Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us
Abstract
1. Introduction
2. The Core of the Body Clock Entrained by Light: Following the Right Path
3. The Retina-Brain Network: The Route to Synchronize (or Desynchronize) Circadian Rhythms and to Regulate Behavior by Light
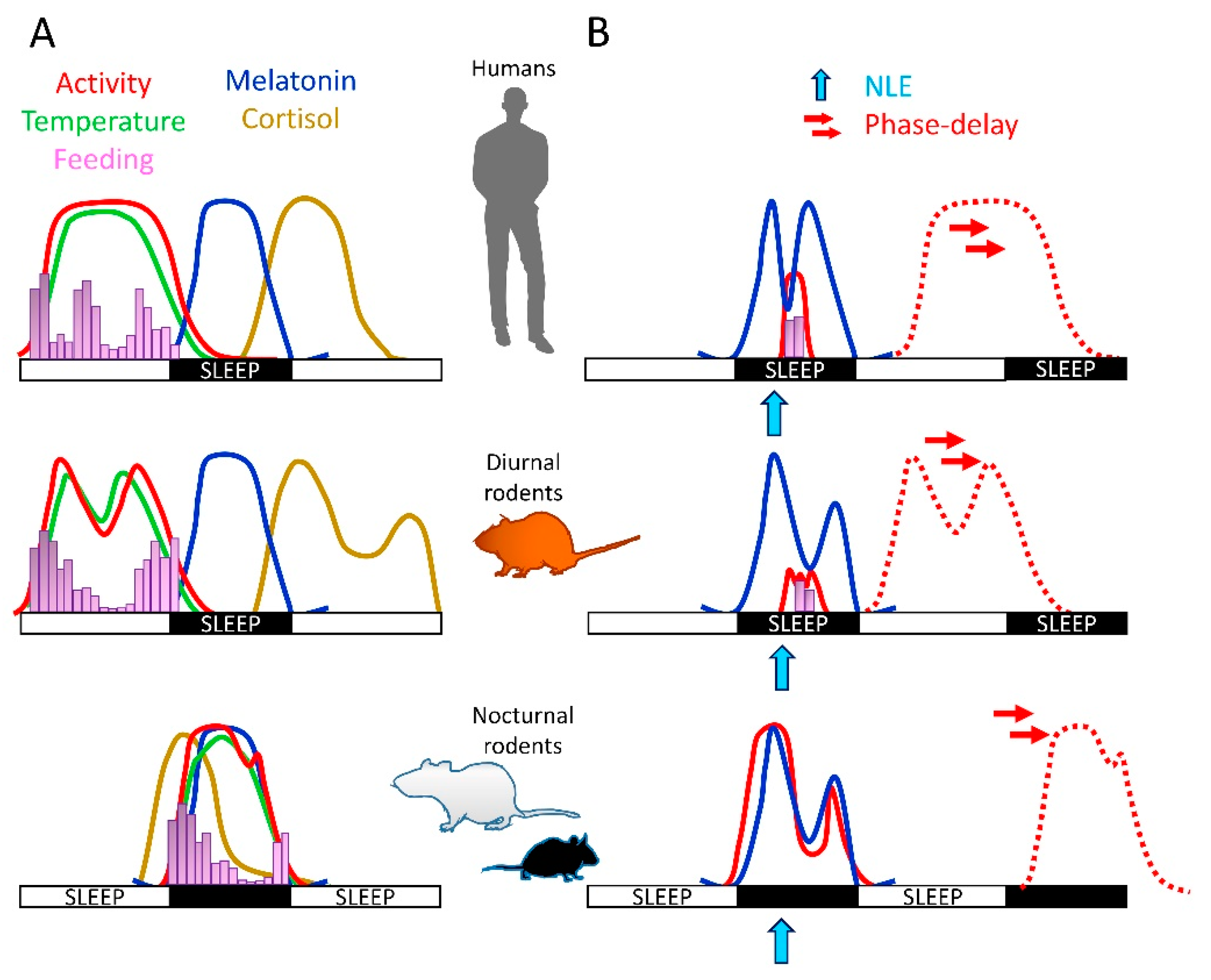
4. Light Effects on Physiology: Differences between Diurnal and Nocturnal Species
5. Nighttime Light Effects on Sleep and Circadian Biology of Diurnal Mammals
6. Nighttime Light Effects on Mood of Diurnal Mammals
7. Nighttime Light Effects on Eating and Metabolism of Diurnal Mammals
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittendrigh, C.S. Temporal organization: Reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 1993, 55, 16–54. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G.; Roenneberg, T. Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 2008, 18, R784–R794. [Google Scholar] [CrossRef]
- Prayag, A.S.; Najjar, R.P.; Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal. Res. 2019, 66, e12562. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.M.; Do, M.T.H.; Dacey, D.; Lucas, R.; Hattar, S.; Matynia, A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: From form to function. J. Neurosci. 2011, 31, 16094–16101. [Google Scholar] [CrossRef]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.-I.; Yanagawa, Y.; Yamanaka, A.; Honma, S. Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J. Physiol. Sci. 2018, 68, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Edwards, M.D.; Smyllie, N.J.; Hamnett, R.; Chesham, J.E.; Brancaccio, M.; Maywood, E.S.; Hastings, M.H. The VIP-VPAC2 neuropeptidergic axis is a cellular pacemaking hub of the suprachiasmatic nucleus circadian circuit. Nat. Commun. 2020, 11, 3394. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Simon, T.; Lones, L.; Herzog, E.D. SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System. J. Neurosci. 2018, 38, 7986–7995. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar]
- Mieda, M.; Ono, D.; Hasegawa, E.; Okamoto, H.; Honma, K.-I.; Honma, S.; Sakurai, T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 2015, 85, 1103–1116. [Google Scholar] [CrossRef]
- Collins, B.; Pierre-Ferrer, S.; Muheim, C.; Lukacsovich, D.; Cai, Y.; Spinnler, A.; Herrera, C.G.; Wen, S.; Winterer, J.; Belle, M.D.; et al. Circadian VIPergic Neurons of the Suprachiasmatic Nuclei Sculpt the Sleep-Wake Cycle. Neuron 2020, 108, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Smyllie, N.J.; Patton, A.P. Molecular-genetic Manipulation of the Suprachiasmatic Nucleus Circadian Clock. J. Mol. Biol. 2020, 432, 3639–3660. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–169. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.-W.; Takao, M.; Berson, D.M.; Yau, K.-W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Rios, L.C.; Le, H.; Perez, A.J.; Phan, S.; Bushong, E.A.; Deerinck, T.J.; Liu, Y.H.; Ellisman, M.A.; Lev-Ram, V.; et al. Synaptic Specializations of Melanopsin-Retinal Ganglion Cells in Multiple Brain Regions Revealed by Genetic Label for Light and Electron Microscopy. Cell Rep. 2019, 29, 628–644e6. [Google Scholar] [PubMed]
- Mure, L.S.; Vinberg, F.; Hanneken, A.; Panda, S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 2019, 366, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.-W.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Fogerson, P.M.; Ospri, L.L.; Thomsen, M.B.; Layne, R.M.; Severin, D.; Zhan, J.; Singer, J.H.; Kirkwood, A.; Zhao, H.; et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 2018, 175, 71–84.e18. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xi, Y.; Peng, Y.; Yang, Y.; Huang, X.; Fu, Y.; Tao, Q.; Xiao, J.; Yuan, T.; An, K.; et al. A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy. Neuron 2019, 102, 128–142.e8. [Google Scholar]
- Langel, J.L.; Smale, L.; Esquiva, G.; Hannibal, J. Central melanopsin projections in the diurnal rodent, Arvicanthis niloticus. Front. Neuroanat. 2015, 9, 93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karnas, D.; Hicks, D.; Mordel, J.; Pévet, P.; Meissl, H. Intrinsic photosensitive retinal ganglion cells in the diurnal rodent, Arvicanthis ansorgei. PLoS ONE 2013, 8, e73343. [Google Scholar] [CrossRef][Green Version]
- Li, J.Y.; Schmidt, T.M. Divergent projection patterns of M1 ipRGC subtypes. J. Comp. Neurol. 2018, 526, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Butler, R.; Godinho, S.I.; Couch, Y.; Brown, L.A.; Vasudevan, S.R.; Flanagan, K.C.; Anthony, D.; Churchill, G.C.; Wood, M.J.; et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell 2013, 154, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.H.; Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Garidou-Boof, M.L.; Sicard, B.; Bothorel, B.; Pitrosky, B.; Ribelayga, C.; Simonneaux, V.; Pévet, P.; Vivien-Roels, B. Environmental control and adrenergic regulation of pineal activity in the diurnal tropical rodent, Arvicanthis ansorgei. J. Pineal Res. 2005, 38, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Lenz, S.; Kalsbeek, A.; Pévet, P.; Buijs, R.M. Glutamatergic clock output stimulates melatonin synthesis at night. Eur. J. Neurosci. 2004, 19, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Krauchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.S.; Rajaratnam, S.M.W.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R.; Kenagy, G.J. Diurnally active rodents for laboratory research. Lab. Anim. 2018, 52, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Caldelas, I.; Poirel, V.-J.; Sicard, B.; Pévet, P.; Challet, E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei. Neuroscience 2003, 116, 583–591. [Google Scholar] [CrossRef]
- Albrecht, U.; Sun, Z.S.; Eichele, G.; Lee, C.C. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 1997, 91, 1055–1064. [Google Scholar] [CrossRef]
- Sato, T.; Kawamura, H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus). Neurosci. Res. 1984, 1, 45–52. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Reppert, S.M.; Eagan, S.M.; Moore-Ede, M.C. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983, 274, 184–187. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Edelstein, K.; Hastings, M.H.; Maywood, E.S. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): Implications for nonphotic phase shifting. J. Biol. Rhythm. 2001, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Otalora, B.B.; Hagenauer, M.H.; Rol, M.A.; Madrid, J.A.; Lee, T.M. Period gene expression in the brain of a dual-phasing rodent, the Octodon degus. J. Biol. Rhythm. 2013, 28, 249–261. [Google Scholar] [CrossRef]
- Vosko, A.M.; Hagenauer, M.H.; Hummer, D.L.; Lee, T.M. Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R353–R361. [Google Scholar] [CrossRef][Green Version]
- Feillet, C.A.; Mendoza, J.; Albrecht, U.; Pévet, P.; Challet, E. Forebrain oscillators ticking with different clock hands. Mol. Cell. Neurosci. 2008, 37, 209–221. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359, eaao0318. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.Y.; Jiang, J.; Liu, Z.; Cai, Y.; Huang, T.; Wang, Y.; Liu, Q.; Nie, Y.; Liu, F.; Cheng, J.; et al. BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. Natl. Sci. Rev. 2019, 6, 87–100. [Google Scholar] [CrossRef]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [Google Scholar] [CrossRef]
- Shuboni, D.D.; Cramm, S.L.; Yan, L.; Ramanathan, C.; Cavanaugh, B.L.; Nunez, A.A.; Smale, L. Acute effects of light on the brain and behavior of diurnal Arvicanthis niloticus and nocturnal Mus musculus. Physiol. Behav. 2015, 138, 75–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuboni, D.D.; Cramm, S.L.; Yan, L.; Nunez, A.A.; Smale, L. Acute behavioral responses to light and darkness in nocturnal Mus musculus and diurnal Arvicanthis niloticus. J. Biol. Rhythms. 2012, 27, 299–307. [Google Scholar] [CrossRef]
- Langel, J.; Ikeno, T.; Yan, L.; Nunez, A.A.; Smale, L. Distributions of GABAergic and glutamatergic neurons in the brains of a diurnal and nocturnal rodent. Brain Res. 2018, 1700, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Kaufmann, C.; Leutritz, T.; Arnold, Y.L.; Speck, O.; Ullsperger, M. The human habenula is responsive to changes in luminance and circadian rhythm. Neuroimage 2019, 189, 581–588. [Google Scholar] [CrossRef]
- Mendoza, J. Circadian neurons in the lateral habenula: Clocking motivated behaviors. Pharmacol. Biochem. Behav. 2017, 162, 55–61. [Google Scholar] [CrossRef]
- Namboodiri, V.M.; Rodriguez-Romaguera, J.; Stuber, G.D. The habenula. Curr. Biol. 2016, 26, R873–R877. [Google Scholar] [CrossRef] [PubMed]
- Salaberry, N.L.; Hamm, H.; Felder-Schmittbuhl, M.-P.; Mendoza, J. A suprachiasmatic-independent circadian clock(s) in the habenula is affected by Per gene mutations and housing light conditions in mice. Brain Struct. Funct. 2019, 224, 19–31. [Google Scholar] [CrossRef]
- Zhao, H.; Rusak, B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience 2005, 132, 519–528. [Google Scholar] [CrossRef]
- Guilding, C.; Hughes, A.T.; Piggins, H.D. Circadian oscillators in the epithalamus. Neuroscience 2010, 169, 1630–1639. [Google Scholar] [CrossRef]
- An, K.; Zhao, H.; Miao, Y.; Xu, Q.; Li, Y.-F.; Ma, Y.-Q.; Shi, Y.-M.; Shen, J.-W.; Meng, J.-J.; Yao, Y.-G.; et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 2020, 23, 869–880. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Lemola, S.; Perkinson-Gloor, N.; Brand, S.; Dewald-Kaufmann, J.F.; Grob, A. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J. Youth Adolesc. 2015, 44, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C. Alerting effects of light. Sleep Med. Rev. 2007, 11, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Chinoy, E.D.; Duffy, J.F.; Czeisler, C.A. Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiol. Rep. 2018, 6, e13692. [Google Scholar] [CrossRef]
- Cho, C.H.; Lee, H.-J.; Yoon, H.-K.; Kang, S.-G.; Bok, K.-N.; Jung, K.-Y.; Kim, L.; Lee, E.-I. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol. Int. 2016, 33, 117–123. [Google Scholar] [CrossRef]
- Cho, C.H.; Yoon, H.-K.; Kang, S.-G.; Kim, L.; Lee, E.-I.; Lee, H.-J. Impact of Exposure to Dim Light at Night on Sleep in Female and Comparison with Male Subjects. Psychiatry Investig. 2018, 15, 520–530. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Effect of exposure to evening light on sleep initiation in the elderly: A longitudinal analysis for repeated measurements in home settings. Chronobiol. Int. 2014, 31, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Effect of evening light exposure on sleep in bipolar disorder: A longitudinal analysis for repeated measures in the APPLE cohort. Aust. N. Z. J. Psychiatry 2020, 55, 305–331. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Association between light exposure at night and manic symptoms in bipolar disorder: Cross-sectional analysis of the APPLE cohort. Chronobiol. Int. 2020, 37, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Paksarian, D.; Rudolph, K.E.; Stapp, E.K.; Dunster, G.P.; He, J.; Mennitt, D.; Hattar, S.; Casey, J.A.; James, P.; Merikangas, K.R. Association of Outdoor Artificial Light at Night With Mental Disorders and Sleep Patterns Among US Adolescents. JAMA Psychiatry 2020, 77, 1266. [Google Scholar] [CrossRef]
- Saper, C.B. The neurobiology of sleep. Contin. Lifelong Learn. Neurol. 2013, 19, 19–31. [Google Scholar] [CrossRef]
- Pilorz, V.; Tam, S.K.E.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef]
- Novak, C.M.; Nunez, A.A. Daily rhythms in Fos activity in the rat ventrolateral preoptic area and midline thalamic nuclei. Am. J. Physiol. 1998, 275, R1620–R1626. [Google Scholar] [CrossRef]
- Novak, C.M.; Smale, L.; Nunez, A.A. Rhythms in Fos expression in brain areas related to the sleep-wake cycle in the diurnal Arvicanthis niloticus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1267–R1274. [Google Scholar] [CrossRef]
- Borbely, A.A.; Tobler, I. Manifestations and functional implications of sleep homeostasis. Handb. Clin. Neurol. 2011, 98, 205–213. [Google Scholar]
- Dijk, D.J.; Daan, S. Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J. Comp. Physiol. A 1989, 165, 205–215. [Google Scholar] [CrossRef]
- Kas, M.J.; Edgar, D.M. Crepuscular rhythms of EEG sleep-wake in a hystricomorph rodent, Octodon degus. J. Biol. Rhythm. 1998, 13, 9–17. [Google Scholar] [CrossRef]
- Hubbard, J.; Ruppert, E.; Calvel, L.; Robin-Choteau, L.; Gropp, C.-M.; Allemann, C.; Reibel, S.; Sage-Ciocca, D.; Bourgin, P. Arvicanthis ansorgei, a Novel Model for the Study of Sleep and Waking in Diurnal Rodents. Sleep 2015, 38, 979–988. [Google Scholar]
- Mendoza, J. Circadian insights into the biology of depression: Symptoms, treatments and animal models. Behav. Brain Res. 2019, 376, 112186. [Google Scholar] [CrossRef]
- Wehr, T.A.; Sack, D.; Rosenthal, N.; Duncan, W.; Gillin, J.C. Circadian rhythm disturbances in manic-depressive illness. Fed. Proc. 1983, 42, 2809–2814. [Google Scholar]
- Wirz-Justice, A. Diurnal variation of depressive symptoms. Dialogues Clin. Neurosci. 2008, 10, 337–343. [Google Scholar]
- Wirz-Justice, A. Seasonality in affective disorders. Gen. Comp. Endocrinol. 2018, 258, 244–249. [Google Scholar] [CrossRef]
- Bromundt, V.; Wirz-Justice, A.; Kyburz, S.; Opwis, K.; Dammann, G.; Cajochen, C. Circadian sleep-wake cycles, well-being, and light therapy in borderline personality disorder. J. Pers. Disord. 2013, 27, 680–696. [Google Scholar] [CrossRef]
- Rosenthal, N.E.; Sack, D.A.; Gillin, J.C.; Lewy, A.J.; Goodwin, F.K.; Davenport, Y.; Mueller, P.S.; Newsome, D.A.; Wehr, T.A. Seasonal affective disorder: A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 1984, 41, 72–80. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Lichtsteiner, M.; Feer, H. Diurnal and seasonal variations in human platelet serotonin in man. J. Neural. Transm. 1977, 41, 7–15. [Google Scholar] [CrossRef]
- Kohsaka, M.; Fukuda, N.; Honma, K.; Honma, S.; Morita, N. Seasonal variation of the human circadian rhythms (2) sleep EEG. Jpn. J. Psychiatry Neurol. 1991, 45, 185–186. [Google Scholar]
- Honma, K.; Honma, S.; Kohsaka, M.; Fukuda, N. Seasonal variation in the human circadian rhythm: Dissociation between sleep and temperature rhythm. Am. J. Physiol. 1992, 262, R885–R891. [Google Scholar] [CrossRef]
- Eisenberg, D.P.; Kohn, P.D.; Baller, E.B.; Bronstein, J.A.; Masdeu, J.C.; Berman, K.F. Seasonal effects on human striatal presynaptic dopamine synthesis. J. Neurosci. 2010, 30, 14691–14694. [Google Scholar] [CrossRef]
- Green, N.H.; Jackson, C.R.; Iwamoto, H.; Tackenberg, M.C.; McMahon, D.G. Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Curr. Biol. 2015, 25, 1389–1394. [Google Scholar] [CrossRef]
- Ikeno, T.; Deats, S.P.; Soler, J.; Lonstein, J.S.; Yan, L. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus). Behav. Brain Res. 2016, 300, 77–84. [Google Scholar] [CrossRef]
- Leach, G.; Adidharma, W.; Yan, L. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus). PLoS ONE 2013, 8, e57115. [Google Scholar] [CrossRef]
- Leach, G.; Ramanathan, C.; Langel, J.; Yan, L. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus). Neuroscience 2013, 234, 31–39. [Google Scholar] [CrossRef][Green Version]
- Itzhacki, J.; Clesse, D.; Goumon, Y.; Van Someren, E.J.; Mendoza, J. Light rescues circadian behavior and brain dopamine abnormalities in diurnal rodents exposed to a winter-like photoperiod. Brain Struct. Funct. 2018, 223, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Adidharma, W.; Leach, G.; Yan, L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience 2012, 220, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Cain, S.W.; Burns, A.C.; Acebo, C.; Carskadon, M.A. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J. Clin. Endocrinol. Metab. 2015, 100, 4067–4073. [Google Scholar] [CrossRef]
- Prayag, A.S.; Münch, M.; Aeschbach, D.; Chellappa, S.L.; Gronfier, C. Light Modulation of Human Clocks, Wake, and Sleep. Clocks Sleep 2019, 1, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Exposure to light at night and risk of depression in the elderly. J. Affect. Disord. 2013, 151, 331–336. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Bedroom Light Exposure at Night and the Incidence of Depressive Symptoms: A Longitudinal Study of the HEIJO-KYO Cohort. Am. J. Epidemiol. 2018, 187, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Helbich, M.; Browning, M.H.; Huss, A. Outdoor light at night, air pollution and depressive symptoms: A cross-sectional study in the Netherlands. Sci. Total Environ. 2020, 744, 140914. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Outdoor light at night and the prevalence of depressive symptoms and suicidal behaviors: A cross-sectional study in a nationally representative sample of Korean adults. J. Affect. Disord. 2018, 227, 199–205. [Google Scholar] [CrossRef]
- Fonken, L.K.; Kitsmiller, E.; Smale, L.; Nelson, R.J. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythm. 2012, 27, 319–327. [Google Scholar] [CrossRef]
- Fonken, L.K.; Haim, A.; Nelson, R.J. Dim light at night increases immune function in Nile grass rats, a diurnal rodent. Chronobiol. Int. 2012, 29, 26–34. [Google Scholar] [CrossRef]
- Hu, H.; Cui, Y.; Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020, 21, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int. J. Obes. 2016, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A.J.L. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.N.; Zee, P.C.; Shalman, D.; Malkani, R.G.; Kang, J.; Reid, K.J. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS ONE 2016, 11, e0155601. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Ambient Light Exposure and Changes in Obesity Parameters: A Longitudinal Study of the HEIJO-KYO Cohort. J. Clin. Endocrinol. Metab. 2016, 101, 3539–3547. [Google Scholar] [CrossRef]
- Obayashi, K.; Yamagami, Y.; Kurumatani, N.; Saeki, K. Bedroom lighting environment and incident diabetes mellitus: A longitudinal study of the HEIJO-KYO cohort. Sleep Med. 2020, 65, 1–3. [Google Scholar] [CrossRef]
- McFadden, E.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am. J. Epidemiol. 2014, 180, 245–250. [Google Scholar] [CrossRef]
- Park, Y.M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of Exposure to Artificial Light at Night While Sleeping With Risk of Obesity in Women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Porcheret, K.; Wald, L.; Fritschi, L.; Gerkema, M.; Gordijn, M.; Merrrow, M.; Rajaratnam, S.M.; Rock, D.; Sletten, T.L.; Warman, G.; et al. Chronotype and environmental light exposure in a student population. Chronobiol. Int. 2018, 35, 1365–1374. [Google Scholar] [CrossRef]
- Albreiki, M.S.; Middleton, B.; Hampton, S.M. A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr. Connect. 2017, 6, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Albreiki, M.; Middleton, B.; Ebajemito, J.; Hampton, S. The effect of light on appetite in healthy young individuals. Proc. Nutr. Soc. 2015, 74, E4. [Google Scholar] [CrossRef]
- Masis-Vargas, A.; Hicks, D.; Mendoza, J.; Kalsbeek, A. Blue light at night acutely impairs glucose tolerance and increases sugar intake in the diurnal rodent Arvicanthis ansorgei in a sex-dependent manner. Physiol. Rep. 2019, 7, e14257. [Google Scholar] [CrossRef]
- AlBreiki, M.; Middleton, B.; Hampton, S. Are leptin responses influenced by bright light treatment in healthy young individuals. Proc. Nutr. Soc. 2015, 74, E209. [Google Scholar] [CrossRef][Green Version]
- Kalsbeek, A.; Fliers, E.; Romijn, J.A.; La Fleur, S.E.; Wortel, J.; Bakker, O.; Endert, E.; Buijs, R.M. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology 2001, 142, 2677–2685. [Google Scholar] [CrossRef]
- Simon, C.; Gronfier, C.; Schlienger, J.L.; Brandenberger, G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. J. Clin. Endocrinol. Metab. 1998, 83, 1893–1899. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; van Dorp, R.; Foppen, E.; Mendoza, J.; Opperhuizen, A.L.; Fliers, E.; Bisschop, P.H.; Meijer, J.H.; Kalsbeek, A.; Deboer, T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci Rep. 2016, 6, 35662. [Google Scholar] [CrossRef]
- Masis-Vargas, A.; Ritsema, W.I.; Mendoza, J.; Kalsbeek, A. Metabolic Effects of Light at Night are Time- and Wavelength-Dependent in Rats. Obesity 2020, 28 (Suppl. 1), S114–S125. [Google Scholar] [CrossRef]
- Verra, D.M.; Sajdak, B.S.; Merriman, D.K.; Hicks, D. Diurnal rodents as pertinent animal models of human retinal physiology and pathology. Prog. Retin. Eye Res. 2020, 74, 100776. [Google Scholar]
- Niijima, A.; Nagai, K.; Nagai, N.; Akagawa, H. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiol Behav. 1993, 54, 555–561. [Google Scholar] [CrossRef]
- Niijima, A.; Nagai, K.; Nagai, N.; Nakagawa, H. Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. J. Auton. Nerv. Syst. 1992, 40, 155–160. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Gourmelen, S.; Dumont, S.; Sage-Ciocca, D.; Pévet, P.; Challet, E. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J. Physiol. 2012, 590, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, J. Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us. Clocks & Sleep 2021, 3, 236-250. https://doi.org/10.3390/clockssleep3020014
Mendoza J. Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us. Clocks & Sleep. 2021; 3(2):236-250. https://doi.org/10.3390/clockssleep3020014
Chicago/Turabian StyleMendoza, Jorge. 2021. "Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us" Clocks & Sleep 3, no. 2: 236-250. https://doi.org/10.3390/clockssleep3020014
APA StyleMendoza, J. (2021). Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us. Clocks & Sleep, 3(2), 236-250. https://doi.org/10.3390/clockssleep3020014



