Physicochemical and Bacteriological Quality of Public Swimming Pools in the Tamale Metropolis, Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. In Situ Measurement
2.4. Laboratory Test (Membrane Filtration Technique)
2.5. Laboratory Test (Pour Plate Technique)
2.6. Data Analysis
3. Results
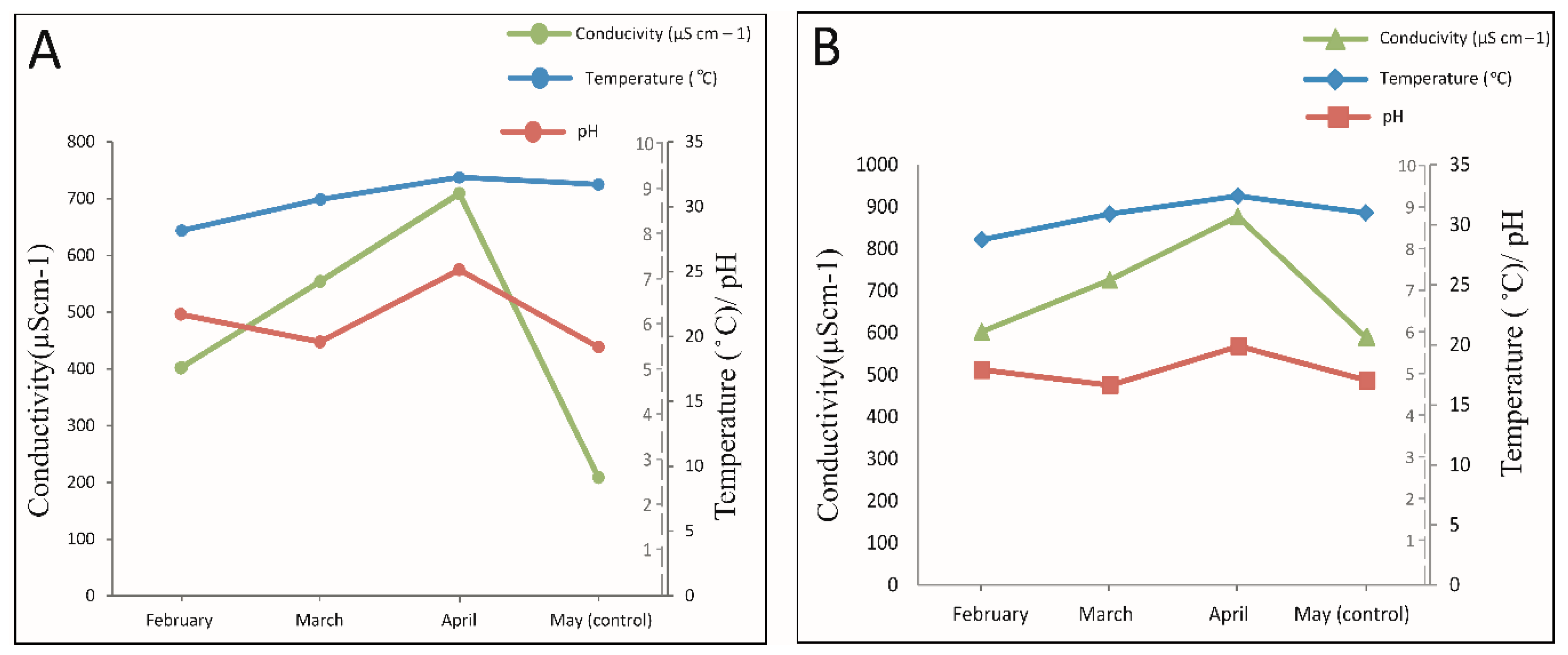
3.1. Physicochemical Parameters
3.2. Relationship between Temperature (°C), pH, and Conductivity (µS cm−1)
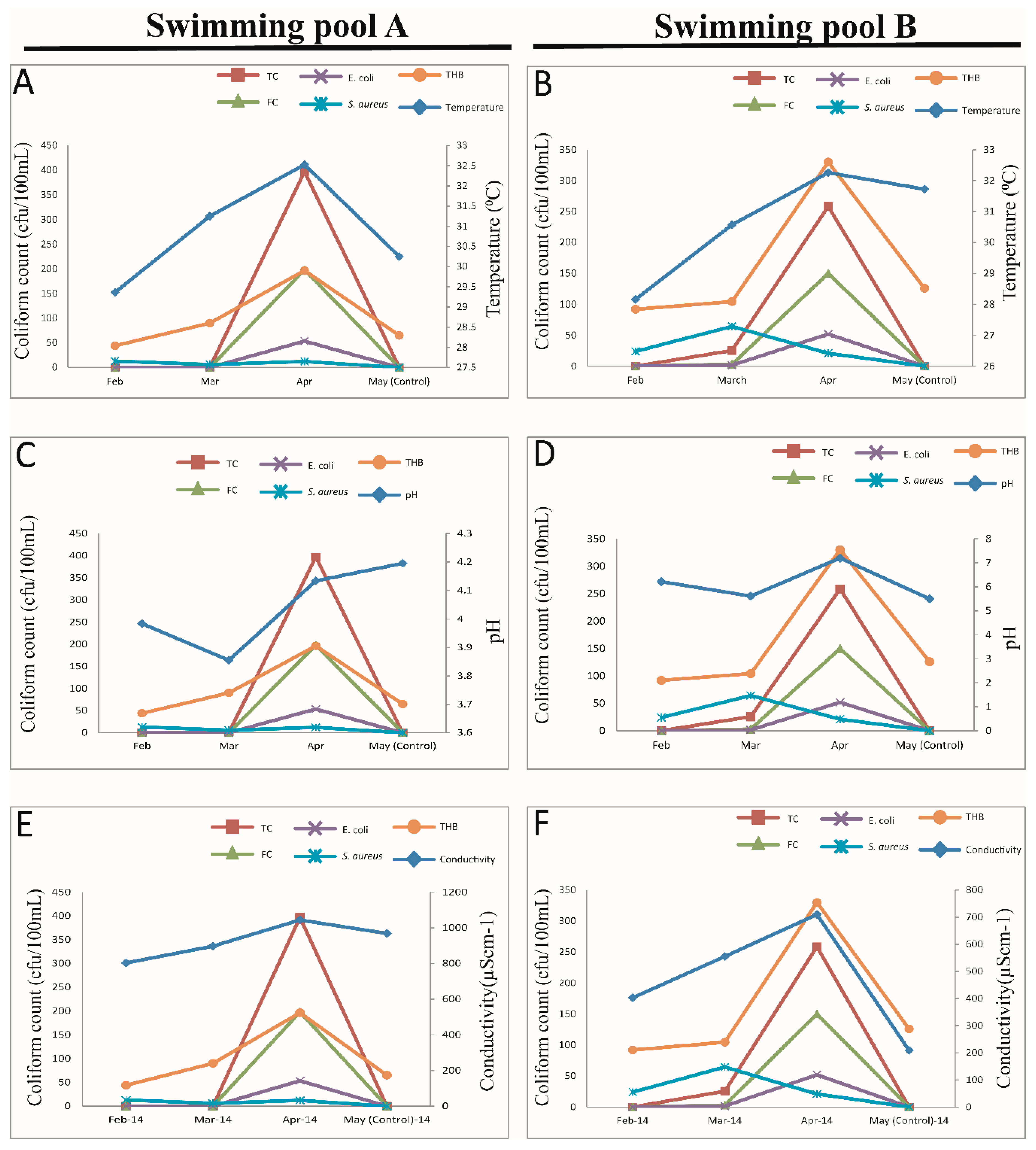
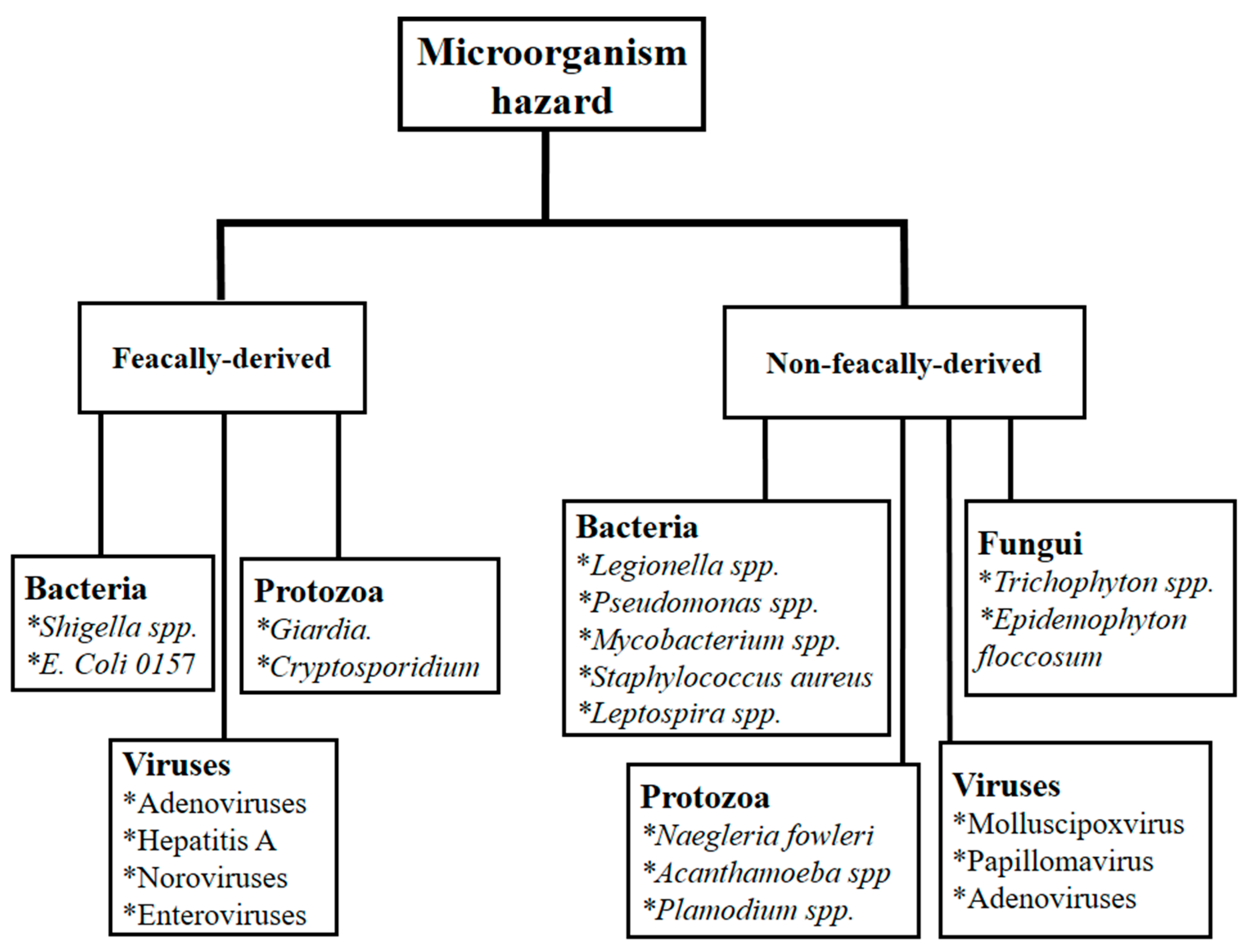
3.3. Isolated Indicator of Microorganism in the Swimming Pool Waters
3.4. Levels of Indicator Microorganism in the Swimming Pool Waters
3.5. Relationship between Physicochemical Parameters and Bacteria Counts
4. Discussion
4.1. Physicochemical
4.2. Microorganismsms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabelli, V.J. Swimming-Associated Illness and Recreational Water Quality Criteria. Water Sci. Technol. 1989, 21, 13–21. [Google Scholar] [CrossRef]
- Alico, R.K.; Dragonjac, M.F. Evaluation of culture media for recovery of Staphylococcus aureus from swimming pools. Appl. Environ. Microbiol. 1986, 51, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.J.; Horsfield, J.; Black, M.A.; Rutherford, K.M.; Fisher, A.; Gemmell, N.J. Histological and transcriptomic effects of 17α-methyltestosterone on zebrafish gonad development. BMC Genom. 2017, 18, 557. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Safe Recreational Water Environments: Swimming Pools and Similar Environments; World Health Organization: Geneva, Switzerland, 2006; Volume 2, p. 146. [Google Scholar]
- Itah, A.Y.; Ekpombok, M.-U.M. Pollution status of swimming pools in south-south zone of south-eastern Nigeria using microbiological and physicochemical indices. Southeast Asian J. Trop. Med. Public Health 2004, 35, 488–493. [Google Scholar]
- Mwajuma, J.J. Physicochemical and Bacteriological Quality of Water, and Antimicrobial Susceptibility of Pathogenic Isolates from Selected Water Sources in Samburu South; School of Pure and Applied Sciences, Kenyatta University: Nairobi City, Kenya, 2010; Unpublished work. [Google Scholar]
- Masoud, G.; Abbass, A.; Abaza, A.; Hazzah, W. Bacteriological quality of some swimming pools in Alexandria with special reference to Staphylococcus aureus. Environ. Monit. Assess. 2016, 188, 412. [Google Scholar] [CrossRef]
- South Australia Health Commission. Standard for the Operation of Swimming Pools and Spa Pools: Supplement C; South Australia Health Commission: Adelaide, South Australia, 2013.
- Health Protection NSW. Public Swimming Pool and Spa Pool Advisory Document—Environmental Health; NSW Health: Sydney, Australia, 2013.
- Amala, S.E.; Aleru, C.P. Bacteriological Quality of Swimming Pools Water in Port Harcourt Metropolis. Nat. Sci. 2016, 8, 79–84. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- WHO. Faecal Pollution and Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Sato, M.; Sanchez, P.; Alves, M.; Stoppe, N.; Martins, M. Evaluation of culture media for Candida albicans and Staphylococcus aureus recovery in swimming pools. Water Res. 1995, 29, 2412–2416. [Google Scholar] [CrossRef]
- Sabath, N.; Itescu, Y.; Feldman, A.; Meiri, S.; Mayrose, I.; Valenzuela, N. Sex determination, longevity, and the birth and death of reptilian species. Ecol. Evol. 2016, 6, 5207–5220. [Google Scholar] [CrossRef]
- Brewster, D.H.; Brown, M.I.; Robertson, D.; Houghton, G.L.; Bimson, J.; Sharp, J.C.M. An outbreak of Escherichia coliO157 associated with a children’s paddling pool. Epidemiol. Infect. 1994, 112, 441–447. [Google Scholar] [CrossRef]
- Hildebrand, J.M.; Maguire, H.C.; Holliman, R.E.; Kangesu, E. An outbreak of Escherichia coli O157 infection linked to paddling pools. Commun. Dis. Rep. CDR Rev. 1996, 6, 33–36. [Google Scholar]
- Friedman, M.S.; Roels, T.; Koehler, J.E.; Feldman, L.; Bibb, W.F.; Blake, P. Escherichia coli O157:H7 Outbreak Associated with an Improperly Chlorinated Swimming Pool. Clin. Infect. Dis. 1999, 29, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yang, J.-S.; Han, M.; Choi, J.-Y. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci. Total Environ. 2010, 408, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Robinton, E.D.; Mood, E.W. A quantitative and qualitative appraisal of microbial pollution of water by swimmers: A preliminary report. Epidemiol. Infect. 1966, 64, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Indabawa, I.I.; Ali, S.; Mukhtar, M.D. Assessment of Microbiological and Physico-Chemical Quality of Some Swimming Pools within Kano Metropolis, Kano Nigeria. In Proceedings of the 3rd International Conference on Biological, Chemical and Environmental Sciences, Kuala Lumpur, Malaysia, 21–22 September 2015. [Google Scholar]
- Shittu, O.; Olaitan, J.; Amusa, T. Physico-chemical and bacteriological analyses of water used for drinking and swimming purposes in abeokuta, nigeria. Afr. J. Biomed. Res. 2010, 11, 285–290. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection byproducts in swimming pool: Occurrences, implications and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef] [PubMed]
- Chango, G.; Palacio, E.; Cerdà, V. Potentiometric chip-based multipumping flow system for the simultaneous determination of fluoride, chloride, pH, and redox potential in water samples. Talanta 2018, 186, 554–560. [Google Scholar] [CrossRef]
- Sila, O.N.; Nzung’A, S.O. Physico-chemical and bacteriological quality of water sources in rural settings, a case study of Kenya, Africa. Sci. Afr. 2019, 2, 2. [Google Scholar] [CrossRef]
- U.S EPA. Safe Drinking Water Act (SDWA); U.S EPA: Washington, DC, USA, 2003.
- Kay, D.; Fawell, J. Standards for Recreational Water Quality; Foundation for Water Research: Buckinghamshire, UK, 2007. [Google Scholar]
- Blostein, J. Shigellosis from swimming in a park pond in Michigan. Public Health Rep. 1991, 106, 317–322. [Google Scholar]
- Wade, T.J.; Pai, N.; Eisenberg, J.N.S.; Colford, J.M. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ. Health Perspect. 2003, 111, 1102–1109. [Google Scholar] [CrossRef]
- Sueiro, R.A.; Araujo, M.; Santos, C.J.; Gómes, M.J.; Garrido, M.J. Evaluation of Coli-ID and MUG Plus media for recovering Escherichia coli and other coliform bacteria from groundwater samples. Water Sci. Technol. 2001, 43, 213–216. [Google Scholar] [CrossRef]
- Favero, M.S.; Drake, C.H.; Randall, G.B. Use of Staphylococci as Indicators of Swimming Pool Pollution. Public Health Rep. 1964, 79, 61. [Google Scholar] [CrossRef]
- Lechevallier, M.W.; Seidler, R.J. Staphylococcus aureus in rural drinking water. Appl. Environ. Microbiol. 1980, 39, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Mavridou, A.; Pappa, O.; Papatzitze, O.; Blougoura, A.; Drossos, P. An overview of pool and spa regulations in Mediterranean countries with a focus on the tourist industry. J. Water Health 2014, 12, 359–371. [Google Scholar] [CrossRef] [PubMed]
| Month | Swimming Pool ‘A’ | Swimming Pool ‘B’ |
|---|---|---|
| Feb – 14 | 29.4 ± 2.4 | 28.2 ± 1.7 |
| Mar – 14 | 31.3 ± 1.4 | 30.6 ± 1.0 |
| Apr – 14 | 32.5 ± 0.0 | 32.3 ± 0.2 |
| May (Control) – 14 | 30.3 ± 1.0 | 31.7 ± 0.5 |
| Mean | 30.8 ± 0.9 | 30.7 ±0.8 |
| p-value (t-test) | 0.8889 | |
| Month | Swimming Pool ‘A’ | Swimming Pool ‘B’ |
|---|---|---|
| Feb. | 3.98 ± 0.51 | 6.21 ± 0.36 |
| Mar. | 3.86 ± 0.52 | 5.60 ± 1.28 |
| Apr. | 4.13 ± 0.02 | 7.20 ± 0.62 |
| May (Control) | 4.19 ± 0.28 | 5.49 ± 0.01 |
| Mean | 4.04 ± 0.26 | 6.13 ± 0.57 |
| p-value (t-test) | 0.0019 | |
| Month | Swimming Pool ‘A’ | Swimming Pool ‘B’ |
|---|---|---|
| Feb. – 14 | 803 ± 151 | 403 ± 32 |
| Mar. – 14 | 897 ± 38 | 555 ± 71 |
| Apr. – 14 | 1044 ± 97 | 710 ± 61 |
| May (Control) – 14 | 968 ± 57 | 209 ± 20 |
| Mean | 928 ± 86 | 469 ± 41 |
| p-value (t-test) | 0.0083 | |
| Source | Total Coliform (TC) | Faecal Coliform (FC) | Escherichia coli | Staphylococcus aureus | Total Heterotrophic Bacteria (THB) (cfu/mL) |
|---|---|---|---|---|---|
| Pool ‘A’ | 99 ± 199 | 50 ± 99 | 14 ± 27 | 8 ± 6 | 99 ± 68 |
| Pool ‘B’ | 71 ± 126 | 7 ± 12 | 13 ± 26 | 27 ± 27 | 163 ± 112 |
| TC | FC | E. coli | S. aureus | THB (cfu/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | No. of Samples | ‘A’ | ‘B’ | ‘A’ | ‘B’ | ‘A’ | ‘B’ | ‘A’ | ‘B’ | ‘A’ | ‘B’ |
| Feb. | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 24 | 44 | 92 |
| Mar. | 2 | 0 | 26 | 0 | 3 | 0 | 2 | 6 | 65 | 90 | 105 |
| Apr. | 2 | 397 | 259 | 198 | 26 | 54 | 52 | 12 | 21 | 197 | 330 |
| May (Control) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 65 | 126 |
| p-Value | 0.8179 | 0.4282 | 1.0000 | 0.2051 | 0.3645 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapha, U.F.; Abobi, S.M.; Quarcoo, G. Physicochemical and Bacteriological Quality of Public Swimming Pools in the Tamale Metropolis, Ghana. J 2020, 3, 236-249. https://doi.org/10.3390/j3020018
Mustapha UF, Abobi SM, Quarcoo G. Physicochemical and Bacteriological Quality of Public Swimming Pools in the Tamale Metropolis, Ghana. J. 2020; 3(2):236-249. https://doi.org/10.3390/j3020018
Chicago/Turabian StyleMustapha, Umar Farouk, Seth Mensah Abobi, and Gerard Quarcoo. 2020. "Physicochemical and Bacteriological Quality of Public Swimming Pools in the Tamale Metropolis, Ghana" J 3, no. 2: 236-249. https://doi.org/10.3390/j3020018
APA StyleMustapha, U. F., Abobi, S. M., & Quarcoo, G. (2020). Physicochemical and Bacteriological Quality of Public Swimming Pools in the Tamale Metropolis, Ghana. J, 3(2), 236-249. https://doi.org/10.3390/j3020018







