62, 2.94) | 0.78 (0.32, 1.92) | 0.96 (0.39, 2.36) | ref. | 0.001 |
| Model 2 | 2.73 (1.2, 6.19) | 1.13 (0.46, 2.74) | 0.66 (0.23, 1.88) | 0.72 (0.26, 1.99) | ref. | 0.01 |
| Model 3 | 2.48 (1.04, 5.92) | 1.05 (0.41, 2.69) | 0.63 (0.2, 1.95) | 0.71 (0.25, 2.02) | ref. | 0.03 |
| Model 4 | 2.25 (0.95, 5.32) | 0.99 (0.39, 2.5) | 0.6 (0.2, 1.83) | 0.67 (0.23, 1.9) | ref. | 0.04 |
| Bilirubin | ||||||
| N (%) b | 38 (2.9) | 24 (1.5) | 34 (3.3) | 36 (3.6) | 37 (2.5) | |
| OR (95% CI) | ||||||
| Model 1 | 1.38 (0.79, 2.43) | 0.7 (0.29, 1.66) | 1.58 (0.86, 2.93) | 1.73 (0.89, 3.33) | ref. | 0.78 |
| Model 2 | 1.31 (0.63, 2.72) | 0.69 (0.23, 2.11) | 1.59 (0.7, 3.59) | 1.79 (0.79, 4.06) | ref. | 0.51 |
| Model 3 | 1.29 (0.63, 2.65) | 0.68 (0.22, 2.15) | 1.58 (0.68, 3.67) | 1.78 (0.77, 4.12) | ref. | 0.46 |
| Model 4 | 1.35 (0.63, 2.89) | 0.7 (0.21, 2.3) | 1.57 (0.65, 3.77) | 1.77 (0.74, 4.28) | ref. | 0.59 |
| Abnormal Levels of Liver Enzymes a | Mesor | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Mesor, median (IQR) | 3.1 (2.9–3.3) | 3.6 (3.5–3.7) | 4.0 (3.9–4.1) | 4.4 (4.3–4.6) | 5.2 (4.9–5.6) | |
| ALT | ||||||
| N (%) b | 143 (11) | 117 (9) | 139 (11) | 128 (10) | 131 (11) | |
| OR (95% CI) | ||||||
| Model 1 | 1.17 (0.87, 1.59) | 0.92 (0.67, 1.28) | 1.15 (0.92, 1.45) | 0.99 (0.74, 1.33) | ref. | 0.49 |
| Model 2 | 1.33 (0.87, 2.05) | 1.04 (0.65, 1.67) | 1.36 (0.96, 1.92) | 1.12 (0.76, 1.66) | ref. | 0.27 |
| Model 3 | 1.24 (0.78, 1.97) | 1 (0.6, 1.65) | 1.32 (0.92, 1.9) | 1.1 (0.73, 1.67) | ref. | 0.49 |
| Model 4 | 1.2 (0.75, 1.93) | 0.99 (0.6, 1.65) | 1.31 (0.89, 1.91) | 1.14 (0.74, 1.76) | ref. | 0.63 |
| AST | ||||||
| N (%) b | 154 (11) | 112 (7.9) | 139 (11) | 126 (10) | 132 (10) | |
| OR (95% CI) | ||||||
| Model 1 | 1.02 (0.75, 1.38) | 0.71 (0.49, 1.04) | 1.03 (0.75, 1.42) | 1.02 (0.68, 1.52) | ref. | 0.31 |
| Model 2 | 1.21 (0.79, 1.84) | 0.84 (0.53, 1.34) | 1.27 (0.82, 1.96) | 1.12 (0.67, 1.86) | ref. | 0.79 |
| Model 3 | 1.14 (0.73, 1.77) | 0.82 (0.5, 1.33) | 1.25 (0.79, 1.96) | 1.11 (0.65, 1.88) | ref. | 0.91 |
| Model 4 | 1.16 (0.74, 1.82) | 0.83 (0.5, 1.36) | 1.25 (0.79, 1.99) | 1.13 (0.65, 1.95) | ref. | 0.99 |
| ALP | ||||||
| N (%) b | 69 (5) | 32 (2) | 29 (1.9) | 30 (1.8) | 47 (3.5) | |
| OR (95% CI) | ||||||
| Model 1 | 1.26 (0.83, 1.93) | 0.51 (0.26, 1.02) | 0.49 (0.28, 0.84) | 0.45 (0.24, 0.84) | ref. | 0.13 |
| Model 2 | 1.47 (0.79, 2.72) | 0.69 (0.28, 1.69) | 0.65 (0.35, 1.24) | 0.50 (0.21, 1.22) | ref. | 0.08 |
| Model 3 | 1.29 (0.67, 2.48) | 0.64 (0.26, 1.56) | 0.62 (0.32, 1.23) | 0.48 (0.19, 1.22) | ref. | 0.16 |
| Model 4 | 1.4 (0.68, 2.87) | 0.66 (0.25, 1.74) | 0.66 (0.33, 1.33) | 0.51 (0.2, 1.33) | ref. | 0.15 |
| GGT | ||||||
| N (%) b | 146 (11) | 119 (7.4) | 131 (9.6) | 132 (8.9) | 122 (9.7) | |
| OR (95% CI) | ||||||
| Model 1 | 1.12 (0.79, 1.58) | 0.73 (0.54, 0.97) | 0.96 (0.7, 1.30) | 0.85 (0.61, 1.19) | ref. | 0.77 |
| Model 2 | 1.33 (0.89, 2.00) | 0.89 (0.62, 1.29) | 1.12 (0.76, 1.67) | 0.91 (0.62, 1.32) | ref. | 0.20 |
| Model 3 | 1.22 (0.8, 1.85) | 0.85 (0.58, 1.26) | 1.08 (0.72, 1.63) | 0.88 (0.59, 1.31) | ref. | 0.37 |
| Model 4 | 1.18 (0.75, 1.87) | 0.84 (0.58, 1.24) | 1.06 (0.71, 1.59) | 0.91 (0.61, 1.37) | ref. | 0.54 |
| Albumin | ||||||
| N (%) b | 60 (3.6) | 40 (2.7) | 37 (2.5) | 33 (2.1) | 27 (1.6) | |
| OR (95% CI) | ||||||
| Model 1 | 2.43 (1.15, 5.13) | 1.74 (0.69, 4.37) | 1.63 (0.76, 3.5) | 1.24 (0.61, 2.52) | ref. | 0.03 |
| Model 2 | 2.22 (0.95, 5.18) | 1.81 (0.63, 5.14) | 1.73 (0.76, 3.95) | 1.2 (0.52, 2.76) | ref. | 0.06 |
| Model 3 | 1.99 (0.83, 4.77) | 1.71 (0.58, 5.07) | 1.65 (0.72, 3.81) | 1.17 (0.51, 2.72) | ref. | 0.10 |
| Model 4 | 1.8 (0.71, 4.59) | 1.62 (0.52, 5.02) | 1.58 (0.65, 3.86) | 1.18 (0.5, 2.79) | ref. | 0.18 |
| Bilirubin | ||||||
| N (%) b | 34 (3) | 37 (3.2) | 26 (1.5) | 31 (3) | 41 (3.5) | |
| OR (95% CI) | ||||||
| Model 1 | 0.96 (0.54, 1.69) | 0.99 (0.59, 1.67) | 0.46 (0.22, 0.95) | 0.97 (0.49, 1.92) | ref. | 0.92 |
| Model 2 | 0.81 (0.4, 1.67) | 0.98 (0.51, 1.86) | 0.43 (0.17, 1.08) | 1.03 (0.45, 2.34) | ref. | 0.56 |
| Model 3 | 0.81 (0.38, 1.69) | 0.97 (0.5, 1.88) | 0.43 (0.17, 1.1) | 1.03 (0.44, 2.38) | ref. | 0.54 |
| Model 4 | 0.86 (0.39, 1.89) | 0.99 (0.48, 2.05) | 0.44 (0.17, 1.16) | 1.02 (0.43, 2.45) | ref. | 0.72 |
| Abnormal Levels of Liver Enzymes a | Amplitude: Mesor | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Amplitude:Mesor, median (IQR) | 1.1 (0.9–1.3) | 1.7 (1.6–1.8) | 2.1 (2.0–2.3) | 2.8 (2.6–3.0) | 3.7 (3.5–4.0) | |
| ALT | ||||||
| N (%) b | 145 (12) | 128 (10) | 124 (9.8) | 134 (10) | 127 (10) | |
| OR (95% CI) | ||||||
| Model 1 | 1.31 (0.93, 1.85) | 1.07 (0.77, 1.48) | 0.97 (0.67, 1.41) | 0.98 (0.70, 1.37) | ref. | 0.12 |
| Model 2 | 1.45 (0.95, 2.23) | 1.19 (0.81, 1.75) | 1.05 (0.67, 1.64) | 1.02 (0.62, 1.67) | ref. | 0.05 |
| Model 3 | 1.34 (0.85, 2.11) | 1.14 (0.76, 1.72) | 1.02 (0.64, 1.62) | 1.01 (0.6, 1.69) | ref. | 0.13 |
| Model 4 | 1.38 (0.86, 2.22) | 1.18 (0.79, 1.76) | 1.04 (0.64, 1.69) | 1.03 (0.6, 1.75) | ref. | 0.08 |
| AST | ||||||
| N (%) b | 186 (15) | 116 (8.6) | 109 (8.2) | 123 (9) | 129 (11) | |
| OR (95% CI) | ||||||
| Model 1 | 1.48 (0.98, 2.24) | 0.82 (0.54, 1.23) | 0.80 (0.50, 1.28) | 0.88 (0.65, 1.20) | ref. | 0.17 |
| Model 2 | 1.64 (1.02, 2.62) | 0.93 (0.59, 1.48) | 0.87 (0.54, 1.40) | 0.99 (0.67, 1.47) | ref. | 0.10 |
| Model 3 | 1.53 (0.93, 2.52) | 0.92 (0.56, 1.49) | 0.85 (0.52, 1.38) | 0.99 (0.65, 1.5) | ref. | 0.18 |
| Model 4 | 1.64 (0.99, 2.72) | 0.92 (0.57, 1.51) | 0.87 (0.53, 1.44) | 0.99 (0.65, 1.51) | ref. | 0.11 |
| ALP | ||||||
| N (%) b | 68 (4.7) | 44 (3.3) | 33 (2.1) | 34 (2.4) | 28 (1.9) | |
| OR (95% CI) | ||||||
| Model 1 | 2.21 (1.19, 4.10) | 1.58 (0.90, 2.77) | 0.95 (0.46, 1.95) | 1.15 (0.53, 2.49) | ref. | 0.01 |
| Model 2 | 2.07 (0.88, 4.85) | 1.82 (0.89, 3.72) | 1.25 (0.52, 3.03) | 1.45 (0.50, 4.14) | ref. | 0.03 |
| Model 3 | 1.93 (0.82, 4.51) | 1.74 (0.82, 3.69) | 1.22 (0.5, 2.97) | 1.43 (0.47, 4.31) | ref. | 0.046 |
| Model 4 | 2.06 (0.84, 5.02) | 1.74 (0.8, 3.78) | 1.18 (0.45, 3.06) | 1.44 (0.47, 4.43) | ref. | 0.04 |
| GGT | ||||||
| N (%) b | 193 (15) | 117 (8) | 112 (8.3) | 109 (8.3) | 119 (8.3) | |
| OR (95% CI) | ||||||
| Model 1 | 1.77 (1.23, 2.55) | 0.88 (0.63, 1.22) | 0.87 (0.61, 1.22) | 0.90 (0.61, 1.32) | ref. | 0.01 |
| Model 2 | 1.75 (1.13, 2.71) | 0.92 (0.60, 1.39) | 1.08 (0.71, 1.64) | 0.95 (0.58, 1.55) | ref. | 0.02 |
| Model 3 | 1.63 (1.04, 2.55) | 0.88 (0.57, 1.36) | 1.05 (0.67, 1.64) | 0.94 (0.57, 1.57) | ref. | 0.04 |
| Model 4 | 1.68 (1.03, 2.73) | 0.91 (0.57, 1.45) | 1.07 (0.68, 1.69) | 0.96 (0.56, 1.64) | ref. | 0.04 |
| Albumin | ||||||
| N (%) b | 82 (5.8) | 30 (2) | 30 (1.9) | 21 (1.5) | 34 (2.1) | |
| OR (95% CI) | ||||||
| Model 1 | 2.67 (1.41, 5.08) | 0.87 (0.44, 1.72) | 0.77 (0.36, 1.64) | 0.62 (0.32, 1.2) | ref. | 0.01 |
| Model 2 | 1.99 (0.86, 4.59) | 0.76 (0.34, 1.71) | 0.69 (0.29, 1.66) | 0.56 (0.26, 1.17) | ref. | 0.09 |
| Model 3 | 1.84 (0.76, 4.47) | 0.73 (0.31, 1.73) | 0.67 (0.27, 1.69) | 0.54 (0.25, 1.17) | ref. | 0.12 |
| Model 4 | 1.83 (0.75, 4.46) | 0.66 (0.26, 1.68) | 0.68 (0.27, 1.71) | 0.54 (0.24, 1.22) | ref. | 0.14 |
| Bilirubin | ||||||
| N (%) b | 36 (3) | 27 (2.2) | 36 (2.6) | 40 (3.9) | 30 (2.3) | |
| OR (95% CI) | ||||||
| Model 1 | 1.56 (0.85, 2.88) | 1.12 (0.52, 2.39) | 1.46 (0.81, 2.64) | 2.09 (1.03, 4.24) | ref. | 0.76 |
| Model 2 | 1.47 (0.64, 3.38) | 1.09 (0.42, 2.82) | 1.4 (0.65, 3.02) | 1.9 (0.73, 4.92) | ref. | 0.92 |
| Model 3 | 1.45 (0.63, 3.37) | 1.08 (0.41, 2.88) | 1.4 (0.63, 3.08) | 1.89 (0.71, 5.04) | ref. | 0.95 |
| Model 4 | 1.49 (0.61, 3.64) | 1.1 (0.39, 3.08) | 1.35 (0.6, 3.04) | 1.91 (0.68, 5.32) | ref. | 0.89 |
| Abnormal Levels of Liver Enzymes a | Acrophase | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Acrophase, median (IQR) | 13.3 (12.8–13.7) | 14.3 (14.1–14.4) | 14.9 (14.7–15.0) | 15.6 (15.4–15.9) | 17.2 (16.6–18.0) | |
| ALT | ||||||
| N (%) b | 122 (9.7) | 119 (10) | 136 (10) | 142 (11) | 139 (11) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.97 (0.67, 1.41) | 0.96 (0.65, 1.42) | 1.03 (0.71, 1.49) | 0.99 (0.65, 1.51) | 0.92 |
| Model 2 | ref. | 1.02 (0.65, 1.58) | 0.9 (0.58, 1.42) | 0.96 (0.62, 1.48) | 0.95 (0.59, 1.55) | 0.73 |
| Model 3 | ref. | 1.03 (0.64, 1.65) | 0.91 (0.57, 1.45) | 0.94 (0.61, 1.46) | 0.93 (0.56, 1.55) | 0.61 |
| Model 4 | ref. | 1.03 (0.65, 1.65) | 0.92 (0.57, 1.5) | 0.99 (0.62, 1.58) | 0.94 (0.56, 1.57) | 0.71 |
| AST | ||||||
| N (%) b | 134 (11) | 123 (9.3) | 128 (9.3) | 133 (10) | 145 (11) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.93 (0.61, 1.43) | 0.95 (0.69, 1.31) | 1.04 (0.72, 1.5) | 1.13 (0.74, 1.71) | 0.41 |
| Model 2 | ref. | 0.99 (0.59, 1.67) | 0.95 (0.63, 1.42) | 0.98 (0.65, 1.47) | 1.07 (0.67, 1.7) | 0.81 |
| Model 3 | ref. | 0.98 (0.57, 1.67) | 0.94 (0.62, 1.42) | 0.96 (0.64, 1.43) | 1.03 (0.64, 1.66) | 0.97 |
| Model 4 | ref. | 0.99 (0.57, 1.73) | 0.95 (0.62, 1.47) | 0.98 (0.63, 1.52) | 1.06 (0.66, 1.71) | 0.81 |
| ALP | ||||||
| N (%) b | 52 (3.6) | 35 (3.2) | 32 (1.9) | 36 (2.1) | 52 (3.1) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.87 (0.48, 1.58) | 0.53 (0.27, 1.03) | 0.62 (0.35, 1.11) | 1.03 (0.69, 1.55) | 0.34 |
| Model 2 | ref. | 1.07 (0.54, 2.13) | 0.68 (0.29, 1.59) | 0.66 (0.33, 1.33) | 0.99 (0.61, 1.62) | 0.31 |
| Model 3 | ref. | 1.09 (0.55, 2.16) | 0.69 (0.3, 1.59) | 0.66 (0.32, 1.37) | 0.95 (0.58, 1.56) | 0.25 |
| Model 4 | ref. | 1.08 (0.52, 2.24) | 0.71 (0.29, 1.71) | 0.61 (0.28, 1.3) | 0.94 (0.52, 1.71) | 0.24 |
| GGT | ||||||
| N (%) b | 122 (9.2) | 122 (8.6) | 123 (8.6) | 120 (9.1) | 163 (11) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.87 (0.61, 1.26) | 0.89 (0.59, 1.35) | 0.98 (0.68, 1.41) | 1.34 (0.94, 1.91) | 0.08 |
| Model 2 | ref. | 0.97 (0.62, 1.53) | 0.96 (0.57, 1.62) | 0.89 (0.56, 1.41) | 1.2 (0.79, 1.82) | 0.52 |
| Model 3 | ref. | 0.99 (0.61, 1.59) | 0.97 (0.56, 1.68) | 0.88 (0.55, 1.42) | 1.17 (0.75, 1.83) | 0.66 |
| Model 4 | ref. | 1 (0.62, 1.63) | 1 (0.58, 1.74) | 0.89 (0.55, 1.46) | 1.2 (0.77, 1.87) | 0.58 |
| Albumin | ||||||
| N (%) b | 38 (2) | 29 (2) | 38 (2.6) | 38 (2.6) | 54 (3.7) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.91 (0.46, 1.8) | 1.22 (0.62, 2.39) | 1.27 (0.68, 2.37) | 2.05 (1.22, 3.42) | 0.003 |
| Model 2 | ref. | 1 (0.47, 2.09) | 1.38 (0.58, 3.3) | 1.28 (0.58, 2.81) | 1.65 (0.87, 3.14) | 0.11 |
| Model 3 | ref. | 1.03 (0.47, 2.24) | 1.42 (0.59, 3.42) | 1.28 (0.57, 2.86) | 1.62 (0.84, 3.12) | 0.13 |
| Model 4 | ref. | 1.03 (0.47, 2.27) | 1.41 (0.56, 3.58) | 1.33 (0.58, 3.05) | 1.56 (0.75, 3.27) | 0.16 |
| Bilirubin | ||||||
| N (%) b | 24 (2.2) | 27 (2.1) | 42 (3.6) | 33 (2.6) | 43 (3.9) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 1 (0.52, 1.92) | 1.7 (0.82, 3.5) | 1.11 (0.53, 2.33) | 1.44 (0.73, 2.85) | 0.25 |
| Model 2 | ref. | 0.95 (0.38, 2.38) | 1.86 (0.77, 4.45) | 1.19 (0.48, 2.94) | 1.59 (0.67, 3.75) | 0.16 |
| Model 3 | ref. | 0.95 (0.37, 2.41) | 1.85 (0.76, 4.53) | 1.18 (0.47, 2.97) | 1.58 (0.66, 3.8) | 0.16 |
| Model 4 | ref. | 0.94 (0.36, 2.43) | 1.81 (0.72, 4.58) | 1.16 (0.44, 3.04) | 1.54 (0.63, 3.8) | 0.18 |
| Abnormal Levels of Liver Enzymes a | Interdaily Stability | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Interdaily stability, median (IQR) | 0.4 (0.3, 0.4) | 0.5 (0.5, 0.5) | 0.6 (0.6, 0.6) | 0.7 (0.7, 0.7) | 0.8 (0.7, 0.8) | |
| ALT | ||||||
| N (%) b | 136 (11) | 145 (12) | 138 (9.9) | 114 (9) | 131 (11) | |
| OR (95% CI) | ||||||
| Model 1 | 0.93 (0.61, 1.42) | 1 (0.63, 1.59) | 0.83 (0.55, 1.27) | 0.76 (0.53, 1.09) | ref. | 0.84 |
| Model 2 | 0.84 (0.5, 1.4) | 1.01 (0.57, 1.78) | 0.85 (0.52, 1.38) | 0.75 (0.52, 1.1) | ref. | 0.91 |
| Model 3 | 0.79 (0.47, 1.35) | 0.96 (0.53, 1.74) | 0.81 (0.48, 1.35) | 0.73 (0.49, 1.08) | ref. | 0.74 |
| Model 4 | 0.79 (0.46, 1.37) | 0.93 (0.51, 1.71) | 0.81 (0.48, 1.37) | 0.74 (0.5, 1.09) | ref. | 0.67 |
| AST | ||||||
| N (%) b | 167 (13) | 159 (12) | 132 (8.8) | 104 (7.9) | 109 (9.2) | |
| OR (95% CI) | ||||||
| Model 1 | 1.45 (1.04, 2.01) | 1.27 (0.9, 1.79) | 0.92 (0.62, 1.36) | 0.84 (0.62, 1.12) | ref. | 0.01 |
| Model 2 | 1.32 (0.82, 2.12) | 1.26 (0.76, 2.08) | 0.95 (0.58, 1.56) | 0.79 (0.55, 1.13) | ref. | 0.09 |
| Model 3 | 1.26 (0.77, 2.05) | 1.21 (0.73, 2.02) | 0.92 (0.55, 1.53) | 0.78 (0.53, 1.14) | ref. | 0.12 |
| Model 4 | 1.33 (0.8, 2.21) | 1.23 (0.72, 2.1) | 0.94 (0.55, 1.6) | 0.8 (0.55, 1.17) | ref. | 0.11 |
| ALP | ||||||
| N (%) b | 73 (5.1) | 33 (2.1) | 37 (2.2) | 29 (2.5) | 34 (2.4) | |
| OR (95% CI) | ||||||
| Model 1 | 3.06 (1.88, 5) | 1.11 (0.6, 2.06) | 1.13 (0.69, 1.87) | 1.14 (0.6, 2.17) | ref. | 0.01 |
| Model 2 | 2.56 (1.26, 5.21) | 1.18 (0.54, 2.62) | 1.18 (0.6, 2.32) | 1.15 (0.47, 2.78) | ref. | 0.03 |
| Model 3 | 2.38 (1.15, 4.95) | 1.11 (0.49, 2.49) | 1.14 (0.56, 2.33) | 1.09 (0.44, 2.71) | ref. | 0.04 |
| Model 4 | 2.59 (1.19, 5.66) | 1.16 (0.5, 2.65) | 1.08 (0.47, 2.52) | 1.06 (0.42, 2.67) | ref. | 0.04 |
| GGT | ||||||
| N (%) b | 159 (12) | 137 (9.9) | 132 (8.9) | 111 (8) | 116 (8.5) | |
| OR (95% CI) | ||||||
| Model 1 | 1.87 (1.29, 2.71) | 1.42 (0.95, 2.13) | 1.21 (0.83, 1.75) | 0.99 (0.69, 1.42) | ref. | 0.001 |
| Model 2 | 1.72 (1.05, 2.81) | 1.49 (0.93, 2.39) | 1.11 (0.74, 1.69) | 0.99 (0.64, 1.53) | ref. | 0.02 |
| Model 3 | 1.63 (1, 2.65) | 1.42 (0.86, 2.35) | 1.07 (0.7, 1.63) | 0.96 (0.61, 1.51) | ref. | 0.02 |
| Model 4 | 1.6 (0.93, 2.77) | 1.35 (0.79, 2.29) | 1.07 (0.68, 1.67) | 0.98 (0.62, 1.55) | ref. | 0.04 |
| Albumin | ||||||
| N (%) b | 69 (4.9) | 45 (2) | 36 (2.9) | 29 (2) | 21 (1.2) | |
| OR (95% CI) | ||||||
| Model 1 | 5.65 (2.85, 11.19) | 2.12 (0.93, 4.83) | 2.9 (1.34, 6.28) | 1.78 (0.72, 4.36) | ref. | <0.001 |
| Model 2 | 5.09 (1.94, 13.39) | 1.96 (0.66, 5.81) | 2.98 (1.04, 8.58) | 1.8 (0.61, 5.35) | ref. | 0.001 |
| Model 3 | 4.7 (1.7, 13.03) | 1.82 (0.58, 5.76) | 2.81 (0.93, 8.45) | 1.7 (0.54, 5.35) | ref. | 0.002 |
| Model 4 | 4.43 (1.56, 12.58) | 1.68 (0.53, 5.29) | 2.52 (0.8, 7.89) | 1.71 (0.54, 5.43) | ref. | 0.004 |
| Bilirubin | ||||||
| N (%) b | 47 (3.5) | 39 (4.4) | 33 (2.9) | 30 (2.1) | 23 (1.5) | |
| OR (95% CI) | ||||||
| Model 1 | 1.72 (0.89, 3.33) | 2.37 (1, 5.62) | 1.63 (0.89, 2.99) | 1.3 (0.61, 2.76) | ref. | 0.02 |
| Model 2 | 1.43 (0.66, 3.12) | 2.3 (0.88, 6.01) | 1.55 (0.77, 3.13) | 1.16 (0.49, 2.74) | ref. | 0.06 |
| Model 3 | 1.42 (0.64, 3.15) | 2.29 (0.86, 6.1) | 1.54 (0.76, 3.15) | 1.16 (0.48, 2.77) | ref. | 0.07 |
| Model 4 | 1.4 (0.63, 3.13) | 2.36 (0.85, 6.58) | 1.55 (0.73, 3.27) | 1.14 (0.46, 2.83) | ref. | 0.07 |
| Abnormal Levels of Liver Enzymes a | Intradaily Variability | p Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Intradaily variability, median (IQR) | 0.4 (0.4, 0.5) | 0.6 (0.5, 0.6) | 0.7 (0.6, 0.7) | 0.8 (0.8, 0.8) | 1.0 (0.9, 1.1) | |
| ALT | ||||||
| N (%) b | 135 (11) | 123 (9.3) | 141 (11) | 152 (12) | 113 (9) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.79 (0.52, 1.18) | 0.97 (0.64, 1.49) | 1.06 (0.7, 1.62) | 0.81 (0.58, 1.14) | 0.81 |
| Model 2 | ref. | 0.99 (0.62, 1.59) | 1.15 (0.71, 1.87) | 1.35 (0.83, 2.2) | 1.01 (0.69, 1.47) | 0.35 |
| Model 3 | ref. | 0.98 (0.6, 1.59) | 1.1 (0.67, 1.83) | 1.29 (0.78, 2.14) | 0.93 (0.63, 1.36) | 0.67 |
| Model 4 | ref. | 1.01 (0.61, 1.67) | 1.15 (0.69, 1.92) | 1.31 (0.79, 2.18) | 0.97 (0.65, 1.44) | 0.53 |
| AST | ||||||
| N (%) b | 153 (12) | 112 (8.3) | 124 (9.7) | 135 (9.2) | 147 (11) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.7 (0.47, 1.04) | 0.86 (0.57, 1.29) | 0.79 (0.54, 1.17) | 0.97 (0.68, 1.4) | 0.94 |
| Model 2 | ref. | 0.87 (0.53, 1.44) | 1.04 (0.62, 1.72) | 0.99 (0.58, 1.7) | 1.32 (0.75, 2.34) | 0.28 |
| Model 3 | ref. | 0.87 (0.52, 1.45) | 1 (0.59, 1.68) | 0.95 (0.53, 1.69) | 1.22 (0.68, 2.17) | 0.44 |
| Model 4 | ref. | 0.87 (0.51, 1.48) | 1.03 (0.6, 1.77) | 0.98 (0.55, 1.74) | 1.3 (0.71, 2.38) | 0.32 |
| ALP | ||||||
| N (%) b | 38 (2.8) | 30 (2) | 34 (2.4) | 33 (1.6) | 71 (5.2) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.63 (0.34, 1.15) | 0.71 (0.35, 1.47) | 0.48 (0.25, 0.92) | 1.61 (0.97, 2.68) | 0.15 |
| Model 2 | ref. | 0.81 (0.37, 1.8) | 1.21 (0.55, 2.69) | 0.69 (0.3, 1.59) | 2.38 (1.2, 4.71) | 0.03 |
| Model 3 | ref. | 0.79 (0.34, 1.85) | 1.14 (0.49, 2.65) | 0.64 (0.27, 1.52) | 2.09 (1.02, 4.27) | 0.06 |
| Model 4 | ref. | 0.8 (0.35, 1.82) | 1.06 (0.45, 2.46) | 0.68 (0.28, 1.66) | 2.44 (1.16, 5.13) | 0.04 |
| GGT | ||||||
| N (%) b | 120 (9.3) | 122 (8.3) | 134 (8.9) | 141 (10) | 138 (10) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 0.8 (0.61, 1.06) | 0.83 (0.59, 1.15) | 0.98 (0.77, 1.26) | 1.01 (0.73, 1.41) | 0.52 |
| Model 2 | ref. | 1.02 (0.73, 1.42) | 1.06 (0.73, 1.56) | 1.36 (0.99, 1.87) | 1.42 (0.96, 2.1) | 0.04 |
| Model 3 | ref. | 1 (0.71, 1.41) | 1.01 (0.68, 1.51) | 1.28 (0.92, 1.77) | 1.29 (0.89, 1.88) | 0.09 |
| Model 4 | ref. | 1.04 (0.73, 1.48) | 1.06 (0.71, 1.58) | 1.32 (0.93, 1.87) | 1.36 (0.89, 2.09) | 0.08 |
| Albumin | ||||||
| N (%) b | 20 (1.5) | 30 (1.8) | 28 (1.7) | 46 (3.5) | 76 (4.3) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 1.07 (0.46, 2.51) | 0.94 (0.37, 2.38) | 2.07 (0.87, 4.9) | 2.72 (1.23, 6.03) | 0.001 |
| Model 2 | ref. | 1.54 (0.55, 4.37) | 1.36 (0.44, 4.21) | 3.2 (1.01, 10.07) | 3.34 (1.05, 10.68) | 0.01 |
| Model 3 | ref. | 1.51 (0.52, 4.39) | 1.27 (0.4, 4.06) | 3.01 (0.91, 10.02) | 2.97 (0.91, 9.72) | 0.01 |
| Model 4 | ref. | 1.54 (0.5, 4.74) | 1.16 (0.33, 4.08) | 2.82 (0.83, 9.57) | 3 (0.86, 10.49) | 0.02 |
| Bilirubin | ||||||
| N (%) b | 25 (1.7) | 32 (2.5) | 32 (2.7) | 36 (4.4) | 47 (3) | |
| OR (95% CI) | ||||||
| Model 1 | ref. | 1.78 (0.75, 4.22) | 2.11 (1.05, 4.23) | 3.49 (1.49, 8.14) | 2.21 (0.98, 5) | 0.003 |
| Model 2 | ref. | 2.1 (0.62, 7.16) | 2.21 (0.78, 6.28) | 4.05 (1.13, 14.51) | 2.12 (0.59, 7.59) | 0.05 |
| Model 3 | ref. | 2.1 (0.6, 7.37) | 2.21 (0.76, 6.41) | 4.03 (1.08, 15.01) | 2.11 (0.57, 7.85) | 0.06 |
| Model 4 | ref. | 2.11 (0.55, 8.1) | 2.31 (0.74, 7.2) | 4.15 (1.04, 16.46) | 2.16 (0.54, 8.69) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeung, C.H.C.; Bauer, C.; Xiao, Q. Associations between Rest–Activity Rhythms and Liver Function Tests: The US National Health and Nutrition Examination Survey, 2011–2014. Clocks & Sleep 2023, 5, 667-685. https://doi.org/10.3390/clockssleep5040045
Yeung CHC, Bauer C, Xiao Q. Associations between Rest–Activity Rhythms and Liver Function Tests: The US National Health and Nutrition Examination Survey, 2011–2014. Clocks & Sleep. 2023; 5(4):667-685. https://doi.org/10.3390/clockssleep5040045
Chicago/Turabian StyleYeung, Chris Ho Ching, Cici Bauer, and Qian Xiao. 2023. "Associations between Rest–Activity Rhythms and Liver Function Tests: The US National Health and Nutrition Examination Survey, 2011–2014" Clocks & Sleep 5, no. 4: 667-685. https://doi.org/10.3390/clockssleep5040045
APA StyleYeung, C. H. C., Bauer, C., & Xiao, Q. (2023). Associations between Rest–Activity Rhythms and Liver Function Tests: The US National Health and Nutrition Examination Survey, 2011–2014. Clocks & Sleep, 5(4), 667-685. https://doi.org/10.3390/clockssleep5040045






