Use of Biowaste for Sodium Removal in Mediterranean Irrigation Water: A Sustainable Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biowaste
2.2. Irrigation Water Samples
2.3. Factor Design: Central Composite Rotatable Design (CCRD)
2.4. Sorption Kinetics
2.5. Sorption Isotherms
2.6. Scanning Electron Microscopy and EDX Analysis
3. Results
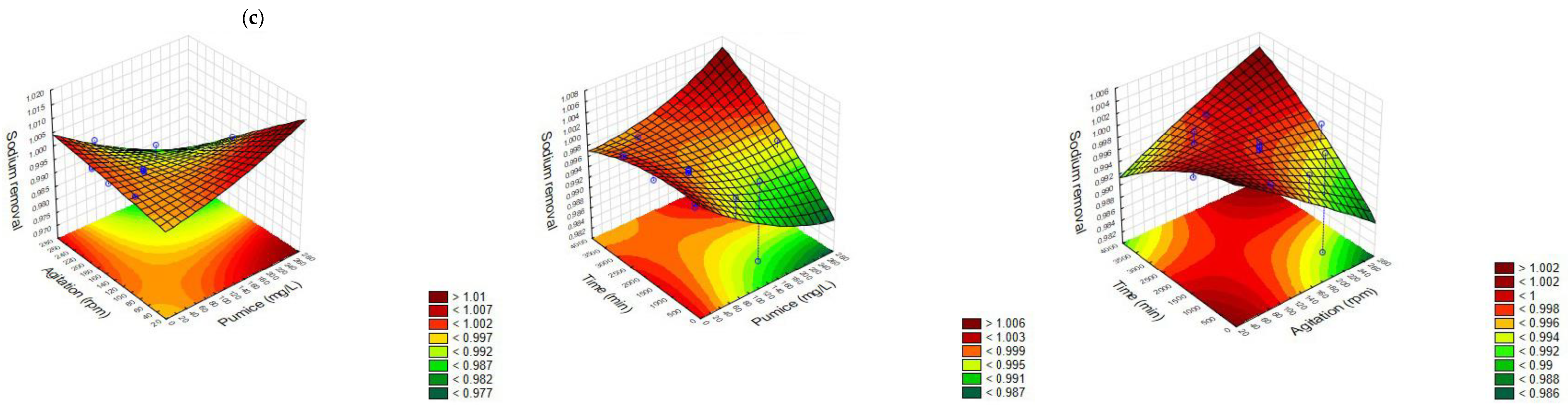
3.1. Central Composite Rotatable Design (CCRD)
3.1.1. Response Surface Analysis
3.1.2. Statistical Validation of the Models
3.2. Sorption Kinetic Study
3.3. Sorption Isotherm
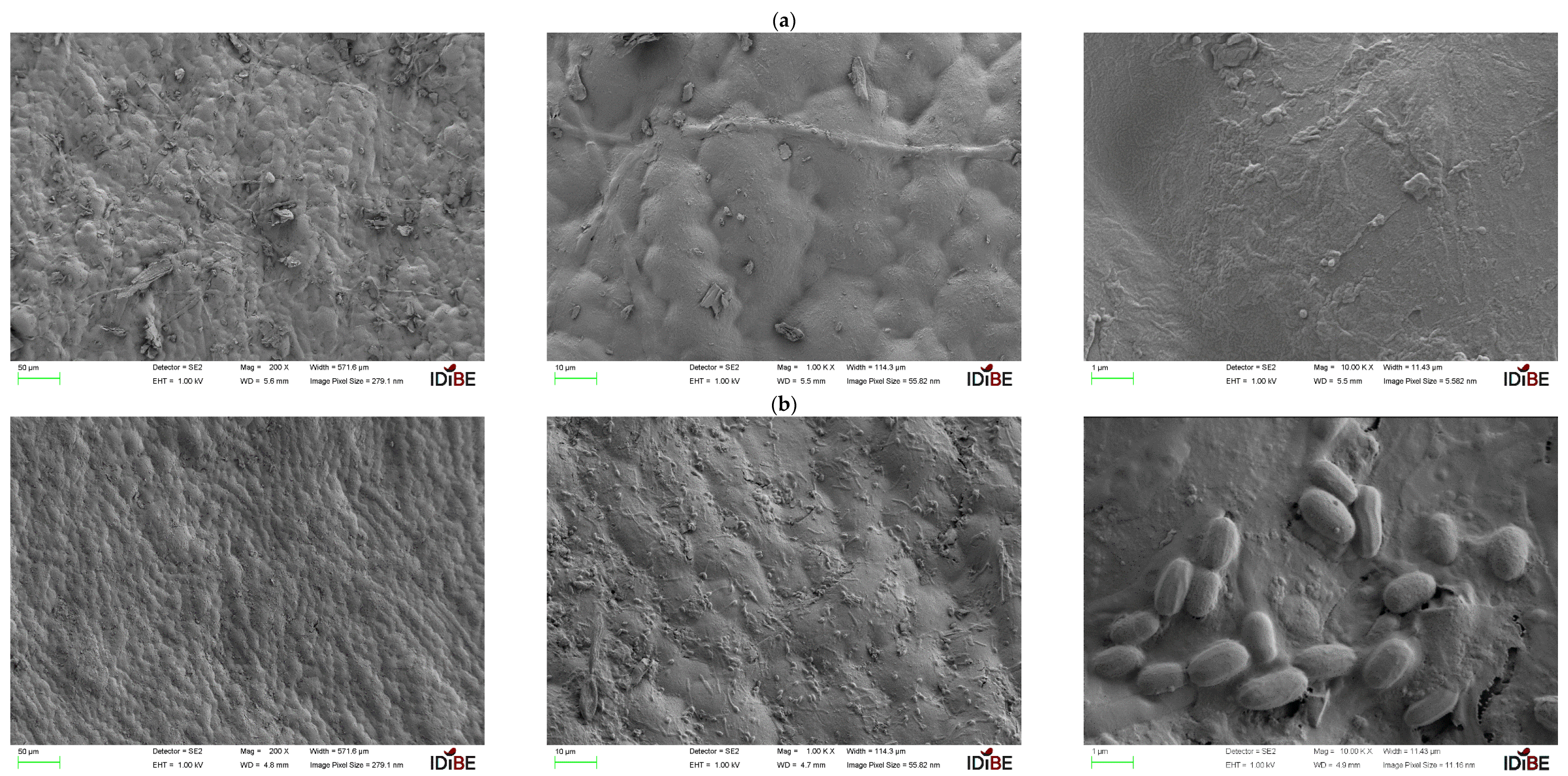
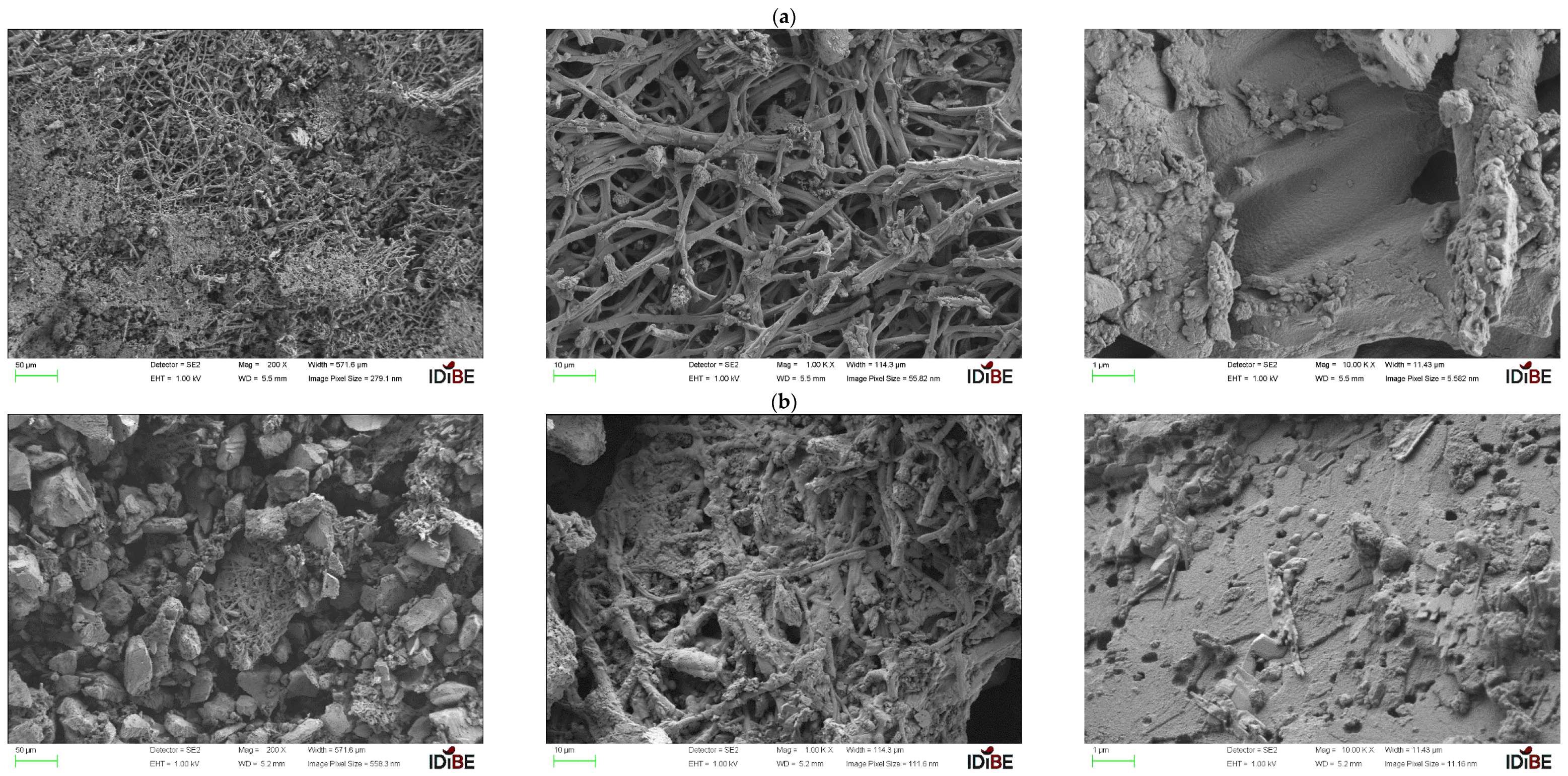
3.4. Scanning Electron Microscopy (SEM) and EDX Analysis
3.4.1. Almond Shell
3.4.2. Eggshells
3.4.3. Pumice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noto, L.V.; Cipolla, G.; Pumo, D.; Francipane, A. Climate Change in the Mediterranean Basin (Part II): A Review of Challenges and Uncertainties in Climate Change Modeling and Impact Analyses. Water Resour. Manag. 2023, 37, 2307–2323. [Google Scholar] [CrossRef]
- Labrousse, C.; Ludwig, W.; Pinel, S.; Sadaoui, M.; Toreti, A.; Lacquement, G. Declining Water Resources in Response to Global Warming and Changes in Atmospheric Circulation Patterns over Southern Mediterranean France. Hydrol. Earth Syst. Sci. 2022, 26, 6055–6071. [Google Scholar] [CrossRef]
- Prada, J.; Dinis, L.-T.; Soriato, E.; Vandelle, E.; Soletkin, O.; Uysal, Ş.; Dihazi, A.; Santos, C.; Santos, J.A. Climate Change Impact on Mediterranean Viticultural Regions and Site-Specific Climate Risk-Reduction Strategies. Mitig. Adapt. Strat. Glob. Chang. 2024, 29, 52. [Google Scholar] [CrossRef]
- Eekhout, J.; de Vente, J. The Impacts of Future Climate Change on Water Security in the Mediterranean Basin. In Proceedings of the Copernicus Meetings, Vienna, Austria, 24–28 April 2023. [Google Scholar]
- Hrour, Y.; Thomas, Z.; Fovet, O.; Sebari, K.; Rousseau-Gueutin, P. Changes in Precipitation and Discharge in a Mediterranean Catchment as a Response to Climate Change and Human Activities. J. Water Clim. Chang. 2022, 13, 3253–3273. [Google Scholar] [CrossRef]
- Villani, L.; Castelli, G.; Yimer, E.A.; Chawanda, C.J.; Nkwasa, A.; Van Schaeybroeck, B.; Penna, D.; van Griensven, A.; Bresci, E. Impacts of Climate Change and Vegetation Response on Future Aridity in a Mediterranean Catchment. Agric. Water Manag. 2024, 299, 108878. [Google Scholar] [CrossRef]
- Tas, I.; Yildirim, Y.E.; Gokalp, Z. The effect of excessive sodium-containing irrigation waters on soil infiltration rate. CTNS 2022, 11, 19–28. [Google Scholar] [CrossRef]
- Klopp, H.W.; Arriaga, F.; Bleam, W. Influence of Exchangeable Sodium and Clay Mineralogy on Soil Water Retention and Hydraulic Conductivity. J. Soil Water Conserv. 2020, 75, 755–764. [Google Scholar] [CrossRef]
- Sharma, A.; Devi, Y.B.; Meetei, T.T. A Review on Impact of Salt Stress in Soil Health and Its Suitable Control Measure. ECJ 2022, 23, 412–424. [Google Scholar] [CrossRef]
- Farahani, E.; Emami, H.; Keller, T. Impact of Monovalent Cations on Soil Structure. Part II. Results of Two Swiss Soils. Int. Agrophysics 2018, 32, 69–80. [Google Scholar] [CrossRef]
- Islam, M.S. Irrigation Water Quality. In Hydrogeochemical Evaluation and Groundwater Quality; Islam, M.S., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 223–280. ISBN 978-3-031-44304-6. [Google Scholar]
- Rodríguez-Espinosa, T.; Pérez Gimeno, A.; Almendro Candel, M.B.; Gómez Lucas, I.; Navarro-Pedreño, J. Low-Quality Irrigation Water Treated Using Waste Biofilters. Water 2023, 15, 2464. [Google Scholar] [CrossRef]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation Water Quality—A Contemporary Perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Mabee, W.E. Chapter 4—Conceptualizing the Circular Bioeconomy. In Circular Economy and Sustainability; Stefanakis, A., Nikolaou, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 53–69. ISBN 978-0-12-819817-9. [Google Scholar]
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S. Biomaterials: A Sustainable Solution for a Circular Economy. Eng. Proc. 2023, 59, 133. [Google Scholar] [CrossRef]
- Vural Gursel, I.; Elbersen, B.; Meesters, K.P.H.; van Leeuwen, M. Defining Circular Economy Principles for Biobased Products. Sustainability 2022, 14, 12780. [Google Scholar] [CrossRef]
- Rahal, Z.; Khechekhouche, A.; Barkat, A.; Sergeevna, S.A.; Hamza, C. Adsorption of Sodium in an Aqueous Solution in Activated Date Pits. Indones. J. Sci. Technol. 2023, 8, 387–412. [Google Scholar] [CrossRef]
- Lima, R.R.C.; de Lima, P.D.S.; Greati, V.R.; de Sousa, P.B.F.; Medeiros, G.V.S. Sodium-Modified Vermiculite for Calcium Ion Removal from Aqueous Solution. Ind. Eng. Chem. Res. 2019, 58, 9380–9389. [Google Scholar] [CrossRef]
- Wan, J.; Wang, H.-T.; Li, X.; Zhang, C.-X.; Wu, X.-S.; Sun, B. Removal of Several Types of Sodium and Potassium Salts from Aqueous Solutions Based on Their Natural Fixation. Desalination Water Treat. 2019, 169, 133–151. [Google Scholar] [CrossRef]
- Schorr, M. Desalination: Trends and Technologies; BoD—Books on Demand. IntechOpen: Rijeka, Croatia, 2011; Available online: https://www.intechopen.com/books/51 (accessed on 28 October 2024)ISBN 978-953-307-311-8.
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse Osmosis Pretreatment Technologies and Future Trends: A Comprehensive Review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Djellabi, R.; Vaccari, M.; Prasad, S.; Aminabhavi, T.M.; Rtimi, S. Emerging Technologies and Sustainable Strategies for Municipal Solid Waste Valorization: Challenges of Circular Economy Implementation. J. Clean. Prod. 2023, 423, 138708. [Google Scholar] [CrossRef]
- El Mashad, H.M.; Edalati, A.; Zhang, R.; Jenkins, B.M. Production and Characterization of Biochar from Almond Shells. Clean Technol. 2022, 4, 854–864. [Google Scholar] [CrossRef]
- Andrews, E.M.; Tabassum, M.; Galatis, E.G.; Yao, E.H.; Gaudin, A.C.M.; Lazcano, C.; Brown, P.H.; Khalsa, S.D.S. Almond Hull and Shell Organic Matter Amendments Increase Microbial Biomass and Multifunctionality in Orchard Soil and the Undisturbed Organic Layer. Appl. Soil Ecol. 2024, 197, 105321. [Google Scholar] [CrossRef]
- Maaloul, N.; Oulego, P.; Rendueles, M.; Ghorbal, A.; Díaz, M. Compuesto de Biopolímero a Partir de Nanocristales de Celulosa de Cáscara de Almendra (Prunus dulcis) Como Adsorbentes Eficaces Para Iones Cu2+ de Soluciones Acuosas. J. Environ. Chem. Eng. 2021, 9, 105139. [Google Scholar] [CrossRef]
- Ajala, E.O.; Eletta, O.A.A.; Ajala, M.A.; Oyeniyi, S.K. Characterization and Evaluation of Chicken Eggshell for Use as a Bio-Resource. Arid. Zone J. Eng. Technol. Environ. 2018, 14, 26–40. [Google Scholar]
- Abdelgalil, S.A.; Kaddah, M.M.Y.; Abo-Zaid, G.A. Eggshell Waste Bioprocessing for Sustainable Acid Phosphatase Production and Minimizing Environmental Hazards. J. Biol. Eng. 2024, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Q.; Wang, T.; Jin, Z.; Luo, T.; Huang, J.; Xu, G.; Zhan, Y.; Wang, H. Pumice as Biological Carriers Improve Impact Load Resistance of UASB Reactors During the Treatment of Raw Incineration Leachates. Pol. J. Environ. Stud. 2022, 31, 1975–1983. [Google Scholar] [CrossRef]
- Karimaian, K.A.; Amrane, A.; Kazemian, H.; Panahi, R.; Zarrabi, M. Retention of Phosphorous Ions on Natural and Engineered Waste Pumice: Characterization, Equilibrium, Competing Ions, Regeneration, Kinetic, Equilibrium and Thermodynamic Study. Appl. Surf. Sci. 2013, 284, 419–431. [Google Scholar] [CrossRef]
- Ismail, A.; El-Shafey, O.; Amr, M.; El-Maghraby, M. Pumice Characteristics and Their Utilization on the Synthesis of Mesoporous Minerals and on the Removal of Heavy Metals. Int. Sch. Res. Not. 2014, 1, 259379. [Google Scholar] [CrossRef]
- Helard, D.; Indah, S.; Sari, C.M.; Mariesta, H. The Adsorption and Regeneration of Natural Pumice as Low-Cost Adsorbent for Nitrate Removal From Water. J. Geosci. Eng. Environ. Technol. 2018, 3, 86–93. [Google Scholar] [CrossRef]
- Gunst, R.F.; Mason, R.L. Fractional Factorial Design. WIREs Comput. Stat. 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Pashley, N.E.; Bind, M.-A.C. Causal Inference for Multiple Treatments Using Fractional Factorial Designs. Can. J. Stat. 2023, 51, 444–468. [Google Scholar] [CrossRef]
- Oyejola, B.; Nwanya, J. Selecting the Right Central Composite Design. Int. J. Stat. Appl. 2015, 5, 21–30. [Google Scholar]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Sadeghalvad, B.; Khorshidi, N.; Azadmehr, A.; Sillanpää, M. Sorption, Mechanism, and Behavior of Sulfate on Various Adsorbents: A Critical Review. Chemosphere 2021, 263, 128064. [Google Scholar] [CrossRef]
- Alaei Shahmirzadi, M.A.; Hosseini, S.S.; Luo, J.; Ortiz, I. Significance, Evolution and Recent Advances in Adsorption Technology, Materials and Processes for Desalination, Water Softening and Salt Removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, J. A Critical Review of Various Adsorbents for Selective Removal of Nitrate from Water: Structure, Performance and Mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-918-5. [Google Scholar]
- Aggelopoulos, C.A.; Moschopoulou, E.; Klepetsanis, P.G.; Tsakiroglou, C.D. Valorization of Fruit Wastes (Pistachio Shells) as Adsorbent for the Removal of Zn from Aqueous Solutions under Adverse Acidic Conditions. Desalination Water Treat. 2017, 74, 174–183. [Google Scholar] [CrossRef]
- Ghorbani, B.; Pourvaezi, R.; Mirzaei, S.J. The Impact of Rice Husk, Activated Carbon, Almond Shell, and Sand Filters on Some Physical and Chemical Properties of Aqueous Salt Solution. Desalination Water Treat. 2016, 57, 10878–10885. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Milea, D.; Muratore, N.; Pettignano, A.; Sammartano, S. A Critical Approach to the Toxic Metal Ion Removal by Hazelnut and Almond Shells. Environ. Sci. Pollut. Res. 2018, 25, 4238–4253. [Google Scholar] [CrossRef]
- Ben Arfi, R.; Ghorbal, A. Advancements in Utilizing Almond-Shell-Based Materials for the Adsorptive Removal of Hazardous Pollutants from Water: A 10-Year Review. Euro-Mediterr. J. Environ. Integr. 2024, 9, 545–568. [Google Scholar] [CrossRef]
- Talukder, P.; Sultana, R.; Naim, M.R.; Turzo, P.I.; Naher, U.H.B. Optimization of Batch Process Parameters for Chromium (VI) Removal from Synthetic Wastewater Using Eggshell–Clay Composite. Discov. Appl. Sci. 2024, 6, 291. [Google Scholar] [CrossRef]
- Mensah, K.; Mahmoud, H.; Fujii, M.; Samy, M.; Shokry, H. Dye Removal Using Novel Adsorbents Synthesized from Plastic Waste and Eggshell: Mechanism, Isotherms, Kinetics, Thermodynamics, Regeneration, and Water Matrices. Biomass Convers. Bioref. 2024, 14, 12945–12960. [Google Scholar] [CrossRef]
- Babalola, B.M.; Wilson, L.D. Valorization of Eggshell as Renewable Materials for Sustainable Biocomposite Adsorbents—An Overview. J. Compos. Sci. 2024, 8, 414. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Ogundola, D.O.; Popoola, L.T.; Bernard, E.; Udeagbara, S.G.; Ogunyemi, A.T.; Olateju, I.I.; Zainul, R. Potassium Permanganate–Modified Eggshell Biosorbent for the Removal of Diclofenac from Liquid Environment: Adsorption Performance, Isotherm, Kinetic, and Thermodynamic Analyses. Environ. Monit. Assess. 2024, 196, 802. [Google Scholar] [CrossRef] [PubMed]
- Shahinuzzaman, M.; Akter, T.; Abdur, R.; Uddin, J.; Chowdhury, F.; Gafur, M.A.; Aziz, S.; Shaikh, M.A.A.; Jamal, M.S.; Hossain, M. Carbon Dioxide Minimization Using Sodium and Potassium Impregnated Calcium Oxide Sorbent Derived from Waste Eggshell. React. Kinet. Mech. Catal. 2024, 137, 359–374. [Google Scholar] [CrossRef]
- Çifçi, D.İ.; Meriç, S. A Review on Pumice for Water and Wastewater Treatment. Desalination Water Treat. 2016, 57, 18131–18143. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of Linear and Nonlinear Equations of Pseudo-First Order and Pseudo-Second Order Kinetic Models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Solute Adsorption at Solid/Solution Interfaces: A Theoretical Development of the Empirical Pseudo-First and Pseudo-Second Order Kinetic Rate Equations, Based on Applying the Statistical Rate Theory of Interfacial Transport. J. Phys. Chem. B 2006, 110, 16514–16525. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Hou, W. Pseudo-Second-Order Kinetic Equation for Describing the Effect of Sorbent and Sorbate Concentrations. Langmuir 2024, 40, 3559–3568. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial Behavior of Intraparticle Diffusion Model Used in the Description of Adsorption Kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L. From Langmuir Kinetics to First- and Second-Order Rate Equations for Adsorption. Langmur 2008, 24, 11625–11630. [Google Scholar] [CrossRef]
- Sheindorf, C.; Rebhun, M.; Sheintuch, M. A Freundlich-Type Multicomponent Isotherm. J. Colloid Interface Sci. 1981, 79, 136–142. [Google Scholar] [CrossRef]
- Johnson, R.D.; Arnold, F.H. The Temkin Isotherm Describes Heterogeneous Protein Adsorption. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1995, 1247, 293–297. [Google Scholar] [CrossRef]
- Gil, A.; Grange, P. Application of the Dubinin-Radushkevich and Dubinin-Astakhov Equations in the Characterization of Microporous Solids. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 39–50. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich Equation and the Underlying Microscopic Adsorption Description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- De Vargas Brião, G.; Hashim, M.A.; Chu, K.H. The Sips Isotherm Equation: Often Used and Sometimes Misused. Sep. Sci. Technol. 2023, 58, 884–892. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; Debord, J.; Harel, M.; Salvestrini, S.; Bollinger, J.-C. The Jovanović Adsorption Isotherm in Water Contaminant Research: Unmasking Spurious Versions and Spotlighting the Real Thing. Chem. Eng. Sci. 2023, 281, 119127. [Google Scholar] [CrossRef]
- Quiñones, I.; Guiochon, G. Extension of a Jovanovic–Freundlich Isotherm Model to Multicomponent Adsorption on Heterogeneous Surfaces. J. Chromatogr. A 1998, 796, 15–40. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Choi, J.W.; Sillanpää, M.; Mtei, K.M. Optimization of Desorption Parameters Using Response Surface Methodology for Enhanced Recovery of Arsenic from Spent Reclaimable Activated Carbon: Eco-Friendly and Sorbent Sustainability Approach. Ecotoxicol. Environ. Saf. 2024, 280, 116550. [Google Scholar] [CrossRef]
- Dokoumetzidis, A.; Magin, R.; Nacheras, P. Fractional Kinetics in Multi-Compartmental Systems. J. Pharmacokinet. Pharmacodyn. 2010, 37, 507–524. [Google Scholar] [CrossRef]
- Siemens, A.M.; Dynes, J.J.; Chang, W. Sodium Adsorption by Reusable Zeolite Adsorbents: Integrated Adsorption Cycles for Salinised Groundwater Treatment. Environ. Technol. 2021, 42, 3083–3094. [Google Scholar] [CrossRef]
- Aguilar-Rosero, J.; Urbina-López, M.E.; Rodríguez-González, B.E.; León-Villegas, S.X.; Luna-Cruz, I.E.; Cárdenas-Chávez, D.L. Development and Characterization of Bioadsorbents Derived from Different Agricultural Wastes for Water Reclamation: A Review. Appl. Sci. 2022, 12, 2740. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Lapolli, F.R.; Nagel-Hassemer, M.E.; Lobo-Recio, M.Á. Optimization of Fe and Mn Removal from Coal Acid Mine Drainage (AMD) with Waste Biomaterials: Statistical Modeling and Kinetic Study. Waste Biomass Valoriz. 2020, 11, 1143–1157. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Legua, P.; Lidón, V.; Conesa, A.; Martínez-Nicolás, J.J.; Melgarejo, P. Evaluation of Agricultural Soil-Improving Zeolite for Improving Irrigation Water Quality. Appl. Sci. 2024, 14, 418. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural Waste Peels as Versatile Biomass for Water Purification—A Review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Lim, S.-F.; Karim, S.K.A.; Chua, S.N.D.; Lim, B.-H. Agricultural Waste-Derived Adsorbents for Decontamination of Heavy Metals. In Integrated Natural Resources Management; Wang, L.K., Wang, M.-H.S., Hung, Y.-T., Shammas, N.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 371–391. ISBN 978-3-030-55172-8. [Google Scholar]
- Awogbemi, O.; Kallon, D.V.V. Progress in Agricultural Waste Derived Biochar as Adsorbents for Wastewater Treatment. Appl. Surf. Sci. Adv. 2023, 18, 100518. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núñez-Delgado, A.; et al. Agricultural Biomass/Waste as Adsorbents for Toxic Metal Decontamination of Aqueous Solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Paranjape, P.; Sadgir, P. Heavy Metal Removal Using Plant Origin Biomass and Agricultural Waste-Derived Biomass from Aqueous Media: A Review. Water Conserv. Sci. Eng. 2023, 8, 9. [Google Scholar] [CrossRef]
- Rocha, C.G.; Zaia, D.A.M.; Alfaya, R.V.d.S.; Alfaya, A.A.d.S. Use of Rice Straw as Biosorbent for Removal of Cu(II), Zn(II), Cd(II) and Hg(II) Ions in Industrial Effluents. J. Hazard. Mater. 2009, 166, 383–388. [Google Scholar] [CrossRef]
- Wen, J.; Dong, H.; Zeng, G. Application of Zeolite in Removing Salinity/Sodicity from Wastewater: A Review of Mechanisms, Challenges and Opportunities. J. Clean. Prod. 2018, 197, 1435–1446. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen, L.H.; Nguyen, V.D.; Nguyen, X.H.; Nguyen, T.H.; Nguyen, T.V.; Vigneswaran, S.; Rinklebe, J.; Tran, H.N. Characteristics and Mechanisms of Cadmium Adsorption onto Biogenic Aragonite Shells-Derived Biosorbent: Batch and Column Studies. J. Environ. Manag. 2019, 241, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, T.; Ke, X.; Liu, Y.; Song, Y.; Shang, X.; Li, J.; Han, Q. A Novel Slag Composite for the Adsorption of Heavy Metals: Preparation, Characterization and Mechanisms. Environ. Res. 2023, 216, 114442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of Ionic Strength on Removal of Toxic Pollutants from Aqueous Media with Multifarious Adsorbents: A Review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef] [PubMed]
- Akpomie, K.G.; Conradie, J. Banana Peel as a Biosorbent for the Decontamination of Water Pollutants. A Review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Farouq, R.; Yousef, N. Equilibrium and Kinetics Studies of Adsorption of Copper (II) Ions on Natural Biosorbent. Int. J. Chem. Eng. Appl. 2015, 6, 319–324. [Google Scholar] [CrossRef]
- Jovanovic, M.; Rajic, N.; Obradovic, B. Novel Kinetic Model of the Removal of Divalent Heavy Metal Ions from Aqueous Solutions by Natural Clinoptilolite. J. Hazard. Mater. 2012, 233–234, 57–64. [Google Scholar] [CrossRef]
- Jovanović, B.M.; Rajaković, L.V. New Approach: Waste Materials as Sorbents for Arsenic Removal from Water. J. Environ. Eng. 2010, 136, 1277–1286. [Google Scholar] [CrossRef]




| Run | X1 | X2 | X3 |
|---|---|---|---|
| 1 | 1 | 1 | −1 |
| 2 | 1 | −1 | 1 |
| 3 | −1 | 1 | 1 |
| 4 | −1 | −1 | −1 |
| 5 | 0 | 0 | 0 |
| 6 | 1 | −1 | −1 |
| 7 | 0 | 0 | 0 |
| 8 | 1 | 1 | 1 |
| 9 | −1 | 1 | −1 |
| 10 | −1 | −1 | 1 |
| 11 | −1.682 | 0 | 0 |
| 12 | 1.682 | 0 | 0 |
| 13 | 0 | 1.682 | 0 |
| 14 | 0 | 0 | −1.682 |
| 15 | 0 | 0 | 0 |
| 16 | 0 | −1.682 | 0 |
| 17 | 0 | 0 | 1.682 |
| Sorbent | Content (g L−1) | Agitation (rpm) | Contact Time (min) | References |
|---|---|---|---|---|
| Almond Shell | 0.1–24 | 100–300 | 5–360 | [40,41,42,43] |
| Eggshell | 0.4–175.3 | 100–300 | 60–2880 | [44,45,46,47,48] |
| Pumice | 2–255.36 | 33–266 | 5–3446 | [30,31,49] |
| Sorbent | Content (g L−1) | Agitation (rpm) | Contact Time (min) |
|---|---|---|---|
| Almond Shell | 6.5 | 200 | 360 |
| Eggshell | 50.2 | 200 | 1500 |
| Pumice | 71 | 150 | 3264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Gómez, D.; Maciá-Vázquez, A.A.; Giménez-Valero, C.; Martínez-Nicolás, J.J.; Legua, P.; Melgarejo, P. Use of Biowaste for Sodium Removal in Mediterranean Irrigation Water: A Sustainable Approach. Clean Technol. 2025, 7, 15. https://doi.org/10.3390/cleantechnol7010015
Núñez-Gómez D, Maciá-Vázquez AA, Giménez-Valero C, Martínez-Nicolás JJ, Legua P, Melgarejo P. Use of Biowaste for Sodium Removal in Mediterranean Irrigation Water: A Sustainable Approach. Clean Technologies. 2025; 7(1):15. https://doi.org/10.3390/cleantechnol7010015
Chicago/Turabian StyleNúñez-Gómez, Dámaris, Alejandro Andy Maciá-Vázquez, Carlos Giménez-Valero, Juan José Martínez-Nicolás, Pilar Legua, and Pablo Melgarejo. 2025. "Use of Biowaste for Sodium Removal in Mediterranean Irrigation Water: A Sustainable Approach" Clean Technologies 7, no. 1: 15. https://doi.org/10.3390/cleantechnol7010015
APA StyleNúñez-Gómez, D., Maciá-Vázquez, A. A., Giménez-Valero, C., Martínez-Nicolás, J. J., Legua, P., & Melgarejo, P. (2025). Use of Biowaste for Sodium Removal in Mediterranean Irrigation Water: A Sustainable Approach. Clean Technologies, 7(1), 15. https://doi.org/10.3390/cleantechnol7010015








