The Effects of General Anaesthesia and Light on Behavioural Rhythms and GABAA Receptor Subunit Expression in the Mouse SCN
Abstract
:1. Introduction
2. Results
2.1. Effects of GA and Light on Locomotor Rhythms of C57 Mice
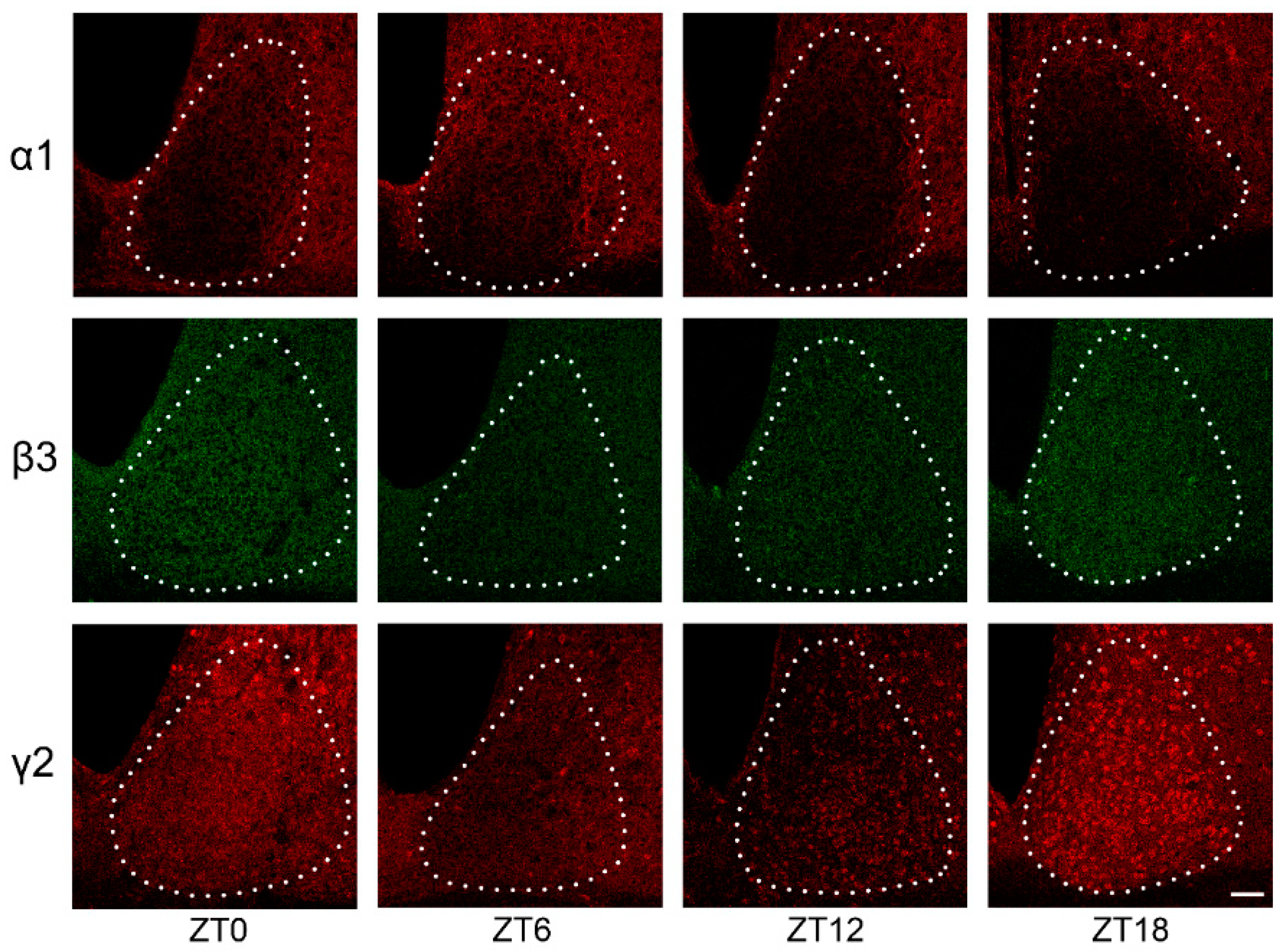
2.2. GABAAR Subunit Expression in the SCN under LD Conditions
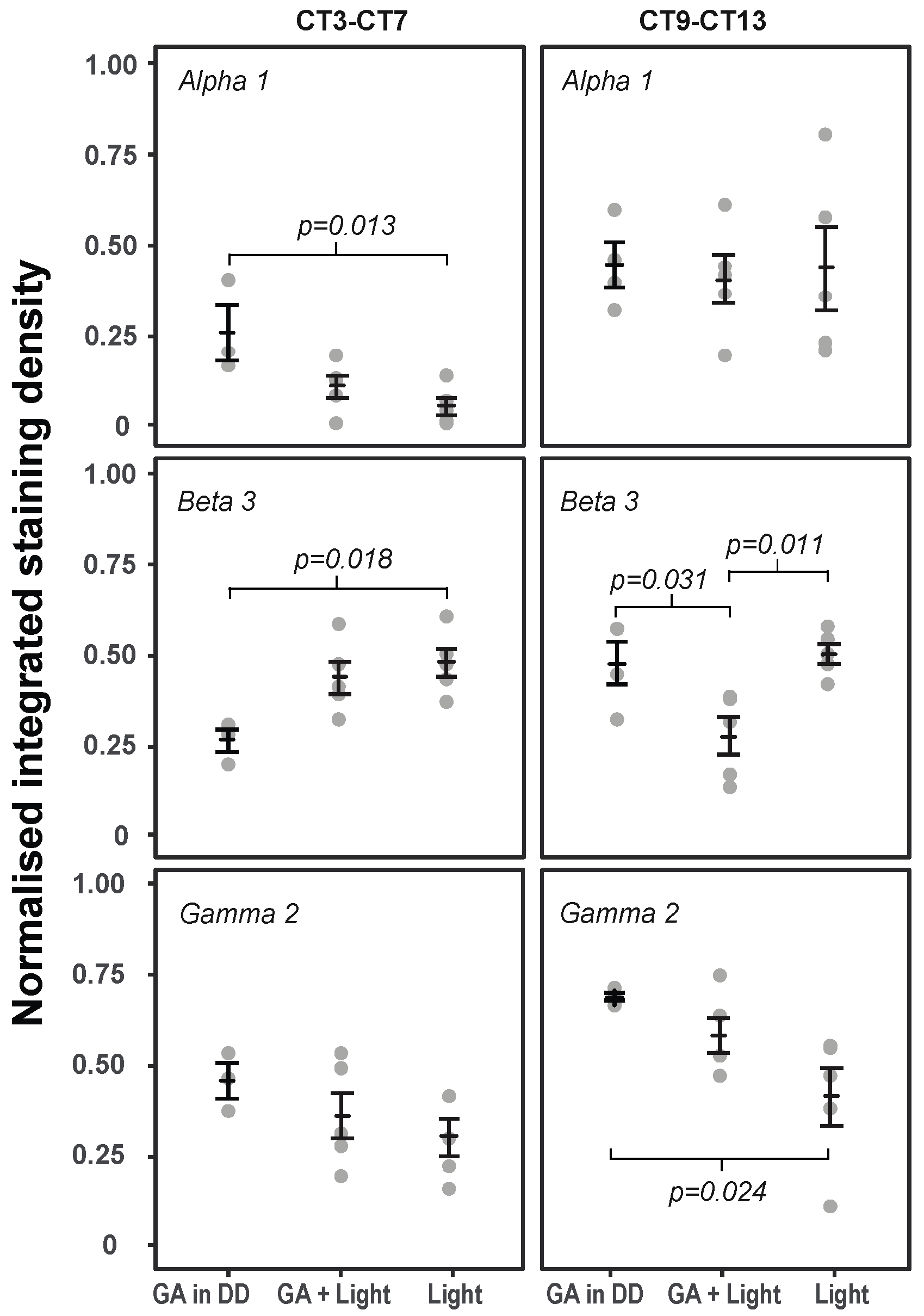
2.3. The Effects of GA and Light on GABAAR Subunit Expression in the SCN at Times of Maximal Behavioural Phase Shifts (CT9–CT13)
2.4. The Effects of GA and Light on GABAAR Subunit Expression in the SCN at Times of Minimal Behavioural Phase Shifts (CT3–CT7)
3. Discussion
4. Materials and Methods
4.1. Behavioural Experiments
4.2. Behavioural Analysis
4.3. Immunohistochemistry Experiments
4.4. Immunohistochemistry
4.5. Imaging and Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and Solutions for Achieving Health, Welfare, and Economic Development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- Gottschalk, A.; Van Aken, H.; Zenz, M.; Standl, T. Ist Anästhesie Gefährlich? Dtsch. Arztebl. 2011, 108, 469–474. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Warman, G.R.; Sleigh, J.; Ludin, N.M.; Cheeseman, J.F. How Does General Anaesthesia Affect the Circadian Clock? Sleep Med. Rev. 2018, 37, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rampes, S.; Ma, K.; Divecha, Y.A.; Alam, A.; Ma, D. Postoperative Sleep Disorders and Their Potential Impacts on Surgical Outcomes. J. Biomed. Res. 2020, 34, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Song, B.; Zhu, J. Sleep Disturbances after General Anesthesia: Current Perspectives. Front. Neurol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Cheeseman, J.F.; Winnebeck, E.C.; Millar, C.D.; Kirkland, L.S.; Sleigh, J.; Goodwin, M.; Pawley, M.D.M.; Bloch, G.; Lehmann, K.; Menzel, R.; et al. General Anesthesia Alters Time Perception by Phase Shifting the Circadian Clock. Proc. Natl. Acad. Sci. USA 2012, 109, 7061–7066. [Google Scholar] [CrossRef] [Green Version]
- Ludin, N.M.; Cheeseman, J.F.; Merry, A.F.; Millar, C.D.; Warman, G.R. The Effects of the General Anaesthetic Isoflurane on the Honey Bee (Apis Mellifera) Circadian Clock. Chronobiol. Int. 2016, 33, 128–133. [Google Scholar] [CrossRef]
- Li, N.; Stanewsky, R.; Popay, T.; Warman, G.; Cheeseman, J. The Effect of General Anaesthesia on Circadian Rhythms in Behaviour and Clock Gene Expression of Drosophila Melanogaster. Clocks Sleep 2020, 2, 32. [Google Scholar] [CrossRef]
- Ludin, N.M.; Orts-Sebastian, A.; Cheeseman, J.F.; Chong, J.; Merry, A.F.; Cumin, D.; Yamazaki, S.; Pawley, M.D.M.; Warman, G.R. General Anaesthesia Shifts the Murine Circadian Clock in a Time-Dependant Fashion. Clocks Sleep 2021, 3, 6. [Google Scholar] [CrossRef]
- Dallmann, R.; Debruyne, J.P.; Weaver, D.R. Photic Resetting and Entrainment in CLOCK-Deficient Mice. J. Biol. Rhythm. 2011, 26, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Iijima, N.; Higo, S.; Aikawa, S.; Matsuo, I.; Takumi, K.; Sakamoto, A.; Ozawa, H. Epigenetic Suppression of Mouse Per2 Expression in the Suprachiasmatic Nucleus by the Inhalational Anesthetic, Sevoflurane. PLoS ONE 2014, 9, e87319. [Google Scholar] [CrossRef]
- Ehlen, J.C.; Novak, C.M.; Karom, M.C.; Gamble, K.L.; Paul, K.N.; Albers, H.E. GABAA Receptor Activation Suppresses Period 1 MRNA and Period 2 MRNA in the Suprachiasmatic Nucleus during the Mid-Subjective Day. Eur. J. Neurosci. 2006, 23, 3328–3336. [Google Scholar] [CrossRef]
- Garcia, P.; Kolesky, S.; Jenkins, A. General Anesthetic Actions on GABAA Receptors. Curr. Neuropharmacol. 2010, 8, 2–9. [Google Scholar] [CrossRef]
- Sieghart, W.; Savic, M.M. International Union of Basic and Clinical Pharmacology. CVI: GABAA Receptor Subtype-and Function-Selective Ligands: Key Issues in Translation to Humans. Pharmacol. Rev. 2018, 70, 836–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, R.W.; Sieghart, W. GABAA Receptors: Subtypes Provide Diversity of Function and Pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Song, Z.G.; Huang, D.X.; Gao, H.; Wang, Q.; Wang, Z.P. A Single Nucleotide Polymorphism in GABAA Receptor Isoforms Is Potentially Responsible for Isoflurane Sensitivity in Mice. Genet. Mol. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Weir, C.J.; Mitchell, S.J.; Lambert, J.J. Role of GABA A Receptor Subtypes in the Behavioural Effects of Intravenous General Anaesthetics. Br. J. Anaesth. 2017, 119, i167–i175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lor, C.; Perouansky, M.; Pearce, R.A. Isoflurane Potentiation of GABAa Receptors Is Reduced but Not Eliminated by the Β3(N265m) Mutation. Int. J. Mol. Sci. 2020, 21, 9534. [Google Scholar] [CrossRef] [PubMed]
- Speigel, I.; Bichler, E.K.; García, P.S. The Influence of Regional Distribution and Pharmacologic Specificity of GABAAR Subtype Expression on Anesthesia and Emergence. Front. Syst. Neurosci. 2017, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, Y.; Li, R.; Wang, C.; Jia, N.; Zhao, C.; Wen, A.; Xiong, L. Propofol Regulates the Surface Expression of GABAA Receptors: Implications in Synaptic Inhibition. Anesth. Analg. 2015, 121, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Zurek, A.A.; Yu, J.; Wang, D.S.; Haffey, S.C.; Bridgwater, E.M.; Penna, A.; Lecker, I.; Lei, G.; Chang, T.; Salter, E.W.R.; et al. Sustained Increase in A5GABAa Receptor Function Impairs Memory after Anesthesia. J. Clin. Investig. 2014, 124, 5437–5441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, B.F.; Andretic, R.; Heller, H.C.; Carter, D.B.; Kilduff, T.S. GABAA, GABAC, and NMDA Receptor Subunit Expression in the Suprachiasmatic Nucleus and Other Brain Regions. Mol. Brain Res. 1995, 28, 239–250. [Google Scholar] [CrossRef]
- Gao, B.; Fritschy, J.M.; Moore, R.Y. GABAA-Receptor Subunit Composition in the Circadian Timing System. Brain Res. 1995, 700, 142–156. [Google Scholar] [CrossRef]
- Alò, R.; Avolio, E.; Di Vito, A.; Carelli, A.; Facciolo, R.M.; Canonaco, M. Distinct α Subunit Variations of the Hypothalamic GABAAreceptor Triplets (Aβγ) Are Linked to Hibernating State in Hamsters. BMC Neurosci. 2010, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, H.E.; Walton, J.C.; Gamble, K.L.; McNeill, J.K.; Hummer, D.L. The Dynamics of GABA Signaling: Revelations from the Circadian Pacemaker in the Suprachiasmatic Nucleus. Front. Neuroendocrinol. 2017, 44, 35–82. [Google Scholar] [CrossRef] [Green Version]
- Ono, D.; Honma, K.; Yanagawa, Y.; Yamanaka, A.; Honma, S. Role of GABA in the Regulation of the Central Circadian Clock of the Suprachiasmatic Nucleus. J. Physiol. Sci. 2018, 68, 333–343. [Google Scholar] [CrossRef]
- Colwell, C.S.; Kaufman, C.M.; Menaker, M.; Ralph, M.R. Light-Induced Phase Shifts and Fos Expression in the Hamster Circadian System: The Effects of Anesthetics. J. Biol. Rhythm. 1993, 8, 179–188. [Google Scholar] [CrossRef]
- Walton, J.C.; McNeill, J.K.; Oliver, K.A.; Elliott Albers, H. Temporal Regulation of GABAA Receptor Subunit Expression: Role in Synaptic and Extrasynaptic Communication in the Suprachiasmatic Nucleus. eNeuro 2017, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of Beta Amyloid 1-42 (Aβ 1-42)-Induced Basal Forebrain Cholinergic Damage by a Non-Classical Estrogen Signaling Activator in Vivo. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABA A Receptor Subunit Expression Changes in the Human Alzheimer’s Disease Hippocampus, Subiculum, Entorhinal Cortex and Superior Temporal Gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef]
- Palpagama, T.H.; Sagniez, M.; Kim, S.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. GabaA Receptors Are Well Preserved in the Hippocampus of Aged Mice. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, S.; Guo, C.; Pashkovski, S.L.; Osorno, T.; Gillis, W.F.; Krauss, J.M.; Nyitrai, H.; Flaquer, I.; El-Rifai, M.; Datta, S.R.; et al. Cerebellum-Specific Deletion of the GABAA Receptor δ Subunit Leads to Sex-Specific Disruption of Behavior. Cell Rep. 2020, 33, 108338. [Google Scholar] [CrossRef] [PubMed]
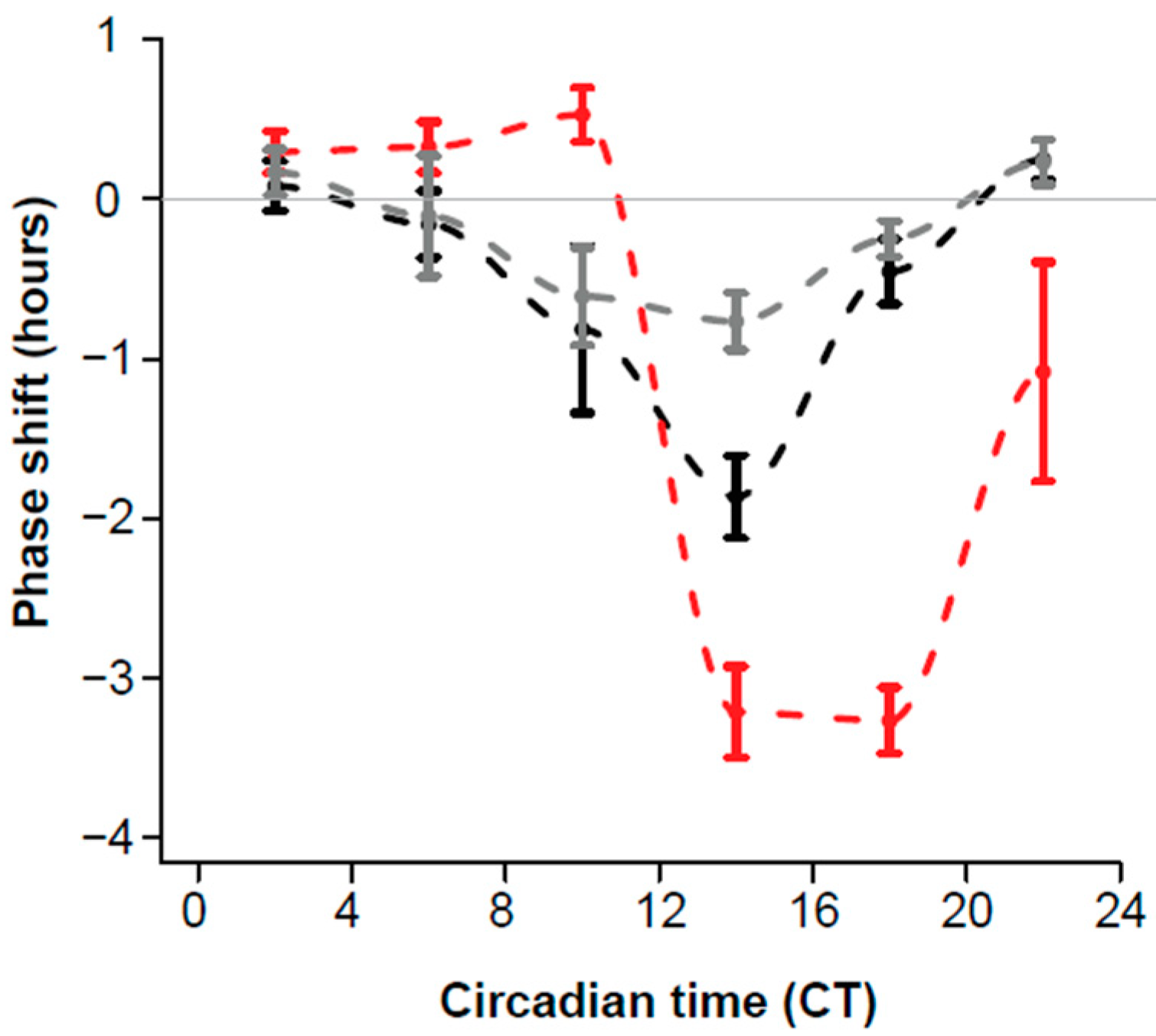

| Light | GA+Light | GA in DD (Ludin et al., 2021) | |||
|---|---|---|---|---|---|
| CT (range) | n | Shift (SE) (h) | n | Shift (SE) (h) | Shift (SE) (h) |
| 2 (0.4–3.8) | 11 | 0.30 (0.13) | 7 | 0.08 (0.16) | 0.17 (0.14) |
| 6 (4.1–7.9) | 11 | 0.33 (0.16) | 10 | −0.16 (0.21) | −0.11 (0.38) |
| 10 (8.0-–2.0) | 8 | 0.53 (0.17) | 9 | −0.82 (0.52) | −0.61 (0.31) |
| 14 (14.2–15.9) | 6 | −3.22 (0.29) | 6 | −1.87 (0.26) | −0.77 (0.18) |
| 18 (16–19.6) | 15 | −3.27 (0.21) | 14 | −0.45 (0.20) | −0.25 (0.11) |
| 22 (20.2–24) | 7 | −1.08 (0.69) | 14 | 0.25 (0.12) | 0.23 (0.14) |
| ZT0 | ZT6 | ZT12 | ZT18 | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | |
| Alpha 1 | 5 | 0.462 (0.015) | 6 | 0.542 (0.025) 1,2 | 5 | 0.411 (0.042) 1 | 6 | 0.361 (0.029) 2 |
| Beta 3 | 2 | 0.728 (0.057) 3 | 4 | 0.400 (0.066) 3 | 4 | 0.456 (0.057) | 5 | 0.568 (0.065) |
| Gamma 2 | 3 | 0.633 (0.019) | 4 | 0.426 (0.046) | 6 | 0.412 (0.066) 4 | 6 | 0.609 (0.025) 4 |
| Treatment | Light | GA + Light | GA in DD | |||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | |
| Alpha 1 | 5 | 0.440 (0.114) | 5 | 0.409 (0.067) | 4 | 0.449 (0.059) |
| Beta 3 | 5 | 0.526 (0.027) 1 | 5 | 0.284 (0.052) 1,2 | 4 | 0.486 (0.061) 2 |
| Gamma 2 | 5 | 0.423 (0.083) 3 | 5 | 0.592 (0.051) | 4 | 0.698 (0.009) 3 |
| Treatment | Light | GA + Light | GA in DD | |||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | n | Mean (SE) | |
| Alpha 1 | 5 | 0.056 (0.024) 1 | 5 | 0.113 (0.031) | 3 | 0.261 (0.073) 1 |
| Beta 3 | 5 | 0.486 (0.039) 2 | 5 | 0.445 (0.045) | 3 | 0.271 (0.034) 2 |
| Gamma 2 | 5 | 0.313 (0.051) | 5 | 0.371 (0.065) | 3 | 0.467 (0.046) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, J.; Cheeseman, J.F.; Pawley, M.D.M.; Kwakowsky, A.; Warman, G.R. The Effects of General Anaesthesia and Light on Behavioural Rhythms and GABAA Receptor Subunit Expression in the Mouse SCN. Clocks & Sleep 2021, 3, 482-494. https://doi.org/10.3390/clockssleep3030034
Chong J, Cheeseman JF, Pawley MDM, Kwakowsky A, Warman GR. The Effects of General Anaesthesia and Light on Behavioural Rhythms and GABAA Receptor Subunit Expression in the Mouse SCN. Clocks & Sleep. 2021; 3(3):482-494. https://doi.org/10.3390/clockssleep3030034
Chicago/Turabian StyleChong, Janelle, James Frederick Cheeseman, Matthew D. M. Pawley, Andrea Kwakowsky, and Guy R. Warman. 2021. "The Effects of General Anaesthesia and Light on Behavioural Rhythms and GABAA Receptor Subunit Expression in the Mouse SCN" Clocks & Sleep 3, no. 3: 482-494. https://doi.org/10.3390/clockssleep3030034







