Asymptotic Relative Efficiency of Parametric and Nonparametric Survival Estimators
Abstract
1. Introduction
2. Notation and Estimators
2.1. The Kaplan–Meier Estimator
The Parametric Survival Estimator
3. Asymptotic Relative Efficiency
4. ARE as Correlation between Estimates
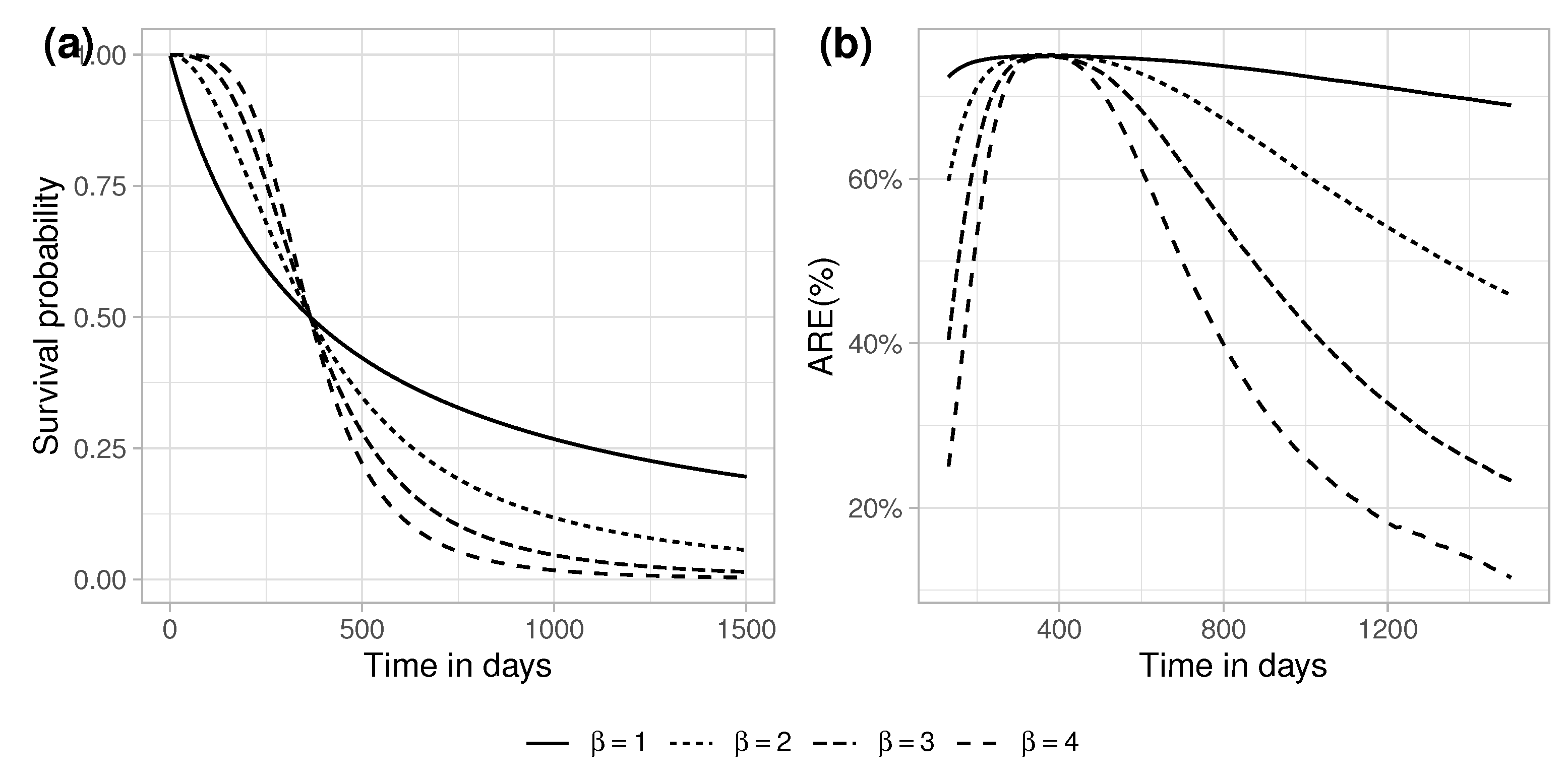
5. ARE for Exponential Survival and Exponential Censoring
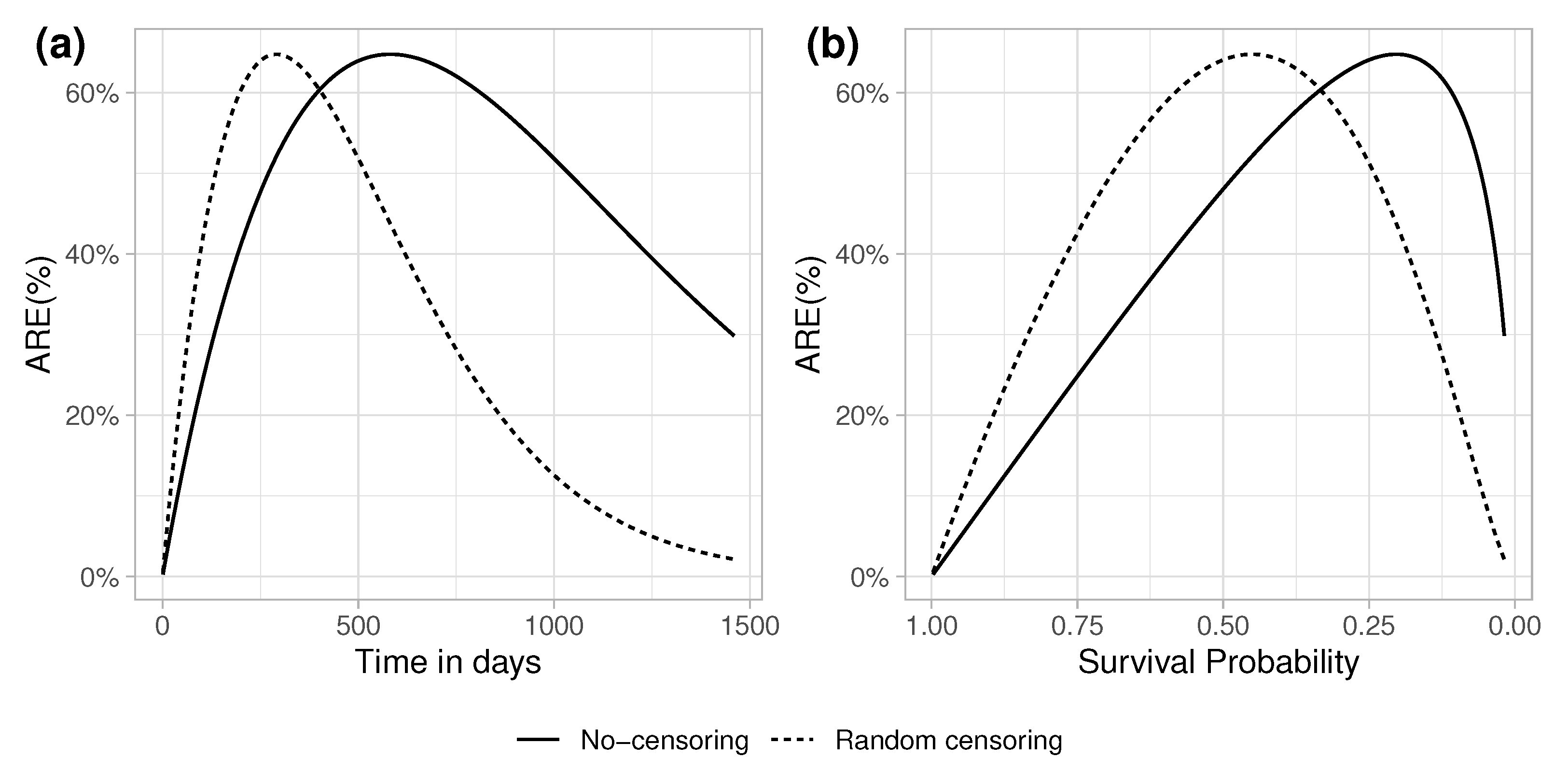
5.1. No-Censoring
5.1.1. Random Censoring Times
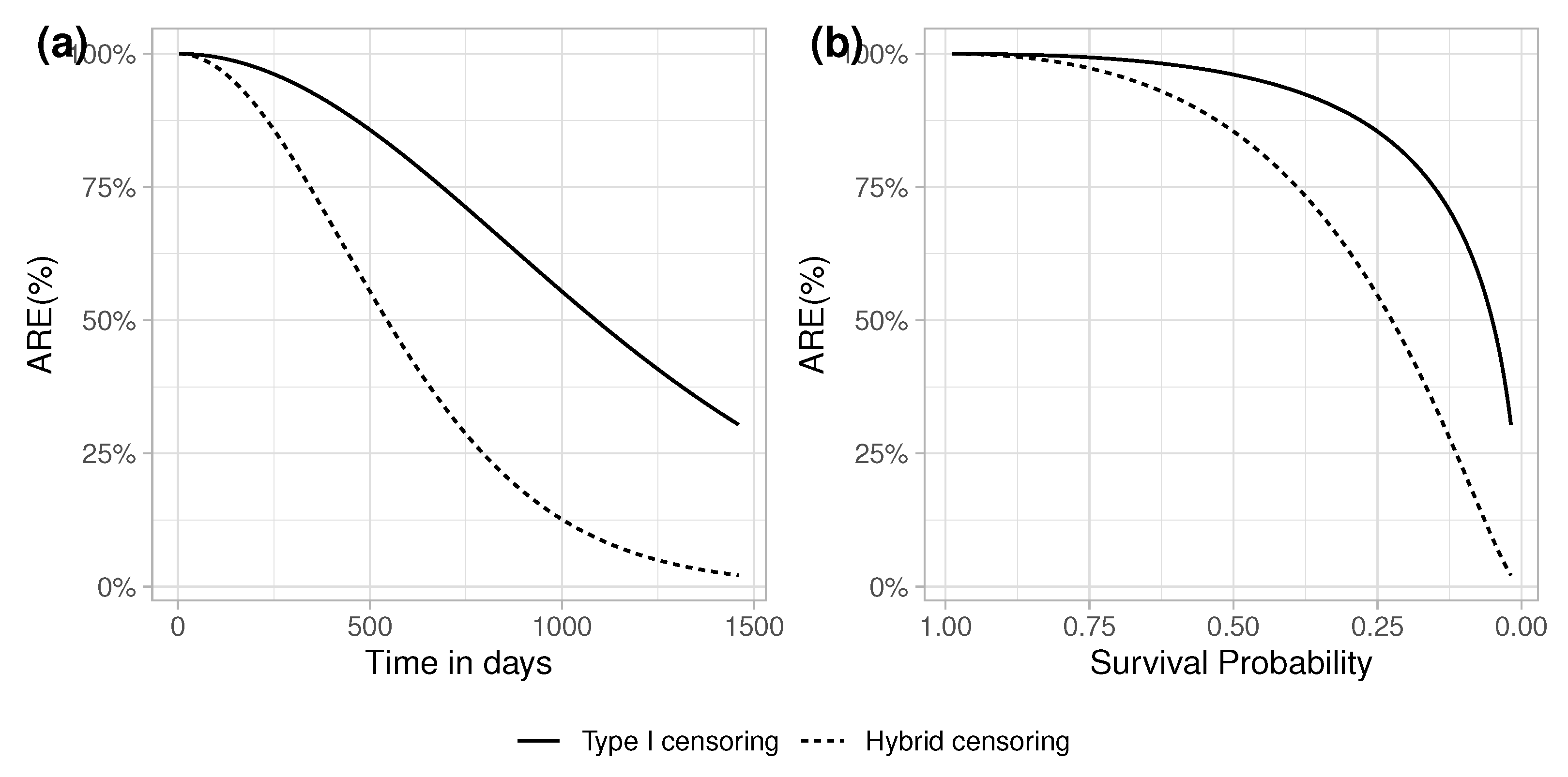
5.1.2. Type I or Fixed Censoring
5.1.3. Hybrid Type I and Random Censoring
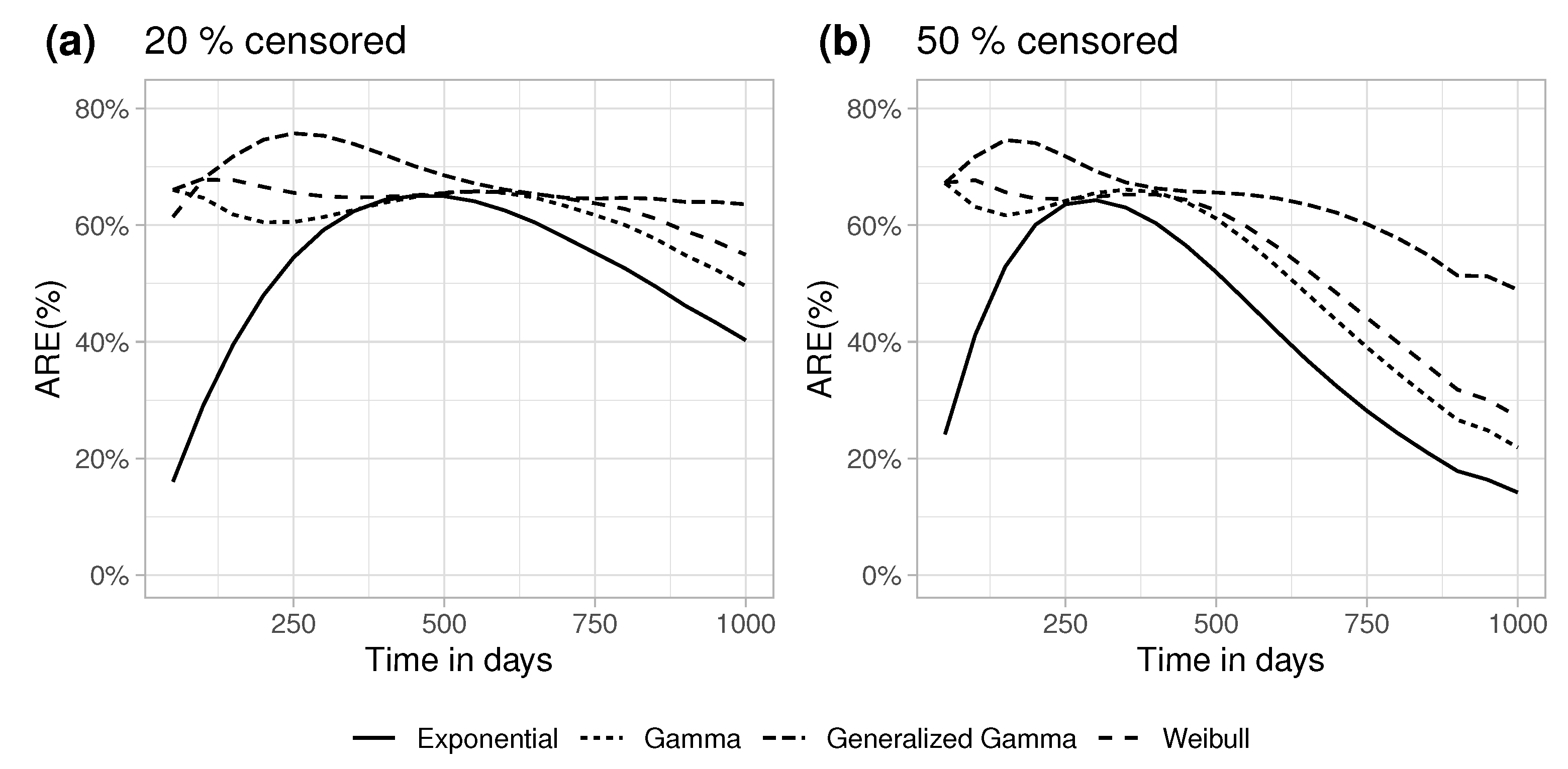
5.2. Efficiency in Finite Samples
6. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Covariance and Correlation between Estimators of Survival

Appendix B. ARE under Hybrid Censoring

Appendix C. Multi-Dimensional Parametric Models
Appendix D. Correlation between Kaplan–Meier Survival Estimates at Different Time Points
References
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Hjort, N.L. On inference in parametric survival data models. Int. Stat. Rev./Rev. Int. Stat. 1992, 60, 355–387. [Google Scholar] [CrossRef]
- Miller, R.G., Jr. What price Kaplan–Meier? Biometrics 1983, 39, 1077–1081. [Google Scholar] [CrossRef]
- Klein, J.P.; Moeschberger, M.L. The robustness of several estimators of the survivorship function with randomly censored data. Commun. Stat.-Simul. Comput. 1989, 18, 1087–1112. [Google Scholar] [CrossRef]
- Aranda-Ordaz, F.J. Relative efficiency of the Kaplan–Meier estimator under contamination: Relative efficiency of the kaplan-meier. Commun. Stat.-Simul. Comput. 1987, 16, 987–997. [Google Scholar] [CrossRef]
- Meier, P.; Karrison, T.; Chappell, R.; Xie, H. The price of Kaplan–Meier. J. Am. Stat. Assoc. 2004, 99, 890–896. [Google Scholar] [CrossRef]
- Jullum, M.; Hjort, N.L. What price semiparametric Cox regression? Lifetime Data Anal. 2019, 25, 406–438. [Google Scholar]
- Cheng, P.E.; Lin, G.D. Maximum likelihood estimation of a survival function under the Koziol-Green proportional hazards model. Stat. Probab. Lett. 1987, 5, 75–80. [Google Scholar]
- Klein, J.P. Small sample moments of some estimators of the variance of the Kaplan–Meier and Nelson-Aalen estimators. Scand. J. Stat. 1991, 18, 333–340. [Google Scholar]
- Zheng, G.; Gastwirth, J.L. On the Fisher information in randomly censored data. Stat. Probab. Lett. 2001, 52, 421–426. [Google Scholar] [CrossRef]
- Zheng, G.; Balakrishnan, N. Fisher Information: Nonstandard Situations. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Nadarajah, S. Reliability for lifetime distributions. Math. Comput. Model. 2003, 37, 683–688. [Google Scholar] [CrossRef]
- Serfling, R. Asymptotic Relative Efficiency in Estimation. Int. Encycl. Stat. Sci. 2011, 23, 68–72. [Google Scholar]
- Nemes, S.; Gustavsson, A.; Taib, Z. Variance Inflation Due to Censoring in Survival Probability Estimates. Statistica 2020, 80, 395–412. [Google Scholar]
- Cramér, H. Mathematical Methods of Statistics; Princeton University Press: Princeton, NJ, USA, 1946. [Google Scholar]
- Cox, D.R. Principles of Statistical Inference; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Peña, E.A.; Rohatgi, V.K. Small sample and efficiency results for the Nelson-Aalen estimator. J. Stat. Plan. Inference 1993, 37, 193–202. [Google Scholar] [CrossRef]
- Mardia, K.V.; Southworth, H.R.; Taylor, C.C. On bias in maximum likelihood estimators. J. Stat. Plan. Inference 1999, 76, 31–39. [Google Scholar] [CrossRef]
- Xuemao Zhang, S.P.; Wang, Y.G. Small sample bias correction or bias reduction? Commun. Stat.-Simul. Comput. 2021, 50, 1165–1177. [Google Scholar] [CrossRef]
- Geurts, J. Some Small–Sample Non–Proportional Hazards Resulis For The Kaplan–Meier Estimator. Stat. Neerl. 1985, 39, 1–13. [Google Scholar] [CrossRef]
- Meier, P. Estimation of a distribution function from incomplete observations. J. Appl. Probab. 1975, 12, 67–87. [Google Scholar] [CrossRef]
- Noether, G.E. On a theorem of Pitman. Ann. Math. Stat. 1955, 26, 64–68. [Google Scholar] [CrossRef]
- Van Eeden, C. The relation between Pitman’s asymptotic relative efficiency of two tests and the correlation coefficient between their test statistics. Ann. Math. Stat. 1963, 34, 1442–1451. [Google Scholar] [CrossRef]
- Jullum, M.; Hjort, N.L. Parametric or nonparametric: The FIC approach. Stat. Sin. 2017, 27, 951–981. [Google Scholar] [CrossRef][Green Version]
- Nemes, S.; Gustavsson, A.; Jauhiainen, A. Focused Information Criterion for Restricted Mean Survival Times: Non-Parametric or Parametric Estimators. Entropy 2022, 24, 713. [Google Scholar] [CrossRef] [PubMed]
- Gebski, V.; Garès, V.; Gibbs, E.; Byth, K. Data maturity and follow-up in time-to-event analyses. Int. J. Epidemiol. 2018, 47, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Boos, D.; Stefanski, L. Essential Statistical Inference: Theory and Methods; Springer: New York, NY, USA, 2013. [Google Scholar]
- Reid, N. Influence Functions for Censored Data. Ann. Stat. 1981, 9, 78–92. [Google Scholar] [CrossRef]
- Marshall, A.W.; Olkin, I. Life Distributions; Springer: Berlin/Heidelberg, Germany, 2007; Volume 13. [Google Scholar]
- Efron, B. Logistic regression, survival analysis, and the Kaplan–Meier curve. J. Am. Stat. Assoc. 1988, 83, 414–425. [Google Scholar] [CrossRef]
| N | t = 50 | t = 180 | t = 365 |
|---|---|---|---|
| ARE | 12.78 | 38.15 | 58.20 |
| 25 | 16.35 (27.92%) | 40.63 (19.4%) | 59.15 (7.45%) |
| 50 | 14.89 (16.49%) | 39.32 (9.15%) | 58.67 (3.70%) |
| 75 | 14.03 (9.77%) | 39.09 (7.35%) | 58.48 (2.21%) |
| 100 | 13.65 (6.79%) | 38.65 (3.91%) | 58.31 (0.88%) |
| 125 | 13.57 (6.17%) | 38.69 (4.22%) | 58.51 (2.44%) |
| 150 | 13.35 (4.45%) | 38.61 (3.59%) | 58.41 (1.66%) |
| 200 | 13.22 (3.43%) | 38.44 (2.26%) | 58.34 (1.11%) |
| 250 | 13.11 (2.57%) | 38.27 (0.93%) | 58.30 (0.37%) |
| 1000 | 12.89 (0.85%) | 38.23 (0.62%) | 58.23 (0.25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, S. Asymptotic Relative Efficiency of Parametric and Nonparametric Survival Estimators. Stats 2023, 6, 1147-1159. https://doi.org/10.3390/stats6040072
Nemes S. Asymptotic Relative Efficiency of Parametric and Nonparametric Survival Estimators. Stats. 2023; 6(4):1147-1159. https://doi.org/10.3390/stats6040072
Chicago/Turabian StyleNemes, Szilárd. 2023. "Asymptotic Relative Efficiency of Parametric and Nonparametric Survival Estimators" Stats 6, no. 4: 1147-1159. https://doi.org/10.3390/stats6040072
APA StyleNemes, S. (2023). Asymptotic Relative Efficiency of Parametric and Nonparametric Survival Estimators. Stats, 6(4), 1147-1159. https://doi.org/10.3390/stats6040072





