Interval-Censored Regression with Non-Proportional Hazards with Applications
Abstract
1. Introduction
2. The Proposed Model
- Exact failure time .
- Right-censored .
- Interval-censored .
3. Estimation
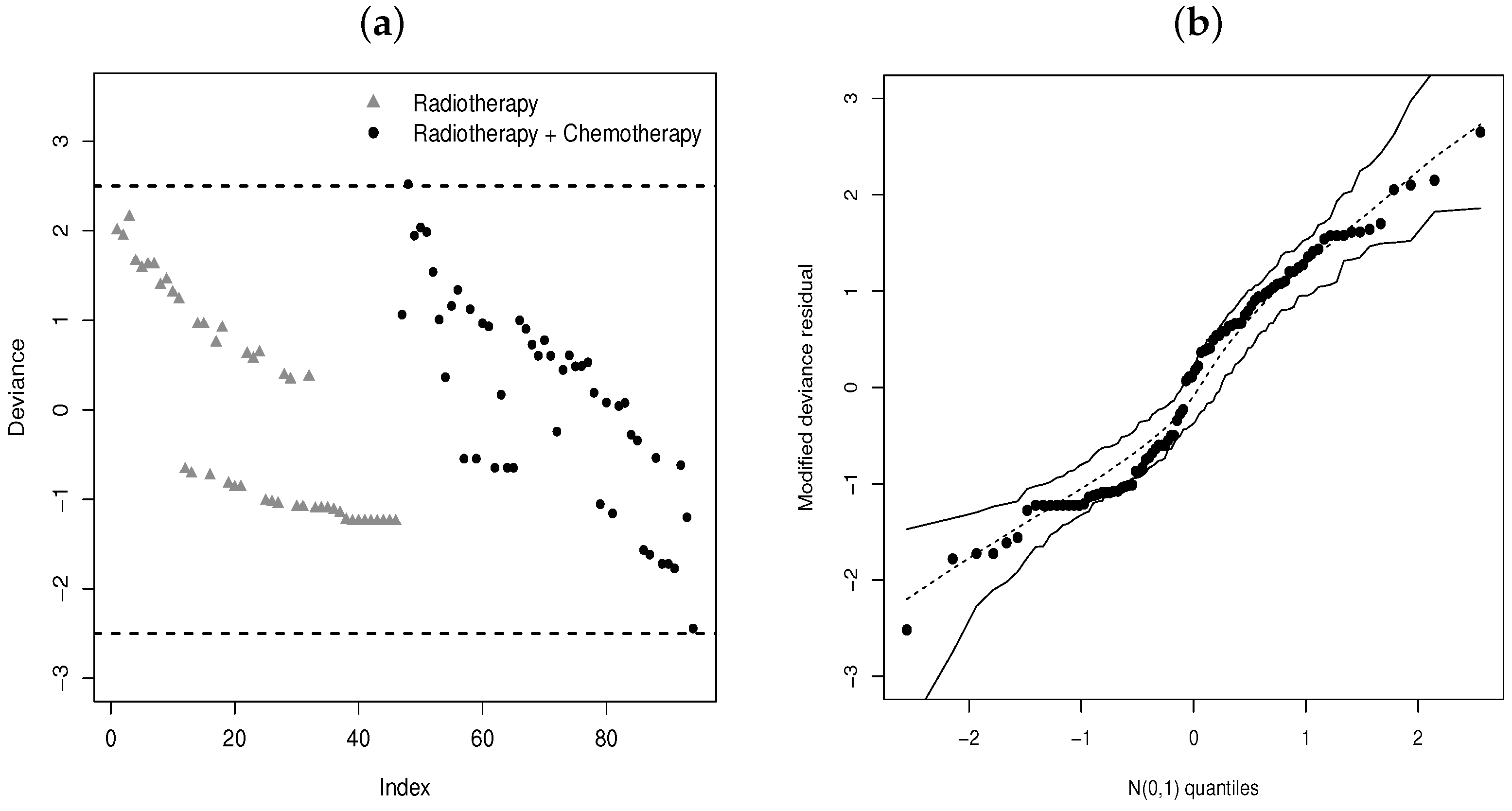
4. Modified Deviance Residuals
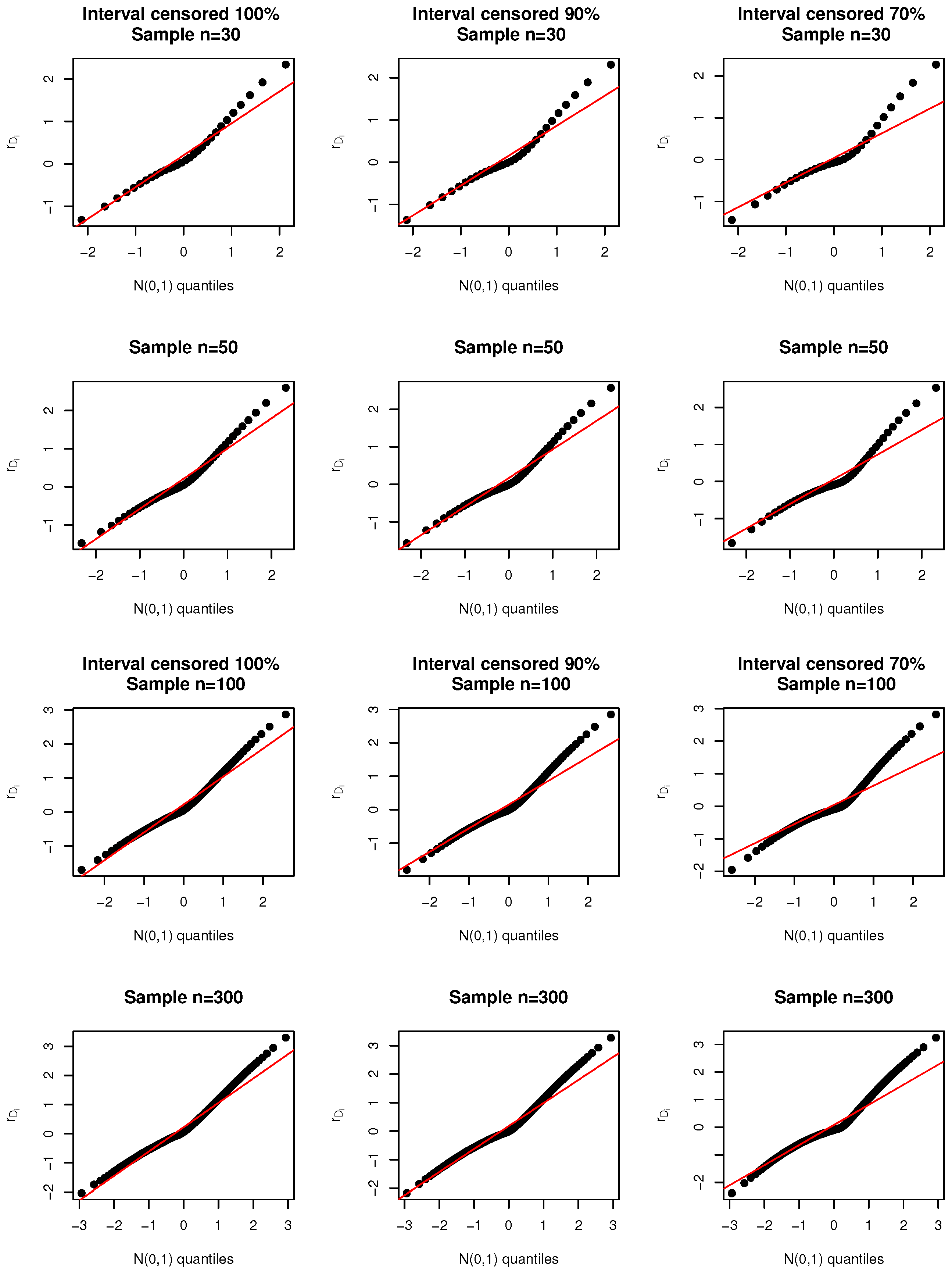
Simulation Studies
- Deviance residual
- (a)
- The coefficients are fixed at , , and ;
- (b)
- The explanatory variable is generated from the binomial distribution with a success probability equal to 0.5 and 1, and and ) are computed;
- (c)
- The variable is generated from , and the logarithm time from (4);
- (d)
- The logarithm censoring time is generated from a uniform distribution by fixing until right-censoring percentages of 0, 10%, or 30%;
- (e)
- The interval length is generated from a uniform distribution;
- (f)
- The limits of the log interval time are and , where k is the length of the randomly chosen interval.
- 2.
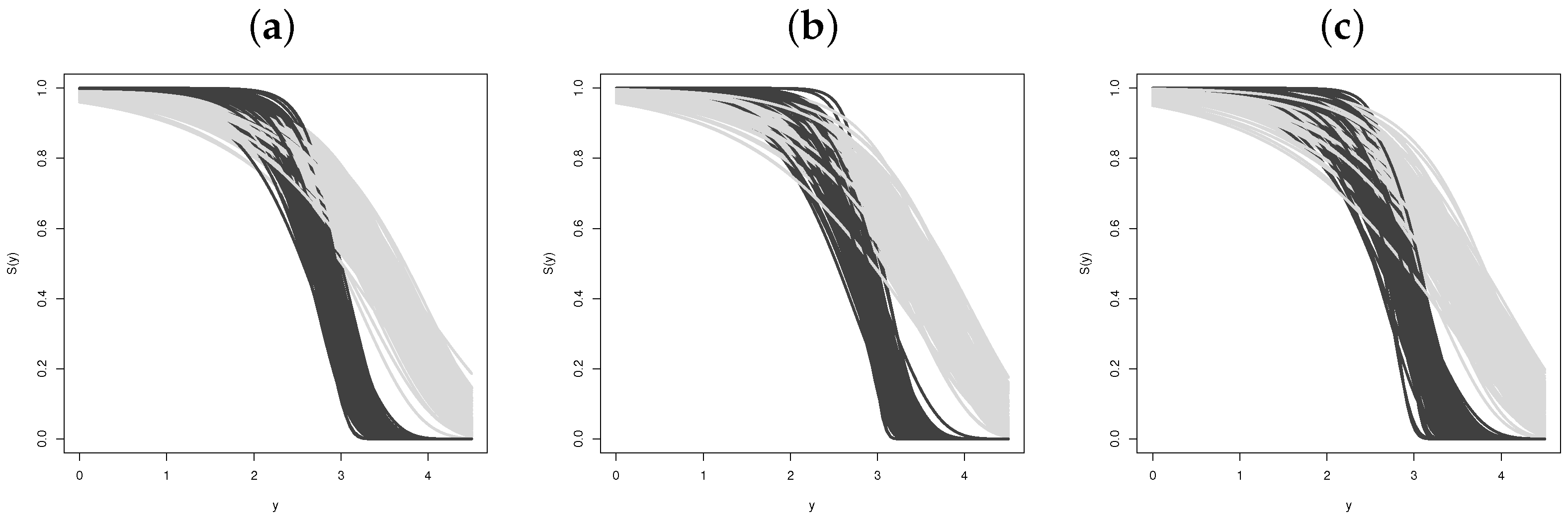
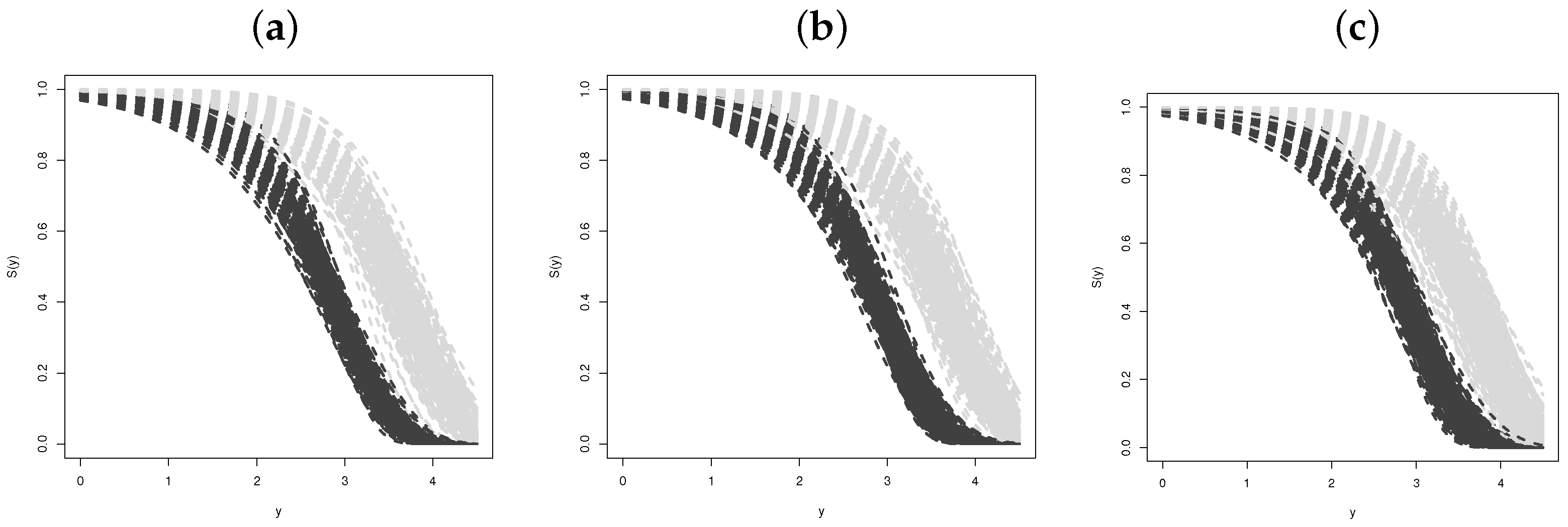
- Estimating the survival function
- (a)
- Effects of the covariables on the parameters and simultaneously:
- i
- Initial values , , and ;
- ii
- Estimate the survival function bywhere is generated from a binomial distribution with a success probability 0.5 and 1.
- (b)
- Effects of the covariables on the parameter :
- i
- Initial values , , and , where the initial value for is the estimate given in Table 2;
- ii
- Estimate the survival function bywhere is generated from a binomial distribution with a success probability equal to 0.5 and 1.
5. Applications
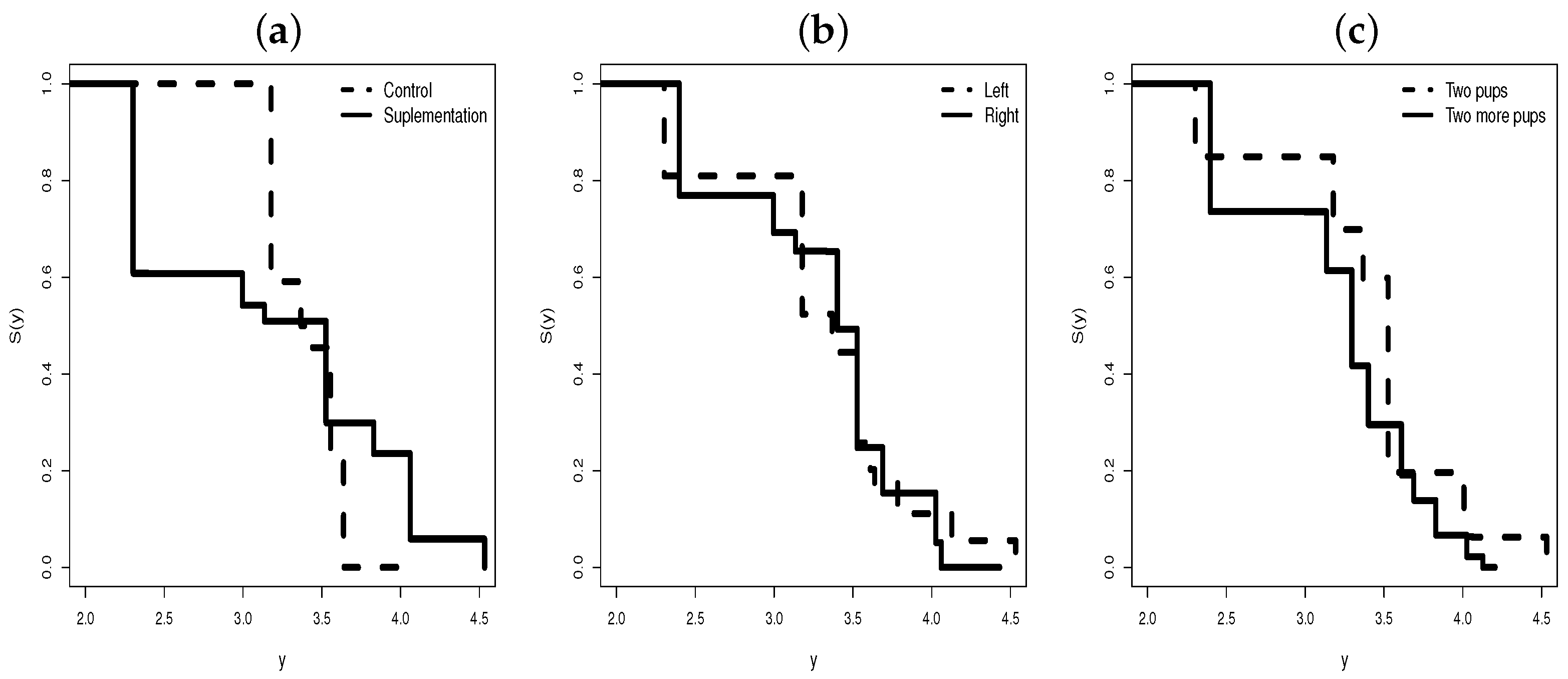
5.1. Regression for the Supplementation Animal Data
- : Logarithm time after delivery until the first ovulation;
- : Treatment (0 = control, 1 = supplementation);
- : Ovary (0 = right, 1 = left);
- : Number of pups (0 = two pups, 1 = two more pups).
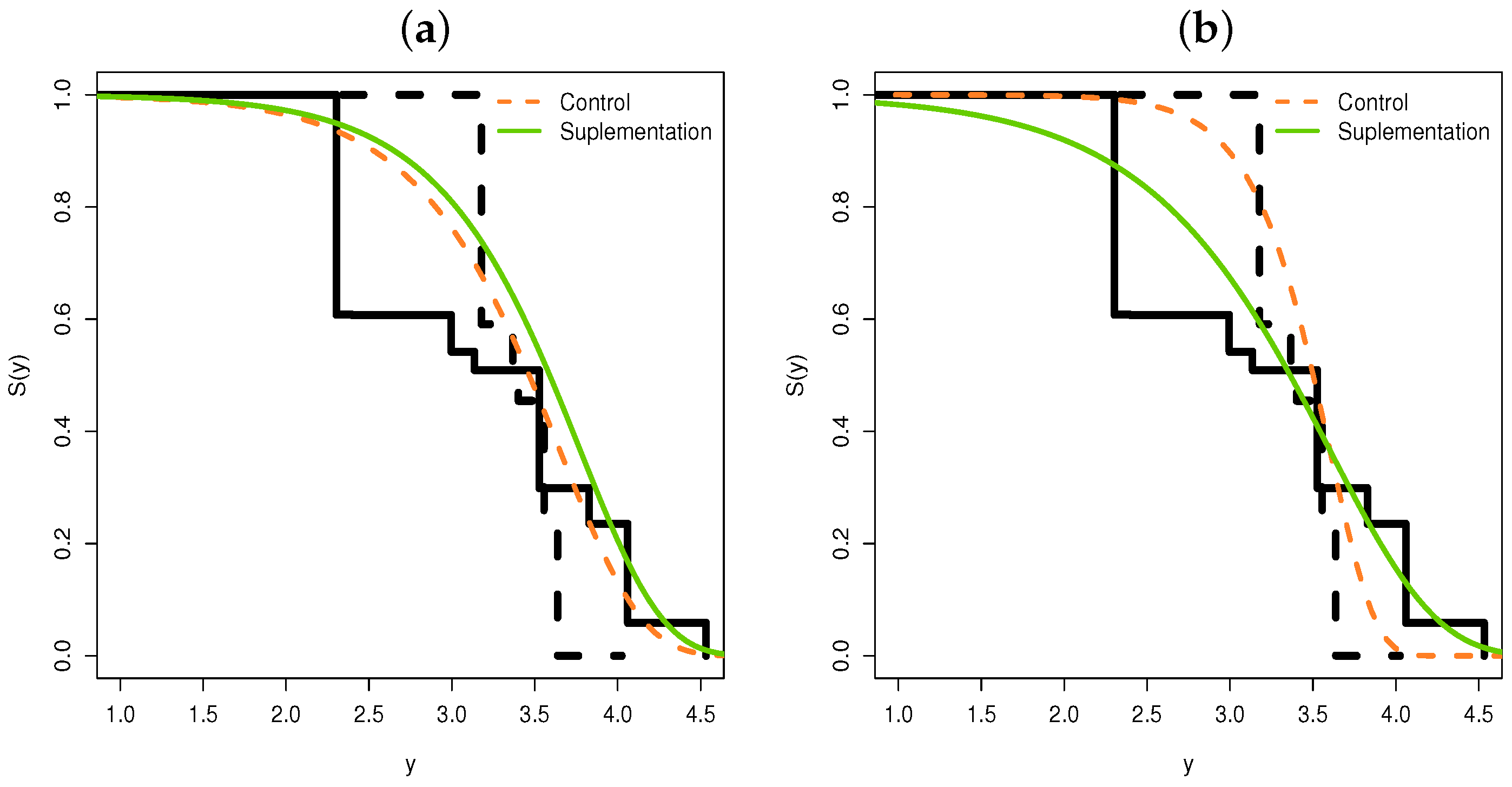
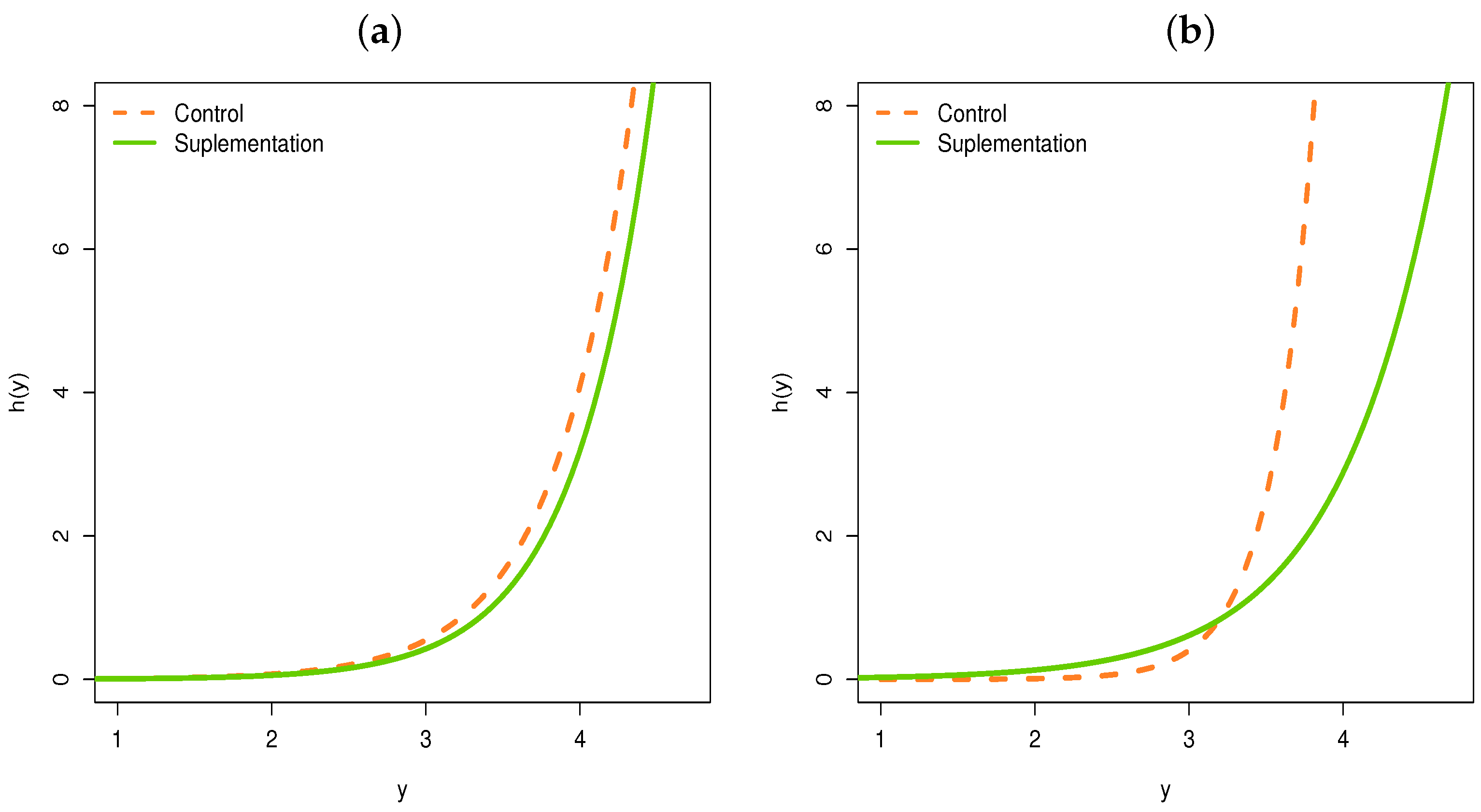
- The levels of the control and supplementation of the treatment are different, explaining the variability in the log ovulation time.
- From Figure 7b, we note that before days (approximately), the treatment control level has a longer survival time than the supplementation level.
- After 33 days, we note the opposite, i.e., the survival time of the supplementation level is longer than the control level in relation to the time of ovulation.
- Thus, if the supplement is applied at longer intervals, the supplement level would have a longer survival time compared with the control.
- We can also note that this change in 33 days is captured by the systematic part of the parameter .
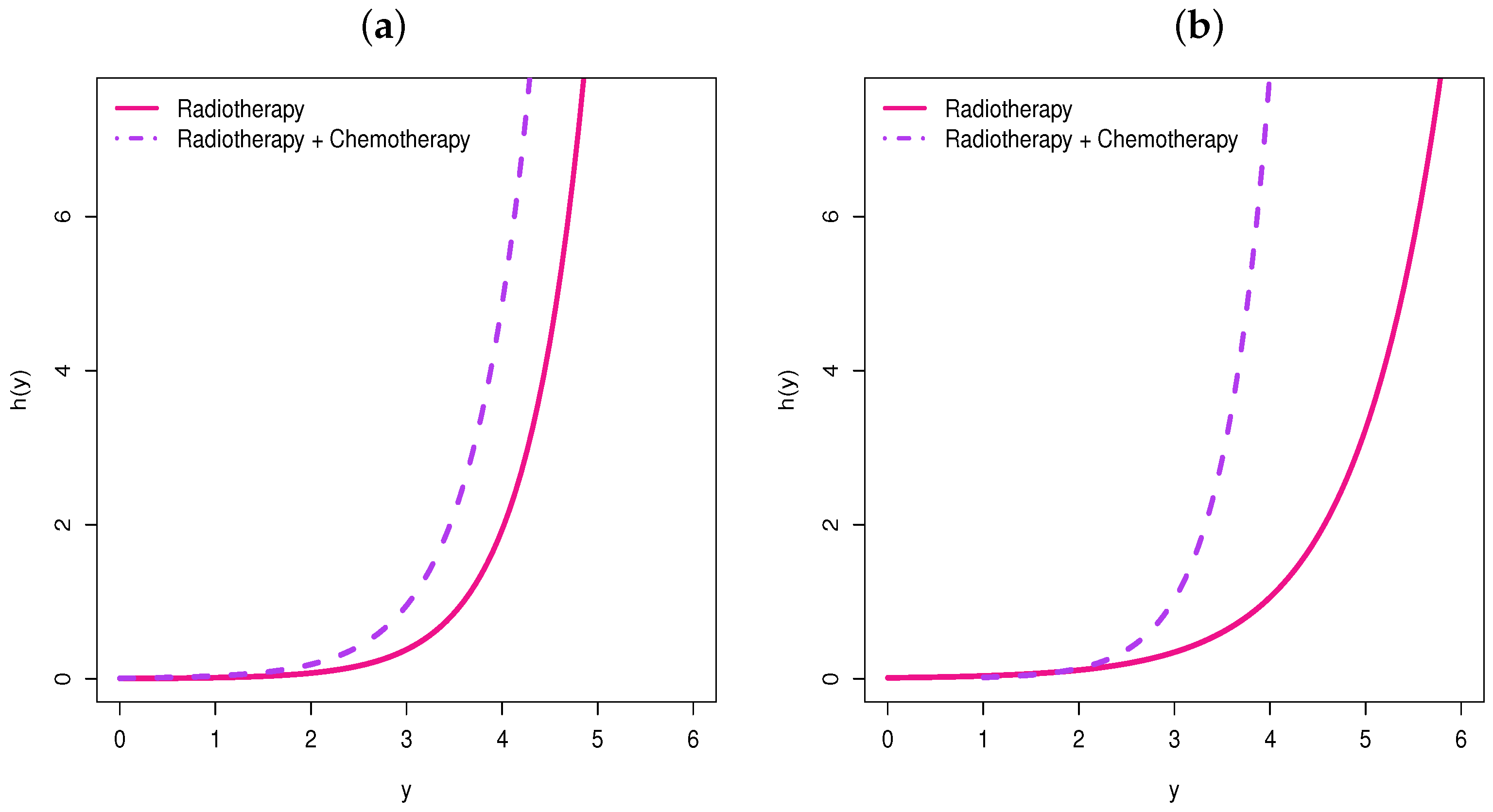
5.2. Regression for Breast Cancer Data
- : Logarithm of time (in months) to first appearance of moderate or severe breast retraction;
- : Type of treatment (0 = radiotherapy and chemotherapy, 1 = radiotherapy).
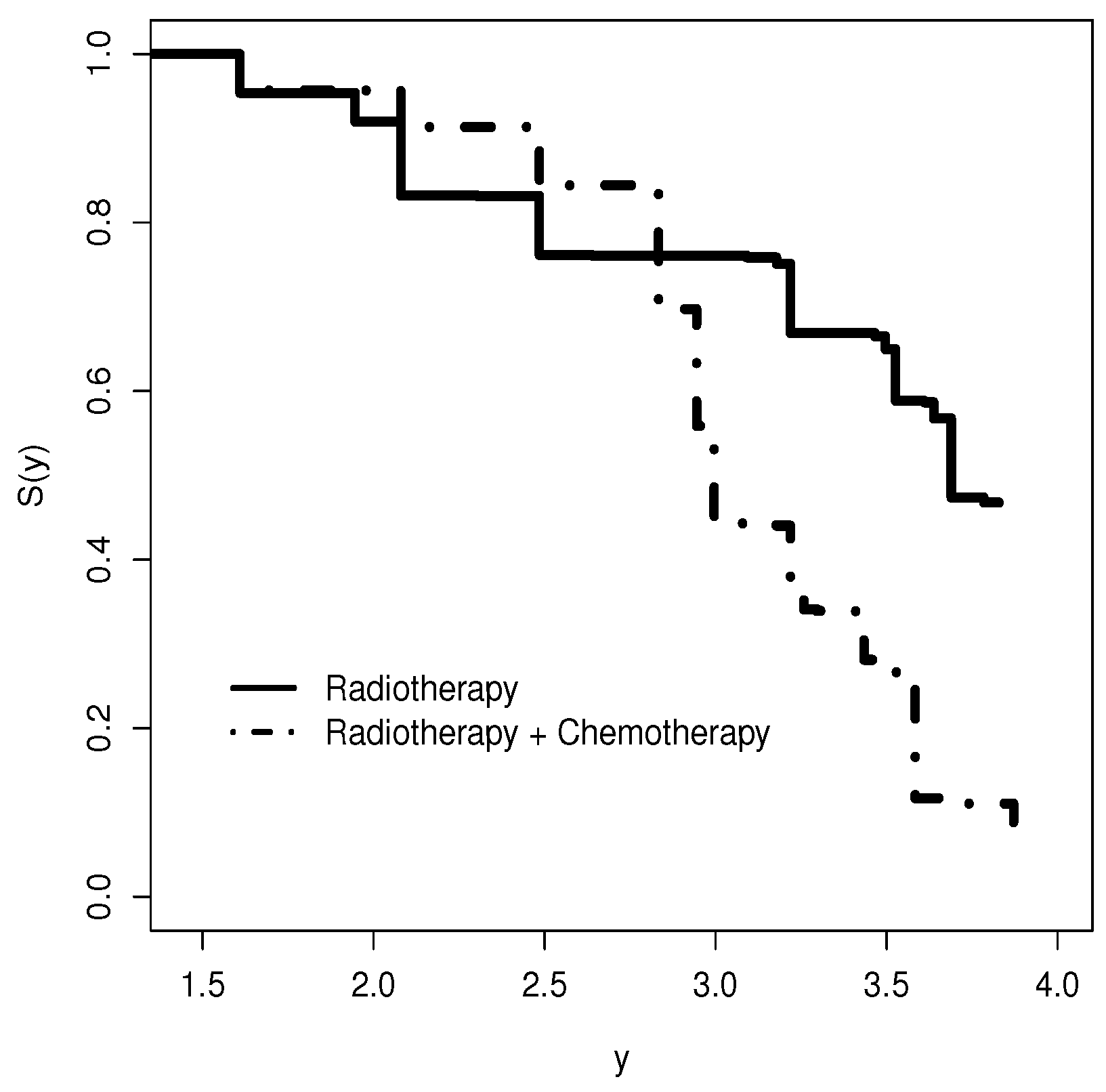
- There is a significant difference between the levels of radiotherapy and chemotherapy and radiotherapy in terms of the covariable treatment in relation to the log time of the first appearance of moderate or severe breast retraction.
- There is a significant difference between the levels of radiotherapy and chemotherapy and radiotherapy in terms of the covariable treatment in relation to the variability of the logarithm of the time of the first appearance of moderate or severe breast retraction.
- We note from Figure 11b that before months (approximately), the radiotherapy and chemotherapy level of treatment has a longer survival time than the radiotherapy level, but this difference is not significant.
- After 12 months, we note the opposite, i.e., the survival time of the radiotherapy level is longer than that of the radiotherapy and chemotherapy level in relation to the time of the first appearance of moderate or severe breast retraction.
- So, we note that 12 months of applying radiotherapy and chemotherapy to the patient makes them less immune.
- We can also note that this change after 12 months is captured by the systematic part of .
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashimoto, E.M.; Ortega, E.M.; Cancho, V.G.; Cordeiro, G.M. The log-exponentiated Weibull regression model for interval-censored data. Comput. Stat. Data Anal. 2010, 54, 1017–1035. [Google Scholar] [CrossRef]
- Betensky, R.A.; Lindsey, J.C.; Ryan, L.M.; Wand, M. A local likelihood proportional hazards model for interval censored data. Stat. Med. 2002, 21, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.L.; Gómez, G. A semiparametric hierarchical method for a regression model with an interval-censored covariate. Aust. N. Z. J. Stat. 2005, 47, 351–364. [Google Scholar] [CrossRef]
- Komárek, A.; Lesaffre, E. The regression analysis of correlated interval-censored data: Illustration using accelerated failure time models with flexible distributional assumptions. Stat. Model. 2009, 9, 299–319. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Calsavara, V.F.; Silva, F.I.; Alves, F.A.; Vivas, A.P. Use of interval-censored survival data as an alternative to Kaplan-Meier survival curves: Studies of oral lesion occurrence in liver transplants and cancer recurrence. Appl. Cancer Res. 2018, 38, 16. [Google Scholar] [CrossRef]
- Finkelstein, D.M. A proportional hazards model for interval-censored failure time data. Biometrics 1986, 42, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Heller, G. Proportional hazards regression with interval censored data using an inverse probability weight. Lifetime Data Anal. 2011, 17, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, T.; Sun, J. Cox regression analysis of dependent interval-censored failure time data. Comput. Stat. Data Anal. 2016, 103, 79–90. [Google Scholar] [CrossRef]
- Ahn, S.; Lim, J.; Paik, M.C.; Sacco, R.L.; Elkind, M.S. Cox model with interval-censored covariate in cohort studies. Biom. J. 2018, 60, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Yadav, J.S.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Ansel, G.; Strickman, N.E.; Wang, H.; Cohen, S.A.; et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2008, 358, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Dummer, R.; Gutzmer, R.; Lorigan, P.; Kim, K.B.; Nyakas, M.; Arance, A.; Liszkay, G.; Schadendorf, D.; Cantarini, M.; et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: A phase 2 double-blind randomised study. Lancet Oncol. 2013, 14, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q. A Weibull multi-state model for the dependence of progression-free survival and overall survival. Stat. Med. 2015, 34, 2497–2513. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Brodziak, A.; Sobczuk, P.; Dendek, C.; Labochka, D.; Korniluk, J.; Bartnik, E.; Szczylik, C. Metastatic tumor burden and loci as predictors of first line sunitinib treatment efficacy in patients with renal cell carcinoma. Sci. Rep. 2019, 9, 7754. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.F. Statistical Models and Methods for Lifetime Data; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 362. [Google Scholar]
- Leiva, V.; Barros, M.; Paula, G.A.; Galea, M. Influence diagnostics in log-Birnbaum–Saunders regression models with censored data. Comput. Stat. Data Anal. 2007, 51, 5694–5707. [Google Scholar] [CrossRef]
- Ortega, E.M.; Paula, G.A.; Bolfarine, H. Deviance residuals in generalised log-gamma regression models with censored observations. J. Stat. Comput. Simul. 2008, 78, 747–764. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M.; Fleming, T.R. Martingale-based residuals for survival models. Biometrika 1990, 77, 147–160. [Google Scholar] [CrossRef]
- Farrington, C. Residuals for proportional hazards models with interval-censored survival data. Biometrics 2000, 56, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data; Springer: Berlin/Heidelberg, Germany, 2003; Volume 1230. [Google Scholar]
- Beadle, G.F.; Silver, B.; Botnick, L.; Hellman, S.; Harris, J.R. Cosmetic results following primary radiation therapy for early breast cancer. Cancer 1984, 54, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Beadle, G.F.; Come, S.; Henderson, I.C.; Silver, B.; Hellman, S.; Harris, J.R. The effect of adjuvant chemotherapy on the cosmetic results after primary radiation treatment for early stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, E.M.; Ortega, E.M.; Cordeiro, G.M.; Cancho, V.G. A new long-term survival model with interval-censored data. Sankhya B 2015, 77, 207–239. [Google Scholar] [CrossRef]
- Yang, C.; Diao, L.; Cook, R. Survival trees for current status data. In Proceedings of the Survival Prediction-Algorithms, Challenges and Applications, Palo Alto, CA, USA, 22–24 March 2021; pp. 83–94. [Google Scholar]



| n | Parameters | Actual Values | 0% | 10% | 30% |
|---|---|---|---|---|---|
| 30 | 3.00 | 2.9158 (0.0284) | 2.9313 (0.0279) | 2.9354 (0.0352) | |
| 0.72 | 0.7444 (0.0879) | 0.7286 (0.0965) | 0.7275 (0.1245) | ||
| −0.71 | −1.1733 (0.8250) | −1.2731 (1.3701) | −1.4154 (2.2010) | ||
| 0.60 | 0.8623 (0.7849) | 0.9578 (1.3332) | 1.0628 (2.2225) | ||
| 50 | 3.00 | 2.9260 (0.0114) | 2.9304 (0.0132) | 2.9411 (0.0172) | |
| 0.72 | 0.7416 (0.0456) | 0.7427 (0.0573) | 0.7375 (0.0714) | ||
| −0.71 | −1.0239 (0.1120) | −1.0507 (0.2259) | −1.0612 (0.3456) | ||
| 0.60 | 0.7701 (0.1679) | 0.7848 (0.2642) | 0.7950 (0.3919) | ||
| 100 | 3.00 | 2.9401 (0.0055) | 2.9443 (0.00964) | 2.9571 (0.0086) | |
| 0.72 | 0.7408 (0.0216) | 0.7372 (0.0275) | 0.7302 (0.0353) | ||
| −0.71 | −0.9714 (0.0390) | −0.9680 (0.0382) | −0.9461 (0.0429) | ||
| 0.60 | 0.7502 (0.0554) | 0.7479 (0.0597) | 0.7319 (0.0678) | ||
| 300 | 3.00 | 2.9437 (0.0018) | 2.9468 (0.0022) | 2.9603 (0.0028) | |
| 0.72 | 0.7438 (0.0075) | 0.7436 (0.0086) | 0.7398 (0.0117) | ||
| −0.71 | −0.9381 (0.0098) | −0.9263 (0.0102) | −0.8997 (0.0112) | ||
| 0.60 | 0.7335 (0.0156) | 0.7257 (0.0170) | 0.7101 (0.0204) |
| One Component for | Two Components for and | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | -Value | B | Estimate | SE | -Value |
| 3.3311 | 0.1053 | <0.0001 | 3.3329 | 0.0852 | <0.0001 | ||
| 0.5648 | 0.1738 | 0.0016 | 0.7193 | 0.2418 | 0.0037 | ||
| 0.6129 | 0.0721 | - | −0.7088 | 0.1407 | <0.0001 | ||
| 0.5952 | 0.2558 | 0.0221 | |||||
| = 286.0 | AIC = 292.0 | = 280.2 | AIC = 288.2 | ||||
| Only One Component for | Two Components for and | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | -Value | Parameter | Estimate | SE | -Value |
| 3.649 | 0.159 | <0.001 | 3.601 | 0.105 | <0.001 | ||
| 0.123 | 0.146 | 0.401 | -0.002 | 0.154 | 0.991 | ||
| 0.045 | 0.145 | 0.759 | 0.230 | 0.131 | 0.085 | ||
| −0.186 | 0.144 | 0.202 | −0.132 | 0.131 | 0.320 | ||
| 0.497 | 0.062 | - | −1.304 | 0.275 | <0.001 | ||
| 0.867 | 0.274 | 0.003 | |||||
| −0.304 | 0.276 | 0.276 | |||||
| 0.293 | 0.313 | 0.353 | |||||
| = 157.700 | AIC = 167.700 | = 148.000 | AIC = 164.000 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prataviera, F.; Hashimoto, E.M.; Ortega, E.M.M.; Savian, T.V.; Cordeiro, G.M. Interval-Censored Regression with Non-Proportional Hazards with Applications. Stats 2023, 6, 643-656. https://doi.org/10.3390/stats6020041
Prataviera F, Hashimoto EM, Ortega EMM, Savian TV, Cordeiro GM. Interval-Censored Regression with Non-Proportional Hazards with Applications. Stats. 2023; 6(2):643-656. https://doi.org/10.3390/stats6020041
Chicago/Turabian StylePrataviera, Fábio, Elizabeth M. Hashimoto, Edwin M. M. Ortega, Taciana V. Savian, and Gauss M. Cordeiro. 2023. "Interval-Censored Regression with Non-Proportional Hazards with Applications" Stats 6, no. 2: 643-656. https://doi.org/10.3390/stats6020041
APA StylePrataviera, F., Hashimoto, E. M., Ortega, E. M. M., Savian, T. V., & Cordeiro, G. M. (2023). Interval-Censored Regression with Non-Proportional Hazards with Applications. Stats, 6(2), 643-656. https://doi.org/10.3390/stats6020041








