Seasonal Variation in the Responsiveness of the Melanopsin System to Evening Light: Why We Should Report Season When Collecting Data in Human Sleep and Circadian Studies
Abstract
1. Introduction
2. Results
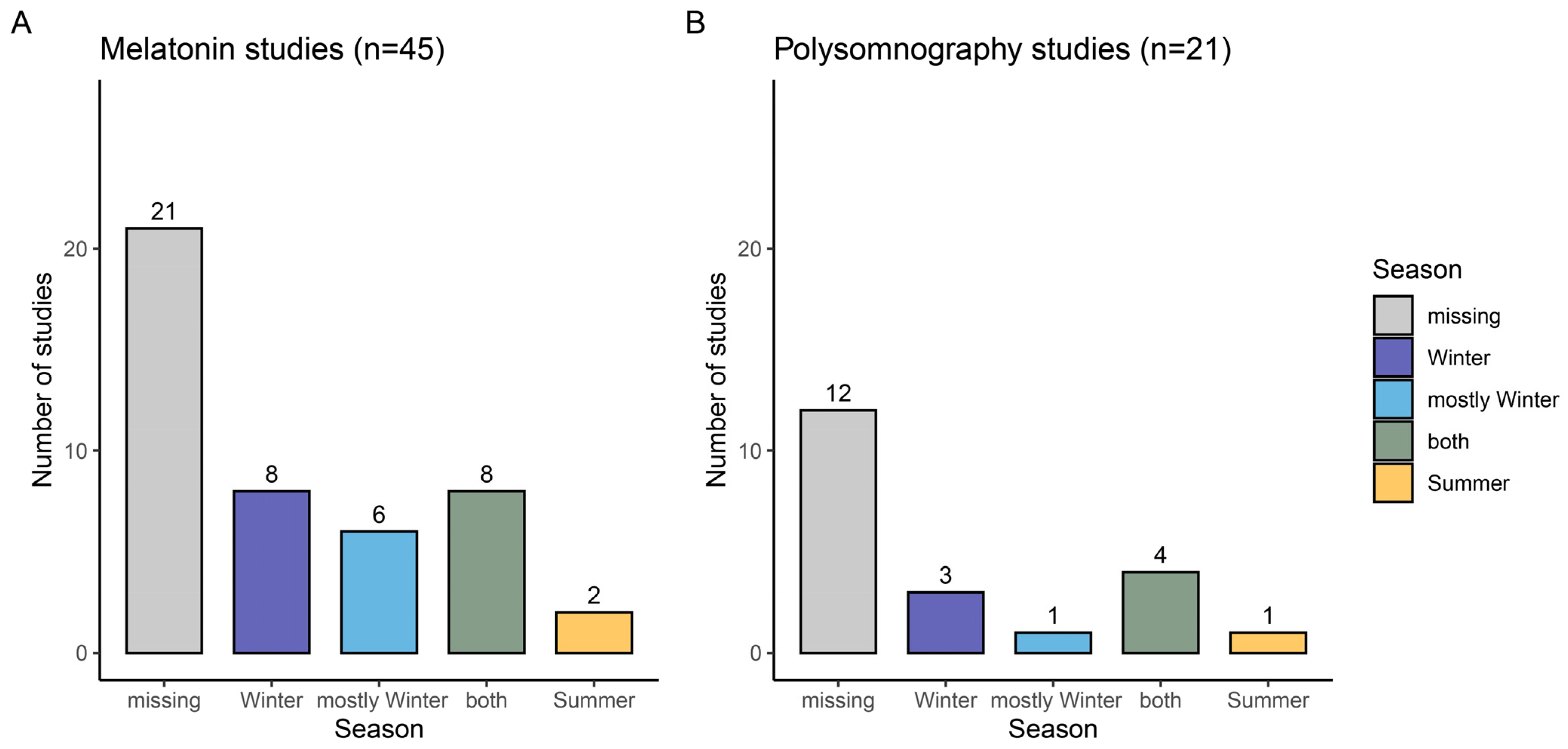
2.1. Reporting of Season in Sleep and Circadian Research on Evening Light Effects
2.1.1. Evening Melatonin Concentrations
2.1.2. Polysomnographically Assessed Night-Time Sleep
2.2. Seasonal Sensitivity to Evening Light without Controlling Light History
2.2.1. Solar Irradiance and Self-Reported Light History
2.2.2. Seasonal Dependent Light Sensitivity
3. Discussion
3.1. Seasonal Variations in Humans
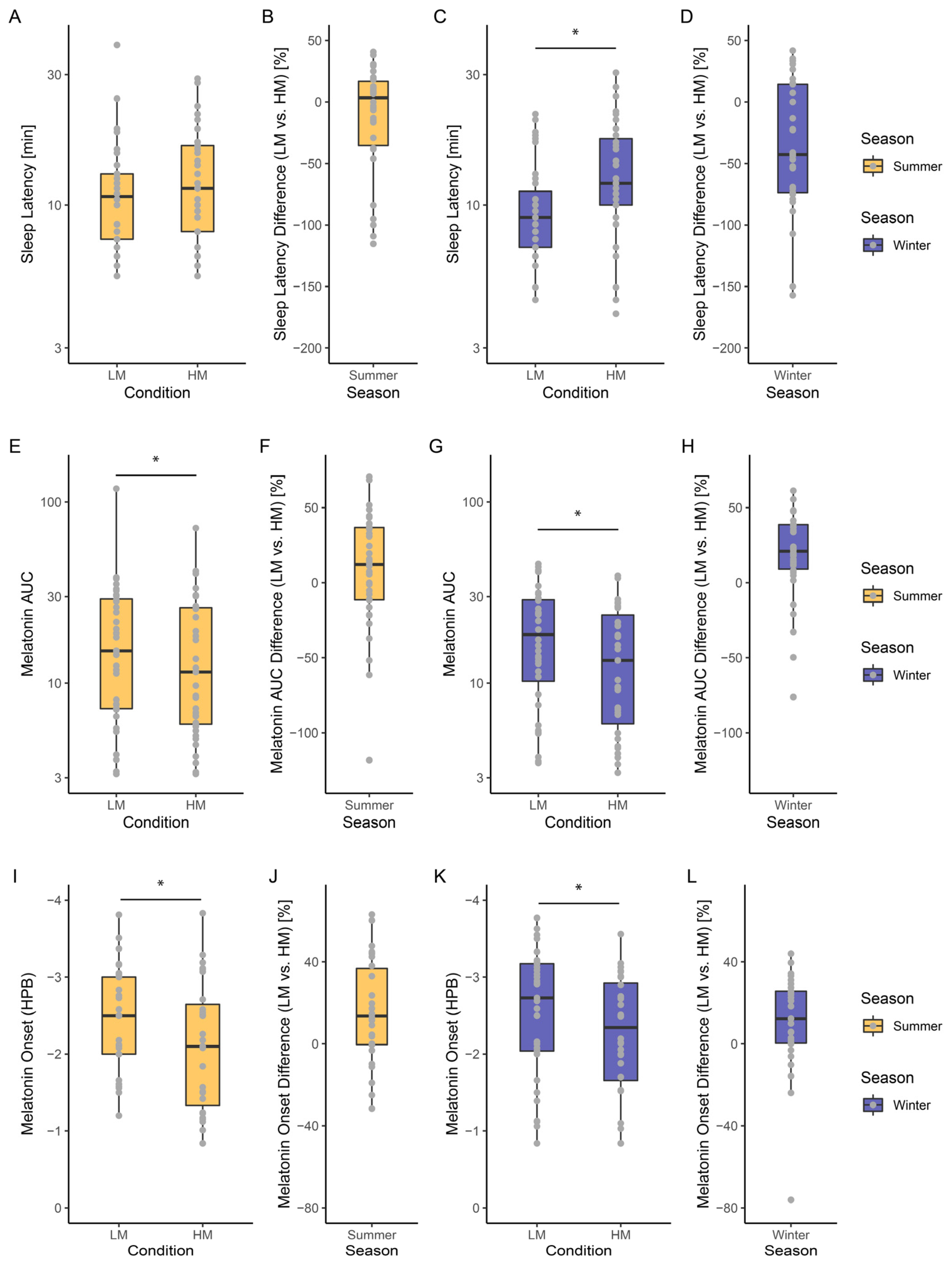
3.2. Seasonal Variation in Light-Induced Melatonin Suppression
3.3. Seasonal Variation of Light-Induced Changes in Melatonin Circadian Phase
3.4. Seasonal Variation in Sleep Architecture and Its Modification by Light
3.5. Suggestions for Improving the Reproducibility of Studies Investigating Non-Visual Effects of Light in Humans
- Studies investigating not only the non-visual effects of light in humans but also any endpoint under potential seasonal influence should report the season, seasonal distribution, and time of day of the respective measurement.
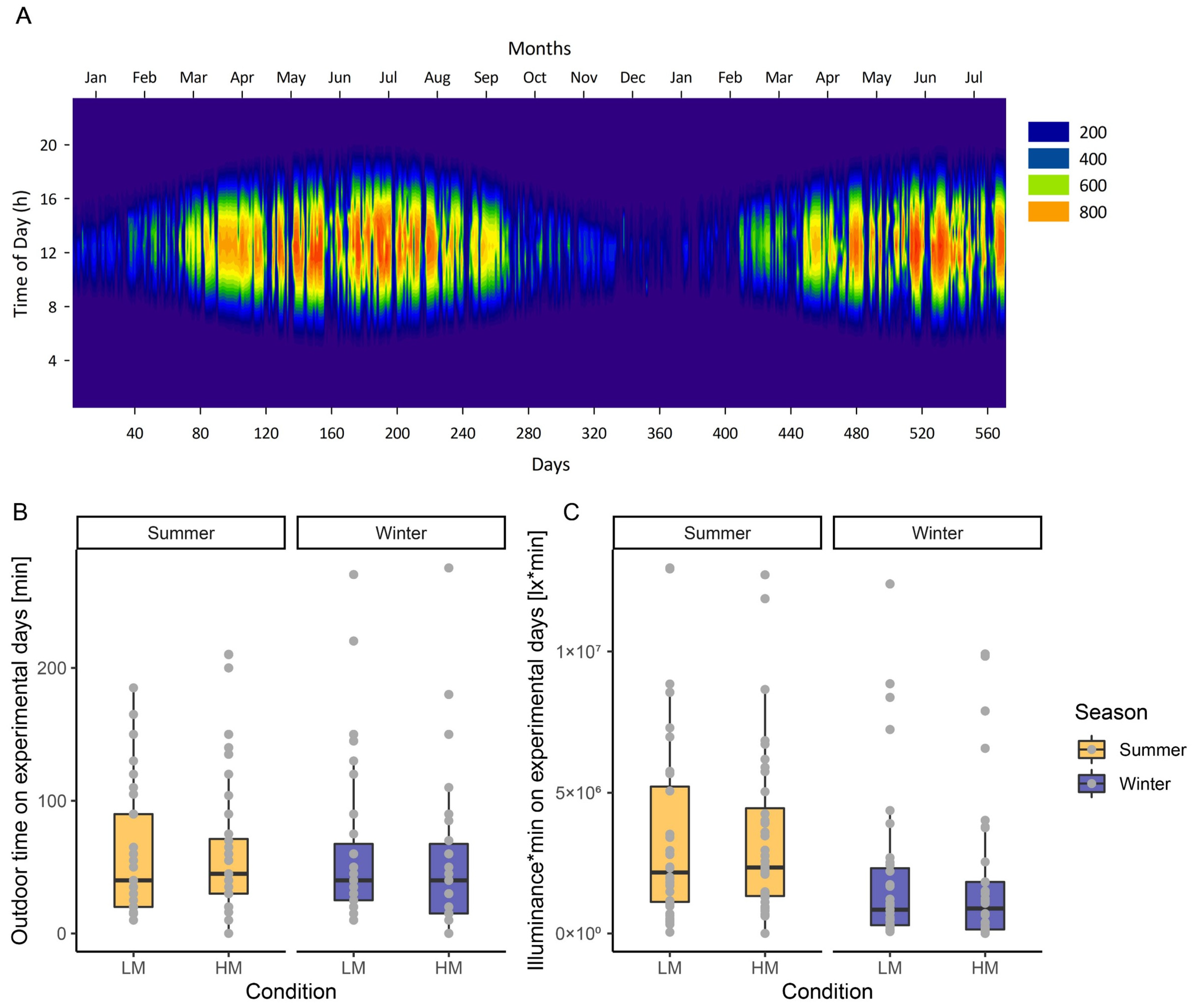
- The assessment and reporting of subjective individual light history (i.e., time spent outdoors) is a relatively simple method, together with weather conditions. The assessment should also include the time of day when outdoor activities took place. In our study, for example, subjectively reported time spent outdoors showed relatively small differences in summer and winter. This is not unlikely, given that most of our participants were students and the study was conducted during the COVID-19 pandemic.
- Taking into account the solar irradiance from local weather stations and combining this with individual time and duration spent outdoors improves the prediction of individual light history, and, for example, our study showed significant differences between summer and winter months. The most accurate is the objective assessment of individual light history by light sensors, which can be worn on different parts of the body (e.g., wrist-worn, on eye-level attached to spectacle frames or worn at the chest), the more advanced of which also provide spectral characterization of light (for an overview, see [109]) (Figure 4).
- One strategy for eliminating the effects of prior light history, if desired, is to keep participants in the laboratory on study days and control the light situation, thus reducing variance by eliminating the bias caused by variations in prior light exposure. In most constant routine studies of the effects of evening light on melatonin (e.g., [38]), illuminance was drastically reduced compared to daylight (<100 lx vs. 1000 lx (overcast day)—100,000 lx (direct sunlight)) when participants remained in the laboratory throughout the experimental day. It should be noted, however, that the ecological validity of these strictly light-controlled studies is compromised by the fact that reducing light exposure during the day increases sensitivity to evening light [15,54]. This should be kept in mind when comparing the results of different study designs.
4. Conclusions and Summary
5. Materials and Methods
5.1. Review
5.1.1. Search Melatonin Studies
5.1.2. Search Polysomnographically Assessed Sleep
5.1.3. Collected Data
5.2. Seasonal Dependent Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, D.C.; Moore, R.Y.; Reppert, S.M. (Eds.) Suprachiasmatic Nucleus: The Mind’s Clock; Oford University Press: New York, NY, USA, 1991; ISBN 978-0-19-506250-2. [Google Scholar]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Gordijn Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Bailes, H.J.; Lucas, R.J. Human Melanopsin Forms a Pigment Maximally Sensitive to Blue Light ( λ max ≈ 479 Nm) Supporting Activation of Gq/11 and Gi/o Signalling Cascades. Proc. R. Soc. B 2013, 280, 20122987. [Google Scholar] [CrossRef]
- Zerbini, G.; Winnebeck, E.C.; Merrow, M. Weekly, Seasonal, and Chronotype-dependent Variation of Dim-light Melatonin Onset. J. Pineal Res. 2021, 70, e12723. [Google Scholar] [CrossRef]
- Seidler, A.; Weihrich, K.S.; Bes, F.; De Zeeuw, J.; Kunz, D. Seasonality of Human Sleep: Polysomnographic Data of a Neuropsychiatric Sleep Clinic. Front. Neurosci. 2023, 17, 1105233. [Google Scholar] [CrossRef]
- Münch, M.; Ladaique, M.; Roemer, S.; Hashemi, K.; Kawasaki, A. Melanopsin-Mediated Acute Light Responses Measured in Winter and in Summer: Seasonal Variations in Adults with and without Cataracts. Front. Neurol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Kawasaki, A.; Wisniewski, S.; Healey, B.; Pattyn, N.; Kunz, D.; Basner, M.; Münch, M. Impact of Long-Term Daylight Deprivation on Retinal Light Sensitivity, Circadian Rhythms and Sleep during the Antarctic Winter. Sci. Rep. 2018, 8, 16185. [Google Scholar] [CrossRef]
- Spitschan, M.; Woelders, T. The Method of Silent Substitution for Examining Melanopsin Contributions to Pupil Control. Front. Neurol. 2018, 9, 941. [Google Scholar] [CrossRef]
- Prayag, A.S.; Najjar, R.P.; Gronfier, C. Melatonin Suppression Is Exquisitely Sensitive to Light and Primarily Driven by Melanopsin in Humans. J. Pineal Res. 2019, 66, e12562. [Google Scholar] [CrossRef]
- Giménez, M.C.; Stefani, O.; Cajochen, C.; Lang, D.; Deuring, G.; Schlangen, L.J.M. Predicting Melatonin Suppression by Light in Humans: Unifying Photoreceptor-based Equivalent Daylight Illuminances, Spectral Composition, Timing and Duration of Light Exposure. J. Pineal Res. 2022, 72, e12786. [Google Scholar] [CrossRef]
- Schöllhorn, I.; Stefani, O.; Lucas, R.J.; Spitschan, M.; Slawik, H.C.; Cajochen, C. Melanopic Irradiance Defines the Impact of Evening Display Light on Sleep Latency, Melatonin and Alertness. Commun. Biol. 2023, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Coomans, C.P.; Ramkisoensing, A.; Meijer, J.H. The Suprachiasmatic Nuclei as a Seasonal Clock. Front. Neuroendocrinol. 2015, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Amritwar, A.; Hong, L.E.; Mohyuddin, I.; Brown, T.; Postolache, T.T. Daily and Seasonal Variation in Light Exposure among the Old Order Amish. Int. J. Environ. Res. Public Health 2020, 17, 4460. [Google Scholar] [CrossRef] [PubMed]
- Hébert, M.; Martin, S.K.; Lee, C.; Eastman, C.I. The Effects of Prior Light History on the Suppression of Melatonin by Light in Humans. J. Pineal Res. 2002, 33, 198–203. [Google Scholar] [CrossRef]
- Smith, K.A.; Schoen, M.W.; Czeisler, C.A. Adaptation of Human Pineal Melatonin Suppression by Recent Photic History. J. Clin. Endocrinol. Metab. 2004, 89, 3610–3614. [Google Scholar] [CrossRef]
- Owen, J.; Arendt, J. Melatonin Suppression in Human Subjects by Bright and Dim Light in Antarctica: Time and Season-Dependent Effects. Neurosci. Lett. 1992, 137, 181–184. [Google Scholar] [CrossRef]
- Chang, A.-M.; Scheer, F.A.J.L.; Czeisler, C.A.; Aeschbach, D. Direct Effects of Light on Alertness, Vigilance, and the Waking Electroencephalogram in Humans Depend on Prior Light History. Sleep 2013, 36, 1239–1246. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Duffy, J.F.; Czeisler, C.A. Unrestricted Evening Use of Light-Emitting Tablet Computers Delays Self-Selected Bedtime and Disrupts Circadian Timing and Alertness. Physiol. Rep. 2018, 6, e13692. [Google Scholar] [CrossRef]
- Rångtell, F.H.; Ekstrand, E.; Rapp, L.; Lagermalm, A.; Liethof, L.; Búcaro, M.O.; Lingfors, D.; Broman, J.-E.; Schiöth, H.B.; Benedict, C. Two Hours of Evening Reading on a Self-Luminous Tablet vs. Reading a Physical Book Does Not Alter Sleep after Daytime Bright Light Exposure. Sleep Med. 2016, 23, 111–118. [Google Scholar] [CrossRef]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.M.; Archer, S.N.; Dijk, D.-J. The Spectral Composition of Evening Light and Individual Differences in the Suppression of Melatonin and Delay of Sleep in Humans: Artificial Evening Light Suppresses Melatonin and Delays Sleep. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Wood, B.; Plitnick, B.; Rea, M.S. The Impact of Watching Television on Evening Melatonin Levels: Impact of Watching Television on Evening Melatonin. J. Soc. Inf. Disp. 2013, 21, 417–421. [Google Scholar] [CrossRef]
- Kräuchi, K.; Cajochen, C.; Danilenko, K.V.; Wirz-Justice, A. The Hypothermic Effect of Late Evening Melatonin Does Not Block the Phase Delay Induced by Concurrent Bright Light in Human Subjects. Neurosci. Lett. 1997, 232, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Pelzl, M.A.; Kann, P.H.; Koehler, U.; Betz, M.; Hildebrandt, O.; Cassel, W. The Effects of Prolonged Single Night Session of Videogaming on Sleep and Declarative Memory. PLoS ONE 2019, 14, e0224893. [Google Scholar] [CrossRef]
- Thompson, A.; Jones, H.; Marqueze, E.; Gregson, W.; Atkinson, G. The Effects of Evening Bright Light Exposure on Subsequent Morning Exercise Performance. Int. J. Sport. Med. 2014, 36, 101–106. [Google Scholar] [CrossRef]
- Lack, L.; Wright, H. The Effect of Evening Bright Light in Delaying the Circadian Rhythms and Lengthening the Sleep of Early Morning Awakening Insomniacs. Sleep 1993, 16, 436–443. [Google Scholar] [CrossRef]
- Souman, J.L.; Borra, T.; de Goijer, I.; Schlangen, L.J.M.; Vlaskamp, B.N.S.; Lucassen, M.P. Spectral Tuning of White Light Allows for Strong Reduction in Melatonin Suppression without Changing Illumination Level or Color Temperature. J. Biol. Rhythm. 2018, 33, 420–431. [Google Scholar] [CrossRef]
- Knaier, R.; Schäfer, J.; Rossmeissl, A.; Klenk, C.; Hanssen, H.; Höchsmann, C.; Cajochen, C.; Schmidt-Trucksäss, A. Prime Time Light Exposures Do Not Seem to Improve Maximal Physical Performance in Male Elite Athletes, but Enhance End-Spurt Performance. Front. Physiol. 2017, 8, 264. [Google Scholar] [CrossRef]
- Höhn, C.; Schmid, S.R.; Plamberger, C.P.; Bothe, K.; Angerer, M.; Gruber, G.; Pletzer, B.; Hoedlmoser, K. Preliminary Results: The Impact of Smartphone Use and Short-Wavelength Light during the Evening on Circadian Rhythm, Sleep and Alertness. Clocks Sleep 2021, 3, 5. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Earl, C.R.; Shaw, P.F.; Royles, P.; Carbone, F.; Webb, H. Phase Delay of the Rhythm of 6-Sulphatoxy Melatonin Excretion by Artificial Light. J. Pineal Res. 1987, 4, 315–320. [Google Scholar] [CrossRef]
- Wahnschaffe, A.; Haedel, S.; Rodenbeck, A.; Stoll, C.; Rudolph, H.; Kozakov, R.; Schoepp, H.; Kunz, D. Out of the Lab and into the Bathroom: Evening Short-Term Exposure to Conventional Light Suppresses Melatonin and Increases Alertness Perception. Int. J. Mol. Sci. 2013, 14, 2573–2589. [Google Scholar] [CrossRef]
- van der Lely, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Götz, T.; Cajochen, C. Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef]
- Spitschan, M.; Lazar, R.; Yetik, E.; Cajochen, C. No Evidence for an S Cone Contribution to Acute Neuroendocrine and Alerting Responses to Light. Curr. Biol. 2019, 29, R1297–R1298. [Google Scholar] [CrossRef] [PubMed]
- Ritter, P.; Wieland, F.; Skene, D.J.; Pfennig, A.; Weiss, M.; Bauer, M.; Severus, E.; Güldner, H.; Sauer, C.; Soltmann, B.; et al. Melatonin Suppression by Melanopsin-Weighted Light in Patients with Bipolar I Disorder Compared to Healthy Controls. J. Psychiatry Neurosci. 2020, 45, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lasko, T.A.; Kripke, D.F.; Elliot, J.A. Melatonin Suppression by Illumination of Upper and Lower Visual Fields. J. Biol. Rhythm. 1999, 14, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Xhrouet, M.; Hamacher, M.; Delloye, E.; LeGoff, C.; Cavalier, E.; Collette, F.; Vandewalle, G. Light Exposure via a Head-Mounted Device Suppresses Melatonin and Improves Vigilant Attention without Affecting Cortisol and Comfort: Head-Mounted Light, Melatonin, Vigilance, & Comfort. Psych. J. 2018, 7, 163–175. [Google Scholar] [CrossRef]
- Weng, M.; Schöllhorn, I.; Kazhura, M.; Cardini, B.B.; Stefani, O. Impact of Evening Light Exposures with Different Solid Angles on Circadian Melatonin Rhythms, Alertness, and Visual Comfort in an Automotive Setting. Clocks Sleep 2022, 4, 47. [Google Scholar] [CrossRef]
- Chang, A.-M.; Santhi, N.; St Hilaire, M.; Gronfier, C.; Bradstreet, D.S.; Duffy, J.F.; Lockley, S.W.; Kronauer, R.E.; Czeisler, C.A. Human Responses to Bright Light of Different Durations: Light DRC in Humans. J. Physiol. 2012, 590, 3103–3112. [Google Scholar] [CrossRef]
- Schmid, S.R.; Höhn, C.; Bothe, K.; Plamberger, C.P.; Angerer, M.; Pletzer, B.; Hoedlmoser, K. How Smart Is It to Go to Bed with the Phone? The Impact of Short-Wavelength Light and Affective States on Sleep and Circadian Rhythms. Clocks Sleep 2021, 3, 40. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High Sensitivity and Interindividual Variability in the Response of the Human Circadian System to Evening Light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef]
- Allen, A.E.; Hazelhoff, E.M.; Martial, F.P.; Cajochen, C.; Lucas, R.J. Exploiting Metamerism to Regulate the Impact of a Visual Display on Alertness and Melatonin Suppression Independent of Visual Appearance. Sleep 2018, 41, zsy100. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.-M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening Use of Light-Emitting eReaders Negatively Affects Sleep, Circadian Timing, and next-Morning Alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Wirz-Justice, A.; Krauchi, K.; Cajochen, C.; Danilenko, K.V.; Renz, C.; Weber, J.M. Evening Melatonin and Bright Light Administration Induce Additive Phase Shifts in Dim Light Melatonin Onset. J. Pineal Res. 2004, 36, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Evening Light Exposure to Computer Screens Disrupts Human Sleep, Biological Rhythms, and Attention Abilities. Chronobiol. Int. 2017, 34, 855–865. [Google Scholar] [CrossRef]
- Cajochen, C.; Jud, C.; Münch, M.; Kobialka, S.; Wirz-Justice, A.; Albrecht, U. Evening Exposure to Blue Light Stimulates the Expression of the Clock Gene PER2 in Humans. Eur. J. Neurosci. 2006, 23, 1082–1086. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening Exposure to a Light-Emitting Diodes (LED)-Backlit Computer Screen Affects Circadian Physiology and Cognitive Performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef]
- Jones, M.J.; Peeling, P.; Dawson, B.; Halson, S.; Miller, J.; Dunican, I.; Clarke, M.; Goodman, C.; Eastwood, P. Evening Electronic Device Use: The Effects on Alertness, Sleep and next-Day Physical Performance in Athletes. J. Sport. Sci. 2018, 36, 162–170. [Google Scholar] [CrossRef]
- Jo, H.; Park, H.R.; Choi, S.J.; Lee, S.-Y.; Kim, S.J.; Joo, E.Y. Effects of Organic Light-Emitting Diodes on Circadian Rhythm and Sleep. Psychiatry Investig. 2021, 18, 471–477. [Google Scholar] [CrossRef]
- Harada, T. Effects of Evening Light Conditions on Salivary Melatonin of Japanese Junior High School Students. J. Circadian Rhythm. 2005, 2, 4. [Google Scholar] [CrossRef]
- Bunnell, D.E.; Treiber, S.P.; Phillips, N.H.; Berger, R.J. Effects of Evening Bright Light Exposure on Melatonin, Body Temperature and Sleep. J. Sleep Res. 1992, 1, 17–23. [Google Scholar] [CrossRef]
- Knaier, R.; Schäfer, J.; Rossmeissl, A.; Klenk, C.; Hanssen, H.; Höchsmann, C.; Cajochen, C.; Schmidt-Trucksäss, A. Effects of Bright and Blue Light on Acoustic Reaction Time and Maximum Handgrip Strength in Male Athletes: A Randomized Controlled Trial. Eur. J. Appl. Physiol. 2017, 117, 1689–1696. [Google Scholar] [CrossRef]
- Wright, H.R.; Lack, L.C. Effect of Light Wavelength on Suppression and Phase Delay of the Melatonin Rhythm. Chronobiol. Int. 2001, 18, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Te Kulve, M.; Schlangen, L.J.M.; Van Marken Lichtenbelt, W.D. Early Evening Light Mitigates Sleep Compromising Physiological and Alerting Responses to Subsequent Late Evening Light. Sci. Rep. 2019, 9, 16064. [Google Scholar] [CrossRef] [PubMed]
- Nagare, R.; Plitnick, B.; Figueiro, M. Does the iPad Night Shift Mode Reduce Melatonin Suppression? Light. Res. Technol. 2019, 51, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Léon, L.; Collomb, S.; Kawasaki, A. Comparison of Acute Non-Visual Bright Light Responses in Patients with Optic Nerve Disease, Glaucoma and Healthy Controls. Sci. Rep. 2015, 5, 15185. [Google Scholar] [CrossRef]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Comparing the Response to Acute and Chronic Exposure to Short Wavelength Lighting Emitted from Computer Screens. Chronobiol. Int. 2018, 35, 90–100. [Google Scholar] [CrossRef]
- Moderie, C.; Van Der Maren, S.; Dumont, M. Circadian Phase, Dynamics of Subjective Sleepiness and Sensitivity to Blue Light in Young Adults Complaining of a Delayed Sleep Schedule. Sleep Med. 2017, 34, 148–155. [Google Scholar] [CrossRef]
- Lovato, N.; Lack, L. Circadian Phase Delay Using the Newly Developed Re-Timer Portable Light Device. Sleep Biol. Rhythm. 2016, 14, 157–164. [Google Scholar] [CrossRef]
- van der Lely, S.; Frey, S.; Garbazza, C.; Wirz-Justice, A.; Jenni, O.G.; Steiner, R.; Wolf, S.; Cajochen, C.; Bromundt, V.; Schmidt, C. Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. J. Adolesc. Health 2015, 56, 113–119. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Bromundt, V.; Frey, S.; Steinemann, A.; Schmidt, C.; Schlote, T.; Goldblum, D.; Cajochen, C. Association of Intraocular Cataract Lens Replacement With Circadian Rhythms, Cognitive Function, and Sleep in Older Adults. JAMA Ophthalmol. 2019, 137, 878. [Google Scholar] [CrossRef]
- Nowozin, C.; Wahnschaffe, A.; Rodenbeck, A.; de Zeeuw, J.; Hädel, S.; Kozakov, R.; Schöpp, H.; Münch, M.; Kunz, D. Applying Melanopic Lux to Measure Biological Light Effects on Melatonin Suppression and Subjective Sleepiness. CAR 2017, 14, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Saletu, B.; Dietzel, M.; Lesch, O.M.; Musalek, M.; Walter, H.; Grünberger, J. Effect of Biologically Active Light and Partial Sleep Deprivation on Sleep, Awakening and Circadian Rhythms in Normals. Eur. Neurol. 1986, 25, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Drennan, M.; Kripke, D.F.; Gillin, J.C. Bright Light Can Delay Human Temperature Rhythm Independent of Sleep. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1989, 257, R136–R141. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.; Campbell, S.S. Timed Exposure to Bright Light Improves Sleep and Alertness during Simulated Night Shifts. Sleep 1991, 14, 511–516. [Google Scholar] [CrossRef]
- Cajochen, C.; Dijk, D.J.; Borbély, A.A. Dynamics of EEG Slow-Wave Activity and Core Body Temperature in Human Sleep After Exposure to Bright Light. Sleep 1992, 15, 337–343. [Google Scholar] [CrossRef]
- Dumont, M.; Carrier, J. Daytime Sleep Propensity After Moderate Circadian Phase Shifts Induced With Bright Light Exposure. Sleep 1997, 20, 11–17. [Google Scholar] [CrossRef][Green Version]
- Cajochen, C.; Kräuchi, K.; Danilenko, K.V.; Wirz-Justice, A. Evening Administration of Melatonin and Bright Light: Interactions on the EEG during Sleep and Wakefulness. J. Sleep Res. 1998, 7, 145–157. [Google Scholar] [CrossRef]
- Gordijn, M.C.M.; Beersma, D.G.M.; Korte, H.J.; Hoofdakker, R.H. Effects of Light Exposure and Sleep Displacement on Dim Light Melatonin Onset. J. Sleep Res. 1999, 8, 163–174. [Google Scholar] [CrossRef]
- Komada, Y.; Tanaka, H.; Yamamoto, Y.; Shirakawa, S.; Yamazaki, K. Effects of Bright Light Pre-exposure on Sleep Onset Process. Psychiatry Clin. Neurosci. 2000, 54, 365–366. [Google Scholar] [CrossRef]
- Burgess, H.J.; Sletten, T.; Savic, N.; Gilbert, S.S.; Dawson, D. Effects of Bright Light and Melatonin on Sleep Propensity, Temperature, and Cardiac Activity at Night. J. Appl. Physiol. 2001, 91, 1214–1222. [Google Scholar] [CrossRef]
- Kozaki, T.; Kitamura, S.; Higashihara, Y.; Ishibashi, K.; Noguchi, H.; Yasukouchi, A. Effect of Color Temperature of Light Sources on Slow-Wave Sleep. J. Physiol. Anthr. Appl. Hum. Sci. 2005, 24, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Münch, M.; Kobialka, S.; Steiner, R.; Oelhafen, P.; Wirz-Justice, A.; Cajochen, C. Wavelength-Dependent Effects of Evening Light Exposure on Sleep Architecture and Sleep EEG Power Density in Men. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1421–R1428. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Biase, R.D.; Imai, M. Interhemispheric EEG Asymmetries during Unilateral Bright-Light Exposure and Subsequent Sleep in Humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1053–R1060. [Google Scholar] [CrossRef]
- Münch, M.; Scheuermaier, K.D.; Zhang, R.; Dunne, S.P.; Guzik, A.M.; Silva, E.J.; Ronda, J.M.; Duffy, J.F. Effects on Subjective and Objective Alertness and Sleep in Response to Evening Light Exposure in Older Subjects. Behav. Brain Res. 2011, 224, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute Exposure to Evening Blue-Enriched Light Impacts on Human Sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef]
- Hilditch, C.J.; Wong, L.R.; Bathurst, N.G.; Feick, N.H.; Pradhan, S.; Santamaria, A.; Shattuck, N.L.; Flynn-Evans, E.E. Rise and Shine: The Use of Polychromatic Short-wavelength-enriched Light to Mitigate Sleep Inertia at Night Following Awakening from Slow-wave Sleep. J. Sleep Res. 2022, 31, e13558. [Google Scholar] [CrossRef] [PubMed]
- Vethe, D.; Drews, H.J.; Scott, J.; Engstrøm, M.; Heglum, H.S.A.; Grønli, J.; Wisor, J.P.; Sand, T.; Lydersen, S.; Kjørstad, K.; et al. Evening Light Environments Can Be Designed to Consolidate and Increase the Duration of REM-Sleep. Sci. Rep. 2022, 12, 8719. [Google Scholar] [CrossRef]
- Michael, P.R.; Johnston, D.E.; Moreno, W. A Conversion Guide: Solar Irradiance and Lux Illuminance. J. Meas. Eng. 2020, 8, 153–166. [Google Scholar] [CrossRef]
- Dunster, G.P.; Hua, I.; Grahe, A.; Fleischer, J.G.; Panda, S.; Wright, K.P.; Vetter, C.; Doherty, J.H.; De La Iglesia, H.O. Daytime Light Exposure Is a Strong Predictor of Seasonal Variation in Sleep and Circadian Timing of University Students. J. Pineal Res. 2023, 74, e12843. [Google Scholar] [CrossRef]
- Adamsson, M.; Laike, T.; Morita, T. Annual Variation in Daily Light Exposure and Circadian Change of Melatonin and Cortisol Concentrations at a Northern Latitude with Large Seasonal Differences in Photoperiod Length. J. Physiol. Anthr. 2017, 36, 6. [Google Scholar] [CrossRef]
- Nioi, A.; Roe, J.; Gow, A.; McNair, D.; Aspinall, P. Seasonal Differences in Light Exposure and the Associations With Health and Well-Being in Older Adults: An Exploratory Study. HERD 2017, 10, 64–79. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.M. Seasonal Rhythms of Human Nutrient Intake and Meal Pattern. Physiol. Behav. 1991, 50, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Greenberg, G.; Miall, W.E.; Thompson, S.G. Seasonal Variation in Arterial Blood Pressure. BMJ 1982, 285, 919–923. [Google Scholar] [CrossRef]
- Gordon, D.J.; Trost, D.C.; Hyde, J.; Whaley, F.S.; Hannan, P.J.; Jacobs, D.R.; Ekelund, L.G. Seasonal Cholesterol Cycles: The Lipid Research Clinics Coronary Primary Prevention Trial Placebo Group. Circulation 1987, 76, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Aschof, J. Annual Rhythm of Human Reproduction: I. Biology, Sociology, or Both? J. Biol. Rhythm. 1990, 5, 195–216. [Google Scholar] [CrossRef]
- Patten, S.B.; Williams, J.V.A.; Lavorato, D.H.; Bulloch, A.G.M.; Fiest, K.M.; Wang, J.L.; Sajobi, T.T. Seasonal Variation in Major Depressive Episode Prevalence in Canada. Epidemiol. Psychiatr. Sci. 2017, 26, 169–176. [Google Scholar] [CrossRef]
- Kasper, S.; Wehr, T.A.; Bartko, J.J.; Gaist, P.A.; Rosenthal, N.E. Epidemiological Findings of Seasonal Changes in Mood and Behavior. A Telephone Survey of Montgomery County, Maryland. Arch. Gen. Psychiatry 1989, 46, 823–833. [Google Scholar] [CrossRef]
- Lambert, G.; Reid, C.; Kaye, D.; Jennings, G.; Esler, M. Effect of Sunlight and Season on Serotonin Turnover in the Brain. Lancet 2002, 360, 1840–1842. [Google Scholar] [CrossRef]
- Meyer, C.; Muto, V.; Jaspar, M.; Kussé, C.; Lambot, E.; Chellappa, S.L.; Degueldre, C.; Balteau, E.; Luxen, A.; Middleton, B.; et al. Seasonality in Human Cognitive Brain Responses. Proc. Natl. Acad. Sci. USA 2016, 113, 3066–3071. [Google Scholar] [CrossRef]
- Higuchi, S.; Motohashi, Y.; Ishibashi, K.; Maeda, T. Less Exposure to Daily Ambient Light in Winter Increases Sensitivity of Melatonin to Light Suppression. Chronobiol. Int. 2007, 24, 31–43. [Google Scholar] [CrossRef]
- Nathan, P.J.; Burrows, G.D.; Norman, T.R. Melatonin Sensitivity to Dim White Light in Different Seasons. Hum. Psychopharmacol. Clin. Exp. 1999, 14, 53–58. [Google Scholar] [CrossRef]
- Gamlin, P.D.R.; McDougal, D.H.; Pokorny, J.; Smith, V.C.; Yau, K.-W.; Dacey, D.M. Human and Macaque Pupil Responses Driven by Melanopsin-Containing Retinal Ganglion Cells. Vis. Res. 2007, 47, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zeitzer, J.M.; Czeisler, C.A. Decreased Sensitivity to Phase-Delaying Effects of Moderate Intensity Light in Older Subjects. Neurobiol. Aging 2007, 28, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A Phase Response Curve to Single Bright Light Pulses in Human Subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef]
- Illnerová, H.; Zvolsky, P.; Vaněček, J. The Circadian Rhythm in Plasma Melatonin Concentration of the Urbanized Man: The Effect of Summer and Winter Time. Brain Res. 1985, 328, 186–189. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Royles, P. Circadian Rhythms of 6-Sulphatoxy Melatonin, Cortisol and Electrolyte Excretion at the Summer and Winter Solstices in Normal Men and Women. Acta Endocrinol. 1986, 113, 450–456. [Google Scholar] [CrossRef]
- Bojkowski, C.J.; Arendt, J. Annual Changes in 6-Sulphatoxymelatonin Excretion in Man. Acta Endocrinol. 1988, 117, 470–476. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Kobelev, E.; Semenova, E.A.; Aftanas, L.I. Summer-Winter Difference in 24-h Melatonin Rhythms in Subjects on a 5-Workdays Schedule in Siberia without Daylight Saving Time Transitions. Physiol. Behav. 2019, 212, 112686. [Google Scholar] [CrossRef]
- Yetish, G.; Kaplan, H.; Gurven, M.; Wood, B.; Pontzer, H.; Manger, P.R.; Wilson, C.; McGregor, R.; Siegel, J.M. Natural Sleep and Its Seasonal Variations in Three Pre-Industrial Societies. Curr. Biol. 2015, 25, 2862–2868. [Google Scholar] [CrossRef]
- Suzuki, M.; Taniguchi, T.; Furihata, R.; Yoshita, K.; Arai, Y.; Yoshiike, N.; Uchiyama, M. Seasonal Changes in Sleep Duration and Sleep Problems: A Prospective Study in Japanese Community Residents. PLoS ONE 2019, 14, e0215345. [Google Scholar] [CrossRef]
- Wehr, T.A. The Durations of Human Melatonin Secretion and Sleep Respond to Changes in Daylength (Photoperiod). J. Clin. Endocrinol. Metab. 1991, 73, 1276–1280. [Google Scholar] [CrossRef]
- Kohsaka, M.; Fukuda, N.; Honma, K.; Honma, S.; Morita, N. Seasonality in Human Sleep. Experientia 1992, 48, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Askenasy, J.J.M.; Goldstein, R. Does a Subtropical Climate Imply a Seasonal Rhythm in REM Sleep? Sleep 1995, 18, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Kobayashi, T.; Yamamoto, T.; Fukuda, H.; Sasaki, M.; Ohta, T. Persistence of the Circadian Rhythm of REM Sleep: A Variety of Experimental Manipulations of the Sleep-Wake Cycle. Sleep 1981, 4, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Weitzman, E.D.; Moore-Ede, M.C.; Zimmerman, J.C.; Knauer, R.S. Human Sleep: Its Duration and Organization Depend on Its Circadian Phase. Science 1980, 210, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Wurts, S.W.; Edgar, D.M. Circadian and Homeostatic Control of Rapid Eye Movement (REM) Sleep: Promotion of REM Tendency by the Suprachiasmatic Nucleus. J. Neurosci. 2000, 20, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Stefani, O.; Blattner, P.; Gronfier, C.; Lockley, S.; Lucas, R. How to Report Light Exposure in Human Chronobiology and Sleep Research Experiments. Clocks Sleep 2019, 1, 24. [Google Scholar] [CrossRef]
- Hartmeyer, S.; Webler, F.; Andersen, M. Towards a Framework for Light-Dosimetry Studies: Methodological Considerations. Light. Res. Technol. 2022, 147715352211032. [Google Scholar] [CrossRef]
- Cajochen, C.; Stefani, O.; Schöllhorn, I.; Lang, D.; Chellappa, S. Influence of Evening Light Exposure on Polysomnographically Assessed Night-Time Sleep: A Systematic Review with Meta-Analysis. Light. Res. Technol. 2022, 147715352210787. [Google Scholar] [CrossRef]
- Cho, J.R.; Joo, E.Y.; Koo, D.L.; Hong, S.B. Let There Be No Light: The Effect of Bedside Light on Sleep Quality and Background Electroencephalographic Rhythms. Sleep Med. 2013, 14, 1422–1425. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lee, H.-J.; Yoon, H.-K.; Kang, S.-G.; Bok, K.-N.; Jung, K.-Y.; Kim, L.; Lee, E.-I. Exposure to Dim Artificial Light at Night Increases REM Sleep and Awakenings in Humans. Chronobiol. Int. 2016, 33, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Takasu, N.N.; Hashimoto, S.; Yamanaka, Y.; Tanahashi, Y.; Yamazaki, A.; Honma, S.; Honma, K. Repeated Exposures to Daytime Bright Light Increase Nocturnal Melatonin Rise and Maintain Circadian Phase in Young Subjects under Fixed Sleep Schedule. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R1799–R1807. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Beersma, D.G.; Daan, S.; Lewy, A.J. Bright Morning Light Advances the Human Circadian System without Affecting NREM Sleep Homeostasis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1989, 256, R106–R111. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Mead, J.; Roos, C.; Lowis, C.; Griffiths, B.; Mucur, P.; Herf, M.; Nam, S.; Veitch, J.A. luox: Validated reference open-access and open-source web platform for calculating and sharing physiologically relevant quantities for light and lighting. Wellcome Open Res. 2022, 6, 69. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöllhorn, I.; Stefani, O.; Blume, C.; Cajochen, C. Seasonal Variation in the Responsiveness of the Melanopsin System to Evening Light: Why We Should Report Season When Collecting Data in Human Sleep and Circadian Studies. Clocks & Sleep 2023, 5, 651-666. https://doi.org/10.3390/clockssleep5040044
Schöllhorn I, Stefani O, Blume C, Cajochen C. Seasonal Variation in the Responsiveness of the Melanopsin System to Evening Light: Why We Should Report Season When Collecting Data in Human Sleep and Circadian Studies. Clocks & Sleep. 2023; 5(4):651-666. https://doi.org/10.3390/clockssleep5040044
Chicago/Turabian StyleSchöllhorn, Isabel, Oliver Stefani, Christine Blume, and Christian Cajochen. 2023. "Seasonal Variation in the Responsiveness of the Melanopsin System to Evening Light: Why We Should Report Season When Collecting Data in Human Sleep and Circadian Studies" Clocks & Sleep 5, no. 4: 651-666. https://doi.org/10.3390/clockssleep5040044
APA StyleSchöllhorn, I., Stefani, O., Blume, C., & Cajochen, C. (2023). Seasonal Variation in the Responsiveness of the Melanopsin System to Evening Light: Why We Should Report Season When Collecting Data in Human Sleep and Circadian Studies. Clocks & Sleep, 5(4), 651-666. https://doi.org/10.3390/clockssleep5040044






