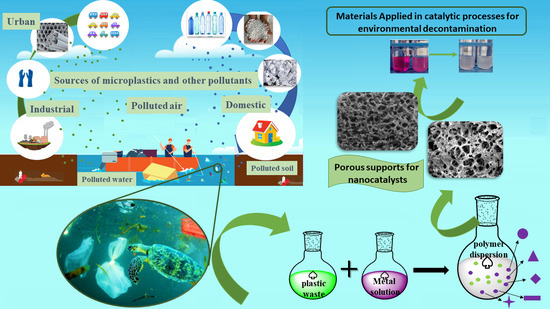
Conversion of Plastic Waste into Supports for Nanostructured Heterogeneous Catalysts: Application in Environmental Remediation
Abstract
1. Introduction
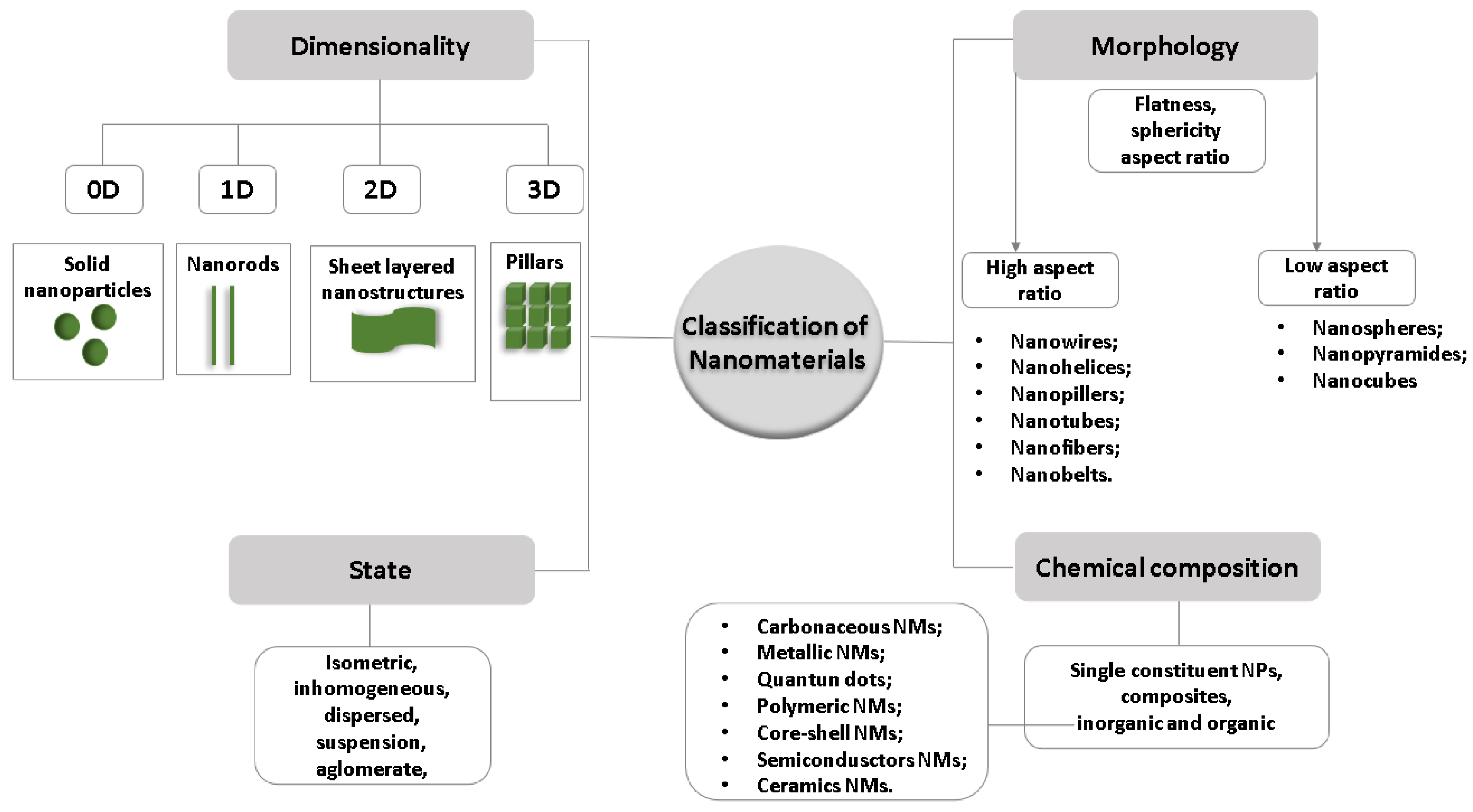
2. Plastic Waste Chemistry
- (I).
- Natural: provide the mechanical foundation for most plants and animals, as do carbohydrates and proteins. Examples of these are biodegradable materials;
- (II).
- Elastomers: have elastic properties, which recover their initial length after tension interruption. Examples of these are tires;
- (III).
- Thermosets: these are plastics that, during manufacture, are moldable at low temperatures and harden when heated, typically via chemical crosslinking reactions. Thus, when heated, they are not recyclable. For this reason, they possess excellent mechanical properties, namely dimensional stability, rigidity, and non-ductility, among others. Examples of these materials are formaldehyde and epoxy resins, among others;
- (IV).
- Thermoplastics: formed by macromolecules. Their main characteristic is that when heated, intermolecular forces are weakened, making them malleable, and when cooled, they solidify. This process is reversible, and this is their main advantage. Most plastics produced today are thermoplastics. There are different types of plastics based on their constituent groups and the type of materials used in their production.
2.1. Types
2.1.1. Polyethylene (PE)
2.1.2. Polypropylene (PP)
2.1.3. Polystyrene (PS)
2.1.4. Poly (Vinyl Chloride) (PVC)
2.1.5. Poly (Ethylene Terephthalate) (PET)
2.1.6. Polyethylene/Polypropylene/Polystyrene (PE/PP/PS)
2.1.7. Low-Density Polyethylene/Polypropylene (LDPE/PP)
2.2. Physicochemical Properties
2.2.1. Molecular Weight
2.2.2. Degree of Crystallinity
2.2.3. Thermal Properties
2.2.4. Electric Properties
2.2.5. Chemical Properties
2.3. Optimization of the Properties of Plastics
3. Plastic Waste and Its Associated Health and Environmental Risks
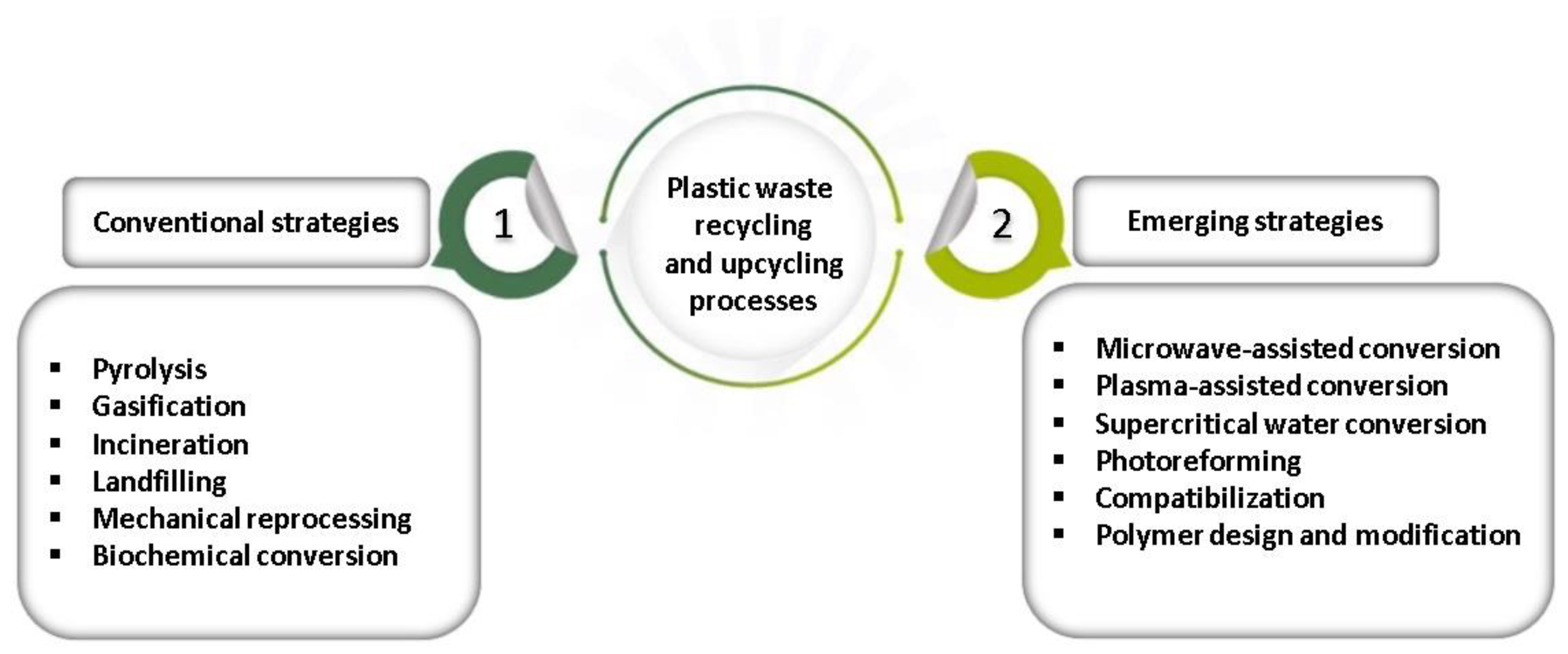
3.1. Technology for Recycling
3.1.1. Mechanical Recycling
3.1.2. Raw Material Recycling
3.1.3. Energy Recycling
3.2. Factors Affecting Plastics Management
3.3. Recycling Issues and the Impact on the Environment
4. Relationship between Nanotechnology and Sustainable Development
4.1. Metallic Nanoparticles and Nanostructured Metal Oxides
4.2. Problems of Use in Suspension
5. Turning Plastic Waste into Valuable Products
5.1. Accumulation of Plastic Waste in the Environment
5.2. Polymeric Composites for Waste Recovery
5.3. Catalysis Opportunities: Supports for Heterogeneous Catalysts
6. Plastics and the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patni, N.; Shah, P.; Agarwal, S.; Singhal, P. Alternate Strategies for Conversion of Waste Plastic to Fuel. ISRN Renew. Energy 2013, 2013, 902053. [Google Scholar] [CrossRef]
- Al Rayaan, M.B. Recent advancements of thermochemical conversion of plastic waste to biofuel-A review. Clean. Eng. Technol. 2021, 2, 100062. [Google Scholar] [CrossRef]
- Yeung, W.S.; Loh, W.W.; Lau, H.H.; Loh, X.J.; Lim, J.Y. Catalysts developed from waste plastics: A versatile system for biomass conversion. Mater. Today Chem. 2021, 21, 100524. [Google Scholar] [CrossRef]
- Istrate, O.M.; Chen, B. Structure and properties of clay/recycled plastic composite. Appl. Clay Sci. 2018, 156, 144–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X. Polymer antibacterial agent immobilized polyethylene films as efficient antibacterial cling film. Mater. Sci. Eng. 2019, 105, 110088. [Google Scholar] [CrossRef]
- Far, H.; Nejadi, S. Experimental investigation on interface shear strength of composite PVC encased macro-synthetic fibre reinforced concrete wall. Structures 2021, 34, 729–737. [Google Scholar] [CrossRef]
- Peitsch, W.K.; Hofmann, I.; Bulkescher, J.; Hergt, M.; Spring, H.; Bleyl, U.; Goerdt, S.; Franke, W.W. Drebrin, an Actin-Binding, Cell-Type Characteristic Protein: Induction and Localization in Epithelial Skin Tumors and Cultured Keratinocyte. J. Investig. Dermatol. 2005, 125, 761–774. [Google Scholar] [CrossRef]
- Sharma, B.; Goswami, Y.; Sharma, S.; Shekhar, S. Inherent roadmap of conversion of plastic waste into energy and its life cycle assessment: A frontrunner compendium. Renew. Sustain. Energy Rev. 2021, 146, 111070. [Google Scholar] [CrossRef]
- Dedman, J.; Christie-Oleza, J.A.; Fernández-Juárez, V.; Echeveste, P. Cell size matters: Nano- and micro-plastics preferentially drive declines of large marine phytoplankton due to coaggregation. J. Hazard. Mater. 2022, 424, 127488. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Eroldoğan, O.T.; Evliyaoğlu, E.; Turchini, G.M.; Wu, X.G. Fish out, plastic in: Global pattern of plastics in commercial fishmeal. Aquacultur 2021, 534, 736316. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Chan, W.P.; Giannis, A.; Lisak, G. Chemical recycling of plastic waste for sustainable material management: A prospective review on catalysts and processes. Renew. Sustain. Energy Rev. 2022, 154, 111866. [Google Scholar] [CrossRef]
- Gaurav, T.; Chetna, T.; Bhashkar, S.B.; Sandeep, P.; Manoj, K.; Sumit, K.; Himani, T.; Sunil, D.; Nanda, G.S. Waste plastic derived graphene sheets as nanofillers to enhance mechanical strength of concrete mixture: An inventive approach to deal with universal plastic waste. Cleaner Eng. Technol. 2021, 5, 100275. [Google Scholar]
- Gaggino, R.; Positieri, M.J.; Irico, P.; Kreiker, J.; Arguello, R.; Sánchez, M.P.A. Ecological Roofing Tiles Made with Rubber and Plastic Waste. J. Adv. Mater. Res. 2013, 844, 458–461. [Google Scholar] [CrossRef]
- Agyeman, S.; Obeng-Ahenkora, N.K.; Assiamah, S.; Twumasi, G. Exploiting recycled plastic waste as an alternative binder for paving blocks production. Case Stud. Const Mater. 2019, 11, e00246. [Google Scholar] [CrossRef]
- Olofinnade, O.; Morawo, A.; Okedairo, O.; Kim, B. Solid waste management in developing countries: Reusing of steel slag aggregate in eco-friendly interlocking concrete paving blocks production. Case Stud. Const Mater. 2021, 14, e00532. [Google Scholar] [CrossRef]
- Chanhoun, M.; Padonou, S.; Adjovi, E.C.; Olodo, E.; Doko, V. Study of the implementation of waste wood, plastics and polystyrenes for various applications in the building industry. Constr. Build. Mater. 2018, 167, 936–941. [Google Scholar] [CrossRef]
- Rajmohan, K.; Ramya, C.; Viswanathan, M.R.; Varjani, S. Plastic pollutants: Waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Barrera-Rendon, E.; Oliva, J.; Rodriguez-Gonzalez, V. Flexible CuS/TiO2 based composites made with recycled bags and polystyrene for the efficient removal of the 4-CP pesticide from drinking wate. Sep. Purif Technol. 2021, 270, 118821. [Google Scholar] [CrossRef]
- De Assis, G.; Skovroinski, E.; Leite, V.D.; Rodrigues, M.O.; Galembeck, A.; Alves, M.F.; De Oliveira, R.J. Conversion of “Waste Plastic” into Photocatalytic Nanofoams for Environmental Remediation. ACS Appl. Mater. Interface 2018, 10, 8077–8085. [Google Scholar] [CrossRef]
- Moura, M.M.M.S.; Lima, V.E.; Neto, A.M.; Lucena, A.L.A.; Napoleão, D.; Duarte, M.M.M.B. Degradation of the mixture of the ketoprofen, meloxicam and tenoxicam drugs using TiO2/metal photocatalysers supported in polystyrene packaging waste Water. Sci. Technol. 2021, 4, 863–876. [Google Scholar] [CrossRef]
- Malara, A. Methods for Recycling Heterogenous Catalyst. Available online: https://encyclopedia.pub/10920 (accessed on 12 November 2021).
- Zhang, H.; Ge, C.; Zhu, C.; Li, Y.; Tian, W.; Cheng, D.; Pan, Z. Deodorizing Properties of Photocatalyst Textiles and Its Effect Analysis. Phys. Procedia 2012, 25, 240–244. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Lan, X.; Hu, Q.; Li, H.; Li, L.; Mao, J. Antifouling strategies based on super-phobic polymer material. Prog. Org. Coat. 2021, 157, 106285. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodriguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunitie. Mater. Res. 2009, 1, 1–39. [Google Scholar] [CrossRef]
- Crawford, R.; Martin, P. Plastics Engineering, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2020. [Google Scholar]
- Bardají, D.K.R.; Moretto, J.A.S.; Furlan, J.P.R.; Stehling, E.G. A mini-review: Current advances in polyethylene bio-degradation. World J. Microbiol. Biotechnol. 2020, 36, 32. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Islam, S.; Haque, M.A.; Rahman, M.; Mithu, M.R. Toxic effects of plastic on human health and environment: A consequences of health risk assessment in Bangladesh. Int. J. Health 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Gahleitner, M.; Paulik, C. Polypropylene and Other Polyolefin. Brydson’s Plast. Mater. 2017, 279–309. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Rosa, D.S.; Guedes, G.F.; Carvalho, L. Processing and thermal, mechanical and morphological characterization of post-consumer polyolefins/thermoplastic starch blend. J. Mater. Sci. 2007, 42, 551–557. [Google Scholar] [CrossRef]
- Andrade, B.T.N.; Bezerra, A.D.S.; Calado, R. Adding value to polystyrene waste by chemically transforming it into sulfonated polystyrene. Matéria (Rio de Janeiro) 2019, 24, 24. [Google Scholar] [CrossRef]
- Sulong, N.H.R.; Mustapa, S.A.S.; Rashid, M.K.A. Application of expanded polystyrene (EPS) in buildings and constructions: A review. J. Appl. Polym. Sci. 2019, 136, 47529. [Google Scholar] [CrossRef]
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.A. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. Int. J. Toxicol. Risk Assess 2019, 5, 1–13. [Google Scholar]
- Ojeda, T. Polymers and the Environment. Polym. Sci. 2013, 2013, 23. [Google Scholar]
- Chowdhury, T.U.; Mahi, M.A.; Haque, K.A.; Rahman, M. A Review On The Use Of Polyethylene Terephthalate (Pet) As Aggregates In Concret. Malay J. Sci. 2018, 37, 118–136. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Proces Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Akshaya, E.; Palaniappan, R.; Sowmya; Rasana, N.; Jayanarayanan, K. Properties of Blends from Polypropylene and Recycled Polyethylene Terephthalate using a Compatibilize. In Materials Today: Proceedings; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 24, pp. 359–368. [Google Scholar]
- Vervoort, S.; den Doelder, J.; Tocha, E.; Genoyer, J.; Walton, K.L.; Hu, Y.; Jeltsch, K. Compatibilization of polypropylene-polyethylene blend. Polym. Eng. Sci. 2017, 58, 460–465. [Google Scholar] [CrossRef]
- Hubo, S.; Delva, L.; Van Damme, N.; Ragaert, K. Blending of recycled mixed polyolefins with recycled poly-propylene: Effect on physical and mechanical propertie. AIP Conf. Proc. 2016, 1779, 140006. [Google Scholar]
- Dorigato, A. Recycling of polymer blend. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of Polypropylene/Polyethylene Blends: Effect of Chain Structure on the Crystallization Behavior. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Aumnate, H.; Spicker, S.; Kiesel, R.; Samadi, M.; Rudolph, N. Recycling of PP/LDPE Blend: Miscibility, Thermal Properties, Rheological Behavior and Crystal Structur. Conf. SPE ANTEC 2016, 2016, 81–88. [Google Scholar]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical Reprocessing of Polyolefin Waste: A Review. Polym. Eng. Sci. 2015, 2899–2909. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.; Nizami, A. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterio. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Kim, S.Y.; Meyer, H.W.; Saalwächter, K.; Zukoski, F. Polymer Dynamics in PEG-Silica Nanocomposites: Effects of Polymer Molecular Weight, Temperature and Solvent Dilution. Macromolecules 2012, 45, 4225–4237. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis in the Analysis of Polymers and Rubber. Encycl. Polym. Sci. Technol. 2015, 2002, 1–33. [Google Scholar] [CrossRef]
- Rogošić, M.; Mencer, H.; Gomzi, Z. Polydispersity index and molecular weight distributions of polymer. Eur. Polym. J. 1996, 32, 1337–1344. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier System. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Stepto, R.F.T. Dispersity in polymer science (IUPAC Recommendations 2009). Pure Appl. Chem. 2009, 81, 351–353. [Google Scholar] [CrossRef]
- Batista, N.L.; Olivier, P.; Bernhart, G.; Rezende, M.; Botelho, E. Correlation between degree of crystallinity, morphology and mechanical properties of PPS/carbon fiber laminate. Mater. Res. 2016, 19, 195–201. [Google Scholar] [CrossRef]
- Uygunoğlu, T.; Güneş, I.; Brostow, W. Physical and Mechanical Properties of Polymer Composites with High Content of Wastes Including Boron. Mater. Res. 2015, 18, 1188–1196. [Google Scholar] [CrossRef]
- Doyle, M.J. On the effect of crystallinity on the elastic properties of semicrystalline polyethylene. Polym. Eng. Sci. 2000, 40, 330–335. [Google Scholar] [CrossRef]
- Molnár, J.; Zuba, Z.; Sepsi, Ö.; Ujhelyi, F.; Erdei, G.; Lenk, S.; Menyhárd, A. Structural investigation of semicrystalline polymer. Polym. Test. 2021, 95, 107098. [Google Scholar] [CrossRef]
- Stein, R.S.; Prud’Homme, R. Origin of polyethylene transparency. J. Polym. Sci. Part B Polym. Lett. 1971, 9, 595–598. [Google Scholar] [CrossRef]
- Crawford, R.J.; Throne, J.L. Rotational molding polymers. Rotat. Molding Technol. 2002, 19–68. [Google Scholar] [CrossRef]
- Jadhav, N.; Gaikwad, V.L.; Nair, K.; Kadam, H. Glass transition temperature: Basics and application in pharmaceutical sector. Asian J. Pharm. 2009, 3, 82. [Google Scholar] [CrossRef]
- Roudaut, G.; Simatos, D.; Champion, D.; Contreras-Lopez, E.; Le Meste, M. Molecular mobility around the glass transition temperature: A mini review. Innov. Food Sci. Emerg. Technol. 2004, 5, 127–134. [Google Scholar] [CrossRef]
- Wang, C.; Kondrashchenko, V.I.; Matseevich, A.V. Prediction of the coefficient of thermal expansion of building materials based on polyvinyl chlorid. J. Phys. Conf. Ser. 2019, 1425, 012094. [Google Scholar] [CrossRef]
- Muthamilselvan, T.; Mondal, T. Thermally Conductive Plastics for Electronic Application. Ref. Modul. Mater. Sci. Mater. Eng. 2021. [Google Scholar] [CrossRef]
- Ouyang, J. Application of intrinsically conducting polymers in flexible electronic. SmartMat 2021, 2, 263–285. [Google Scholar] [CrossRef]
- Da Cunha, B.; Lopes, P.P.; Mayer, F.D.; Hoffmann, R. Assessment of Chemical and Mechanical Properties of Polymers Aiming to Replace the Stainless Steel in Distillation Column. Mater. Res. 2018, 21, 21. [Google Scholar] [CrossRef]
- Baum, B.; Deanin, R.D. Controlled UV Degradation in Plastic. Polym. Technol. Eng. 1973, 2, 1–28. [Google Scholar] [CrossRef]
- Markovičová, L.; Zatkalíková, V. The effect of UV aging on structural polymer. IOP Conf. Ser. Mater. Sci. Eng. 2019, 465, 012004. [Google Scholar] [CrossRef]
- Teska, P.; Dayton, R.; Li, X.; Lamb, J.; Strader, P. Damage to Common healthcare. Polymer Surfaces from UV Exposure. Nano LIFE 2020, 10, 2050001. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustainable. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar]
- Paoli, M.A.D.; Waldman, W.R. Bio-based additives for thermoplastic. Polímeros 2019, 29, e2019030. [Google Scholar] [CrossRef]
- Czogała, J.; Pankalla, E.; Turczyn, R. Recent Attempts in the Design of Efficient PVC Plasticizers with Reduced Migration. Materials 2021, 14, 844. [Google Scholar] [CrossRef]
- Souza, D.H.S.; Andrade, T.; Dias, M.L. Effect of synthetic mica on the thermal properties of poly(lactic acid). Polímeros 2014, 24, 20–24. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Oldenziel, R.; Weber, H. Introduction: Reconsidering Recycling. Contemp. Eur Hist. 2013, 22, 347–370. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.; D’Hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future direction. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Lahtela, V.; Hyvärinen, M.; Kärki, T. Composition of Plastic Fractions in Waste Streams: Toward more. Efficient Recycling and Utilization. Polymers 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.; Ude, A.; Ogunmuyiwa, E.; Zulkifli, R.; Beas, I. An Overview of Plastic Waste Generation and Management in Food Packaging Industrie. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Walker, T.R.; McGuinty, E.; Charlebois, S.; Music, J. Single-use plastic packaging in the Canadian food industry: Consumer behavior and perception Humanit. Soc. Sci. Commun. 2021, 8, 80. [Google Scholar] [CrossRef]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impact. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Chabuk, A.; Al-Ansari, N.; Hussein, H.M.; Kamaleddin, S.; Knutsson, S.; Pusch, R.; Laue, J. Soil Characteristics in Selected Landfill Sites in the Babylon Governorate. Iraq. J. Civil Eng. Archit. 2017, 11, 348–363. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future application. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic waste. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef]
- Jubinville, D.; Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T. Thermo-mechanical recycling of polypropylene for the facile and scalable fabrication of highly loaded wood plastic composite. Compos. Part B Eng. 2021, 219, 108873. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R. Advances and approaches for chemical recycling of plastic waste. J. Appl. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Almeida, D.; Marques, M.D.F. Thermal and catalytic pyrolysis of plastic waste. Polímeros 2016, 26, 44–51. [Google Scholar] [CrossRef]
- Grigore, M.E. Methods of Recycling, Properties and Applications of Recycled Thermoplastic Polymer. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philo Tran R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, H.; Cheng, Z.; Yan, B.; Chen, G.; Wang, S. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Maafa, I.M.; Ahmed, U.; Akhter, P.; Shehzad, N.; Amjad, U.-E.-S.; Hussain, M. Thermal conversion of polystyrene plastic waste to liquid fuel via ethanolysis. Fuel 2020, 279, 118498. [Google Scholar] [CrossRef]
- Power Technology. Turning Waste into Power: The Plastic to Fuel Project. Available online: https://www.powertechnology.com/comment/plastic-to-fuel/ (accessed on 7 December 2021).
- Jha, K.K.; Kannan, T. Recycling of plastic waste into fuel by pyrolysis—A review. Mater. Today Proc. 2021, 37, 3718–3720. [Google Scholar] [CrossRef]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Tomishige, K. Low-Temperature Catalytic Upgrading of Waste Polyolefinic Plastics into Liquid Fuels and Waxes. Appl. Catal. B Environ. 2020, 285, 119805. [Google Scholar] [CrossRef]
- Christopher, F.J.; Kumar, P.S.; Vo, D.-V.N.; Christopher, F.C.; Jayaraman, L. Methods for chemical conversion of plastic wastes into fuels and chemicals. A review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Jha, K.K.; Kannan, T.; Chandradass, J.; Wilson, D.V.H.; Das, A. Analysis and simulation of mini pyrolysis reactor for conversion of plastic waste into fuel. Mater. Today Proc. 2021, 45, 7166–7170. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Kedzierski, M.; Frère, D.; Le Maguer, G.; Bruzaud, S. Why is there plastic packaging in the natural environment? Under-standing the roots of our individual plastic waste management behavior. Sci. Total Environ. 2020, 740, 139985. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC waste. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- North, E.J.; Halden, R.U. Plastics and environmental health: The road ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issue. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.A.A.; Suryanto; Sudarwanto, A.S.; Nugraha, S.; Utomowati, R. Reduce marine debris policy in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 724, 012118. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste–A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Martinez, W. How science and technology developments impact employment and education. Proc. Natl. Acad. Sci. USA 2018, 115, 12624–12629. [Google Scholar] [CrossRef]
- Mitchell, S.; Qin, R.; Zheng, N.; Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Mishra, R.; Militky, J. Future outlook in the context of nanoscale textiles as a technology for the twenty-first century. Nanotechnol. Text. 2019, 387–388. [Google Scholar] [CrossRef]
- Kumar, K.; Chowdhury, A. Nanoscale Heterogeneity in Amorphous and Semi-Crystalline Materials: A Technical Perspective. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulation. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metal 2021, 11, 80. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenge. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- García-Quintero, A.; Palencia, M. A critical analysis of environmental sustainability metrics applied to green synthesis of nanomaterials and the assessment of environmental risks associated with the nanotechnology. Sci. Total Environ. 2021, 793, 148524. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sus-tainable innovation in nanotechnology: State-of-play for smart nanomaterial. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Khalaj, M.; Kamali, M.; Costa, M.E.V.; Capela, I. Green synthesis of nanomaterials—A scientometric assessment. J. Clean. Prod. 2020, 267, 122036. [Google Scholar] [CrossRef]
- De Jesus, R.A.; De Assis, G.; De Oliveira, R.J.; Costa, J.A.S.; Da Silva, M.P.; Bilal, M.; Figueiredo, R.T. Environ-mental remediation potentialities of metal and metal oxide nanoparticles: Mechanistic biosynthesis, influencing factors, and application standpoint. Environ. Technol. Innov. 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Application. J. NanoMater. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review Front. Bioeng. Biotecnol. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant deriva-tives and their new avenues in pharmacological application—An updated report. Saudi Pharm J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100279. [Google Scholar] [CrossRef]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and green synthesis of carbon nanomaterials: A review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Fleischer, T.; Grunwald, A. Making nanotechnology developments sustainable A role for technology assessment? J. Clean. Prod. 2008, 16, 889–898. [Google Scholar] [CrossRef]
- Tom, A.P. Nanotechnology for sustainable water treatment—A review. Mater. Today Proc. 2021, 1–6. [Google Scholar] [CrossRef]
- Diallo, M.S.; Fromer, N.A.; Jhon, M.S. Nanotechnology for sustainable development: Retrospective and outlook. J. Nanoparticle Res. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Moore, K.; Wei, W. Applications of carbon nanomaterials in perovskite solar cells for solar energy conversion. Nano Mater. Sci. 2021, 3, 276–290. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H. Recent advances in carbon nanomaterial-optimized perovskite solar cell. Mater. Today Energy 2021, 21, 100769. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.-J.; Hedau, B.S.; Ha, T.-J. Recent progress in solar cells based on carbon nanomaterial. Sol. Energy 2021, 220, 953–990. [Google Scholar] [CrossRef]
- Qi, H.; Xu, Y.; Hu, P.; Yao, C.; Yang, D. Construction and applications of DNA-based nanomaterials in cancer therapy. Chin. Chem. Lett. 2021, 1–14. [Google Scholar] [CrossRef]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical application. Mater. Today 2021, 2021, 1–27. [Google Scholar] [CrossRef]
- Damodharan, J. Nanomaterials in medicine—An overview. Mater. Today Proc. 2020, 37, 383–385. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensor. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Ahmad, R.; Majhi, S.M.; Zhang, X.; Swager, T.M.; Salama, K.N. Recent progress and perspectives of gas sensors based on vertically oriented ZnO nanomaterial. Adv. Colloid Interface Sci. 2019, 270, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Van Duy, N.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Share, K.; Westover, A.; Li, M.; Pint, L. Surface engineering of nanomaterials for improved energy storage—A review. Chem. Eng. Sci. 2016, 154, 3–19. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, L.; Fang, Z.; Yan, C.; Zhang, X.; Yu, G. Structural Engineering of 2D Nanomaterials for Energy Storage and Catalysis. Adv. Mater. 2018, 30, e1706347. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Saini, V.K.; Bhushan, B. Chapter Nanomaterials for Energy Harvesting and Storage: An Overview. Appl. Nanomater. Agric. Food Sci. Med. 2021, 16, 188–203. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterial. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Zhai, W.; Lu, S.; Liang, J.; He, Z.; Long, H.; Xiong, T.; Sun, H.; He, Q.; et al. Phase Engineering of Nanomaterials for Clean Energy and Catalytic Application. Adv. Energy Mater. 2020, 10, 2002019. [Google Scholar] [CrossRef]
- Khalil, M.; Kadja, G.T.; Ilmi, M.M. Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. J. Ind. Eng. Chem. 2021, 93, 78–100. [Google Scholar] [CrossRef]
- Jangid, P.; Inbaraj, M.P. Applications of nanomaterials in wastewater treatment. Mater. Today Proc. 2021, 43, 2877–2881. [Google Scholar] [CrossRef]
- Alidokht, L.; Anastopoulos, I.; Ntarlagiannis, D.; Soupios, P.; Tawabini, B.; Kalderis, D.; Khataee, A. Recent advances in the application of nanomaterials for the remediation of arsenic-contaminated water and soil. J. Environ. Chem. Eng. 2021, 9, 105533. [Google Scholar] [CrossRef]
- Sridevi, M.; Nirmala, C.; Jawahar, N.; Arthi, G.; Vallinayagam, S.; Sharma, V.K. Role of nanomaterial’s as adsorbent for heterogeneous reaction in waste water treatment. J. Mol. Struct. 2021, 1241, 130596. [Google Scholar] [CrossRef]
- Anwar, J.; Dipak, V.; Shirish, H.; Bharat, A.; Javed, S.; Mika, S. Cellulose-based nanomaterials for water and wastewater treatments: A review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar]
- Cheng, K.; Lim, J.-W.; Adhikari, S. Converting solid biomass waste into nanomaterial for the treatment of hazardous waste. Chemosphere 2021, 285, 131461. [Google Scholar] [CrossRef]
- Tamsilian, Y.; Shirazi, M.; Rad, G.M. Nanomaterial-Incorporated Polymer Composites for Industrial Effluent: From Syn-thesis to Application. Encycl. Mater. Compos. 2021, 1, 998–1012. [Google Scholar]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Naveen Prasad, B.S.; Praveen Kumar, T.R.; Rajasimman, M.; Hosseini-Bandegharaei, A.; et al. A focus to green synthesis of metal/metal based ox-ide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Badri, A.; Slimi, S.; Guergueb, M.; Kahri, H.; Mateos, X. Green synthesis of copper oxide nanoparticles using Prickly Pear peel fruit extract: Characterization and catalytic activity. Inorg. Chem. Commun. 2021, 134, 109027. [Google Scholar] [CrossRef]
- Cruz, D.R.; de Jesus, G.K.; Santos, A.; Silva, W.R.; Wisniewski, A.; Cunha, G.; Romão, L.P. Magnetic nanostructured material as heterogeneous catalyst for degradation of AB210 dye in tannery wastewater by electro-Fenton process. Chemosphere 2021, 280, 130675. [Google Scholar] [CrossRef]
- Liu, D.; Yang, N.; Zeng, Q.; Liu, H.; Chen, D.; Cui, P.; Xu, L.; Hu, C.; Yang, J. Core-shell Ag–Pt nanoparticles: A versatile platform for the synthesis of heterogeneous nanostructures towards catalyzing electrochemical reaction. Chin. Chem. Lett. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Soares, L.G.; Vaz, M.D.O.; Teixeira, S.R.; Alves, A.K. Absorbance determination and photocatalytic production of hydrogen using tungsten and TiO2 oxide nanostructures As catalyst. Clean. Eng. Technol. 2021, 5, 100268. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalyst. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the environment, human exposure pathway, and health effects: A review. Sci. Total. Environ. 2020, 759, 143470. [Google Scholar] [CrossRef]
- Korani, M.; Ghazizadeh, E.; Korani, S.; Hami, Z.; Mohammadi-Bardbori, A. Effects of silver nanoparticles on human health. Eur. J. Nanomed. 2015, 7, 51–62. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Atmianlu, P.A.; Badpa, R.; Aghabalaei, V.; Baghdadi, M. A review on the various beds used for immobilization of nanoparticles: Overcoming the barrier to nanoparticle applications in water and wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106514. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, M.; Shan, L.; Wu, J.; Quan, G.; Cui, L.; Yan, J. Bimetallic FeNi nanoparticles immobilized by bio-mass-derived hierarchically porous carbon for efficient removal of Cr (VI) from aqueous solution. J. Hazard. Mater. 2022, 423, 127098. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspective. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.-S.; Zhang, Y.-D.; Gao, H.-J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Kr, S.D. A review of hierarchical nanostructures of TiO2: Advances and application. Appl. Sur Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Bozon-Verduraz, F.; Fiévet, F.; Piquemal, J.-Y.; Brayner, R.; El Kabouss, K.; Soumare, Y.; Viau, G.; Shafeev, G. Nanoparticles of metal and metal oxides: Some peculiar synthesis methods, size and shape control, application to catalysts preparation. Braz. J. Phys. 2009, 39, 134–140. [Google Scholar] [CrossRef]
- Kanniah, P.; Chelliah, P.; Thangapandi, J.R.; Thangapandi, E.J.S.B.; Kasi, M.; Sivasubramaniam, S. Chapter Benign fabrication of metallic/metal oxide nanoparticles from alga. In Nanobiotechnology for Plant Protection: Agri-Waste and Microbes for Production of Sustainable Nanomaterial; Abd-Elsalam, K.A., Periakaruppan, R., Rajeshkuma, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 465–493. [Google Scholar]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Kudo, S.; Hayashi, J. Chapter 2. An Overview of Metal Oxide Nanostructures. Synth. Inorg. Nanomater. 2018, 19–57. [Google Scholar] [CrossRef]
- Rao, V.N.; Malu, T.J.; Cheralathan, K.K.; Sakar, M.; Pitchaimuthu, S.; Rodríguez-González, V.; Kumari, M.M.; Shankar, M.V. Light-driven transformation of biomass into chemicals using photocatalysts—Vistas and challenge. J. Environ. Manag. 2021, 284, 111983. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.; Kausar, M.A.; Saeed, M.; Gupta, V.K.; Singh, R.; Ramteke, P. Advances in nanomaterials induced biohydrogen production using waste biomas. Bioresou Technol. 2020, 307, 123094. [Google Scholar] [CrossRef]
- Nwosu, U.; Wang, A.; Palma, B.; Zhao, H.; Khan, M.A.; Kibria, M.; Hu, J. Selective biomass photoreforming for valuable chemicals and fuels: A critical review. Renew. Sustain. Energy Rev. 2021, 148, 111266. [Google Scholar] [CrossRef]
- Ma, J.; Jin, D.; Li, Y.; Xiao, D.; Jiao, G.; Liu, Q.; Guo, Y.; Xiao, L.; Chen, X.; Li, X.; et al. Photocatalytic conversion of biomass-based monosaccharides to lactic acid by ultrathin porous oxygen doped carbon nitrid. Appl. Catal. B Environ. 2021, 283, 119520. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The future of semiconductors nanoparticles: Synthesis, properties and application Mater. Sci. Eng. B 2021, 272, 1–24. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nano-particles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Ramalingam, G.; Kathirgamanathan, P.; Ravi, G.; Elangovan, T.; Kumar, B.A.; Manivannan, N.; Kasinathan, K. Quantum Confinement Effect of 2D NanomaterialS; Quantum Dots—Fundamental and Applications, Faten Divsar; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/70534 (accessed on 12 November 2021). [CrossRef]
- Aghamirzaei, M.; Khiabani, M.S.; Hamishehkar, H.; Mokarram, R.R.; Amjadi, M. Antioxidant, antimicrobial and cytotoxic activities of biosynthesized gold nanoparticles (AuNPs) from Chinese lettuce (CL) leave extract (Brassica rapa va pekinensis). Mater. Today Commun. 2021, 29, 102831. [Google Scholar] [CrossRef]
- Ezealigo, U.S.; Ezealigo, B.N.; Aisida, S.O.; Ezema, F.I. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Patil, M.P.; Seong, Y.-A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Synthesis of silver nanoparticles using aqueous extract of Cuscuta japonica seeds and their antibacterial and antioxidant activitie. Inorg. Chem. Commun. 2021, 109035. [Google Scholar] [CrossRef]
- Cizmar, T.; Panzic, I.; Capan, I.; Gajović, A. Nanostructured TiO2 photocatalyst modified with Cu for improved imidacloprid degradation. Appl. Sur. Sci. 2021, 569, 151026. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, T.; Li, L.; Wang, J.; Li, Y.; Zhang, H. Core-shell nanostructure α-Fe2O3/SnO2 binary oxides for the catalytic ox-idation and adsorption of elemental mercury from flue gas. J. Environ. Chem. Eng. 2021, 9, 105137. [Google Scholar] [CrossRef]
- Hoang, T.T.N.; Lin, Y.-S.; Le, T.N.H.; Le, T.K.; Huynh, T.K.X.; Tsai, D.-H. Cu-ZnO@Al2O3 hybrid nanoparticle with enhanced activity for catalytic CO2 conversion to methanol. Adv. Powder Technol. 2021, 32, 1785–1792. [Google Scholar] [CrossRef]
- Nandanapalli, K.R.; Mudusu, D.; Lingandhinne, R.M.R.; Lee, S.W. Passivation layeredependent catalysis of zinc oxide nanostructure. Mater. Today Chem. 2021, 22, 100592. [Google Scholar] [CrossRef]
- Damian, C.; Necolau, M.; Neblea, I.; Vasile, E.; Iovu, H. Synergistic effect of graphene oxide functionalized with SiO2 nanostructures in the epoxy nanocomposite. Appl. Sur Sci. 2020, 507, 145046. [Google Scholar] [CrossRef]
- Minakshi, M.; Blackford, M.; Ionescu, M. Characterization of alkaline-earth oxide additions to the MnO2 cathode in an aqueous secondary battery. J. Alloy. Compd. 2011, 509, 5974–5980. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electro-chemistry of magnesium molybdate hierarchical architectures for high performance sodium device. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, M. Metallic Nanoparticle. Available online: https://sabotin.ung.si/~sstanic/teaching/Seminar/2009/20091214_Dragomir_MetNP.pd (accessed on 8 November 2021).
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef]
- Goniakowski, J. Noguera, Insulating oxide surfaces and nanostructure. Comptes Rendus Phys. 2016, 17, 471–480. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Application. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.; Matin, M.; Hakim, M.; Islam, M. Optical and electrical properties of impurity-less multiferroic bismuth ferrite nanoparticle. Mater. Sci. Eng. B 2021, 275, 115501. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Zhang, X. Recent progress on transition metal oxides as advanced mate-rials for energy conversion and storage. Energy Storage Mater. 2021, 42, 317–369. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A. Nano-Heterostructure Metal Oxide Gas Sensors: Opportunities and Challenge. Ref. Modul. Mater. Sci. Mater. Eng. 2022, 3, 297–301. [Google Scholar]
- Hassan, I.U.; Salim, H.; Naikoo, G.A.; Awan, T.; Dar, R.A.; Arshad, F.; Qurashi, A. A review on recent advances in hi-erarchically porous metal and metal oxide nanostructures as electrode materials for supercapacitors and non-enzymatic glucose sensor. J. Saudi Chem. Soc. 2021, 25, 101228. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Adekoya, J.A.; Ogunniran, K.O.; Siyanbola, T.O.; Dare, E.O.; Revaprasadu, N. Band Structure, Morphology, Functionality, and Size-Dependent Properties of Metal Nanoparticle. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; IntechOpen: London, UK, 2018; pp. 15–42. Available online: https://www.intechopen.com/chapters/58870 (accessed on 12 November 2021). [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. J. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanism. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Nwanya, A.; Razanamahandry, L.; Bashir, A.; Ikpo, O.; Nwanya, S.; Botha, S.; Ntwampe, S.; Ezema, F.I.; Iwuoha, E.; Maaza, M. Industrial textile effluent treatment and antibacterial effectiveness of Zea mays L. Dry husk mediated bio-synthesized copper oxide nanoparticle. J. Hazard. Mater. 2019, 375, 281–289. [Google Scholar] [CrossRef]
- Ragothaman, M.; Mekonnen, B.T.; Palanisamy, T. Synthesis of magnetic Fe–Cr bimetallic nanoparticles from industrial effluents for smart material application. Mater. Chem. Phys. 2020, 253, 123405. [Google Scholar] [CrossRef]
- Jeevanandam, P.; Klabunde, K.J. Chapter 14. Adsorbents. Synthesis, Properties, and Applications of Oxide Nanomaterials; Rodriguez, J.A., Fernández-Garcia, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 383. ISBN 978-0-471-72405-6. [Google Scholar]
- Xu, L.-H.; Patil, D.S.; Yang, J.; Xiao, J. Metal Oxide Nanostructures: Synthesis, Properties, and Application. J. Nanotechnol. 2015, 2015, 1–2. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalyst. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Lofrano, G.; Carotenuto, M.; Libralato, G.; Domingos, R.F.; Markus, A.; Dini, L.; Meric, S. Polymer functionalized nanocomposites for metals removal from water and wastewater: An overview. Water Res. 2016, 92, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.L.; Casman, E.A.; Lowry, G.V.; Lead, J.R.; Viparelli, E.; Baalousha, M. Modeling Nanomaterial Environmental Fate in Aquatic System. Environ. Sci. Technol. 2015, 49, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Soleimani, F.; Bidgoli, N.S.S.; Nezafat, Z.; Orooji, Y.; Baran, T. Recent developments in polymer-supported ruthenium nanoparticles/complexes for oxidation reaction. J. Organomet. Chem. 2021, 933, 121658. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Zhu, H. Health and Environmental Safety of Nanomaterial Polymer Nanocomposites and Other Materials Containing Nanoparticle; Elsevier: Amsterdam, The Netherlands, 2021; eBook; ISBN 9780128205105. [Google Scholar]
- Hu, Z.-P.; Wang, Z.; Yuan, Z.-Y. Cr/Al2O3 catalysts with strong metal-support interactions for stable catalytic dehydro-genation of propane to propylene. Mol. Catal. 2020, 493, 111052. [Google Scholar] [CrossRef]
- Neppolian, B.; Mine, S.; Horiuchi, Y.; Bianchi, C.L.; Matsuoka, M.; Dionysiou, D.D.; Anpo, M. Efficient photocatalytic degradation of organics present in gas and liquid phases using Pt-TiO2/Zeolite (H-ZSM). Chemosphere 2016, 153, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Shang, R.; Heijman, S.G.J.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-T.; Niu, H.; Wu, H.; Zeng, X.-F.; Wang, J.-X.; Chen, J.-F. PVP-stabilized platinum nanoparticles supported on mod-ified silica spheres as efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2021, 46, 25081–25091. [Google Scholar] [CrossRef]
- Karaboğa, S. Tungsten(VI) oxide supported rhodium(0) nanoparticles; highly efficient catalyst for H2 production from dimethylamine borane. Int. J. Hydrogen Energy 2021, 46, 17763–17775. [Google Scholar] [CrossRef]
- Böhm, D.; Beetz, M.; Gebauer, C.; Bernt, M.; Schröter, J.; Kornherr, M.; Zoller, F.; Bein, T.; Fattakhova-Rohlfing, D. Highly conductive titania supported iridium oxide nanoparticles with low overall iridium density as OER catalyst for large-scale PEM electrolysis. Appl. Mater. Today 2021, 24, 101134. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, W.; He, G.; Chi, J.; Duan, Y.; Chen, M.; Tian, M. Facile synthesis of extremely small Ag3PO4 nanoparticles on hierarchical hollow silica sphere (HHSS) for the enhanced visible-light photocatalytic property and stability. Colloids Surf. A 2019, 571, 1–8. [Google Scholar] [CrossRef]
- Orooji, Y.; Akbari, Z.; Nezafat, Z.; Nasrollahzadeh, M.; Kamali, T.A. Recent signs of progress in polymer-supported silver complexes/nanoparticles for remediation of environmental pollutants. J. Mol. Liq. 2021, 329, 115583. [Google Scholar] [CrossRef]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S.; Sahoo, G.C.; Ben Amar, R. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Oton, L.F.; Oliveira, A.C.; de Araujo, J.C.S.; Araujo, R.S.; de Sousa, F.F.; Saraiva, G.D.; Lang, R.; Otubo, L.; Duarte, G.C.D.S.; Campos, A. Selective catalytic reduction of NOx by CO (CO-SCR) over metal-supported nanoparticles dispersed on porous alumina. Adv. Powder Technol. 2020, 31, 464–476. [Google Scholar] [CrossRef]
- Poupart, R.; Grande, D.; Carbonnier, B.; Le Droumaguet, B. Porous polymers and metallic nanoparticles: A hybrid wed-ding as a robust method toward efficient supported catalytic systems. Prog. Polym. Sci. 2019, 96, 21–42. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; Sengupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanoparticle Res. 2012, 14, 1–24. [Google Scholar] [CrossRef]
- Singh, S.; Naik, T.S.S.K.; Anil, A.G.; Dhiman, J.; Kumar, V.; Dhanjal, D.S.; Aguilar-Marcelino, L.; Singh, J.; Ramamurthy, P.C. Micro (nano) plastics in wastewater: A critical review on toxicity risk assessment, behaviour, environmental impact and challenges. Chemosphere 2022, 290, 133169. [Google Scholar] [CrossRef] [PubMed]
- Fakirov, S. A new approach to plastic recycling via the concept of microfibrillar composites. Adv. Ind. Eng. Polym. Res. 2021, 4, 187–198. [Google Scholar] [CrossRef]
- Ronkay, F.; Molnar, B.; Gere, D.; Czigany, T. Plastic waste from marine environment: Demonstration of possible routes for recycling by different manufacturing technologies. Waste Manag. 2021, 119, 101–110. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Zada, M. Effect of carbon supported metals on the tertiary recycling of waste expanded poly-styrene. Process Saf. Environ. Prot. 2015, 96, 149–155. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Ribeiro-Brasil, D.R.G.; Torres, N.R.; Picanço, A.B.; Sousa, D.S.; Ribeiro, V.S.; Brasil, L.S.; Montag, L.F.d.A. Contamination of stream fish by plastic waste in the Brazilian Amazon. Environ. Pollut. 2020, 266, 115241. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.; Townsend, K.; King, R.; Huston, W.; Nash, S.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Horton, A.A.; Barnes, D.K.A. Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems. Sci. Total Environ. 2020, 738, 140349. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Pillai, J. Managing plastic waste—What emerging economies like India can learn from developed nations. Reinf. Plast. 2021, 1–4. [Google Scholar] [CrossRef]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S.; Naik, B. Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. ChemospheRe 2016, 159, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, C.; Wen, Y.; Chen, X.; Zhao, X.; Tang, T.; Holze, R.; Mijowska, E. Highly efficient conversion of waste plastic into thin carbon nanosheets for superior capacitive energy storage. Carbon 2021, 171, 819–828. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Phanisankar, B.; Rao, N.V.; Manikanta, J. Conversion of waste plastic to fuel products. Mater. Today Proc. 2020, 33, 5190–5195. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Jia, X.; Qin, C.; Friedberger, T.; Guan, Z.; Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2016, 2, e1501591. [Google Scholar] [CrossRef]
- Babayemi, J.O.; Nnorom, I.; Osibanjo, O.; Weber, R. Ensuring sustainability in plastics use in Africa: Consumption, waste generation, and projections. Environ. Sci. Eur. 2019, 31, 1–20. [Google Scholar] [CrossRef]
- Amankwa, M.O.; Tetteh, E.K.; Mohale, G.T.; Dagba, G.; Opoku, P. The production of valuable products and fuel from plastic waste in Africa. Discov. Sustain. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the loop for PET, PE and PP waste from house-holds: Influence of material properties and product design for plastic recycling. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Muñoz, M. Plastic waste re-cycling via pyrolysis: A bibliometric survey and literature review. J. Anal. Appl. Pyrolysis 2021, 156, 105265. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Almeshal, I.; Magbool, H.M.; Alabduljabbar, H.; Alyousef, R. Performance of sustainable concrete containing different types of recycled plastic. J. Cleaner Prod. 2021, 328, 129517. [Google Scholar] [CrossRef]
- Mehmandost, N.; Soriano, M.L.; Lucena, R.; Goudarzi, N.; Chamjangali, M.A.; Cardenas, S. Recycled polystyrene-cotton composites, giving a second life to plastic residues for environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103424. [Google Scholar] [CrossRef]
- Karmakar, G.P. Regeneration and Recovery of Plastics. Ref. Modul. Mater. Sci. Mater. Eng. 2020, 1–19. [Google Scholar] [CrossRef]
- Vieira, O.; Ribeiro, R.S.; Diaz de Tuesta, J.L.; Gomes, H.T.; Silva, A.M.T. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chem. Eng. J. 2022, 428, 131399. [Google Scholar] [CrossRef]
- Adamu, M.; Trabanpruek, P.; Jongvivatsakul, P.; Likitlersuang, S.; Iwanami, M. Mechanical performance and optimization of high-volume fly ash concrete containing plastic wastes and graphene nanoplatelets using response surface methodology. Constr. Build. Mater. 2021, 308, 125085. [Google Scholar] [CrossRef]
- Mohan, H.T.; Jayanarayanan, K.; Mini, K. Recent trends in utilization of plastics waste composites as construction materials. Constr. Build. Mater. 2021, 271, 121520. [Google Scholar] [CrossRef]
- Haque, M.S.; Islam, S. Effectiveness of waste plastic bottles as construction material in Rohingya displacement camps. Cleaner Eng. Technol. 2021, 3, 100110. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A. Plastic wastes to construction products: Status, limitations and future perspective. Case Stud. Constr. Mater. 2020, 12, e00330. [Google Scholar] [CrossRef]
- Murts, G.T.; Ram, C.; Gebru, K.A. Fabrication and characterization of cement based floor tiles using eggshell and plastic wastes as a low cost construction materials. Case Stud. Constr. Mater. 2021, 15, e00747. [Google Scholar] [CrossRef]
- Rajesh, S.; Murthy, Z.V.P. Ultrafiltration membranes from waste polyethylene terephthalate and additives: Synthesis and characterization. Química Nova 2014, 37, 653–657. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Ambani, S.K.; Kusumadewi, S.; Sutanto, H.; Widiputri, D.I.; Kartawiria, I.S. Utilization of used polyethylene terephthalate (PET) bottles for the development of ultrafiltration membrane. J. Environ. Chem. Eng. 2020, 8, 104381. [Google Scholar] [CrossRef]
- Maiti, A.; Pandey, A. Polymer and Waste Plastic in Membranes. Ref. Modul. Mater. Sci. Mater. Eng. 2021. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, M.; Han, W.; Li, P. Waste office paper filled polylactic acid composite filaments for 3D printing. Compos. Part B Eng. 2021, 221, 108998. [Google Scholar] [CrossRef]
- Garrido, J.; Sáez, J.; Armesto, J.I.; Espada, A.M.; Silva, D.; Goikoetxea, J.; Lekube, B. 3D printing as an enabling technology to implement maritime plastic Circular Economy. Procedia Manuf. 2020, 51, 635–641. [Google Scholar] [CrossRef]
- Santander, P.; Cruz Sanchez, F.A.; Boudaoud, H.; Camargo, M. Closed loop supply chain network for local and distributed plastic recycling for 3D printing: A MILP-based optimization approach. Resour. Conserv. Recycl. 2020, 154, 104531. [Google Scholar] [CrossRef]
- Maldonado-García, B.; Pal, A.K.; Misra, M.; Gregori, S.; Mohanty, A.K. Sustainable 3D printed composites from recycled ocean plastics and pyrolyzed soy-hulls: Optimization of printing parameters, performance studies and prototypes development. Compos. Part C Open Access 2021, 6, 100197. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Jha, J.N.; Gill, K.S. Utilization of Plastic Wastes for Improving the Sub-Grades in Flexible Pavements. Paving Mater. Pavement Anal. 2010, 320–326. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Jha, J.N.; Gill, K.S.; Shukla, S.K. Utilization of Fly Ash and Waste Recycled Product Reinforced with Plastic Wastes as Construction Materials in Flexible Pavement. In Geo-Congress 2014 Technical Papers, Atlanta, Georgia, 23–26 February 2014; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2014; p. 3890. [Google Scholar]
- Benson, C.H.; Khire, M.V. Reinforcing Sand with Strips of Reclaimed High-Density Polyethylene. J. Geotech. Eng. 1994, 120, 838–855. [Google Scholar] [CrossRef]
- Kumi-Larbi, A.; Yunana, D.; Kamsouloum, P.; Webster, M.; Wilson, D.C.; Cheeseman, C. Recycling waste plastics in de-veloping countries: Use of low-density polyethylene water sachets to form plastic bonded sand blocks. Waste Manag. 2018, 80, 112–118. [Google Scholar] [CrossRef]
- Xiao, D.; Yu, Z.; Qing, S.; Du, S.; Xiao, H. Development of agricultural waste/recycled plastic/waste oil bio-composite wallpaper based on two-phase dye and liquefaction filling technology. Environ. Sci. Pollut. Res. 2020, 27, 2599–2621. [Google Scholar] [CrossRef]
- Galati, A.; Scalenghe, R. Plastic end-of-life alternatives, with a focus on the agricultural sector. Curr. Opin. Chem. Eng. 2021, 32, 100681. [Google Scholar] [CrossRef]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef]
- Pegoretti, A.; Kolarik, J.; Peroni, C.; Migliaresi, C. Recycled poly(ethylene terephthalate)/layered silicate nanocomposites: Morphology and tensile mechanical properties. Polymers 2004, 45, 2751–2759. [Google Scholar] [CrossRef]
- Kráčalík, M.; Pospíšil, L.; Šlouf, M.; Mikešová, J.; Sikora, A.; Šimoník, J.; Fortelný, I. Recycled poly(ethylene terephthalate) reinforced with basalt fibres: Rheology, structure, and utility properties. Polym. Compos. 2008, 29, 437–442. [Google Scholar] [CrossRef]
- Velenturf, A.P.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Bening, C.R.; Pruess, J.T.; Blum, N.U. Towards a circular plastics economy: Interacting barriers and con-tested solutions for flexible packaging recycling. J. Clean. Prod. 2021, 302, 126966. [Google Scholar] [CrossRef]
- Johansen, M.R.; Christensen, T.B.; Ramos, T.M.; Syberg, K. A review of the plastic value chain from a circular economy perspective. J. Environ. Manag. 2022, 302, 113975. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Gall, M.; Wiener, M.; de Oliveira, C.C.; Lang, R.W.; Hansen, E.G. Building a circular plastics economy with informal waste pickers: Recyclate quality, business model, and societal impacts. Resour. Conserv. Recycl. 2020, 156, 104685. [Google Scholar] [CrossRef]
- Bucknall, D.G. Plastics as a materials system in a circular economy. Philos. Trans. R Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190268. [Google Scholar] [CrossRef] [PubMed]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for polymer to polymer recycling from waste: Current trends and op-portunities for improving the circular economy of polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- Delvere, I.; Iltina, M.; Shanbayev, M.; Abildayeva, A.; Kuzhamberdieva, S.; Blumberga, D. Evaluation of Polymer Matrix Composite Waste Recycling Methods. Environ. Clim. Technol. 2019, 23, 168–187. [Google Scholar] [CrossRef]
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite materials based on recycled polyethylene terephthalate and their properties—A comprehensive review. Compos. Part B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Velasquez, E.; Garrido, L.; Guarda, A.; Galotto, M.; de Dicastillo, C.L. Increasing the incorporation of recycled PET on polymeric blends through the reinforcement with commercial nanoclays. Appl. Clay Sci. 2019, 180, 105185. [Google Scholar] [CrossRef]
- Cosnita, M.; Cazan, C.; Duta, A. The influence of inorganic additive on the water stability and mechanical properties of recycled rubber, polyethylene terephthalate, high density polyethylene and wood composites. J. Clean. Prod. 2017, 165, 630–636. [Google Scholar] [CrossRef]
- Kakoria, A.; Chandel, S.S.; Sinha-Ray, S. Novel supersonically solution blown nanofibers from waste PET bottle for PM0.1-2 filtration: From waste to pollution mitigation. Polymers 2021, 234, 124260. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, X.; Xiao, Z.; Liu, R. A Clean and Sustainable Cellulose-Based Composite Film Reinforced with Waste Plastic Polyethylene Terephthalate. Adv. Mater. Sci. Eng. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Aydoğmuş, E.; Arslanoğlu, H.; Dağ, M. Production of waste polyethylene terephthalate reinforced biocomposite with RSM design and evaluation of thermophysical properties. J. Build. Eng. 2021, 44, 103337. [Google Scholar] [CrossRef]
- US EPA, Advancing Sustainable Materials Management: Facts and Figures 2013: Assessing Trends in Material Generation, Recycling and Disposal in the United States. Available online: https://www.epa.gov/sites/production/files/2021-01/documents/2018_tables_and_figures_dec_2020_fnl_508.pdf (accessed on 9 November 2021).
- Pasternak, G.; Ormeno-Cano, N.; Rutkowski, P. Recycled waste polypropylene composite ceramic membranes for ex-tended lifetime of microbial fuel cells. Chem. Eng. J. 2021, 425, 130707. [Google Scholar] [CrossRef]
- Sezgin, H.; Kucukali-Ozturk, M.; Berkalp, O.B.; Yalcin-Enis, I. Design of composite insulation panels containing 100% recycled cotton fibers and polyethylene/polypropylene packaging wastes. J. Clean. Prod. 2021, 304, 127132. [Google Scholar] [CrossRef]
- Martínez-López, M.; Martínez-Barrera, G.; Salgado-Delgado, R.; Gencel, O. Recycling polypropylene and polyethylene wastes in production of polyester based polymer mortars. Constr. Build. Mater. 2021, 274, 121487. [Google Scholar] [CrossRef]
- Lin, A.; Tan, Y.K.; Wang, C.-H.; Kua, H.W.; Taylor, H. Utilization of waste materials in a novel mortar–polymer laminar composite to be applied in construction 3D-printing. Compos. Struct. 2020, 253, 112764. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, Z.; Zhang, X.; Ma, S. Co-recycling of acrylonitrile–butadiene–styrene waste plastic and nonmetal particles from waste printed circuit boards to manufacture reproduction composites. Environ. Technol. 2015, 36, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zulkernain, N.H.; Gani, P.; Chuck Chuan, N.; Uvarajan, T. Utilisation of plastic waste as aggregate in construction materials: A review. Constr. Build. Mater. 2021, 296, 123669. [Google Scholar] [CrossRef]
- Safarpour, M.; Safikhani, A.; Vatanpour, V. Polyvinyl chloride-based membranes: A review on fabrication techniques, applications and future perspectives. Sep. Purif. Technol. 2021, 279, 119678. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Mohammed, I.I.; Mohammed, S.A. Some properties of concrete with plastic aggregate derived from shredded PVC sheets. Constr. Build. Mater. 2019, 201, 232–245. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, D.; Wang, X.; Deng, L.; Fan, X.; Ding, Z.; Makinia, J. Transformation of polyvinyl chloride (PVC) into a versatile and efficient adsorbent of Cu(II) cations and Cr(VI) anions through hydrothermal treatment and sulfonation. J. Hazard. Mater. 2022, 423, 126973. [Google Scholar] [CrossRef]
- Bhran, A.; Shoaib, A.; Elsadeq, D.; El-Gendi, A.; Abdallah, H. Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes. Chin. J. Chem. Eng. 2018, 26, 715–722. [Google Scholar] [CrossRef]
- Hezarjaribi, M.; Bakeri, G.; Sillanpää, M.; Chaichi, M.J.; Akbari, S.; Rahimpour, A. Novel adsorptive PVC nanofibrous/thiol-functionalized TNT composite UF membranes for effective dynamic removal of heavy metal ions. J. Environ. Manag. 2021, 284, 111996. [Google Scholar] [CrossRef]
- Yong, M.; Zhang, Y.; Sun, S.; Liu, W. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling. J. Membr. Sci. 2019, 575, 50–59. [Google Scholar] [CrossRef]
- Aji, M.M.; Narendren, S.; Purkait, M.K.; Katiyar, V. Utilization of waste polyvinyl chloride (PVC) for ultrafiltration membrane fabrication and its characterization. J. Environ. Chem. Eng. 2020, 8, 103650. [Google Scholar] [CrossRef]
- Turner, A. Polystyrene foam as a source and sink of chemicals in the marine environment: An XRF study. Chemosphere 2021, 263, 128087. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Shi, Y.; Chen, Y.; Zhu, S.; Feng, Y.; Lv, Y.; Yang, F.; Liu, M.; Shui, W. Constructing segregated polystyrene composites for excellent fire resistance and electromagnetic wave shielding. J. Colloid Interface Sci. 2022, 606, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Eskander, S.B.; Saleh, H.M.; Tawfik, M.E.; Bayoumi, T.A. Towards potential applications of cement-polymer composites based on recycled polystyrene foam wastes on construction fields: Impact of exposure to water ecologies. Case Stud. Constr. Mater. 2021, 15, e00664. [Google Scholar] [CrossRef]
- Saleh, H.M.; Eskander, S.B. Impact of water flooding on hard cement-recycled polystyrene composite immobilizing ra-dioactive sulfate waste simulate. Constr. Build. Mater. 2019, 222, 522–530. [Google Scholar] [CrossRef]
- Maaroufi, M.; Belarbi, R.; Abahri, K.; Benmahiddine, F. Full characterization of hygrothermal, mechanical and morpho-logical properties of a recycled expanded polystyrene-based mortar. Constr. Build. Mater. 2021, 301, 124310. [Google Scholar] [CrossRef]
- Bouzit, S.; Merli, F.; Sonebi, M.; Buratti, C.; Taha, M. Gypsum-plasters mixed with polystyrene balls for building insula-tion: Experimental characterization and energy performance. Constr. Build. Mater. 2021, 283, 122625. [Google Scholar] [CrossRef]
- Olofinnade, O.; Chandra, S.; Chakraborty, P. Recycling of high impact polystyrene and low-density polyethylene plastic wastes in lightweight based concrete for sustainable construction. In Proceedings of the International Conference & Exposition on Mechanical, Material and Manufacturing Technology (ICE3MT), Hyderabad, India, 9 October 2020; pp. 2151–2156. [Google Scholar] [CrossRef]
- Sow, P.K.; Ishita; Singhal, R. Sustainable approach to recycle waste polystyrene to high-value submicron fibers using so-lution blow spinning and application towards oil-water separation. J. Environ. Chem. Eng. 2020, 8, 102786. [Google Scholar] [CrossRef]
- Machado, N.C.; de Jesus, L.A.; Pinto, P.S.; de Paula, F.G.; Alves, M.O.; Mendes, K.H.; Mambrini, R.V.; Barrreda, D.; Rocha, V.; Santamaría, R.; et al. Waste-polystyrene foams-derived magnetic carbon material for adsorption and redox supercapacitor applications. J. Clean. Prod. 2021, 313, 127903. [Google Scholar] [CrossRef]
- Reynoso, L.E.; Carrizo Romero, Á.B.; Viegas, G.M.; San Juan, G.A. Characterization of an alternative thermal insulation material using recycled expanded polystyrene. Constr. Build. Mater. 2021, 301, 124058. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef]
- Lens-Pechakova, L.S. Recent studies on enzyme-catalysed recycling and biodegradation of synthetic polymers. Adv. Ind. Eng. Polym. Res. 2021, 4, 151–158. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Jacob, M.; Vignesh, N.S.; Varalakshmi, P. Pristine and modified chitosan as solid catalysts for catalysis and biodiesel production: A minireview. Int. J. Biol. Macromol. 2021, 167, 807–833. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Renu Hada, P.; Anita Bhatia, S.K.; Malpani, S.K. Development of green, effective, and cost-efficient perlite supported solid base catalyst and application in condensation reac-tions. Mater. Today Proc. 2021. Available online: https://www.sciencedirect.com/science/article/pii/S2214785321066232 (accessed on 7 November 2021). [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2021, 1–27. [Google Scholar] [CrossRef]
- Guan, Y.; Chaffart, D.; Liu, G.; Tan, Z.; Zhang, D.; Wang, Y.; Li, J.; Ricardez-Sandoval, L. Machine learning in solid heterogeneous catalysis: Recent developments, challenges and perspectives. Chem. Eng. Sci. 2021, 248, 117224. [Google Scholar] [CrossRef]
- Sedghi, R.; Asadi, S.; Heidari, B.; Heravi, M.M. TiO2/polymeric supported silver nanoparticles applied as superior nano-catalyst in reduction reactions. Mater. Res. Bull. 2017, 92, 65–73. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Shokouhimehr, M.; Varma, R.S. Recent developments in palladium (nano)catalysts supported on polymers for selective and sustainable oxidation processes. Coord. Chem. Rev. 2019, 397, 54–75. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; De Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Sandhu, S.; Krishnan, S.; Karim, A.V.; Shriwastav, A. Photocatalytic Denitrification of Water using Polystyrene Immobilized TiO2 as Floating Catalyst. J. Environ. Chem. Eng. 2020, 8, 104471. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Swaminathan, M.; Houas, A. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. 2017, 60, 3–13. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Pan, S.; Zhang, X.; Zhang, W.; Pan, B. Utilization of gel-type polystyrene host for immobilization of nano-sized hydrated zirconium oxides: A new strategy for enhanced phosphate removal. Chemosphere 2021, 263, 127938. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for Recovering Nutrients from Wastewater: A Critical Review. Environ. Eng. Sci. 2019, 36, 1–19. [Google Scholar] [CrossRef]
- Patel, S.B.; Vasava, D.V. Azo functionalized polystyrene supported Copper nanoparticles: An economical and highly efficient catalyst for A3 and KA2 coupling reaction under microwave irradiation. Nano Struct. Nano Objects 2020, 21, 100416. [Google Scholar] [CrossRef]
- Sandoval, C.; Molina, G.; Vargas Jentzsch, P.; Pérez, J.; Muñoz, F. Photocatalytic Degradation of Azo Dyes Over Semi-conductors Supported on Polyethylene Terephthalate and Polystyrene Substrates. J. Adv. Oxid. Technol. 2017, 20, 1–19. [Google Scholar]
- Mossmann, A.; Dotto, G.L.; Hotza, D.; Jahn, S.L.; Foletto, E.L. Preparation of polyethylene–supported zero–valent iron buoyant catalyst and its performance for Ponceau 4R decolorization by photo–Fenton process. J. Environ. Chem. Eng. 2019, 7, 102963. [Google Scholar] [CrossRef]
- Thiam, A.; Brillas, E.; Garrido, J.A.; Rodríguez, R.M.; Sirés, I. Routes for the electrochemical degradation of the artificial food azo-colour Ponceau 4R by advanced oxidation processes. Appl. Catal. B Environ. 2016, 180, 227–236. [Google Scholar] [CrossRef]
- Linda, T.; Muthupoongodi, S.; Sahaya Shajan, X.; Balakumar, S. Photocatalytic Degradation of Congo Red and Crystal Violet Dyes on Cellulose/PVC/ZnO Composites under UV Light Irradiation. Mater. Today Proc. 2016, 3, 2035–2041. [Google Scholar] [CrossRef]
- Bartáček, J.; Drabina, P.; Váňa, J.; Sedlák, M. Recoverable polystyrene-supported catalysts for Sharpless allylic alcohols epoxidations. React. Funct. Polym. 2019, 137, 123–132. [Google Scholar] [CrossRef]
- Vaiano, V.; Chianese, L.; Rizzo, L.; Iervolino, G. Visible light driven oxidation of arsenite to arsenate in aqueous solution using Cu-doped ZnO supported on polystyrene pellets. Catal. Today 2021, 361, 69–76. [Google Scholar] [CrossRef]
- Wang, J.-C.; Li, Y.; Li, H.; Cui, Z.-H.; Hou, Y.; Shi, W.; Zhang, Y.-P. A novel synthesis of oleophylic Fe2O3/polystyrene fibers by γ-Ray irradiation for the enhanced photocatalysis of 4-chlorophenol and 4-nitrophenol degradation. J. Hazard. Mater. 2019, 379, 120806. [Google Scholar] [CrossRef] [PubMed]
- Ayeleru, O.O.; Dlova, S.; Akinribide, O.J.; Ntuli, F.; Kupolati, W.K.; Marina, P.F.; Blencowe, A.; Olubambi, P.A. Challenges of plastic waste generation and management in sub-Saharan Africa: A review. Waste Manag. 2020, 110, 24–42. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- Selvaranjan, K.; Navaratnam, S.; Rajeev, P.; Ravintherakumaran, N. Environmental challenges induced by extensive use of face masks during COVID-19: A review and potential solutions. Environ. Chall. 2021, 3, 100039. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Fayaz, H. Bio-degradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Reports. 2020, 10, 21960. [Google Scholar]

| Types | Structure | Symbology |
|---|---|---|
| * PE | 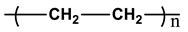 |   |
| PP |  |  |
| PS | 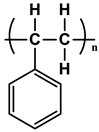 |  |
| PVC |  |  |
| PET | 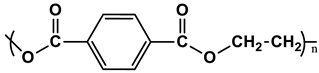 |  |
| LDPE |  | |
| HDPE |  | |
| Others |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Assis, G.C.; de Jesus, R.A.; da Silva, W.T.A.; Ferreira, L.F.R.; Figueiredo, R.T.; de Oliveira, R.J. Conversion of Plastic Waste into Supports for Nanostructured Heterogeneous Catalysts: Application in Environmental Remediation. Surfaces 2022, 5, 35-66. https://doi.org/10.3390/surfaces5010002
de Assis GC, de Jesus RA, da Silva WTA, Ferreira LFR, Figueiredo RT, de Oliveira RJ. Conversion of Plastic Waste into Supports for Nanostructured Heterogeneous Catalysts: Application in Environmental Remediation. Surfaces. 2022; 5(1):35-66. https://doi.org/10.3390/surfaces5010002
Chicago/Turabian Stylede Assis, Geovânia Cordeiro, Roberta Anjos de Jesus, Wélida Tamires Alves da Silva, Luiz Fernando Romanholo Ferreira, Renan Tavares Figueiredo, and Rodrigo José de Oliveira. 2022. "Conversion of Plastic Waste into Supports for Nanostructured Heterogeneous Catalysts: Application in Environmental Remediation" Surfaces 5, no. 1: 35-66. https://doi.org/10.3390/surfaces5010002
APA Stylede Assis, G. C., de Jesus, R. A., da Silva, W. T. A., Ferreira, L. F. R., Figueiredo, R. T., & de Oliveira, R. J. (2022). Conversion of Plastic Waste into Supports for Nanostructured Heterogeneous Catalysts: Application in Environmental Remediation. Surfaces, 5(1), 35-66. https://doi.org/10.3390/surfaces5010002