Fabrication of Efficient and Selective Modified Graphene Paste Sensor for the Determination of Catechol and Hydroquinone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Electrode Preparation
3. Results and Discussion
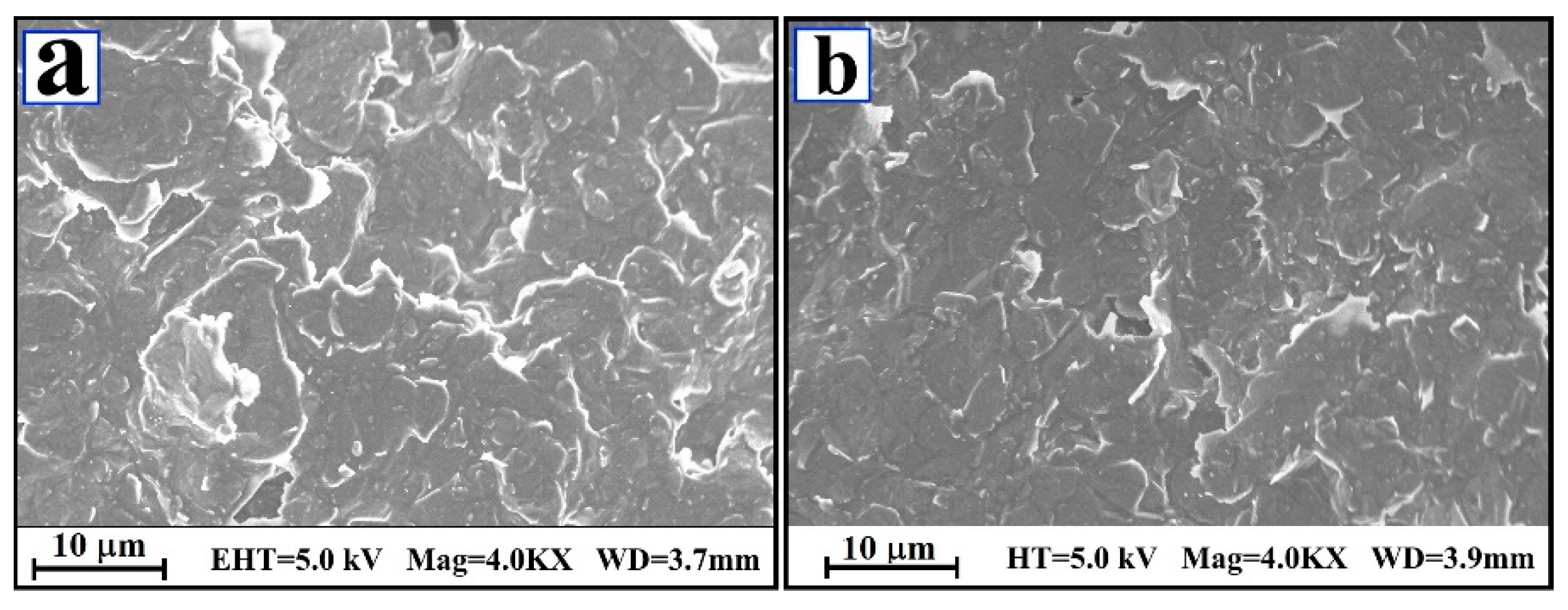
3.1. FE-SEM Inspection
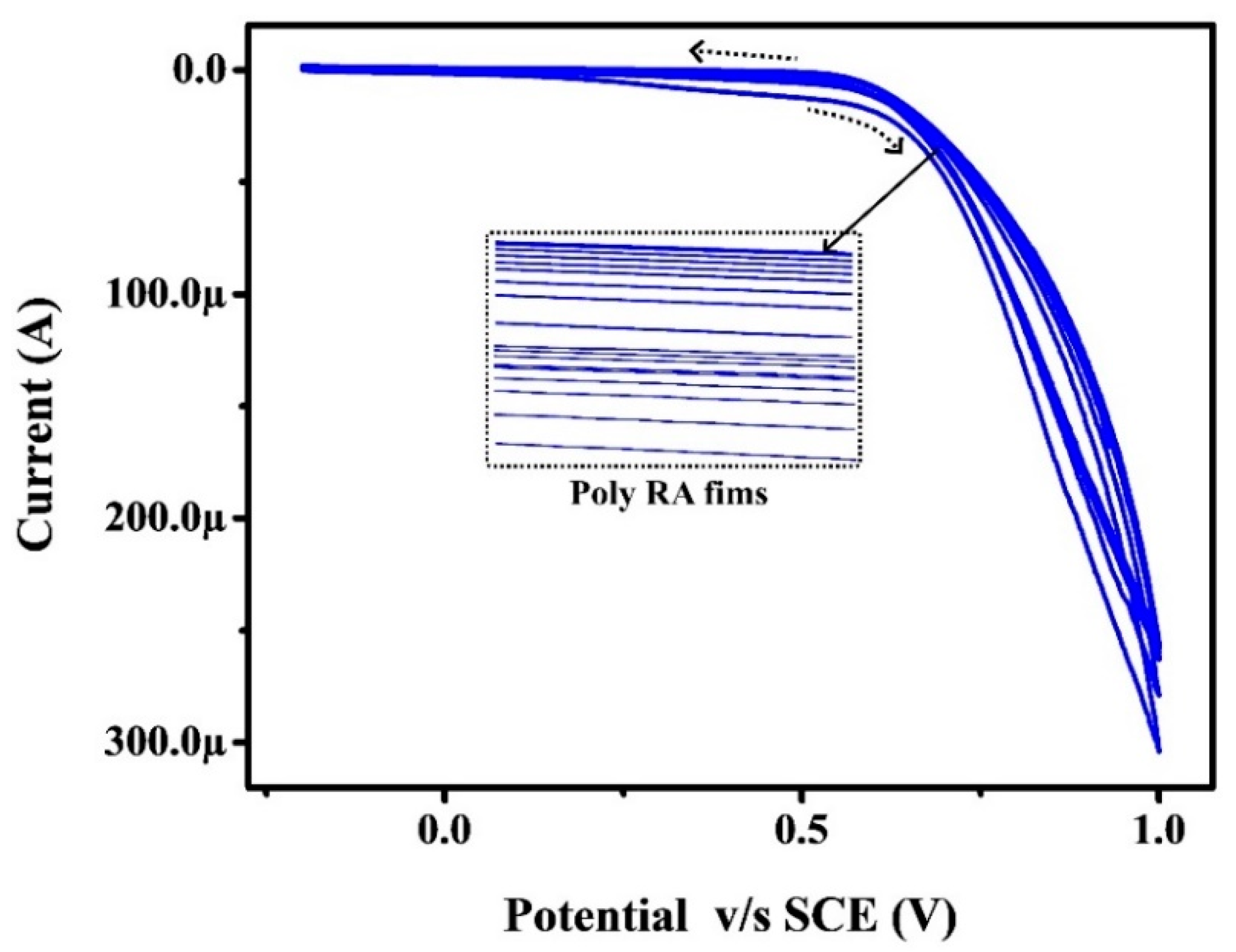
3.2. Electro-Polymerization of RA at BGPE through Pre-Treatment Process
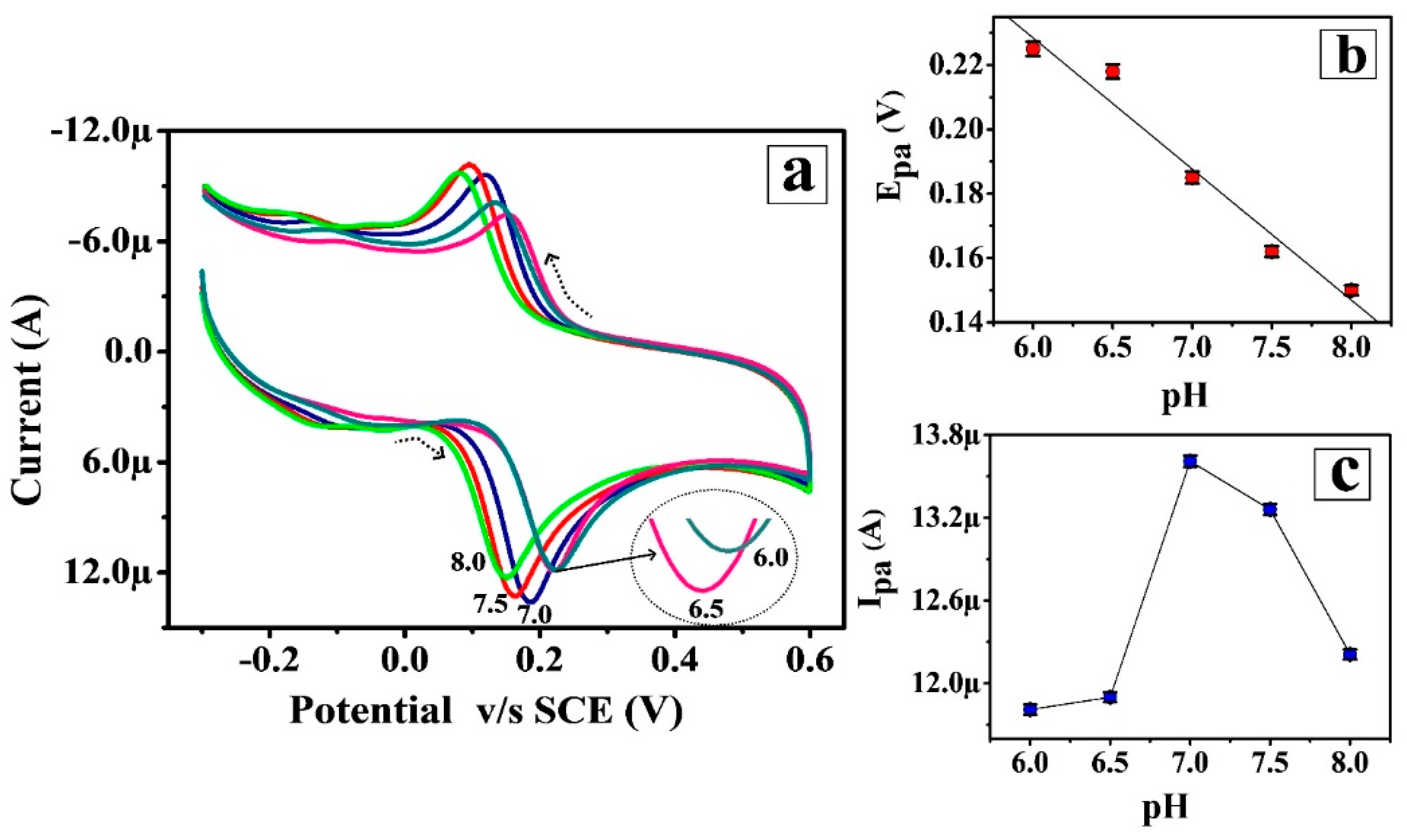
3.3. Impact of Sustaining Electrolyte pH
3.4. CV Response of CC at Poly(RA)MGPE and Surface Area
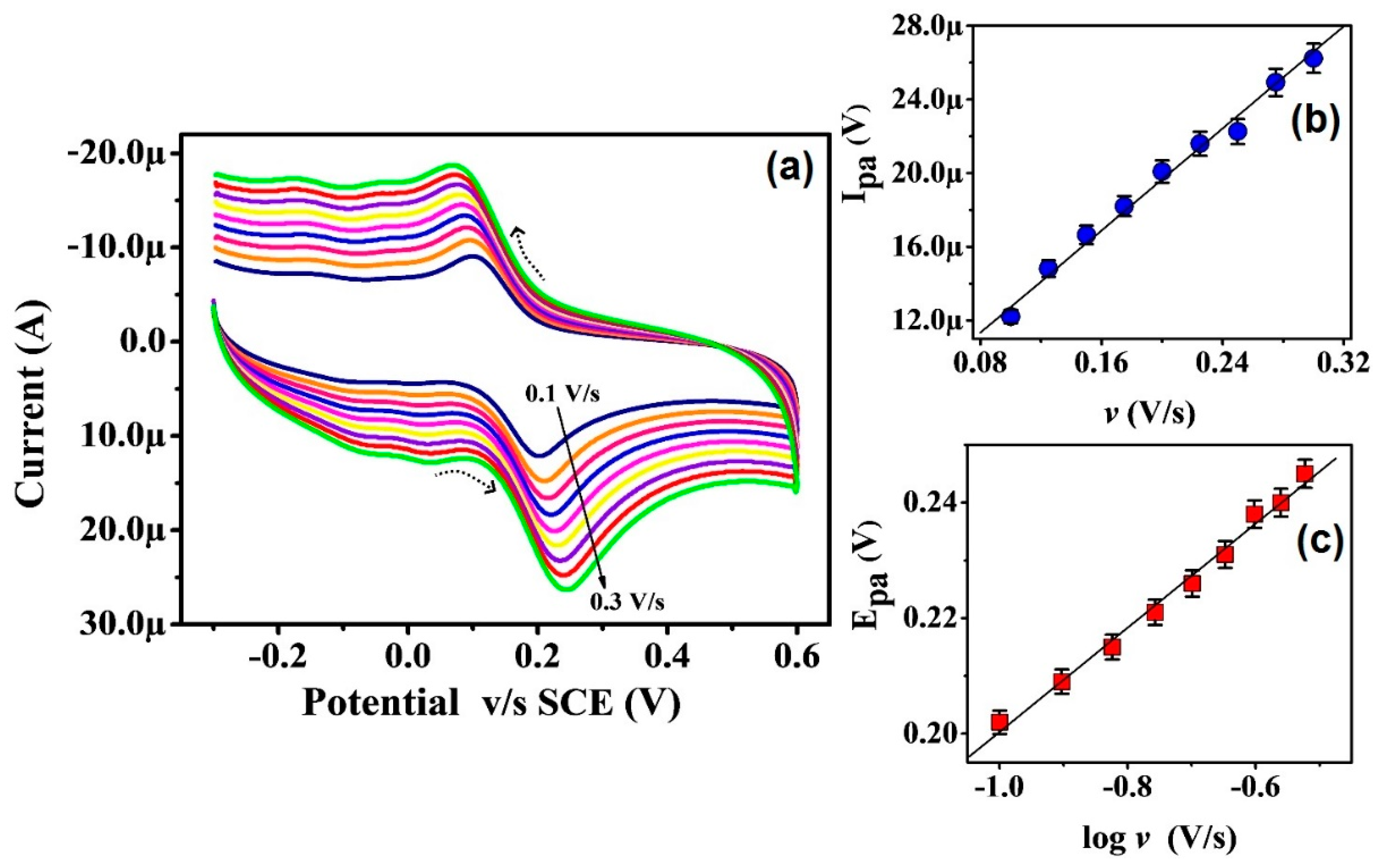
3.5. Scan Rate Impact on Electrode Reaction, Peak Current, and Peak Potential of CC
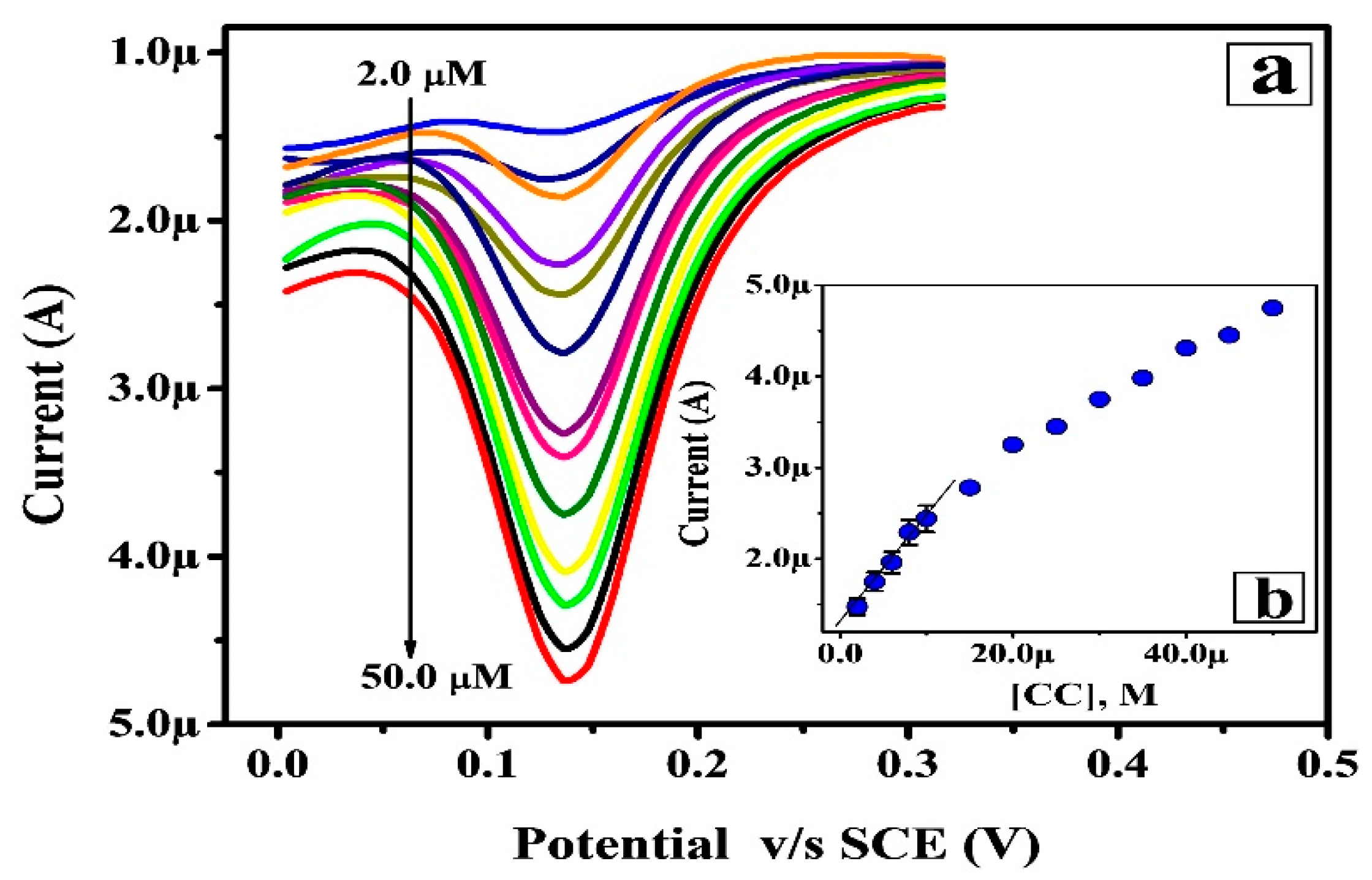
3.6. Limit of Detection and Quantification
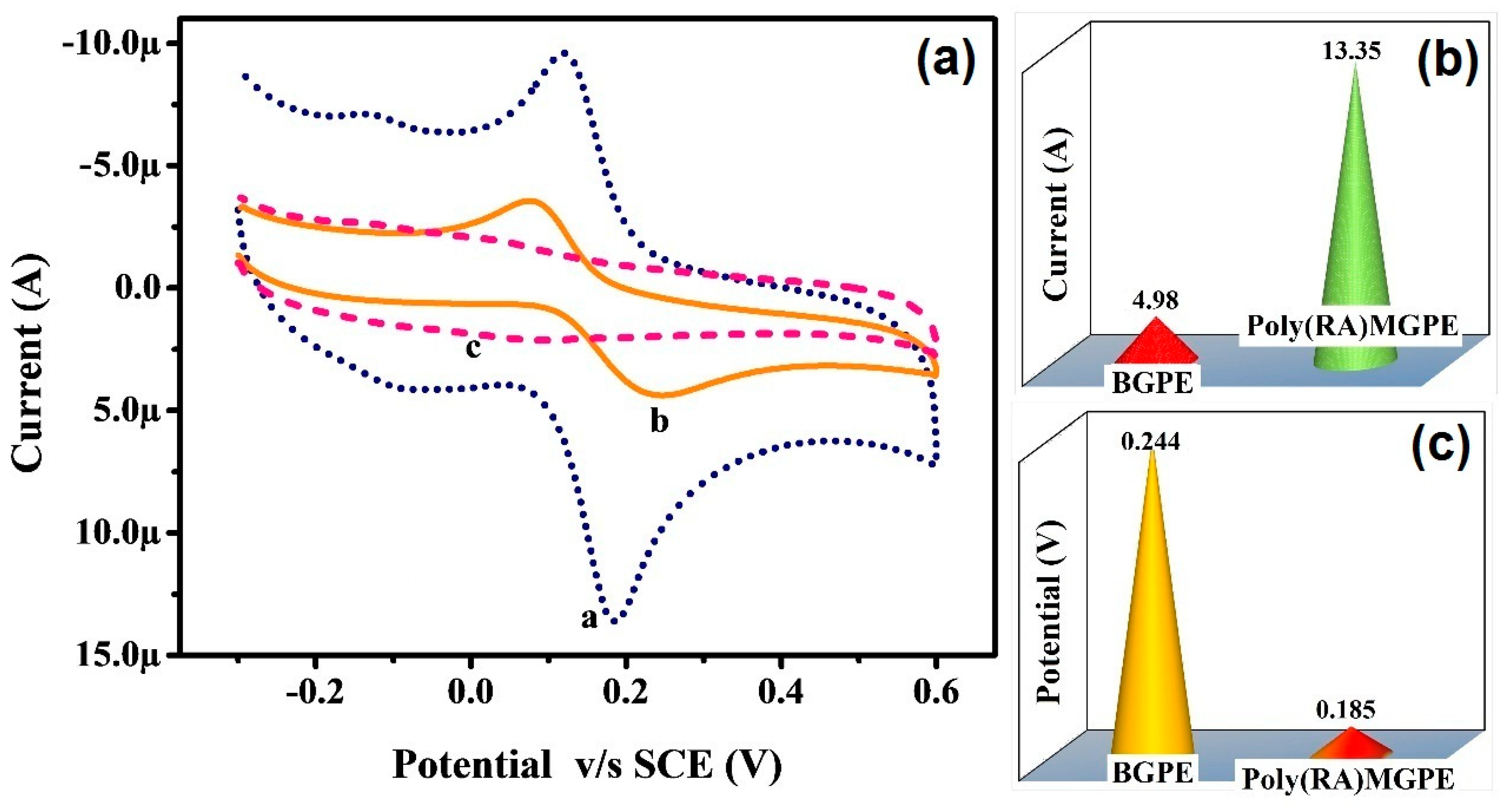
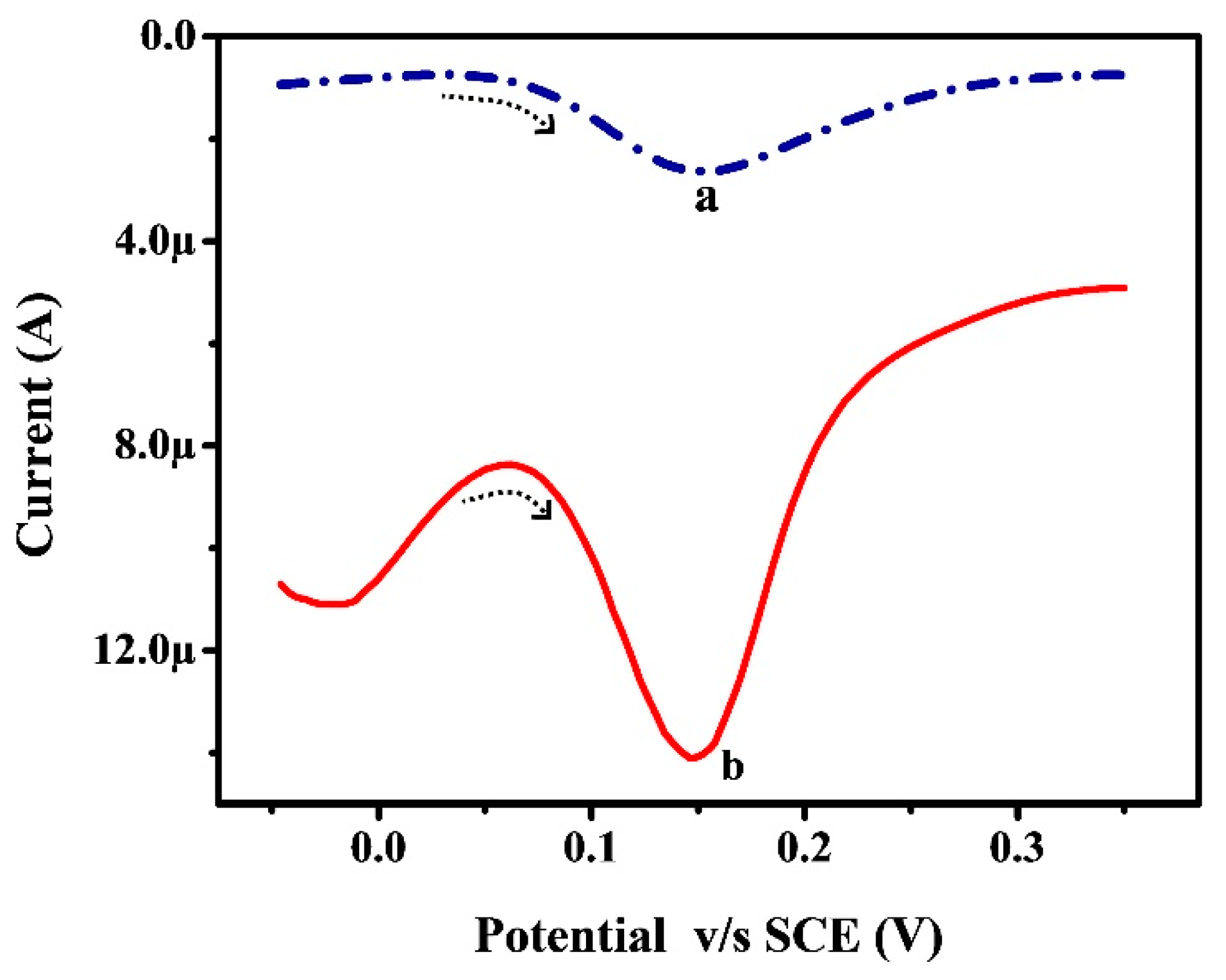
3.7. Oxidative Response of CC at Poly (RA)MGPE and BGPE via DPV
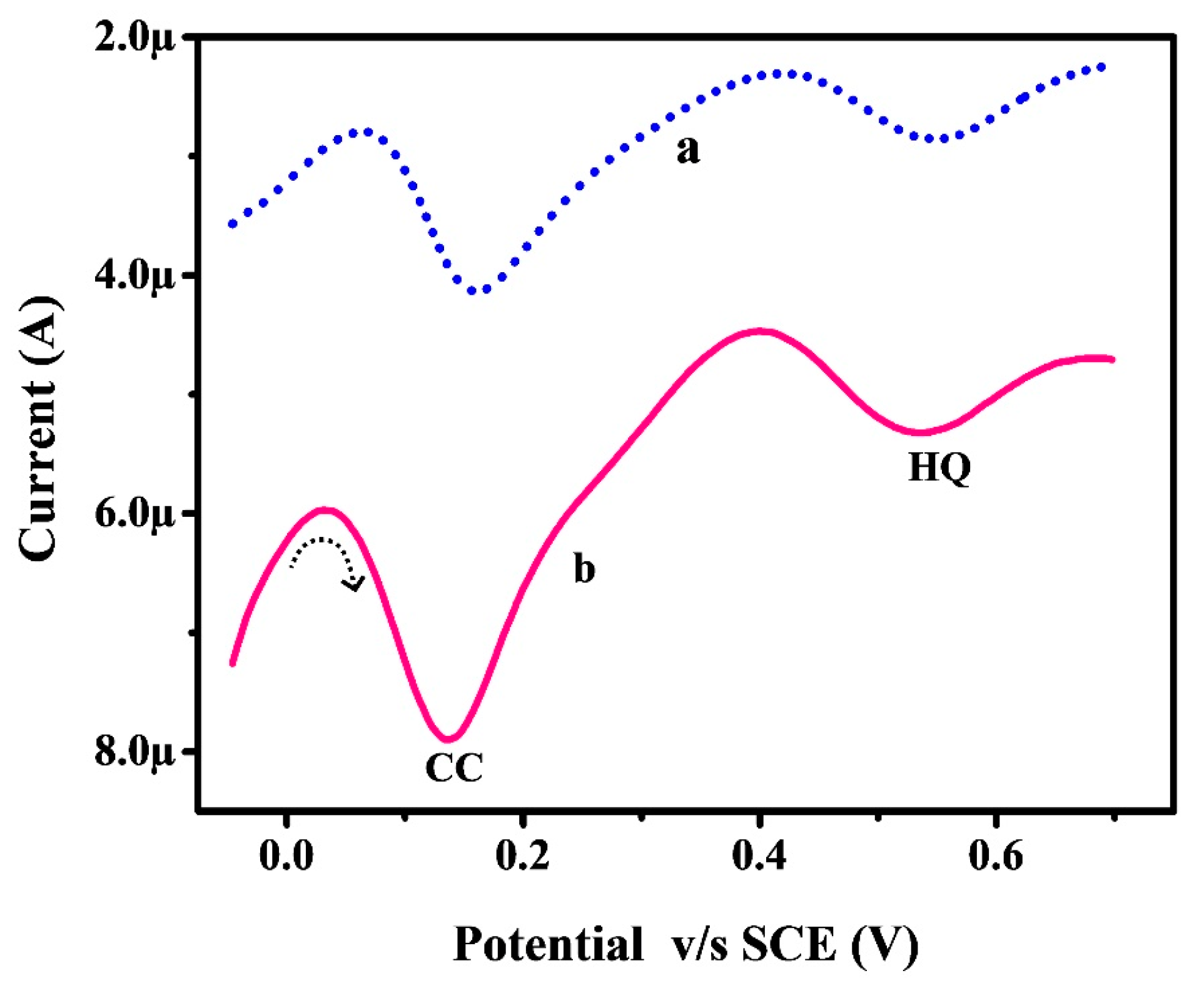
3.8. Selective DPV Analysis of CC with HQ at Poly(RA)MGPE
3.9. Reproducibility, Repeatability, and Stability
3.10. CC Analysis in Water Samples
4. Conclusions
Funding
Conflicts of Interest
References
- Tekalign, K. Cyclic Voltammetric and Electrochemical Simulation on the Electro-Oxidation of Catechol in the Absence of Any Nucleophile: Original Research. Chem. Mater. Res. 2017, 9, 1–12. [Google Scholar]
- Alemu, Y.; Amare, M.; Admassie, S.; Tessema, M. Simultaneous determination of hydroquinone and catechol at poly(p-asa)/MWNTS composite film modified glassy carbon electrode. SINET Ethiop. J. Sci. 2012, 35, 29–40. [Google Scholar]
- Barner, B.A. Catechol. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L., Ed.; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Khala, L.; Rafiee, M. Kinetic study of the oxidation andnitration of catechols in the presence of nitrous acid ionization equilibria. J. Hazard. Mater. 2010, 174, 801–806. [Google Scholar] [CrossRef]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M. E electron: The simplest chemical reagent. Synlett 2007, 3, 503–504. [Google Scholar] [CrossRef] [Green Version]
- Nematollahi, D.; Rafiee, M.; Fotouhi, L. Mechanistic study of homogeneous reactions coupled with electrochemical oxidation of catechols. J. Iran. Chem. Soc. 2009, 6, 448–476. [Google Scholar] [CrossRef]
- Gillner, M.; Moore, G.S.; Cederburg, H.; Gustafsson, K. International program on chemical safety. In Environmental Health Criteria 157; World Health Organization: Geneva, Switzerland, 1994; Available online: http://www.inchem.org/documents/ehc/ehc/ehc157.htm (accessed on 2 June 2020).
- Bhanger, M.I.; Niaz, A.; Shah, A.; Rauf, A. Ultra-trace level determination of hydroquinone in waste photographic solutions by UV-vis spectrophotometry. Talanta 2007, 72, 546–553. [Google Scholar]
- Gao, W.H.; Quigley, C.L. Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J. Chromatogr. A 2011, 1218, 4307–4311. [Google Scholar] [CrossRef]
- Lee, B.L.; Ong, H.Y.; Shi, C.Y.; Ong, C.N. Simultaneous determination of hydroquinone, catechol and phenol in urine using high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. A 1993, 619, 259–266. [Google Scholar] [CrossRef]
- Pistonesi, M.F.; Di Nezio, M.S.; Centurión, M.E.; Palomeque, M.E.; Lista, A.G.; Fernández Band, B.S. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least-squares (PLS). Talanta 2006, 69, 1265–1268. [Google Scholar] [CrossRef]
- Li, S.F.; Li, X.Z.; Xu, J.; Wei, X.W. Flow-injection chemiluminescence determination of polyphenols using luminol–NaIO4–gold nanoparticles system. Talanta 2008, 75, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chi, L.; Yang, Z.; He, P.; Wang, Q.; Fang, Y. Simultaneous determination of dihydroxybenzene and phenylenediamine positional isomers using capillary zone electrophoresis coupled with amperometric detection. J. Sep. Sci. 2009, 32, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 2001, 55, 1039–1046. [Google Scholar] [CrossRef]
- Shakeel, N.; Ahamed, M.I.; Ahmed, A.; Rahman, M.M.; Asiri, A.M. Functionalized magnetic nanoparticle-reduced graphene oxide nanocomposite for enzymatic biofuel cell applications. Int. J. Hydrogen Energy 2019, 44, 28294–28304. [Google Scholar] [CrossRef]
- Anish, K.; Parwaz Khan, A.A.; Rahman, M.M.; Asiri, A.M.; Alamry, K.A. Preparation of polyaniline grafted graphene oxide–WO3 nanocomposite and its application as a chromium(iii) chemi-sensor. RSC Adv. 2015, 5, 105169–105178. [Google Scholar] [CrossRef]
- Chikere, C.O.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Interaction between Amorphous Zirconia Nanoparticles and Graphite: Electrochemical Applications for Gallic Acid Sensing Using Carbon Paste Electrodes in Wine. Nanomaterials 2020, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, T.N.; Sureshkumar, K.; Teixeira, S.R.; Fernandez, C.; Ramakrishnappa, T. Ag Doped Titanium Dioxide Nanocomposite-Modified Glassy Carbon Electrode as Electrochemical Interface for Catechol Sensing. Electroanalysis 2016, 28, 452–461. [Google Scholar] [CrossRef]
- Jahir, A.; Rahman, M.M.; Siddiquey, I.A.; Asiri, A.M.; Hasnat, M.A. Efficient hydroquinone sensor based on zinc, strontium and nickel based ternary metal oxide (TMO) composites by differential pulse voltammetry. Sen. Actuators B Chem. 2018, 256, 383–392. [Google Scholar] [CrossRef]
- Lopes de Macedo, I.Y.; Garcia, L.F.; Ribeiro de Souza, A.; Lima da Silva, A.M.; Fernandez, C.; Santos, M.D.G.; Magalhaes, R.S.; Torres, I.M.S.; de Souza Gila, E. Differential Pulse Voltammetric Determination of Albendazole and Mebendazole in Pharmaceutical Formulations Based on Modified Sonogel Carbon Paste Electrodes with Perovskite-Type LaFeO3 Nanoparticles. J. Electrochem. Soc. 2016, 163, B428–B434. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Alam, M.M.; Asiri, A.M.; Jahir, A.; Rahman, M.M. Efficient hydroquinone sensor development based on Co3O4 nanoparticle. Microchem. J. 2020, 157, 104972. [Google Scholar] [CrossRef]
- Karim, M.R.; Rahman, M.M.; Asiri, A.M.; Hayami, S. Branched Alkylamine–Reduced Graphene Oxide Hybrids as a Dual Proton–Electron Conductor and Organic-Only Water-Splitting Photocatalyst. ACS Appl. Mater. Int. 2020, 12, 10829–10838. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.B.; Rashid, K.; Imran, M.; Fernandez, C.; Cheng-Zong, Y.; Li, S. Synergistic Electronic Pull of Graphene oxide Supported Pd Nanoparticles on Enhancing Catalytic Activity of Electro Deposited Pt Nanoparticles for Methanol Oxidation Reaction. Int. J. Electrochem. Sci. 2016, 11, 6735–6746. [Google Scholar] [CrossRef]
- Manjunatha, J.G.; Kumara Swamy, B.E.; Mamatha, G.P.; Chandra, U.; Niranjana, E.; Sherigara, B.S. Cyclic voltammetric studies of dopamine at lamotrigine and TX-100 modified carbon paste electrode. Int. J. Electrochem. Sci. 2009, 4, 187–196. [Google Scholar]
- Manjunatha, J.G.; Kumara Swamy, B.E.; Deepa, R.; Krishna, V.; Mamatha, G.P.; Chandra, U.; Sharath Shankar, S.; Sherigara, B.S. Electrochemical studies of dopamine at eperisone and cetyltrimethyl ammonium bromide surfactant modified carbon paste electrode: A cyclic voltammetric study. Int. J. Electrochem. Sci. 2009, 4, 662–671. [Google Scholar]
- Manjunatha, J.G.; Kumara Swamy, B.E.; Gilbert, O.; Mamatha, G.P.; Sherigara, B.S. Sensitive voltammetric determination of dopamine at salicylic acid and TX-100, SDS, CTAB modified carbon paste electrode. Int. J. Electrochem. Sci. 2010, 5, 682–695. [Google Scholar]
- Manjunatha, J.G.; Kumara Swamy, B.E.; Mamatha, G.P.; Gilbert, O.; Chandrashekar, B.N.; Sherigara, B.S. Electrochemical studies of dopamine and epinephrine at a poly(tannic acid) modified carbon paste electrode: A cyclic voltammetric study. Int. J. Electrochem. Sci. 2010, 5, 1236–1245. [Google Scholar]
- Manjunatha, J.G.; Deraman, M.; Basri, N.H.; Talib, I.A. Fabrication of poly (Solid Red A) modified carbon nano tube paste electrode and its application for simultaneous determination of epinephrine, uric acid and ascorbic acid. Arab. J. Chem. 2018, 11, 149–158. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A novel poly(glycine) biosensor towards the detection of indigo carmine: A voltammetric study. J. Food Drug Anal. 2017, 26, 292–299. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Electroanalysis of estriol hormone using electrochemical sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Surfactant modified carbon nanotube paste electrode for the sensitive determination of mitoxantrone anticancer drug. J. Electrochem. Sci. Eng. 2017, 7, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, J.G. A new electrochemical sensor based on modified carbon nanotube-graphite mixture paste electrode for voltammetric determination of resorcinol. Asian J. Pharm. Clin. Res. 2017, 10, 295–300. [Google Scholar]
- Manjunatha, J.G.; Deraman, M.; Basri, N.H.; Talib, I.A. Selective detection of dopamine in the presence of uric acid using polymerized phthalo blue film modified carbon paste electrode. Adv. Mater. Res. 2014, 895, 447–451. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Highly sensitive polymer based sensor for determination of the drug mitoxantrone. J. Surf. Sci. Technol. 2018, 34, 74–80. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A novel voltammetric method for the enhanced detection of the food additive tartrazine using an electrochemical sensor. Heliyon 2018, 4, e00986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushpanjali, P.A.; Manjunatha, J.G.; Shreenivas, M.T. The Electrochemical Resolution of Ciprofloxacin, Riboflavin and Estriol Using Anionic Surfactant and Polymer-Modified Carbon Paste Electrode. ChemistrySelect 2020, 4, 13427–13433. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Electrochemical polymerised graphene paste electrode and application to catechol sensing. Open Chem. Eng. J. 2019, 13, 81–87. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Poly(Adenine) Modified Graphene-Based Voltammetric Sensor for the Electrochemical Determination of Catechol, Hydroquinone and Resorcinol. Open Chem. Eng. J. 2020, 14, 52–62. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphite oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Kosynkin, D.V.; Sinitskii, A.; Sun, Z.; Tour, J.M. Lower-Defect Graphene Oxide Nanoribbons from Multiwalled Carbon Nanotubes. ACS Nano 2010, 4, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, J.G.; Kumara Swamy, B.E.; Deraman, M. Electrochemical Studies of Dopamine, Ascorbic Acid and Their Simultaneous Determination at a Poly(Rosaniline) Modified Carbon Paste Electrode: A Cyclic Voltammetric Study. Anal. Bioanal. Electrochem. 2013, 5, 426–438. [Google Scholar]
- Manjunatha, J.G.; Deraman, M.; Basri, N.H.; Nor, N.S.M.; Talib, I.A.; Ataollahi, N. Sodium dodecyl sulfate modified carbon nanotubes paste electrode as a novel sensor for the simultaneous determination of dopamine, ascorbic acid, and uric acid. C. R. Chim. 2014, 17, 465–476. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. A Simple Approach for the Electrochemical Determination of Vanillin at Ionic Surfactant Modified Graphene Paste Electrode. Microchem. J. 2019, 154, 104575. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. Elevated and rapid voltammetric sensing of riboflavin at poly(helianthin dye) blended carbon paste electrode with heterogeneous rate constant elucidation. J. Iran. Chem. Soc. 2020, 17, 1507–1519. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A surfactant enhanced graphene paste electrode as an effective electrochemical sensor for the sensitive and simultaneous determination of catechol and resorcinol. Chem. Data Collect. 2020, 25, 100331. [Google Scholar] [CrossRef]
- Lavirons, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. Fast and enhanced electrochemical sensing of dopamine at cost-effective poly(dl-phenylalanine) based graphite electrode. J. Electroanal. Chem. 2020, 878. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Kumara Swamy, B.E. Electroanalysis of catechol in presence of hydroquinone at poly (calmagite) modified carbon paste electrode: A voltammetric study. Sci. Lett. J. 2016, 5, 236. [Google Scholar]
- Li, M.; Ni, F.; Wang, Y.; Xu, S.; Zhang, D. Sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of resorcinol with a Zn/Al layered double hydroxide film modified glassy carbon electrode. Electroanalysis 2009, 21, 1521–1526. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D. Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal. Chim. Acta 2012, 724, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.S.; Gasparini, B.C.; Magosso, H.A.; Spinelli, A. Electrochemical behavior of hydroquinone and catechol at a silsesquioxane-modified carbon paste electrode. J. Braz. Chem. Soc. 2013, 24, 695–699. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Z.N. Influence of micelles on the electrochemical behaviors of catechol and hydroquinone and their simultaneous determination. Anal. Bioanal. Chem. 2006, 384, 1525–1532. [Google Scholar] [CrossRef] [PubMed]

| Method | Sensor | LOD, µM | Reference |
|---|---|---|---|
| DPV | Poly(calmagite) MCPE | 2.550 | [52] |
| DPV | Zn/Al layered double hydroxide film MGCE | 1.200 | [53] |
| DPV | Graphene oxide and multiwall carbon nanotubes | 1.800 | [54] |
| DPV | Silsesquioxane-modified carbon paste electrode | 10.000 | [55] |
| DPV | Influence of micelles/GCE | 3.000 | [56] |
| DPV | Poly(RA)MGPE | 0.820 | Present work |
| Environmental Sample | Added Concentration (µM) | Found Concentration (µM) | Recovery (%) |
|---|---|---|---|
| Water | 0.120 | 0.117 | 97.50 |
| 0.160 | 0.155 | 96.87 | |
| 0.200 | 0.196 | 98.00 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjunatha, J.G. Fabrication of Efficient and Selective Modified Graphene Paste Sensor for the Determination of Catechol and Hydroquinone. Surfaces 2020, 3, 473-483. https://doi.org/10.3390/surfaces3030034
Manjunatha JG. Fabrication of Efficient and Selective Modified Graphene Paste Sensor for the Determination of Catechol and Hydroquinone. Surfaces. 2020; 3(3):473-483. https://doi.org/10.3390/surfaces3030034
Chicago/Turabian StyleManjunatha, Jamballi G. 2020. "Fabrication of Efficient and Selective Modified Graphene Paste Sensor for the Determination of Catechol and Hydroquinone" Surfaces 3, no. 3: 473-483. https://doi.org/10.3390/surfaces3030034
APA StyleManjunatha, J. G. (2020). Fabrication of Efficient and Selective Modified Graphene Paste Sensor for the Determination of Catechol and Hydroquinone. Surfaces, 3(3), 473-483. https://doi.org/10.3390/surfaces3030034





