Current Review: Alginate in the Food Applications
Abstract
1. Introduction
2. Structures and Properties of Alginates
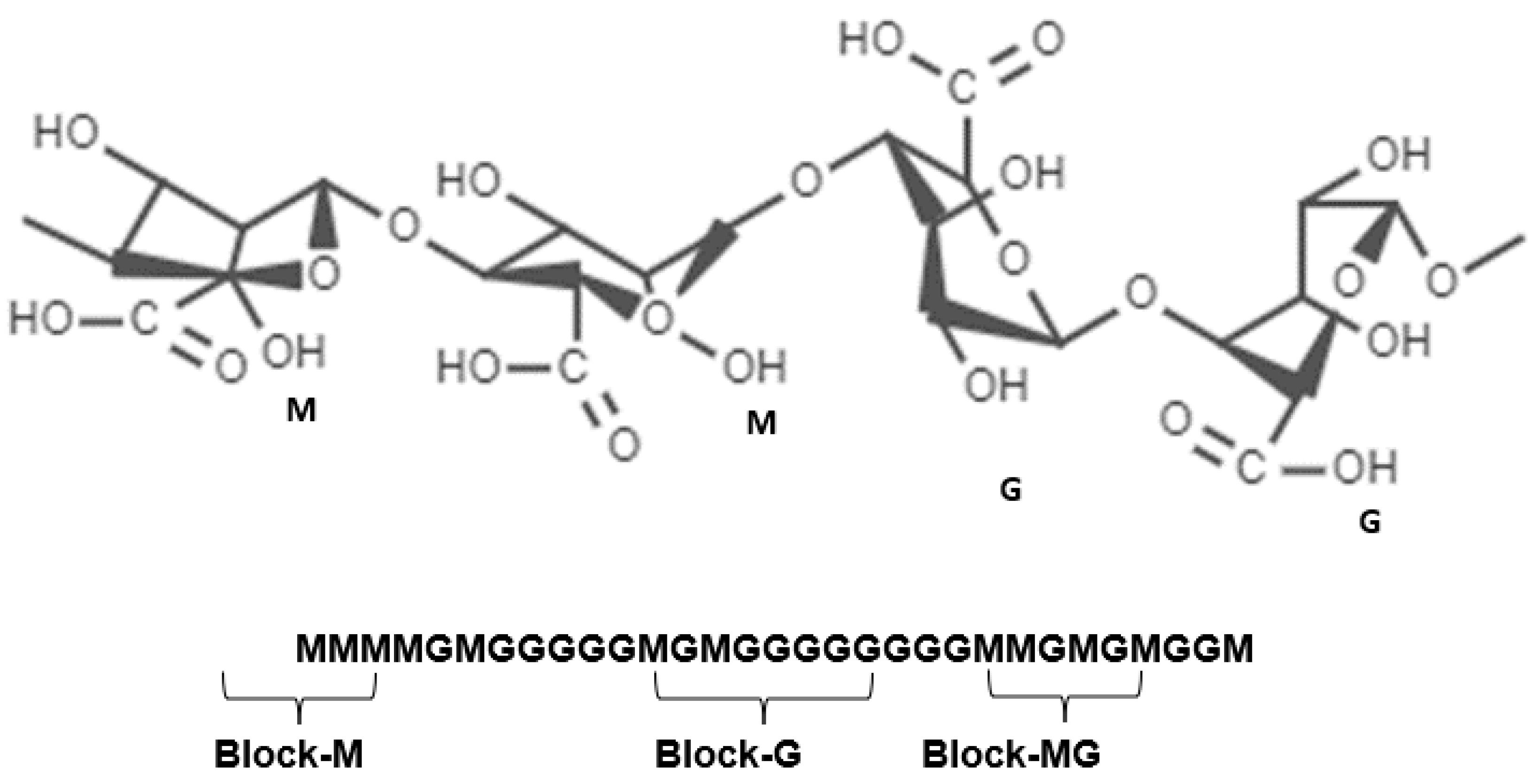
2.1. Structural Features
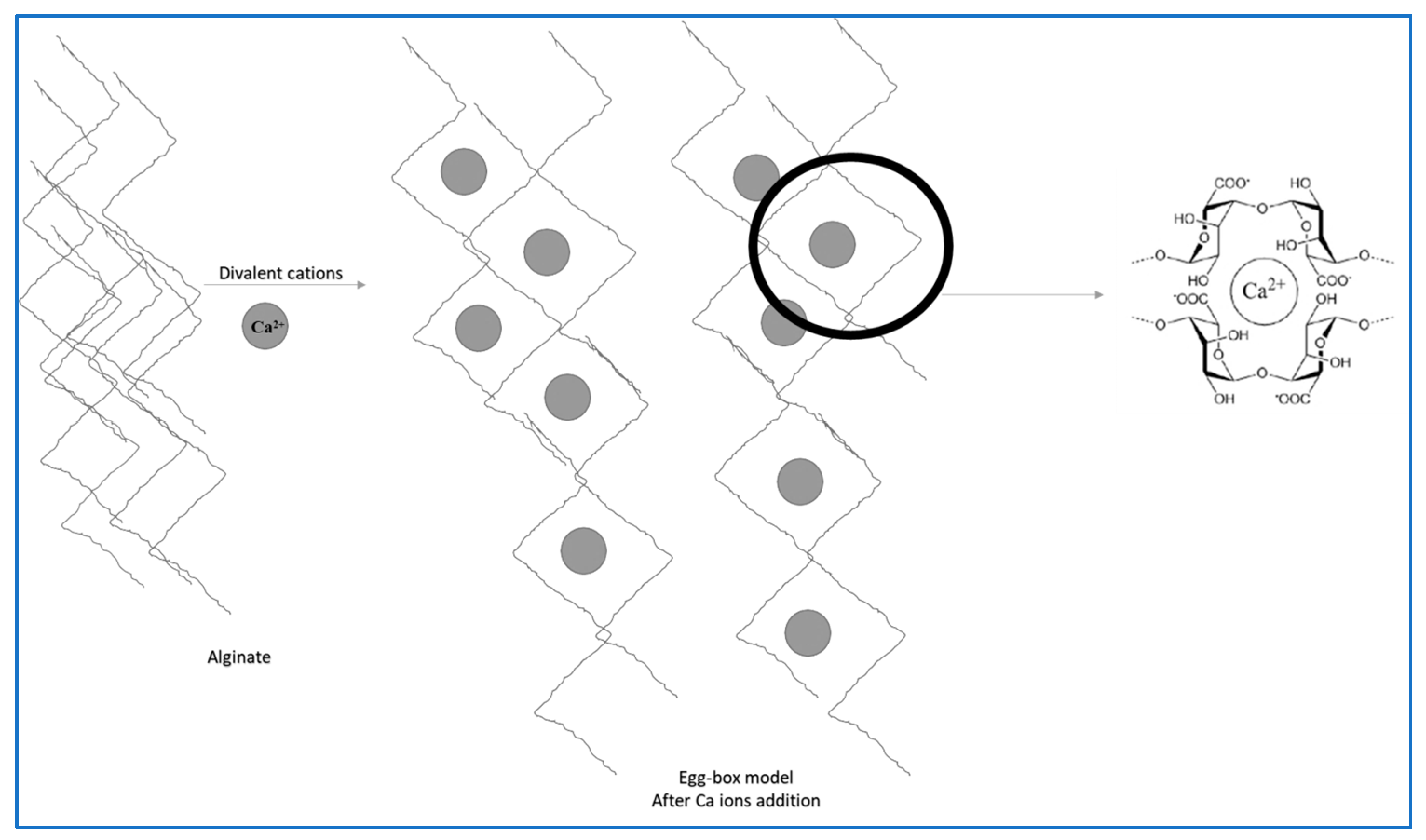
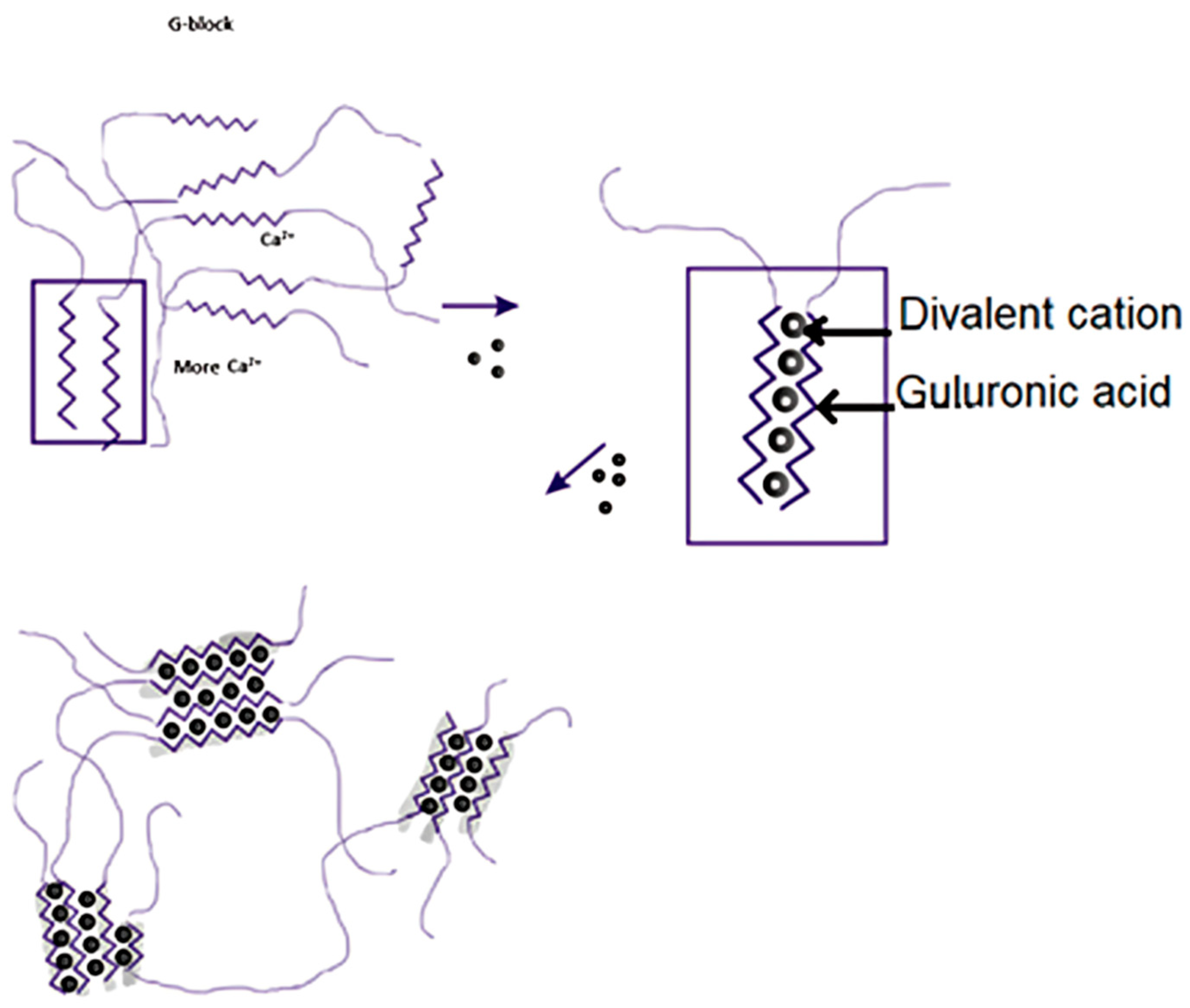
2.2. Alginate Molecule Crosslinking
2.3. Stability of Alginate Solutions
2.4. Alginate Gelling Properties
2.5. Alginate Gel Stability
2.6. Gelation Mechanisms of Alginate
2.6.1. External Gelation
2.6.2. Internal Gelation
2.6.3. Inverse Gelation
2.6.4. Interfacial Gelation
2.6.5. Interrupted Gelation in Multi-Step
2.7. Extraction of Alginate
2.7.1. Producing Low Molecular Weight Alginate
2.7.2. Ultrasound-Assisted Extraction (UAE)
2.7.3. Microwave-Assisted Extraction (MAE)
2.7.4. Pressurized Liquid Extraction (PLE)
2.7.5. Enzymes-Assisted Extraction (EAE)
2.8. Application of Alginate in the Food Industry
2.8.1. Thickening Properties
| Alginate Use Section | Type of Additives | References |
|---|---|---|
| Active food packaging | Alginate films with cottonseed protein hydrolysate. | [72] |
| Alginate-based edible film of fruits and vegetables. | [73] | |
| Alginate and carrageenan selective barrier to CO2 and fats. | [74] | |
| Sulfur–alginate films. | [69] | |
| Alginate films based on soybean oil were applied to sweet cherries. | [75] | |
| The casing of dry fermented sausage (different cations). | [76] | |
| Probiotic edible films. | [77] | |
| Rainbow trout (Oncorhynchus mykiss) filet coting with alginate/tannins. | [78] | |
| Green edible alginate/pectin chocolate and vegetable puff packages. | [79] | |
| Edible coating of alginate/thyme oil effects in fresh cantaloupe. | [80] | |
| UV wall and oxygen permeability of alginate/Cellulose nanocrystals in chicken breast. | [81] | |
| Thickening application | Gelling agents instead of the back-fat ingredient of low-fat frankfurters sausage. | [82] |
| Cold gelation for jelly candy products. | [68] | |
| Using grape oil in alginate/gelatin emulsion instead of pork fat in meat products. | [83] | |
| Delivering cells. | [84] | |
| Capsulation | Betacyanins and polyphenols encapsulation. | [85] |
| Alginate/κ-carrageenan gel beads to release egg yolk immunoglobulin Y (antibody). | [86] | |
| Alginate beads combined with different compounds to deliver tea polyphenols. | [87] | |
| Whey protein and soy protein combination with alginate to encapsulate lycopene. | [88] | |
| Encapsulation of assai pulp oil in chitosan/alginate complexes. | [65] | |
| The natural colorant of anthocyanin and phenolic compounds in alginate beads for beverage application. | [89] | |
| Hydrogel | Linseed oil nano-emulsion to prohibit oxidation. | [90] |
| Selective solid phase extraction of lead ions in food and water samples. | [91] | |
| Imitated fruit pieces for various food products like dairy and bakery products or as topping in food products. | [92] | |
| Colorimetric hydrogel indicator for visual food spoilage monitoring | [93] | |
| extrusion-based 3D food printing | [94] |
2.8.2. Alginate as a Raw Material for Food Packaging: Edible Film and Coating
2.8.3. Alginate as a Chelating Agent
2.8.4. Alginate as an Emulsifier
2.8.5. Emulsified Meat
2.8.6. Encapsulation Properties
Complex Coacervation in Encapsulation of Alginate
Probiotic Encapsulation
Flavor and Aroma Encapsulation
Encapsulation of Oils, Enzymes, and Sensitive Chemicals
2.8.7. Heat Distributor
2.8.8. Fat Absorber
2.8.9. 3D Printing
2.8.10. Antioxidant and Antibacterial Potential
3. Conclusions and Future Trend
Funding
Data Availability Statement
Conflicts of Interest
References
- Bannikova, A.; Evteev, A.; Pankin, K.; Evdokimov, I.; Kasapis, S. Microencapsulation of fish oil with alginate: In-vitro evaluation and controlled release. LWT-Food Sci. Technol. 2018, 90, 310–315. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, R.; Belscak-Cvitanovic, A.; Manojlovic, V.; Komes, D.; Nedovic, V.; Bugarski, B. Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J. Sci. Food Agric. 2012, 92, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation optimization of lemon balm antioxidants in calcium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential food and nutraceutical applications of alginate: A review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef] [PubMed]
- Farahani, Z.K.; Ali, S.M.; Mousavi, E.; Ardebili, S.M.S.; Bakhoda, H. Modification of sodium alginate by octenyl succinic anhydride to fabricate beads for encapsulating jujube extract. Curr. Res. Food Sci. 2022, 5, 157–166. [Google Scholar] [CrossRef]
- BeMiller, J.N. 14—Algins/Alginates. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; Woodhead Publishing: Cambridge, UK; AACC International Press: St. Paul, MN, USA, 2019; pp. 293–301. [Google Scholar] [CrossRef]
- Morrish, C.; Teimouri, S.; Istivan, T.; Kasapis, S. Molecular characterisation of hot moulded alginate gels as a delivery vehicle for the release of entrapped caffeine. Food Hydrocoll. 2020, 109, 106142. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Physicochemical and swelling properties of composite gel microparticles based on alginate and callus cultures pectins with low and high degrees of methylesterification. Int. J. Biol. Macromol. 2020, 164, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Selvan, D.A.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, H.; Kwak, S. Self-Reinforcement of Alginate Hydrogel via Conformational Control. Eur. Polym. J. 2019, 116, 480–487. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2020, 11, 191–199. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Rahimivand, M.; Tafvizi, F.; Noorbazargan, H. Macromolecules Synthesis and characterization of alginate nanocarrier encapsulating Artemisia ciniformis extract and evaluation of the cytotoxicity and apoptosis induction in AGS cell line. Int. J. Biol. Macromol. 2020, 158, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Dragnet, K. Alginates. In Handbook of Hydrocolloids; Springer: Boston, MA, USA, 2000; pp. 379–395. [Google Scholar]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium Concentration Effects on the Mechanical and Biochemical Properties of Chondrocyte-Alginate Constructs. Cell Mol Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjak-Braek, G. Effect of Ca2+, Ba2+, Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Helgerud, T.; Gåserød, O.; Fjæreide, T.; Andersen, P.O.; Larsen, C.K. Alginates. In Food Stabilizers, Thickeners and Gelling Agents; Wiley-Blackwell: Chichester, UK, 2010; pp. 50–72. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation behavior and mechanism of alginate with calcium: Dependence on monovalent counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef] [PubMed]
- Mbizvo, G.K.; Bennett, K.; Simpson, C.R.; Duncan, S.E.; Chin, R.F. Epilepsy-related and other causes of mortality in people with epilepsy: A systematic review of systematic reviews. Epilepsy Res. 2019, 157, 106192. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Doherty, J.V.O.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Chibuike, C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672. [Google Scholar] [CrossRef]
- Whyte, J.N.C.; Southcott, B.A. An extraction procedure for plants: Extracts from the red alga Rhodomela larix. Phytochemistry 1970, 9, 1159–1161. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, H.H.; Yang, Z.N.; Xie, Y. Preparation of low-molecular-weight sodium alginate by ozonation. Carbohydr. Polym. 2021, 251, 117104. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, J.F.; Zhang, X.; Heng, Y.; Chen, W.; Chen, Z.; Wu, X.; Wu, X. Preparation of alginate oligosaccharide and its effects on decay control and quality maintenance of harvested kiwifruit. Carbohydr. Polym. 2020, 242, 116462. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Farmani, B.; Maggi, F.; Morshedloo, M.R. β-Aminobutyric acid treatment confers decay tolerance in strawberry fruit by warranting sufficient cellular energy providing. Sci. Hortic. 2018, 240, 249–257. [Google Scholar] [CrossRef]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Kumar, K.A. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.G. Ultrasound-assisted extraction of sulfated polysaccharide from Nizamuddinia zanardinii: Process optimization, structural characterization, and biological properties. J. Food Process Eng. 2019, 42, e12979. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Yuen, A.K.L.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Liu, R.H. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crops Prod. 2017, 100, 1–11. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sivakumar, M.; Ruckmani, K. Microwave-assisted extraction of polysaccharides from Cyphomandra betacea and its biological activities. Int. J. Biol. Macromol. 2016, 92, 682–693. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2021, 57, 872–885. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Ortega-Barbosa, J.P.; del Pilar Sánchez-Camargo, A.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Pressurized Liquid Extraction of Bioactives. Compr. Foodomics 2021, 754–770. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.D.; Woo, H.C.; Chun, B.S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Franco, C.M.M.; Zhang, W. Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochem. 2016, 51, 1503–1510. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.G.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Simsek, S.; Alam, Z.; Amid, A. Enzyme aided extraction of sulfated polysaccharides from Turbinaria turbinata brown seaweed. Int. Food Res. J. 2017, 24, 1660–1666. [Google Scholar]
- Rostami, Z.; Tabarsa, M.; You, S.G.; Rezaei, M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process Biochem. 2017, 58, 289–297. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural stability and viability of microencapsulated probiotic bacteria: Areview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Sheu, T.Y.; Marshall, R.T. Microentrapment of Lactobacilli in Calcium Alginate Gels. J. Food Sci. 1993, 58, 557–561. [Google Scholar] [CrossRef]
- Bahramparvar, M.; Tehrani, M.M. Application and functions of stabilizers in ice cream. Food Rev. Int. 2011, 27, 389–407. [Google Scholar] [CrossRef]
- Cook, D.J.; Hollowood, T.A.; Linforth, R.S.T.; Taylor, A.J. Correlating instrumental measurements of texture and flavour release with human perception. Int. J. Food Sci. Technol. 2005, 40, 631–641. [Google Scholar] [CrossRef]
- Regand, A.; Goff, H.D. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 2003, 17, 95–102. [Google Scholar] [CrossRef]
- Probst, Y. A review of the nutrient composition of selected Rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Tsai, F.H.; Chiang, P.Y.; Kitamura, Y.; Kokawa, M.; Islam, M.Z. Producing liquid-core hydrogel beads by reverse spherification: Effect of secondary gelation on physical properties and release characteristics. Food Hydrocoll. 2017, 62, 140–148. [Google Scholar] [CrossRef]
- Yuasa, M.; Tagawa, Y.; Tominaga, M. The texture and preference of “mentsuyu (Japanese noodle soup base) caviar” prepared from sodium alginate and calcium lactate. Int. J. Gastron. Food Sci. 2019, 18, 100178. [Google Scholar] [CrossRef]
- Gaikwad, S.A.; Kulthe, A.A.; Suthar, T.R. Characterization of flavoured sweet water balls prepared by basic spherification technique. Int. J. Chem. Stud. 2019, 7, 1714–1718. [Google Scholar]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Applications of alginate as a functional food ingredient. In Biopolymers for Food Design; Academic Press: Cambridge, MA, USA, 2018; pp. 409–429. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Qin, Y. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products. In Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 135–152. [Google Scholar] [CrossRef]
- Kamdem, I.E.; Saidou, C.; Ngassoum, M.B.; Ndjouenkeu, R. Synergistic interactions in dilute aqueous solutions between alginate and tropical vegetal hydrocolloids. Heliyon 2020, 6, e04348. [Google Scholar] [CrossRef] [PubMed]
- de Avelar, M.H.M.; Efraim, P. Alginate/pectin cold-set gelation as a potential sustainable method for jelly candy production. LWT 2020, 123, 109119. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, H.; Rhim, J. Effect of sulfur nanoparticles on properties of alginate-based films for active food packaging applications. Food Hydrocoll. 2020, 110, 106155. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.; de Figueiredo Sousa, H.A.; da Silva, E.R.; Dyszy, F.H.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- De Nobili, M.D.; Soria, M.; Martinefski, M.R.; Tripodi, V.P.; Fissore, E.N.; Rojas, A.M. Stability of L-(+)-ascorbic acid in alginate edible films loaded with citric acid for antioxidant food preservation. J. Food Eng. 2016, 175, 1–7. [Google Scholar] [CrossRef]
- Albert, A.; Salvador, A.; Fiszman, S.M. A film of alginate plus salt as an edible susceptor in microwaveable food. Food Hydrocoll. 2012, 27, 421–426. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-koch, W.; Kumar, M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, E.A.; Dehghannya, J.; Ghanbarzadeh, B. Effect of hydrocolloid type on transfer phenomena during deep-fat frying of coated potato strips: Numerical modeling and experimental analysis. Comput. Electron. Agric. 2018, 154, 382–399. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, T.; Duan, S.; Qin, Z.; Li, C.; Zhang, Z.; Liu, A.; Wu, D.; Chen, H.; Han, G.; et al. Effects of sodium alginate and rice variety on the physicochemical characteristics and 3D printing feasibility of rice paste. LWT 2020, 127, 109360. [Google Scholar] [CrossRef]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, 108184. [Google Scholar] [CrossRef]
- Quesada, H.B.; De Araújo, T.P.; Tait, D.; Angélica, M.; Dornellas, S. Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: A review on composite adsorbents for the removal of water contaminants. Int. J. Biol. Macromol. 2020, 164, 2535–2549. [Google Scholar] [CrossRef] [PubMed]
- Sáez, M.I.; Suárez, M.D.; Martínez, T.F. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT-Food Sci. Technol. 2019, 118, 108767. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Guo, S.; Xie, N.; Yao, L.; Day, S.W.; Howard, S.C.; Graff, J.C.; Gu, T.; Ji, J. Real-time estimation and prediction of mortality caused by COVID-19 with patient information based algorithm. Sci. Total Environ. 2020, 727, 138394. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Kulig, D.; Król-Kilińska, Ż.; Żarowska, B.; Bobak, Ł.; Jarmoluk, A. Biophysico-chemical properties of alginate oligomers obtained by acid and oxidation depolymerization. Polymers 2021, 13, 2258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Stenhoff, A.; Steadman, L.; Nevitt, S.; Benson, L.; White, R.G. Acceptance and commitment therapy and subjective wellbeing: A systematic review and meta-analyses of randomised controlled trials in adults. J. Context. Behav. Sci. 2020, 18, 256–272. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdallah, N.A.; El-shafei, K.; Taw, N.F.; El-sayed, H.S. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 2020, 6, 105605. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Calvo, T.R.A.; Perullini, M.; Santagapita, P.R. Encapsulation of betacyanins and polyphenols extracted from leaves and stems of beetroot in Ca (II)-alginate beads: A structural study. J. Food Eng. 2018, 235, 32–40. [Google Scholar] [CrossRef]
- Gu, L.; McClements, D.J.; Li, J.; Su, Y.; Yang, Y.; Li, J. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Li, Q.; Duan, M.; Hou, D.; Chen, X.; Shi, J.; Zhou, W. Fabrication and Characterization of Ca(II)-Alginate-Based Beads Combined with Different Polysaccharides as Vehicles for Delivery, Release and Storage of Tea Polyphenols. Food Hydrocoll. 2021, 112, 106274. [Google Scholar] [CrossRef]
- Shu, J.; McClements, D.J.; Luo, S.; Ye, J.; Liu, C. Effect of internal and external gelation on the physical properties, water distribution, and lycopene encapsulation properties of alginate-based emulsion gels. Food Hydrocoll. 2023, 139, 108499. [Google Scholar] [CrossRef]
- Al-Harbi, S.A.; Almulaiky, Y.Q. Purification and biochemical characterization of Arabian balsam α-amylase and enhancing the retention and reusability via encapsulation onto calcium alginate/Fe2O3 nanocomposite beads. Int. J. Biol. Macromol. 2020, 160, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Nair, S.; Pandey, K.K. UV resistant wood coating based on zinc oxide and cerium oxide dispersed linseed oil nano-emulsion. Mater. Today Commun. 2022, 30, 103177. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhang, L.; Liu, M.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. A hybrid monolithic column based on layered double hydroxide-alginate hydrogel for selective solid phase extraction of lead ions in food and water samples. Food Chem. 2018, 257, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, B.; Sharma, S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharma Innov. J. 2018, 7, 142–148. [Google Scholar]
- Tang, Q.; Hu, J.; Li, S.; Lin, S.; Tu, Y.; Gui, X. Colorimetric hydrogel indicators based on polyvinyl alcohol/sodium alginate for visual food spoilage monitoring. Int. J. Food Sci. Technol. 2022, 57, 6867–6880. [Google Scholar] [CrossRef]
- Rysenaer, V.B.J.; Ahmadzadeh, S.; Van Bockstaele, F.; Ubeyitogullari, A. An extrusion-based 3D food printing approach for generating alginate-pectin particles. Curr. Res. Food Sci. 2023, 6, 100404. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Vijaya, A.; Mariadoss, A.; Xiaowen, H.; Wang, M. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium alginate-based edible coating containing nanoemulsion of Citrus sinensis essential oil eradicates planktonic and sessile cells of food-borne pathogens and increased quality attributes of tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef]
- Marcos, B.; Gou, P.; Arnau, J.; Guàrdia, M.D.; Comaposada, J. Co-extruded alginate as an alternative to collagen casings in the production of dry-fermented sausages: Impact of coating composition. Meat Sci. 2020, 169, 108184. [Google Scholar] [CrossRef]
- Lim, H.; Ho, K.; Kaur, C.; Singh, S.; Ooi, C.; Tey, B. Pickering emulsion hydrogel as a promising food delivery system: Synergistic effects of chitosan Pickering emulsifier and alginate matrix on hydrogel stability and emulsion delivery. Food Hydrocoll. 2020, 103, 105659. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Jung, S.; Kim, Y.B.; Choi, Y.S. Effects of replacing pork fat with grape seed oil and gelatine/alginate for meat emulsions. Meat Sci. 2020, 163, 108079. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Bastos, H.; Vicente, J.; Corr, C.H.; De Carvalho, M.G.; Garcia-rojas, E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 224, 116447. [Google Scholar] [CrossRef] [PubMed]
- Rezaul, M.; Shishir, I.; Ferdowsi, R.; Rahman, R.T.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Navarro, R.; Arancibia, C.; Herrera, M.L.; Matiacevich, S. Effect of type of encapsulating agent on physical properties of edible films based on alginate and thyme oil. Food Bioprod. Process. 2015, 97, 63–75. [Google Scholar] [CrossRef]
- Atencio, S.; Maestro, A.; Santamaría, E.; Gutiérrez, J.M.; González, C. Encapsulation of ginger oil in alginate-based shell materials. Food Biosci. 2020, 37, 100714. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Pereira, B.C.; Lopes, G.K.; Andrade, C.T. Encapsulation and antioxidant activity of assai pulp oil (Euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Paris, M.J.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Modelling release mechanisms of cinnamon (Cinnamomum zeylanicum) essential oil encapsulated in alginate beads during vapor-phase application. J. Food Eng. 2020, 282, 110024. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, L.; Wu, S.; Dong, L.; Liu, Q.; Wang, Q. Synthesis and characterization of compartmented Ca-alginate/silica self-healing fibers containing bituminous rejuvenator. Constr. Build. Mater. 2018, 190, 623–631. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of immobilized enzymes in food industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Razmjou, A.; Yek, S.M.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef]
- Weng, Y.; Ranaweera, S.; Zou, D.; Cameron, A.; Chen, X.; Song, H.; Zhao, C.X. Alginate particles for enzyme immobilization using spray drying. J. Agric. Food Chem. 2022, 70, 7139–7147. [Google Scholar] [CrossRef]
- Abang, S.; Chan, E.-S.; Poncelet, D. Effects of process variables on the encapsulation of oil in ca-alginate capsules using an inverse gelation technique. J. Microencapsul. 2012, 29, 417–428. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pournaki, S.K.; Aleman, R.S.; Hasani-Azhdari, M.; Marcia, J.; Yadav, A.; Moncada, M. Current Review: Alginate in the Food Applications. J 2024, 7, 281-301. https://doi.org/10.3390/j7030016
Pournaki SK, Aleman RS, Hasani-Azhdari M, Marcia J, Yadav A, Moncada M. Current Review: Alginate in the Food Applications. J. 2024; 7(3):281-301. https://doi.org/10.3390/j7030016
Chicago/Turabian StylePournaki, Shirin Kazemzadeh, Ricardo Santos Aleman, Mehrdad Hasani-Azhdari, Jhunior Marcia, Ajitesh Yadav, and Marvin Moncada. 2024. "Current Review: Alginate in the Food Applications" J 7, no. 3: 281-301. https://doi.org/10.3390/j7030016
APA StylePournaki, S. K., Aleman, R. S., Hasani-Azhdari, M., Marcia, J., Yadav, A., & Moncada, M. (2024). Current Review: Alginate in the Food Applications. J, 7(3), 281-301. https://doi.org/10.3390/j7030016







