Motus Vita Est: Fruit Flies Need to Be More Active and Sleep Less to Adapt to Either a Longer or Harder Life
Abstract
1. Introduction
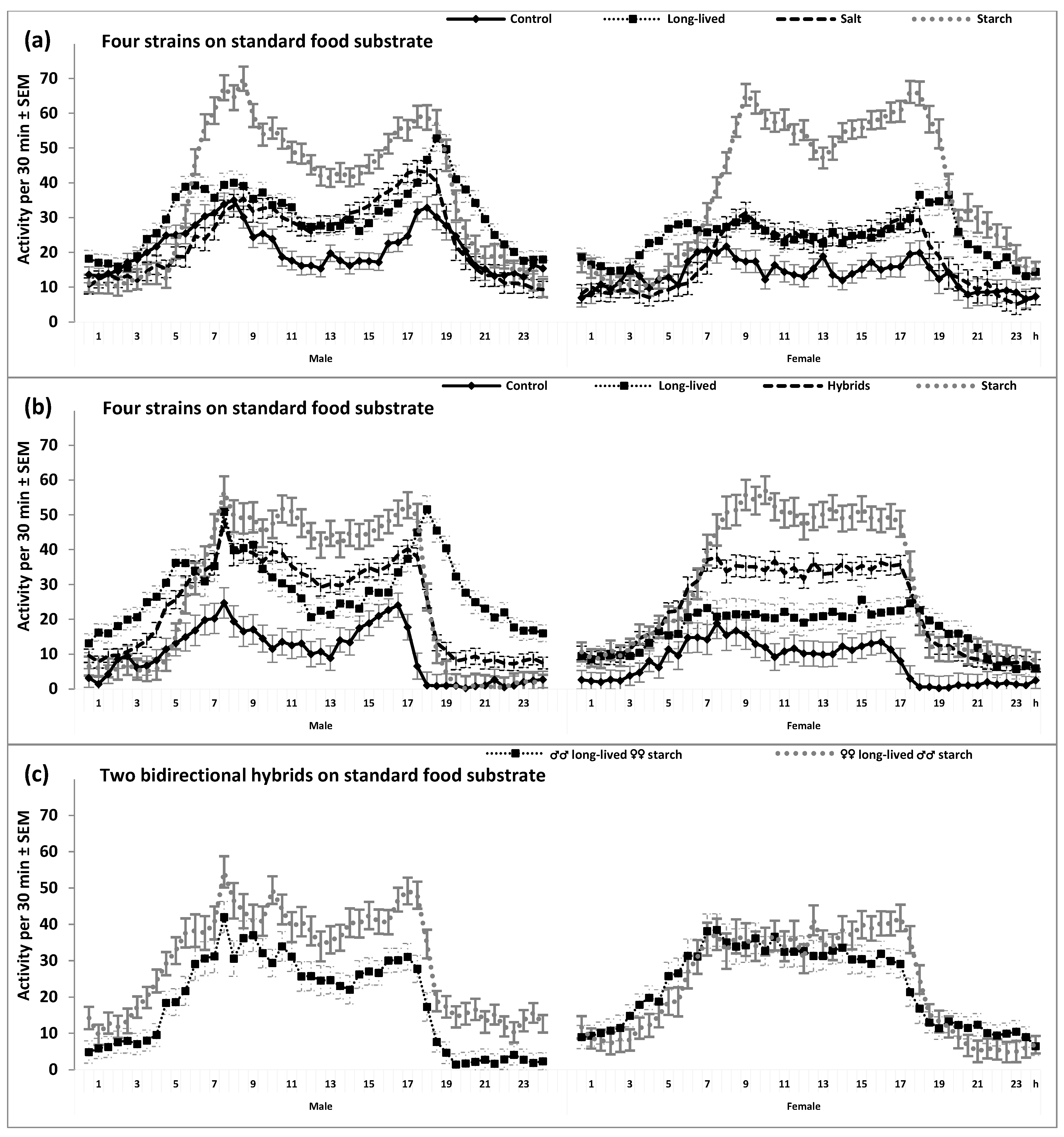
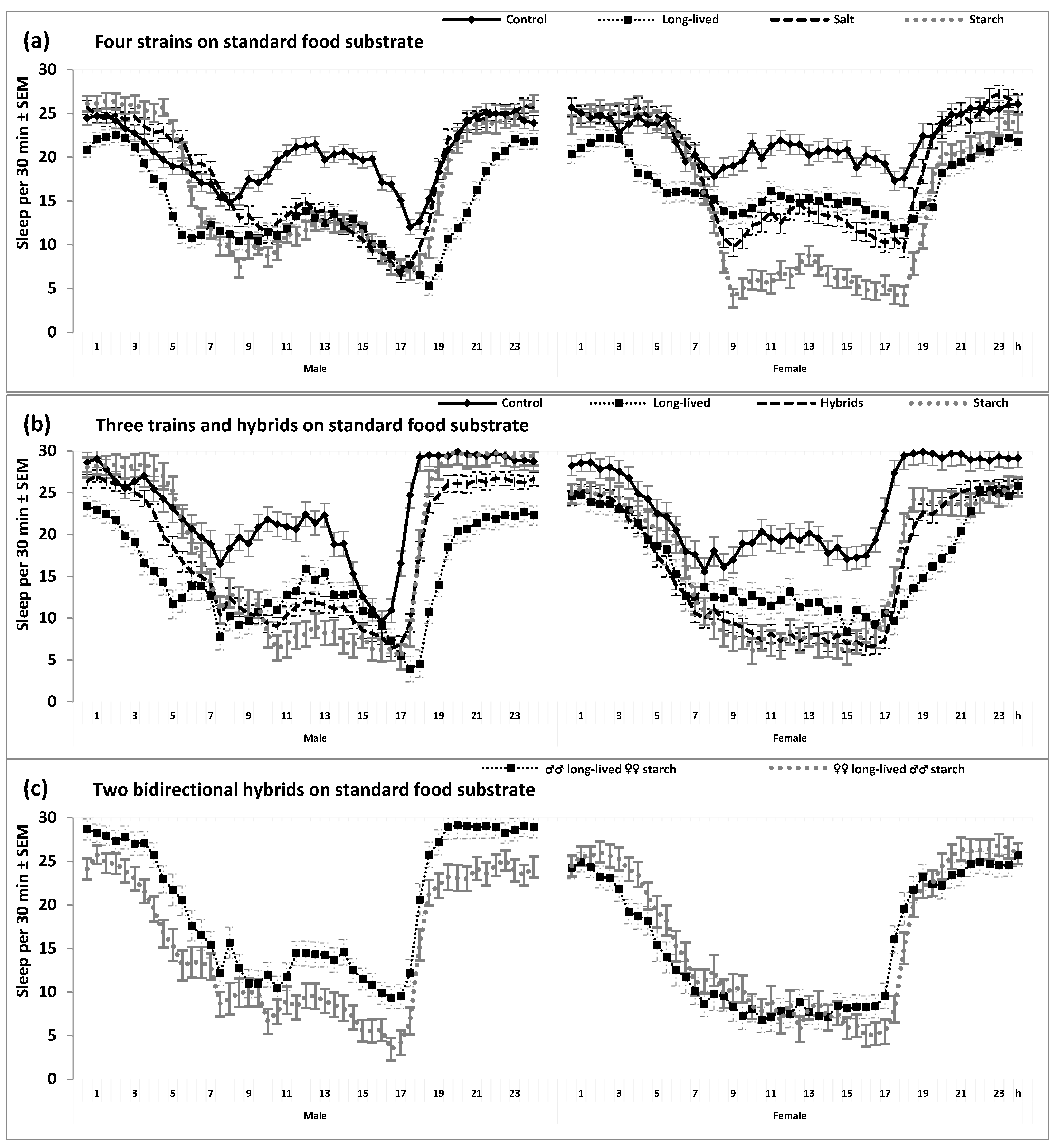
2. Results
3. Discussion
4. Materials and Methods
4.1. Selection of Three Strains
4.2. Testing the 24 h Patterns of Locomotor Activity and Sleep
4.3. Testing Fly Weight, Content of Two Sugars, Longevity, and Fecundity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, H.S. Optimal tactics of reproduction and life history. In Behavioral Ecology: An Evolutionary Approach; Krebs, J.R., Davies, N.B., Eds.; Sinauer: Sunderland, MA, USA, 1978; pp. 411–429. [Google Scholar]
- Daan, S.; Tinbergen, J. Adaptation of life histories. In Behavioural Ecology: An Evolutionary Approach, 4th ed.; Krebs, J.R., Davies, N.B., Eds.; Blackwell: Oxford, UK, 1997; pp. 311–333. [Google Scholar]
- Wu, J.; Yonezawa, T.; Kishino, H. Evolution of Reproductive Life History in Mammals and the Associated Change of Functional Constraints. Genes 2021, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Garland, T., Jr.; Schutz, H.; Chappell, M.A.; Keeney, B.K.; Meek, T.H.; Copes, L.E.; Acosta, W.; Drenowatz, C.; Maciel, R.C.; van Dijk, G.; et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: Human and rodent perspectives. J. Exp. Biol. 2011, 214 Pt 2, 206–229. [Google Scholar] [CrossRef] [PubMed]
- Teske, J.A.; Billington, C.J.; Kotz, C.M. Mechanisms underlying obesity resistance associated with high spontaneous physical activity. Neuroscience 2014, 256, 91–100. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Plasqui, G. Physical activity and human energy expenditure. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 607–613. [Google Scholar] [CrossRef]
- Hayes, J.P.; Garland, T., Jr. The evolution of endothermy: Testing the aerobic capacity model. Evolution 1995, 49, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Gebczyński, A.K.; Konarzewski, M. Locomotor activity of mice divergently selected for basal metabolic rate: A test of hypotheses on the evolution of endothermy. J. Evol. Biol. 2009, 22, 1212–1220. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Yang, K. Gene Therapy for Parkinsons Disease: Progress and Challenges. Curr. Gene Ther. 2005, 5, 71–80. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of Exercise is a Major Cause of Chronic Diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Drenowatz, C. The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription. J. Funct. Morphol. Kinesiol. 2016, 1, 230–239. [Google Scholar] [CrossRef]
- Schoeppe, S.; Alley, S.; Van Lippevelde, W.; Bray, N.A.; Williams, S.L.; Duncan, M.J.; Vandelanotte, C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 127. [Google Scholar] [CrossRef]
- Swallow, J.G.; Koteja, P.; Carter, P.A.; Garland, T., Jr. Food consumption and body composition in mice selected for high wheel-running activity. J. Comp. Physiol. B. 2001, 171, 651–659. [Google Scholar] [CrossRef]
- Sadowska, J.; Gębczyński, A.K.; Konarzewski, M. Metabolic risk factors in mice divergently selected for BMR fed high fat and high carb diets. PLoS ONE 2017, 12, e0172892. [Google Scholar] [CrossRef] [PubMed]
- Pérusse, L.; Tremblay, A.; Leblanc, C.; Bouchard, C. Genetic and environmental influences on level of habitual physical activity and exercise participation. Am. J. Epidemiol. 1989, 129, 1012–1022. [Google Scholar] [CrossRef]
- Bouchard, C.; Dionne, F.T.; Simoneau, J.A.; Boulay, M.R. Genetics of aerobic and anaerobic performances. Exerc. Sport Sci. Rev. 1992, 20, 27–58. [Google Scholar]
- Joosen, A.M.; Gielen, M.; Vlietinck, R.; Westerterp, K.R. Genetic analysis of physical activity in twins. Am. J. Clin. Nutr. 2005, 82, 1253–1259. [Google Scholar] [CrossRef]
- Lightfoot, J.T.; de Geus, E.; Booth, F.W.; Bray, M.S.; Hoed, M.D.; Kaprio, J.; Kelly, S.A.; Pomp, D.; Saul, M.C.; Thomis, M.; et al. Biological/Genetic Regulation of Physical Activity Level: Consensus from GenBioPAC. Med. Sci. Sports Exerc. 2018, 50, 863–873. [Google Scholar] [CrossRef]
- Kunz, I.; Schorr, U.; Klaus, S.; Sharma, A.M. Resting Metabolic Rate and Substrate Use in Obesity Hypertension. Hypertension 2000, 36, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L.; Nagy, T.R.; Hunter, G.R.; Darnell, B.E.; Hensrud, D.D.; Weiss, H.L. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am. J. Clin. Nutr. 2000, 72, 1088–1094. [Google Scholar] [CrossRef]
- Buscemi, S.; Verga, S.; Caimi, G.; Cerasola, G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int. J. Obes. 2005, 29, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free. Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Kannan, U.; Vasudevan, K.; Balasubramaniam, K.; Yerrabelli, D.; Shanmugavel, K.; John, N.A. Effect of Exercise Intensity on Lipid Profile in Sedentary Obese Adults. J. Clin. Diagn. Res. 2014, 8, BC08–BC10. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.L.; Samson, D.R. Sleep in a comparative context: Investigating how human sleep differs from sleep in other primates. Am. J. Phys. Anthr. 2018, 166, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Economy and Endurance in Human Evolution. Curr. Biol. 2017, 27, R613–R621. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.R. The Human Sleep Paradox: The Unexpected Sleeping Habits of Homo sapiens. Annu. Rev. Anthr. 2021, 50, 259–274. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Hyde, R.T.; Wing, A.L.; Hsieh, C.C. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Adami, P.E.; Negro, A.; Lala, N.; Martelletti, P. The role of physical activity in the prevention and treatment of chronic diseases. Clin. Ter. 2010, 161, 537–541. [Google Scholar]
- Reimers, C.D.; Knapp, G.; Reimers, A.K. Does Physical Activity Increase Life Expectancy? A Review of the Literature. J. Aging Res. 2012, 2012, 243958. [Google Scholar] [CrossRef]
- Flatt, T. Ageing: Diet and longevity in the balance. Nature 2009, 462, 989–990. [Google Scholar] [CrossRef]
- Hansen, M.; Flatt, T.; Aguilaniu, H. Reproduction, Fat Metabolism, and Life Span: What Is the Connection? Cell Metab. 2013, 17, 10–19. [Google Scholar] [CrossRef]
- Mackay, T.F.C. The genetic architecture of quantitative traits. Annu. Rev. Genet. 2001, 35, 303–339. [Google Scholar] [CrossRef]
- Koteja, P.; Garland, T., Jr.; Sax, J.K.; Swallow, J.G.; Carter, P.A. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim. Behav. 1999, 58, 1307–1318. [Google Scholar] [CrossRef]
- Prasad, N.G.; Joshi, A. What have two decades of laboratory life-history evolution studies on Drosophila melanogaster taught us? J. Genet. 2003, 82, 45–76. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.K.; Rose, M.R. Experimental evolution with Drosophila. Am. J. Physiol. 2009, 296, R1847–R1854. [Google Scholar]
- Burke, M.K.; Barter, T.T.; Cabral, L.G.; Kezos, J.N.; Phillips, M.A.; Rutledge, G.A.; Phung, K.H.; Chen, R.H.; Nguyen, H.D.; Mueller, L.D.; et al. Rapid divergence and convergence of life-history in experimentally evolved Drosophila melanogaster. Evolution 2016, 70, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. Drosophila melanogaster’s history as a human commensal. Curr. Biol. 2007, 17, R77–R81. [Google Scholar] [CrossRef]
- Stephan, W.; Li, H. The recent demographic and adaptive history of Drosophila melanogaster. Heredity 2007, 98, 65–68. [Google Scholar] [CrossRef]
- Flatt, T. Life-History Evolution and the Genetics of Fitness Components in Drosophila melanogaster. Genetics 2020, 214, 3–48. [Google Scholar] [CrossRef]
- Jordan, K.W.; Carbone, M.A.; Yamamoto, A.; Morgan, T.J.; Mackay, T.F. Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome Biol. 2007, 8, R172. [Google Scholar] [CrossRef]
- Jordan, K.W.; Morgan, T.J.; Mackay, T.F.C. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics 2006, 174, 271–284. [Google Scholar] [CrossRef]
- Greenspan, R.J.; Dierick, H.A. ‘Am not I a fly like thee?’ From genes in fruit flies to behavior in humans. Hum. Mol. Genet. 2004, 13, R267–R273. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Garland, T., Jr.; Carter, P.A. Evolutionary physiology. Annu. Rev. Physiol. 1994, 56, 579–621. [Google Scholar] [CrossRef]
- Charlesworth, B. Causes of natural variation in fitness: Evidence from studies of Drosophila populations. Proc. Natl. Acad. Sci. USA 2015, 112, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.R.; Cabral, L.G.; Philips, M.A.; Rutledge, G.A.; Phung, K.H.; Mueller, L.D.; Greer, L.F. The Great Evolutionary Divide: Two Genomic Systems Biologies of Aging. Interdiscip. Top Gerontol. 2015, 40, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Sharma, V.K. Correlated changes in life history traits in response to selection for faster pre-adult development in the fruit fly Drosophila melanogaster. J. Exp. Biol. 2014, 217 Pt 4 Pt 4, 580–589. [Google Scholar] [CrossRef]
- Vermeulen, C.J.; Loeschcke, V. Longevity and the stress response in Drosophila. Exp. Gerontol. 2007, 42, 153–159. [Google Scholar] [CrossRef]
- Graves, J.L.; Toolson, E.C.; Jeong, C.; Vu, L.N.; Rose, M.R. Desiccation, Flight, Glycogen, and Postponed Senescence in Drosophila melanogaster. Physiol. Zool. 1992, 65, 268–286. [Google Scholar] [CrossRef]
- Wit, J.; Sarup, P.; Lupsa, N.; Malte, H.; Frydenberg, J.; Loeschcke, V. Longevity for free? Increased reproduction with limited trade-offs in Drosophila melanogaster selected for increased life span. Exp. Gerontol. 2013, 48, 349–357. [Google Scholar] [CrossRef]
- Partridge, L.; Fowler, K. Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution 1992, 46, 76–91. [Google Scholar] [CrossRef]
- Chippindale, A.K.; Leroi, A.M.; Kim, S.B.; Rose, M.R. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biol. 1993, 6, 171–193. [Google Scholar] [CrossRef]
- Barnes, A.I.; Wigby, S.; Boone, J.M.; Partridge, L.; Chapman, T. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc. Biol. Sci. 2008, 275, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Arking, R.; Force, A.G.; Dudas, S.P.; Buck, S.; Baker, G.T., III. Factors contributing to the plasticity of the extended longevity phenotypes of Drosophila. Exp. Gerontol. 1996, 31, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Prowse, N.; Pignatelli, P. Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc. Biol. Sci. 1999, 266, 255–261. [Google Scholar] [CrossRef]
- Arking, R.; Buck, S.; Hwangbo, D.-S.; Lane, M. Metabolic Alterations and Shifts in Energy Allocations Are Corequisites for the Expression of Extended Longevity Genes in Drosophila. Ann. N. Y. Acad. Sci. 2002, 959, 251–262; discussion 463–465. [Google Scholar] [CrossRef]
- Buck, S.A.; Arking, R. Metabolic alterations in genetically selected Drosophila strains with different longevities. J. Am. Aging Assoc. 2001, 24, 151–161. [Google Scholar] [CrossRef]
- Khazaeli, A.A.; Van Voorhies, W.; Curtsinger, J.W. Longevity and metabolism in Drosophila melanogaster: Genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics 2005, 169, 231–242. [Google Scholar] [CrossRef]
- Rose, M.R. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 1984, 38, 1004–1010. [Google Scholar] [CrossRef]
- Luckinbill, L.S.; Arking, R.; Clare, M.J.; Cirocco, W.C.; Buck, S.A. Selection for delayed senescence in Drosophila melanogaster. Evolution 1984, 38, 996–1003. [Google Scholar] [CrossRef]
- Deepashree, S.; Shivanandappa, T.; Ramesh, S.R. Life History Traits of an Extended Longevity Phenotype of Drosophila melanogaster. Curr. Aging Sci. 2017, 10, 224–238. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Lenski, R.E.; Ebert, D.; Hollis, B.; Olivieri, I.; Whitlock, M.C. Experimental evolution. Trends Ecol. Evol. 2012, 27, 547–560. [Google Scholar] [CrossRef]
- Hillesheim, E.; Stearns, S.C. Correlated responses in life-history traits to artificial selection for body weight in Drosophila melanogaster. Evolution 1992, 46, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Vijendravarma, R.K.; Narasimha, S.; Kawecki, T.J. Evolution of foraging behaviour in response to chronic malnutrition in Drosophila melanogaster. Proc. Biol. Sci. 2012, 279, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Hoedjes, K.M.; Rodrigues, M.A.; Flatt, T. Amino acid modulation of lifespan and reproduction in Drosophila. Curr. Opin. Insect Sci. 2017, 23, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Keebaugh, E.S.; Tariq, M.; Ja, W.W. Evolutionary responses of Drosophila melanogaster under chronic malnutrition. Front. Ecol. Evol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a Versatile Model Organism in Food and Nutrition Research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef]
- Zajitschek, F.; Georgolopoulos, G.; Vourlou, A.; Ericsson, M.; Zajitschek, S.R.K.; Friberg, U.; Maklakov, A.A. Evolution Under Dietary Restriction Decouples Survival From Fecundity in Drosophila melanogaster Females. J. Gerontol. Ser. A 2019, 74, 1542–1548. [Google Scholar] [CrossRef]
- Lüersen, K.; Röder, T.; Rimbach, G. Drosophila melanogaster in nutrition research—The importance of standardizing experimental diets. Genes Nutr. 2019, 14, 3. [Google Scholar] [CrossRef]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2007, 22, 80–86. [Google Scholar] [CrossRef]
- Rion, S.; Kawecki, T.J. Evolutionary biology of starvation resistance: What we have learned from Drosophila. J. Evol. Biol. 2007, 20, 1655–1664. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span—From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Good, T.P.; Tatar, M. Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. Insect Physiol. 2001, 47, 1467–1473. [Google Scholar] [CrossRef]
- Dmitriew, C.; Rowe, L. The Effects of Larval Nutrition on Reproductive Performance in a Food-Limited Adult Environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef]
- Gray, J.L.; Sokolowski, B.M.; Simpson, J.S. Drosophila as a useful model for understanding the evolutionary physiology of obesity resistance and metabolic thrift. Fly 2021, 15, 47–59. [Google Scholar] [CrossRef]
- Tu, M.-P.; Tatar, M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell 2003, 2, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef]
- Kapahi, P.; Kaeberlein, M.; Hansen, M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev. 2017, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. Contributions of genomics to life-history theory. Nat. Rev. Genet. 2007, 8, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011, 46, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Hoedjes, K.M.; van den Heuvel, J.; Kapun, M.; Keller, L.; Flatt, T.; Zwaan, B.J. Distinct genomic signals of lifespan and life history evolution in response to postponed reproduction and larval diet in Drosophila. Evol. Lett. 2019, 3, 598–609. [Google Scholar] [CrossRef]
- Kraft, T.S.; Venkataraman, V.V.; Wallace, I.J.; Crittenden, A.N.; Holowka, N.B.; Stieglitz, J.; Harris, J.; Raichlen, D.A.; Wood, B.; Gurven, M.; et al. The energetics of uniquely human subsistence strategies. Science 2021, 374, eabf0130. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.; Cremers, T.; Westerink, B.; Van De Zande, L.; Bijlsma, R. Changes in dopamine levels and locomotor activity in response to selection on virgin lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006, 127, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.B.; Su, O.O.; Yang, N.; Bauer, J.H. Sleep-length differences are associated with altered longevity in the fruit fly Drosophila melanogaster. Biol. Open 2020, 9, bio054361. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Rose, M.R.; Bradley, T.J. The respiratory pattern in Drosophila melanogaster selected for desiccation resistance is not associated with the observed evolution of decreased locomotory activity. Physiol. Biochem. Zool. 2004, 77, 10–17. [Google Scholar] [CrossRef]
- Masek, P.; Reynolds, L.A.; Bollinger, W.L.; Moody, C.; Mehta, A.; Murakami, K.; Yoshizawa, M.; Gibbs, A.G.; Keene, A.C. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J. Exp. Biol. 2014, 217 Pt 17, 3122–3132. [Google Scholar] [CrossRef]
- Slocumb, M.E.; Regalado, J.M.; Yoshizawa, M.; Neely, G.G.; Masek, P.; Gibbs, A.G.; Keene, A.C. Enhanced sleep is an evolutionarily adaptive response to starvation stress in Drosophila. PLoS ONE 2015, 10, e0131275. [Google Scholar] [CrossRef]
- May, C.M.; Doroszuk, A.; Zwaan, B.J. The effect of developmental nutrition on life span and fecundity depends on the adult reproductive environment in Drosophila melanogaster. Ecol. Evol. 2015, 5, 1156–1168. [Google Scholar] [CrossRef]
- May, C.M.; van den Heuvel, J.; Doroszuk, A.; Hoedjes, K.M.; Flatt, T.; Zwaan, B.J. Adaptation to developmental diet influences the response to selection on age at reproduction in the fruit fly. J. Evol. Biol. 2019, 32, 425–437. [Google Scholar] [CrossRef]
- Swallow, J.G.; Koteja, P.; Carter, P.A.; Garland, T. Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J. Exp. Biol. 1999, 202 Pt 18, 2513–2520. [Google Scholar] [CrossRef]
- Girard, I.; Swallow, J.G.; Carter, P.A.; Koteja, P.; Rhodes, J.S.; Garland, T. Maternal-care behavior and life-history traits in house mice (Mus domesticus) artificially selected for high voluntary wheel-running activity. Behav. Process. 2002, 57, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Nehrenberg, D.L.; Hua, K.; Garland, T., Jr.; Pomp, D. Exercise, weight loss, and changes in body composition in mice: Phenotypic relationships and genetic architecture. Physiol. Genom. 2011, 43, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, L.; Garland, T., Jr. Mice selectively bred for high voluntary wheel-running behavior conserve more fat despite increased exercise. Physiol. Behav. 2018, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Teske, J.A.; Billington, C.J.; Kuskowski, M.A.; Kotz, C.M. Spontaneous physical activity protects against fat mass gain. Int. J. Obes. 2012, 36, 603–613. [Google Scholar] [CrossRef]
- Speakman, J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005, 208 Pt 9, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Talbot, D.A.; Selman, C.; Snart, S.; McLaren, J.S.; Redman, P.; Krol, E.; Jackson, D.M.; Johnson, M.S.; Brand, M.D. Uncoupled and surviving: Individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004, 3, 87–95. [Google Scholar] [CrossRef]
- Wichlinski, L.J. Adaptive Solutions to the Problem of Vulnerability During Sleep. Evol. Psychol. Sci. 2022, 8, 442–477. [Google Scholar] [CrossRef]
- Bochdanovits, Z.; De Jong, G. Experimental evolution in drosophila melanogaster: Interaction of temperature and food quality selection regimes. Evolution 2003, 57, 1829–1836. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Overgaard, J.; Loeschcke, V.; Mayntz, D. Dietary protein content affects evolution for body size, body fat and viability in Drosophila melanogaster. Biol. Lett. 2011, 7, 269–272. [Google Scholar] [CrossRef]
- Kolss, M.; Vijendravarma, R.K.; Schwaller, G.; Kawecki, T.J. Life-history consequences of adaptation to larval nutritional stress in Drosophila. Evolution 2009, 63, 2389–2401. [Google Scholar] [CrossRef]
- Sakai, T.; Ishida, N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 9221–9225. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Krishnan, P.; Hardin, P.; Amrein, H. Nocturnal Male Sex Drive in Drosophila. Curr. Biol. 2007, 17, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hamasaka, Y.; Suzuki, T.; Hanai, S.; Ishida, N. Evening circadian oscillator as the primary determinant of rhythmic motivation for Drosophila courtship behavior. Genes Cells 2010, 15, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Varma, V.; Saha, S.; Sheeba, V.; Sharma, V.K. Significance of activity peaks in fruit flies, Drosophila melanogaster, under seminatural conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 8984–8989. [Google Scholar] [CrossRef] [PubMed]
- Majercak, J.; Sidote, D.; Hardin, P.E.; Edery, I. How a Circadian Clock Adapts to Seasonal Decreases in Temperature and Day Length. Neuron 1999, 24, 219–230. [Google Scholar] [CrossRef]
- Kistenpfennig, C.; Nakayama, M.; Nihara, R.; Tomioka, K.; Helfrich-Förster, C.; Yoshii, T. A Tug-of-War between Cryptochrome and the Visual System Allows the Adaptation of Evening Activity to Long Photoperiods in Drosophila melanogaster. J. Biol. Rhythm. 2018, 33, 24–34. [Google Scholar] [CrossRef]
- Deppisch, P.; Prutscher, J.M.; Pegoraro, M.; Tauber, E.; Wegener, C.; Helfrich-Förster, C. Adaptation of Drosophila melanogaster to Long Photoperiods of High-Latitude Summers Is Facilitated by the ls-Timeless Allele. J. Biol. Rhythm. 2022, 37, 185–201. [Google Scholar] [CrossRef]
- Zakharenko, L.P.; Petrovskii, D.V.; Putilov, A.A. Larks, owls, swifts, and woodcocks among fruit flies: Differential responses of four heritable chronotypes to long and hot summer days. Nat. Sci. Sleep 2018, 10, 181–191. [Google Scholar] [CrossRef]
- Sujkowski, A.; Bazzell, B.; Carpenter, K.; Arking, R.; Wessells, R.J. Endurance exercise and selective breeding for longevity extend Drosophila healthspan by overlapping mechanisms. Aging (Albany NY) 2015, 7, 535–552. [Google Scholar] [CrossRef]
- Eikelboom, R. Human parallel to voluntary wheel running: Exercise. Anim. Behav. 1999, 57, F11–F12. [Google Scholar] [CrossRef]
- Markov, A.V.; Ivnitsky, S.B.; Kornilova, M.B.; Naimark, E.B.; Shirokova, N.G.; Perfilieva, K.S. Maternal effect obscures adaptation to adverse environments and hinders divergence in Drosophila melanogaster. Zh. Obshch. Biol. 2015, 76, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, E.U.; Naimark, E.B.; Markov, A.V. Adaptation of Drosophila melanogaster to unfavorable growth medium affects lifespan and age-related fecundity. Biochemistry (Moscow) 2016, 81, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, E.U.; Merzlikin, D.S.; Zavialov, A.E.; Maslov, A.A.; Mironova, E.A.; Markov, A.V. Both genes and microbiome modulate the effect of selection for longevity in Drosophila melanogaster. Zh. Obshch. Biol. 2022, 83, 268–287. (In Russian) [Google Scholar]
- Belkina, E.G.; Naimark, E.B.; Gorshkova, A.A.; Markov, A.V. Does adaptation to different diets result in assortative mating? Ambiguous results from experiments on Drosophila. J. Evol. Biol. 2018, 31, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, L.; Petrovskii, D.; Dorogova, N.; Putilov, A. Association between the deleterious effects of high temperature on fertility and sleep in female intra-specific hybrids of Drosophila melanogaster. Insects 2021, 12, 336. [Google Scholar] [CrossRef]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor Activity Level Monitoring Using the Drosophila Activity Monitoring (DAM) System: Figure 1. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5518. [Google Scholar] [CrossRef]
- Donelson, N.C.; Kim, E.Z.; Slawson, J.B.; Vecsey, C.G.; Huber, R.; Griffith, L.C. High-Resolution Positional Tracking for Long-Term Analysis of Drosophila Sleep and Locomotion Using the “Tracker” Program. PLoS ONE 2012, 7, e37250. [Google Scholar] [CrossRef]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Hathiramani, S.S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Models Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef]
- Karpova, E.K.; Eremina, M.A.; Pirozhkova, D.S.; Gruntenko, N.E. Stress-related hormones affect carbohydrate metabolism in Drosophila females. Arch. Insect Biochem. Physiol. 2019, 101, e21540. [Google Scholar] [CrossRef]
| rANOVAs on 48 30 min Intervals of the 24 h Patterns | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | ||||||
| Measure | Activity | Sleep | Activity | Sleep | ||||
| Factor | F | df | F | df | F | df | F | df |
| “Time point” | 109.3 *** | 47/13,677 | 207.0 *** | 47/13,677 | 67.5 *** | 47/8366 | 128.4 *** | 47/8366 |
| “Strain” | 9.4 *** | 3/291 | 14.6 *** | 3/291 | 23.9 *** | 3/179 | 18.4 *** | 3/179 |
| Interaction | 14.3 *** | 141/13,677 | 13.3 *** | 141/13,677 | 15.1 *** | 141/8366 | 13.4 *** | 141/8366 |
| Pairwise comparisons | Sleep (min per 30 min) | |||||||
| Male | Activity | Control | Long- | Salt | Starch | |||
| Strain | Mean | SEM | lived | Mean | SEM | |||
| Control | 21.13 | 2.14 | - | 6.07 *** | 2.30 | 3.48 ** | 20.14 | 0.65 |
| Long-lived | 30.64 | 2.20 | −9.51 *** | - | −3.77 *** | −2.59 | 14.07 | 0.67 |
| Salt | 24.38 | 2.14 | −3.25 | 6.26 | - | 1.18 | 17.84 | 0.65 |
| Starch | 36.70 | 2.35 | −15.57 *** | −6.05 | −12.3 *** | - | 16.66 | 0.71 |
| Activity (per 30 min) | Sleep | |||||||
| Sleep (min per 30 min) | ||||||||
| Female | Activity | Control | Long- | Salt | Starch | Sleep | ||
| Strain | Mean | SEM | lived | Mean | SEM | |||
| Control | 13.60 | 2.65 | - | 5.20 *** | 3.43 ** | 7.16 *** | 22.09 | 0.71 |
| Long-lived | 23.89 | 2.22 | −10.28 ** | - | −1.77 | 1.96 | 16.89 | 0.69 |
| Salt | 17.72 | 2.18 | −4.11 | 6.17 | - | −3.73 ** | 18.67 | 0.70 |
| Starch | 37.96 | 2.18 | −24.35 *** | −14.07 *** | −20.24 *** | - | 14.94 | 0.70 |
| Activity (per 30 min) | Sleep | |||||||
| rANOVAs on 48 30 min Intervals of the 24 h Patterns | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Food Substrates | Strains/Hybrids | |||||||
| Measure | Activity | Sleep | Activity | Sleep | ||||
| Factor | F | df | F | df | F | df | F | df |
| “Time point” | 47.6 *** | 47/7567 | 105.7 *** | 47/7567 | 132.8 *** | 47/11,468 | 281.5 *** | 47/11,468 |
| “Strain” | 11.9 *** | 3/161 | 10.3 *** | 3/161 | 29.7 *** | 3/244 | 32.5 *** | 3/244 |
| Interaction | 13.3 *** | 141/7567 | 15.8 *** | 141/7567 | 15.6 *** | 141/11,468 | 12.1 *** | 141/11,468 |
| Pairwise comparisons | Sleep (min per 30 min) | |||||||
| Adverse food | Activity | Control | Long- | Salt | Starch | |||
| Strain | Mean | SEM | lived | Mean | SEM | |||
| Control | 13.47 | 2.14 | - | 1.77 | 6.94 *** | 4.82 *** | 21.59 | 0.78 |
| Long-lived | 18.16 | 1.87 | −4.52 | - | 5.17 *** | 3.05 ** | 19.70 | 0.68 |
| Salt | 25.90 | 1.90 | −12.74 *** | −8.21 * | - | −2.12 | 14.81 | 0.69 |
| Starch | 28.15 | 1.83 | −14.74 *** | −10.22 ** | −2.01 | - | 16.83 | 0.66 |
| Activity (per 30 min) | Sleep | |||||||
| Sleep (min per 30 min) | ||||||||
| Strains/hybrids | Activity | Control | Long- | Hybrids | Starch | Sleep | ||
| Strain | Mean | SEM | lived | Mean | SEM | |||
| Control | 8.90 | 1.54 | - | 7.72 *** | 6.55 *** | 5.78 *** | 23.30 | 0.61 |
| Long-lived | 22.74 | 1.60 | −14.09 *** | - | −1.17 | −1.95 | 15.61 | 0.64 |
| Hybrids | 23.60 | 1.10 | −14.71 *** | −0.62 | - | −0.77 | 16.74 | 0.44 |
| Starch | 28.09 | 1.57 | −19.19 *** | −4.48 | −5.10 | - | 17.52 | 0.63 |
| Activity (per 30 min) | Sleep | |||||||
| Measurement | Male | Female | Results of Three-Way ANOVAs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Mean | SEM | Mean | SEM | Factor | F | df | p | |
| Fly | Control | 0.856 | 0.036 | 1.427 | 0.033 | “Sex” | 322.4 | 1/241 | <0.001 |
| weight, | Long-lived | 0.748 | 0.037 | 1.144 | 0.034 | “Diet” | 13.9 | 1/241 | <0.001 |
| mg | Salt | 0.741 | 0.033 | 1.132 | 0.035 | “Strain” | 14.9 | 3/241 | <0.001 |
| Starch | 0.830 | 0.035 | 1.218 | 0.032 | Interaction | 3.4 | 3/241 | 0.02 | |
| Glucose, | Control | 4.40 | 0.24 | 6.24 | 0.17 | “Sex” | 90.0 | 1/103 | <0.001 |
| µg/mg fly | Long-lived | 4.14 | 0.23 | 4.95 | 0.19 | “Diet” | 75.3 | 1/103 | <0.001 |
| Salt | 3.61 | 0.16 | 5.52 | 0.17 | “Strain” | 10.3 | 3/103 | <0.001 | |
| Starch | 4.99 | 0.18 | 5.54 | 0.16 | Interaction | 7.3 | 3/103 | <0.001 | |
| Trehalose, | Control | 13.44 | 0.61 | 4.27 | 0.27 | “Sex” | 569.5 | 1/72 | <0.001 |
| µg/mg fly | Long-lived | 14.25 | 0.45 | 9.44 | 0.32 | “Diet” | 84.7 | 1/72 | <0.001 |
| Salt | 14.72 | 0.38 | 7.03 | 0.30 | “Strain” | 36.6 | 3/72 | <0.001 | |
| Starch | 14.75 | 0.32 | 7.13 | 0.29 | Interaction | 11.5 | 3/72 | <0.001 | |
| Trehalose- | Control | 2.96 | 0.34 | 0.76 | 0.15 | “Sex” | 269.8 | 1/72 | <0.001 |
| glucose | Long-lived | 3.93 | 0.25 | 1.91 | 0.18 | “Diet” | 6.8 | 1/72 | <0.05 |
| ratio | Salt | 5.27 | 0.21 | 1.37 | 0.16 | “Strain” | 18.6 | 3/72 | <0.001 |
| Starch | 3.63 | 0.18 | 1.25 | 0.16 | Interaction | 13.6 | 3/72 | <0.001 | |
| Pairwise comparisons | Fly weight | Long- | Glucose, µg/mg fly | ||||||
| Strain | Mean | SEM | Control | lived | Salt | Starch | Mean | SEM | |
| Fly weight, | Control | 1.142 | 0.025 | 0.56 * | 0.87 ** | 0.27 | 5.32 | 0.15 | |
| mg and | Long-lived | 0.946 | 0.025 | 0.144 *** | 0.32 | −0.28 | 4.55 | 0.15 | |
| Glucose, | Salt | 0.937 | 0.024 | 0.149 *** | 0.006 | −0.60 ** | 4.56 | 0.12 | |
| µg/mg fly | Starch | 1.024 | 0.024 | 0.145 *** | 0.001 | 0.005 | 5.26 | 0.12 | |
| Fly weight, mg | Glucose | ||||||||
| Trehalose | Long- | Trehalose-glucose ratio | |||||||
| Strain | Mean | SEM | Control | lived | Salt | Starch | Mean | SEM | |
| Trehalose, | Control | 7.33 | 0.27 | −1.26 *** | −1.68 *** | −1.22 *** | 1.49 | 0.15 | |
| µg/mg fly and | Long-lived | 11.84 | 0.27 | −4.85 *** | −0.42 | 0.04 | 2.92 | 0.15 | |
| Trehalose– | Salt | 9.59 | 0.23 | −3.92 *** | 0.93 * | 0.45 | 2.67 | 0.13 | |
| Glucose | Starch | 10.94 | 0.22 | −4.47 *** | 0.38 | −0.55 | 2.44 | 0.12 | |
| ratio | Trehalose, µg/mg fly | Ratio | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharenko, L.P.; Petrovskii, D.V.; Bobrovskikh, M.A.; Gruntenko, N.E.; Yakovleva, E.Y.; Markov, A.V.; Putilov, A.A. Motus Vita Est: Fruit Flies Need to Be More Active and Sleep Less to Adapt to Either a Longer or Harder Life. Clocks & Sleep 2023, 5, 98-115. https://doi.org/10.3390/clockssleep5010011
Zakharenko LP, Petrovskii DV, Bobrovskikh MA, Gruntenko NE, Yakovleva EY, Markov AV, Putilov AA. Motus Vita Est: Fruit Flies Need to Be More Active and Sleep Less to Adapt to Either a Longer or Harder Life. Clocks & Sleep. 2023; 5(1):98-115. https://doi.org/10.3390/clockssleep5010011
Chicago/Turabian StyleZakharenko, Lyudmila P., Dmitrii V. Petrovskii, Margarita A. Bobrovskikh, Nataly E. Gruntenko, Ekaterina Y. Yakovleva, Alexander V. Markov, and Arcady A. Putilov. 2023. "Motus Vita Est: Fruit Flies Need to Be More Active and Sleep Less to Adapt to Either a Longer or Harder Life" Clocks & Sleep 5, no. 1: 98-115. https://doi.org/10.3390/clockssleep5010011
APA StyleZakharenko, L. P., Petrovskii, D. V., Bobrovskikh, M. A., Gruntenko, N. E., Yakovleva, E. Y., Markov, A. V., & Putilov, A. A. (2023). Motus Vita Est: Fruit Flies Need to Be More Active and Sleep Less to Adapt to Either a Longer or Harder Life. Clocks & Sleep, 5(1), 98-115. https://doi.org/10.3390/clockssleep5010011








