Abstract
This review aims to enhance the understanding of the fundamentals, applications, and future directions in hydrogen production techniques. It highlights that the hydrogen economy depends on abundant non-dispatchable renewable energy from wind and solar to produce green hydrogen using excess electricity. The approach is not limited solely to existing methodologies but also explores the latest innovations in this dynamic field. It explores parameters that influence hydrogen production, highlighting the importance of adequately controlling the temperature and concentration of the electrolytic medium to optimize the chemical reactions involved and ensure more efficient production. Additionally, a synthesis of the means of transport and materials used for the efficient storage of hydrogen is conducted. These factors are essential for the practical feasibility and successful deployment of technologies utilizing this energy resource. Finally, the technological innovations that are shaping the future of sustainable use of this energy resource are emphasized, presenting a more efficient alternative compared to the fossil fuels currently used by society. In this context, concrete examples that illustrate the application of hydrogen in emerging technologies are highlighted, encompassing sectors such as transportation and the harnessing of renewable energy for green hydrogen production.
1. Introduction
Recently, a worldwide race has begun to produce electrical energy from hydrogen. Obtained through a renewable energy source, green hydrogen has been the main highlight within this context. The option for the same is because hydrogen has a high calorific value, and its reaction releases about 2.5 times more energy than the combustion of a fossil hydrocarbon (diesel, gasoline, propane, and methane, among others) [1].
The production of hydrogen involves the breakdown of water molecules into hydrogen and oxygen. One of the simplest ways to separate the two chemical elements from water is through an electrochemical process, electrolysis. Electrolysis has been perfected over the centuries and would have emerged from the pioneering work of Humphry Davy (1778) [2], who, by passing an electric current through potassium carbonate, obtained potassium; Alessandro Volta, at the end of 1799 [3], invented the electric battery through his creation of an electrochemical cell formed by discs of zinc and copper separated by cotton soaked in a saline solution and presented it to the Royal Society of London in 1800 [4]; and John Frederic Daniell (1836) [5], with copper and zinc electrodes that were in individual cells, which he called a saline bridge, connecting two vats and increasing the efficiency of the battery.
Besides its use in generating electrical energy, hydrogen can be transformed into other energy carriers like methanol [6], ammonia [7], and synthetic liquids [8], expanding the possibilities of applications [9]. From an energy perspective, hydrogen production is extremely advantageous [10]. However, costs are still relatively high [11]. According to estimates published in 2020 by the International Renewable Energy Agency (IRENA) [12], it is expected to become increasingly attractive as the cost of electricity generated from renewable energy sources continues to fall. For instance, IRENA reports that the costs of producing electricity generated from solar photovoltaic and wind systems have already dropped by 80% and 40%, respectively, in the last decade, with the expectation that these trends will continue. At present, the cost of green hydrogen is about USD 6 per kg, and it is anticipated to reduce to USD 1 to USD 2 per kg in the 2030s [12]. The reduction of electricity costs from renewable energy sources is not the only factor that needs improvement. Research also needs to be expanded and improved regarding electrolyzers and electrolytes used in electrolysis [13]. The electrolyzers are the electrodes that, immersed in an electrolytic solution, conduct the process of separating hydrogen and oxygen through the passage of an electric current [14]. Therefore, they must withstand high current intensities, be good conductors, and be resistant to wear and corrosion [15]. The electrolytes are the solutions where chemical reactions occur.
However, in addition to advancements in hydrogen production techniques, the transportation method and choice of storage type can greatly influence the final cost of the product. Transportation involves a process of transmission and distribution until the product reaches the end consumer.
This transportation can be conducted in gaseous form, which requires compressing the hydrogen to high pressures and subsequently storing it in reinforced containers to ensure safety and compliance with market regulations [16]. In this context, compact storage forms of hydrogen are more cost-effective for transportation, as diffuse forms tend to be more expensive due to their larger volume [17].
Another option is the transportation of liquefied hydrogen, which allows for compact storage in cryogenic tanks.
Finally, transporting hydrogen using solid-state storage methods offers high capacity and safety, although each method has its own advantages and limitations. Activated carbon is cost-effective but has moderate storage capacity and contamination issues [18]. Carbon nanotubes are lightweight and stable but face challenges with dimensional control and structural damage [19]. Metal–organic frameworks (MOFs) adsorb hydrogen quickly but are sensitive to moisture and temperature fluctuations [20]. Metal hydrides provide high capacity and safety but are costly and have slow absorption rates [21].
In this work, we will discuss the most common transportation techniques, including pipelines, tanker trucks, ships, and trains. We will also examine how the choice of storage methods directly influences the available transportation options. Therefore, integrated planning is crucial to maximize the efficiency, safety, and economic viability of this energy source.
Furthermore, hydrogen is categorized into three color classifications: green, blue, and grey, which indicate its environmental impact. This classification primarily reflects the energy sources and technologies used in hydrogen production. Green hydrogen is entirely environmentally benevolent and relies only on renewable energy. Grey hydrogen, on the other hand, is derived from fossil fuels and, as a result, can have a negative environmental impact during its production phase. Blue hydrogen can be considered in between green and grey hydrogen in terms of environmental impact. Finally, there is also blue hydrogen, which is produced from fossil fuels as grey hydrogen is, but instead of this one, the production of blue hydrogen promotes carbon dioxide capturing, reducing the overall carbon emissions of the production process.
Hydrogen can be produced through various technologies, all of which require feedstocks and energy sources, including non-renewable fossil fuels and renewable options like biomass, wind, and solar energy. In terms of environmental impact and toxic emissions, the hydrogen production technologies derived from renewable sources are more environmentally friendly than the other methods, but such production routes are less competitive when considering the overall efficiency, cost, and maturity levels. The most common hydrogen production technology is natural gas steam reforming, accompanied by oil reforming and gasification. As a result, most of the hydrogen produced globally still comes from fossil fuels rather than renewable energy. The use of biomass waste to produce hydrogen has also been profoundly investigated and tested, but this approach still entails considerable technological challenges to be used on a large scale. The water-splitting electrolysis and thermolysis technologies are continuously evolving. However, these methodologies are still facing important challenges to be applied in large-scale industrial applications.
Biological-based technology can use organic waste and produce hydrogen through biochemical action, but they are in the early stages of design and implementation and are evaluated merely on laboratory-scale experiments. Large-scale manufacturing deals with large volumes and amounts, and the amount of generated hydrogen via microorganisms is much lower. This fact spurges its industrial exploration.
In view of the above, the main objective of this review is to present and discuss the fundamentals, applications, and perspectives of the various techniques employed in hydrogen production. Additionally, the parameters that affect the production and physical means of transport and materials used for hydrogen storage are presented and discussed. Finally, examples of hydrogen utilization in emerging technologies are provided. Figure 1 shows a schematic diagram, representing some of the best-known methods of hydrogen production.
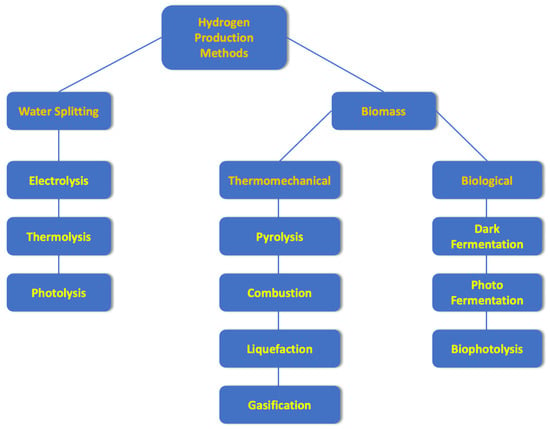
Figure 1.
Scheme illustrating hydrogen production methods.
In the following chapter, a brief description of these types of hydrogen production processes is made.
2. Hydrogen Production Methods
2.1. Water Splitting
The water-splitting process involves breaking the water molecule to produce hydrogen. This method is driven by various chemical reactions that require high temperatures to occur. A liquid medium, called an electrolyte in this case, is generally involved in a closed cycle that consumes only water, producing hydrogen and oxygen. Within this context, electrolysis, thermolysis, and photolysis stand out.
2.1.1. Electrolysis
Electrolysis is a physicochemical process that uses electrical energy regardless of its source to initiate a chemical reaction that results in the separation of the elements within a molecule. In fact, water electrolysis is one of the most effective methods for hydrogen production, as it utilizes water and produces only pure oxygen as a byproduct. Additionally, electrolysis can harness energy from sustainable resources such as solar, wind, and biomass. However, currently, only 4% of hydrogen production can be achieved using water electrolysis, primarily due to economic factors [22].
In an electrolytic cell operating under constant pressure and temperature, the energy needed for the reaction is dictated by the change in enthalpy (ΔH). The electrical energy component is represented by Gibbs Free Energy (ΔG), whereas the thermal energy component is the product of temperature (T) and the change in entropy (ΔS). Equation (1) expresses the Gibbs Free Energy:
At constant pressure and temperature (P = 1 atm, T = 298 K), for example, a water molecule has enthalpy (ΔH = +285.840 kJ/mol) and entropy (ΔS = +163.15 J/mol·K) values, resulting in ΔG = +273.22 kJ/mol. Due to the endothermic nature of the enthalpy change and the positive Gibbs Free Energy change, the reaction is considered non-spontaneous. In summary, the electrolysis process is endothermic, and its reaction is non-spontaneous [23]. Energy consumption is directly related to pressure and temperature. Consequently, changes in these parameters impact the voltages in the process. Increasing the temperature in the process results in a reduction in the amount of electrical energy required for electrolysis [24]. Table 1 provides an overview of the typical specifications for production processes in alkaline electrolysis, proton exchange membrane (PEM) electrolysis, and solid oxide electrolyzers [25].

Table 1.
Typical production processes in alkaline electrolysis, proton exchange membrane, and solid oxide electrolyzer [25].
Alkaline Electrolyzer
This type is frequently utilized in large-scale production systems across various industries for hydrogen generation through cells. Alkaline electrolyzer cells are categorized into two groups based on the electrolyte used. The first employs potassium hydroxide, while the second uses sodium hydroxide, with the latter being replaceable by sodium chloride. The electrolysis process through alkaline electrolyzers is a mature, reliable, and safe technology, able to generate hydrogen with a purity level reaching 99.8% [26]. Typically, these cells consist of an alkaline solution, a pair of electrodes, and a microporous separator, usually comprising about 30% of the weight in potassium hydroxide or sodium hydroxide, as illustrated in Figure 2.
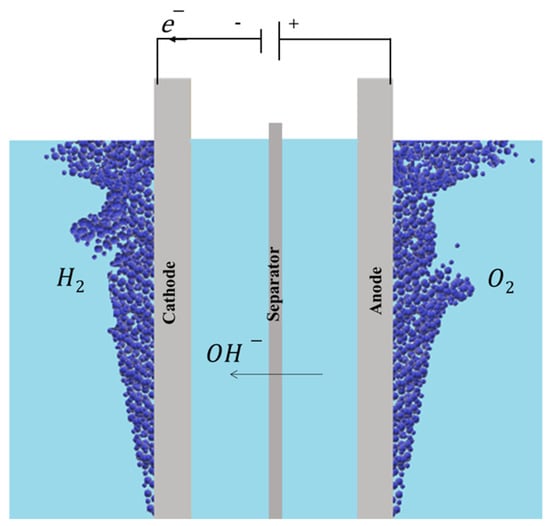
Figure 2.
Simplified alkaline electrolysis cell.
Reactants generally migrate from the bulk to the interface, while products move from the interface to the bulk. At the same time, changes in the concentrations of reactants and products at the interface lead to variations in the thermophysical properties of the electrolyte, particularly density. These variations, in turn, generate buoyant forces that depend on their orientation relative to gravitational acceleration. This experimental investigation focuses on alkaline water electrolysis, where the electrolyte is a dilute potassium hydroxide solution that dissociates into K+ and OH− ions. As a result, oxygen gas is produced at the anode and hydrogen gas at the cathode, according to the electrochemical reactions described by Equations (2) and (3), respectively:
and at the cathode:
Typically, alkaline cells operate at low temperatures (60–80 °C), and the energy consumption for H2 production is around 4.5–5.5 kWh/Nm3, with an efficiency of 60%, as they operate at low current densities (<250 mA/cm2) [27,28]. One of the primary advantages of alkaline water electrolysis compared to other electrolysis technologies is that alkaline electrodes can be crafted from abundant and inexpensive materials. Simple iron or nickel steel electrodes are utilized for hydrogen production (cathode), and nickel is employed in the production of oxygen (anode) [23].
Proton-Exchange Membrane
The Proton-Exchange Membrane was developed in mid-1966 to overcome the disadvantages of alkaline electrolysis. It utilizes solid polymeric sulfonated membranes, such as Nafion, as proton-conducting electrolytes. This process is characterized by its low gas permeability, thin thickness (0.2 mm), high proton conductivity, the ability to operate at high pressures, and low temperatures (20–80 °C) [22].
The cell is composed of a membrane positioned between two plate-shaped electrodes, known as the cathode and the anode. These plates are exposed to a flow of water and gas. When the system is powered through the electrodes, it initiates the separation of water molecules. At the anode, water molecules are broken down into oxygen and hydrogen ions. Subsequently, these ions, along with the electrons traveling through the electrical circuit, traverse the polymeric membrane to reach the cathode [29]. Moreover, the authors Liu et al. [30] developed ordered structures within membrane electrode assembly for proton-exchange membrane water electrolyzers. They developed a hybrid membrane electrode assembly with a cone-shaped configuration, which involved embedding titania nanoparticles into a Nafion emulsion to create a rough-surfaced Nafion array. The hybrid ordered membrane electrode assembly attained an average surface roughness of around 3.4 nm, which was approximately 2.6-fold greater than that of the ordered membrane electrode assembly without titania nanoparticles, and it had a current density reaching around 2.5 A.cm−2 at 2 V with 14.4 µg cm−2 iridium catalyst loading. Furthermore, Jin et al. [31] suggested that employing advanced air-breathing proton-exchange membrane fuel cells with a condensing-tower-like curved flow field could be an effective approach for hydrogen production. The authors enhanced air diffusion and fuel cell performance by using this novel flow field design, which eliminates the need for an additional fan, thus making the fuel cell more compact and reducing internal power consumption. Their results, including the polarization curve and galvanostatic discharge tests, demonstrated that the curved flow field improved air diffusion into the proton exchange membrane fuel cell, thereby boosting its performance. The four-layer stack with the curved cathode flow field achieved a peak power of 2.35 W (120 W/kg). Figure 3 schematically represents a proton-exchange membrane fuel cell.
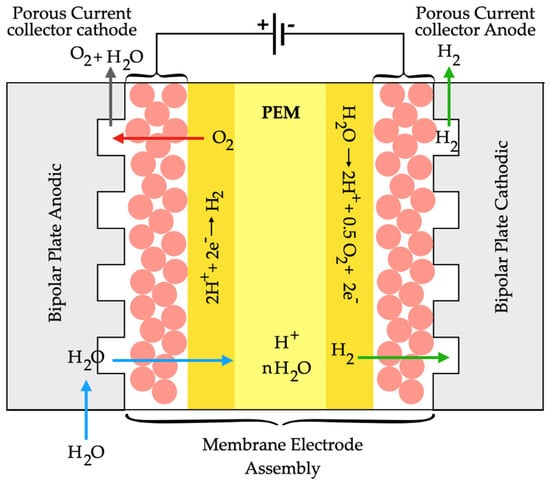
Figure 3.
Scheme of a proton-exchange membrane fuel cell for hydrogen production.
Solid Oxide Electrolyzer
The Solid Oxide Electrolyzer was first introduced in the 1980s, capturing significant attention due to its ability to produce exceptionally pure hydrogen with heightened efficiency. They achieve this by operating at elevated temperatures (500–850 °C) and under pressure, utilizing water in the form of steam. When compared to alkaline and Proton-Exchange Membrane methods, the solid oxide electrolyzers outperform them in terms of efficiency, primarily due to their higher operating temperatures [22]. The solid oxide electrolyzer technology utilizes solid ion-conductive ceramics as an electrolyte, enabling operation at higher temperatures. However, one of the main challenges lies in the high degradation of materials due to these elevated temperatures. In the solid oxide electrolyzer, the electrodes (anode and cathode) are separated by a membrane, and its principle is the reverse of the proton-exchange membrane [32]. Figure 4 shows the scheme of a solid oxide electrolyzer.
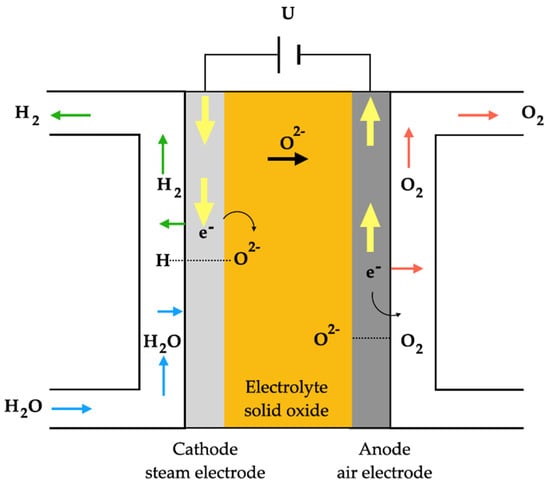
Figure 4.
Typical solid oxide electrolyzer functioning principle.
2.1.2. Thermolysis
The water thermolysis process requires extremely high temperatures, which can impact costs and production levels. The following chemical reaction, as represented by Equation (4), illustrates the necessary conditions for generating a substantial quantity of hydrogen using this method:
According to Bockris et al. [33], only 10% of the water decomposes at this temperature. It is also crucial to prevent the recombination of oxygen and hydrogen after cooling. Marshall and Blencoe [34] emphasize that the thermal decomposition of water is energetically unfavorable.
According to Silva [35], the production of hydrogen through the direct decomposition of the water molecule requires the search for materials whose behavior is stable across the operating temperature ranges. Despite the various options available, they all face similar challenges. For example, materials like graphite, tungsten, or tungsten carbide tend to oxidize when exposed to hydrogen and oxygen at high temperatures. The stability of the oxides is known at these temperatures. However, the effect of hydrogen on them is not well understood. According to the literature, ceramic materials, such as boron nitride (melting point of 2973 K), can be useful for these applications if their oxidation is controlled [25].
Based on an assessment in energy and economic terms [28], the feasibility of electrolysis and thermolysis was compared. In the case of thermolysis, the authors emphasize that, due to chemical processes occurring at high temperatures, it is not possible to avoid heat rejection unless heat pumps are used to recycle this energy. However, the additional investment costs will be higher compared to a power plant with the same internal efficiency capable of powering an equivalent electrolysis process.
Bockris et al. [33] go beyond the conclusions of Bidard [36] and propose the discontinuation of thermolysis as a method for hydrogen production, a suggestion that was further supported by a subsequent study conducted by Perkins and Weimar [37]. This latter study does not foresee the economic viability of water thermolysis, due to the temperature requirements, materials, and processes necessary to separate hydrogen and oxygen. Faced with these challenges, researchers were motivated to explore alternative methods capable of decomposing the water molecule, opting for approaches that involve significantly lower temperatures, such as the use of thermochemical cycles [38,39].
However, more recently, and according to Lee et al. [40], to address the issue of the substantial amount of thermal energy required, pairs of metal oxides/halides and metal oxides can be used to lower the maximum temperature needed below 1000 K.
2.1.3. Photolysis
Photolysis specifically refers to the direct breaking of water molecules using light energy and differs from photocatalysis, as will be seen in Section 2.2.2, since the former involves the use of a catalyst, usually a semiconductor, to enhance the efficiency of water separation. Photolysis typically refers to the decomposition of water into hydrogen and oxygen using light energy. This can be achieved through direct or indirect photolysis.
Direct photolysis leverages the photosynthetic abilities of algae and cyanobacteria to decompose water into hydrogen and oxygen [41]. These microorganisms absorb solar light, undergo water-splitting reactions, and, through enzymes such as hydrogenases and nitrogenases, produce biohydrogen [42,43].
Under anaerobic conditions, some microorganisms release electrons, converting hydrogen ions into hydrogen gas [44]. The extracted protons and electrons are recombined by a chloroplast hydrogenase, resulting in hydrogen gas with a purity level of up to 98% [45]. However, oxygen production also occurs, which can inhibit hydrogen production [46].
Researchers are working to optimize microorganisms, redirecting more solar energy towards hydrogen production. Additives like sulfate can suppress oxygen production but also inhibit hydrogen production mechanisms [47]. Although water is the primary source, direct photolysis encounters challenges, including the requirement for a large area to capture adequate light and the difficulty of maintaining continuous hydrogen production in the presence of oxygen.
Indirect photolysis is a two-phase process in which the generation of biohydrogen and oxygen occurs in distinct stages. The production of biohydrogen originates from intracellular reserves such as glycogen and starch found in cyanobacteria and microalgae [48]. Cyanobacteria, also known as blue–green algae, are commonly employed in this process. Indirect photolysis begins with carbon dioxide fixation by cyanobacteria, using solar light to generate cellular substances and oxygen [49]. Subsequently, the cellular substance is utilized in the production of biohydrogen [50]. Finally, in the field of water-splitting hydrogen production technologies, innovative forms of hydrolysis are being tested. That is the case of the work conducted by the researchers Hammad et al. [51], in which they investigated aluminum hydrolysis as a technological solution for hydrogen production enhanced by sodium hydride. The oxide protective layer around aluminum limits its hydrolytic activity. To mitigate this limitation, the sodium hydride was incorporated to form the core–shell structure of aluminum-based sodium hydride fuel powder through simple hand mixing. The new core–shell structure of aluminum-based sodium hydride solid fuel powder showed the best hydrolytic performance among the aluminum-based materials and produced 98% hydrogen yield at a 1 to 0.9 M ratio of sodium hydride and aluminum in water. The authors verified that the hydrolytic activity consisted of sodium hydride hydrolytic splitting and aluminum hydrolysis. It was verified that the sodium hydride was the key element for supporting the complete aluminum hydrolysis and attaining a superior hydrogen yield when compared to that obtained with calcium hydride. Table 2 summarizes the efficiency, scalability, benefits, and disadvantages of water-splitting processes to produce hydrogen.

Table 2.
Efficiency, scalability, benefits, and disadvantages of hydrogen-producing water-splitting processes.
2.2. Biomass
The use of biomass can represent an efficient and cost-effective alternative for hydrogen production. Within this context, two categories stand out: thermochemical and biological.
Thermochemical hydrogen production involves processes that use heat to decompose biomass and, thus, generate hydrogen. Thermochemical methods include pyrolysis, combustion, liquefaction, and gasification.
Biological hydrogen production involves using microorganisms, such as bacteria and algae, to ferment or photosynthesize biomass and generate hydrogen as a byproduct. The following are part of the biological methods: dark fermentation, photo fermentation, and biophotolysis.
Both approaches present specific challenges and advantages, which will be discussed in the sequence.
2.2.1. Thermochemical
The primary thermochemical processes encompass pyrolysis, combustion, liquefaction, and gasification. The thermochemical approach involves generating hydrogen and hydrogen-rich gases through the conversion of biomass [52]. Subsequent sections will delve into the discussion of these processes.
Pyrolysis
Typically, biomass is heated to temperatures ranging from 650 K to 800 K at pressures of 0.1–0.5 MPa in an oxygen-free environment, which facilitates the production of liquid oils. Alongside gaseous compounds and solid charcoal [53], several critical factors influence this process, including the type of feedstock, catalyst, temperature, and residence time during biomass pyrolysis [54]. Biomass pyrolysis techniques are generally classified into two categories: (I) slow pyrolysis technique and (II) fast (or flash) pyrolysis.
Slow pyrolysis is less commonly used because it primarily produces charcoal.
In contrast, fast pyrolysis involves rapidly heating biomass to elevated temperatures in the absence of air, resulting in the generation of a vapor that condenses into a dark brown, fluid bio-liquid [55]. The products of fast pyrolysis can be categorized into gaseous, liquid, and solid phases:
- Gaseous products, such as hydrogen, methane, carbon monoxide, and carbon dioxide, are considered due to the organic nature of the biomass in the pyrolysis technique.
- Liquid products, including oil and tar, remain in a liquefied form at room temperature, such as acetic acid, and acetone, among others.
- Solid products comprise char, pure carbon, and other inert materials.
Oil products, in addition to gasified products, undergo further separation based on their solubility. The soluble fraction is processed to increase the hydrogen content for hydrogen production, whereas the insoluble fraction is typically used in adhesive formulations. Achieving a maximum yield of 90% hydrogen in pyrolysis is possible when using nickel as the catalyst [56]. Key control parameters in the pyrolysis process for hydrogen production encompass temperature, heating and cooling rates, residence period, and the nature of the catalyst [57]. These parameters can be adjusted according to the selected reactor types and heat-transfer modes [58]. Conversely, the production of hydrogen from pyrolysis can be categorized into three main groups.
The initial group involves catalytic pyrolysis employing continuous feeding and a fluidized bed reactor, evaluated based on the catalyst weight/biomass ratio. This method produces more than 50% mole of hydrogen in the total gas composition.
The second group focuses on the catalytic steam reforming of pyrolysis liquids (bio-oil) through pyrolytic reactions, aiming to optimize the nitrogen and oxygen levels in the bio-oil. Although both the conversion rate and hydrogen yield tend to decrease as the reaction proceeds, the hydrogen content stays above 80% after reforming at 850 °C [59].
The third group involves recent investigations into in-line steam reforming of volatiles from biomass fast pyrolysis. These studies examine the impacts of reforming factors such as temperature, space–time, and steam/biomass ratio. This approach addresses operational challenges related to bio-oil handling by implementing a continuous two-step pyrolysis-reforming technique.
Additionally, studies by authors like Rabah and Eldighiby [60] demonstrate that inorganic salts such as carbonates, chlorides, and chromates promote the pyrolysis reaction rate. Extensive research has also been conducted on various catalysts, including nickel catalysts [61], Y-type zeolite [62], K2CO3, and Na2CO3 [63], as well as various metal oxides like silica, alumina, titania [64], and Cr2O3 [65], to assess their impact on hydrocarbon decomposition in tar. More effective catalysts, such as ruthenium and rhodium, have been explored, although noble metals are costly and less commonly investigated [66]. Furthermore, various types of feedstocks have been explored, such as agricultural residues [67], post-consumer wastes like synthetic polymers [68], mixed biomass, and rapeseed [69], for hydrogen production from pyrolysis. Utilizing fluidized bed reactors mitigates the deposition of char and coke on the catalyst surface, thereby diminishing the influence on reforming performance [70]. Figure 5 illustrates the generic diagram of the pyrolysis process for hydrogen generation. Figure 6 schematically represents the pyrolysis for hydrogen generation involving hydrogen iodide decomposition, Bunsen reaction, and sulfuric acid decomposition.
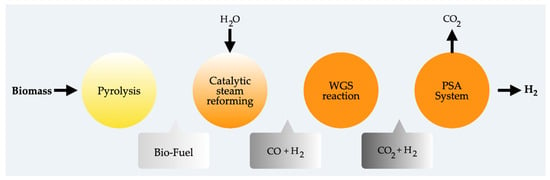
Figure 5.
Main steps of hydrogen production by pyrolysis using biomass.
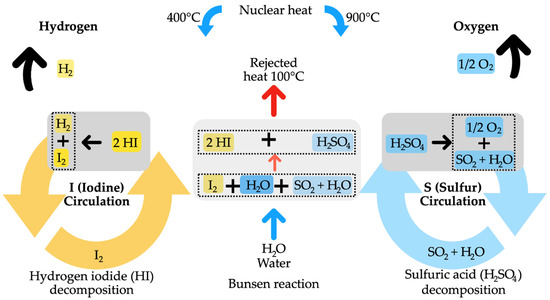
Figure 6.
Pyrolysis for hydrogen production with hydrogen iodide decomposition, Bunsen reaction, and sulfuric acid decomposition.
Combustion
The process of combustion entails the direct combustion of biomass raw materials in the presence of air to derive heat from the chemical energy of biomass, mechanical power, or electricity using various equipment like stoves, boilers, furnaces, or steam turbines.
The production of hydrogen can result from combustion reactions, which generally occur in industrial processes involving hydrocarbons as fuels. Hydrogen can be obtained through the steam-reforming process, a chemical reaction between a hydrocarbon, such as methane, and water vapor, resulting in hydrogen and carbon monoxide. This reaction is often used on a large scale in industry to produce hydrogen. However, it is important to note that this method is not a “clean” way of producing hydrogen, as it generates by-products such as carbon monoxide, which are harmful to the environment.
According to Agrafiotis et al. [71] and Zhai et al. [72], until the year 2010, steam methane reforming (SMR) was the main source of hydrogen production in the United States, accounting for 95% of the production of this element. In global terms, about 72% of global hydrogen production in 2020 was obtained through SMR of natural gas without carbon dioxide capture, representing 60% of the 90 million tons of total hydrogen production capacity [73].
As mentioned previously, this process involves the reaction of fossil fuels, mainly natural gas, which also ends up producing carbon monoxide. In this process, methane reacts with water vapor to produce carbon monoxide and hydrogen through an endothermic process that requires temperatures close to 900 °C [74,75]. Furthermore, carbon dioxide is also formed in this same process, leading it to be known as “gray hydrogen”. If the carbon dioxide is captured during the process, it is called “blue hydrogen”, which is less polluting.
Additionally, Wu et al. [76] developed an innovative technology for promoting hydrogen production through formaldehyde reforming using oxide-derived copper nanowires at room temperature. The research team implemented a two-step method to create oxide-derived copper nanowires on a copper mesh surface, resulting in a monolithic catalyst that significantly enhanced hydrogen production from the reforming of formaldehyde and water. Their findings confirmed that the specialized oxide nanostructure notably improved the reforming performance of copper and that hydrogen production had a linear relationship with oxygen pressure. They reported a 36-fold increase in the hydrogen generation rate compared to conditions without oxygen. Density functional theory calculations revealed that formaldehyde molecules adsorb on the copper surface only when adjacent to adsorbed oxygen, with the hydrogen release process having the most significant impact on the rate.
Liquefaction
Liquefying hydrogen is a complex and energy-demanding process, which is crucial for realizing the full potential of hydrogen as a clean and efficient energy carrier. This industrial procedure plays a pivotal role in applications ranging from space exploration to fuel cells and industrial processes, necessitating the need for a detailed understanding of its underlying principles. In fact, hydrogen liquefaction is a multidimensional process encompassing compression, purification, cooling, expansion, and condensation [77].
The compression process starts with increasing the pressure of gaseous hydrogen. This step is essential as it reduces the hydrogen’s volume, facilitating its subsequent cooling and liquefaction. Efficient compression is vital for ensuring the overall energy efficiency of the process. Before entering the liquefaction phase, hydrogen often undergoes purification steps to eliminate impurities and trace contaminants. This is imperative for ensuring the quality and safety of liquefied hydrogen, particularly as it may be used in sensitive applications such as fuel cells.
The hydrogen is then subjected to a cooling process where it is brought to extremely low temperatures. The liquefaction typically occurs close to absolute zero, around −253 °C. Achieving and maintaining these temperatures requires the use of advanced cryogenic technologies and equipment. Moreover, the cooling process involves controlled expansion of the gas through a valve. The Joule–Thomson effect, whereby a gas experiences a temperature change upon expansion, contributes significantly to further cooling. This effect is instrumental in achieving the low temperatures necessary for liquefaction. As the temperature decreases, hydrogen transitions from the gaseous phase to the liquid phase. Liquid hydrogen is then collected and stored in cryogenic containers designed to maintain extremely low temperatures. This phase change is a critical aspect of the liquefaction process.
The liquefied hydrogen, now in a compact and easily transportable form, is stored in cryogenic tanks. These tanks are designed to minimize heat transfer, preventing the liquid hydrogen from vaporizing. Specialized containers equipped with advanced insulation systems are used for transportation, ensuring that the liquefied hydrogen remains in its liquid state during transit.
Advances in technology are continually enhancing the efficiency of the hydrogen liquefaction processes. These include innovations in hybrid liquefaction methods and improved insulation materials, all contributing to the broader goal of establishing hydrogen as a versatile and sustainable energy carrier. As global interest in hydrogen as a clean energy solution grows, continuous research and technological advancements in hydrogen liquefaction will be crucial in defining the future of sustainable energy [78]. Figure 7 illustrates the hydrogen production process through liquefaction.
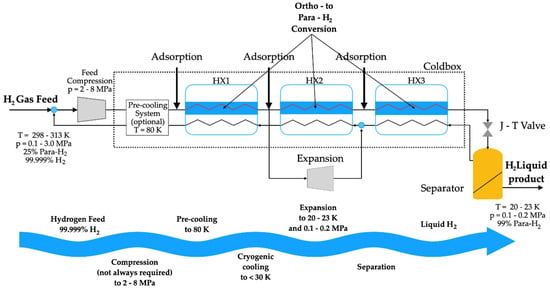
Figure 7.
Main steps of the hydrogen liquefaction process.
Figure 8 illustrates the hydrothermal liquefaction treatment to produce, among other products, green hydrogen.
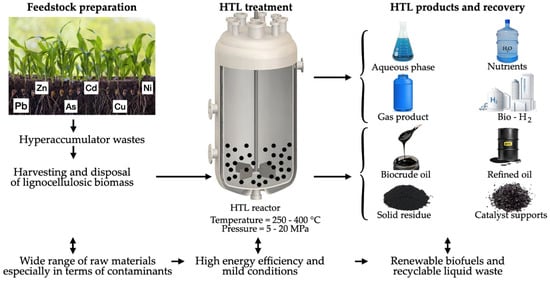
Figure 8.
Hydrothermal liquefaction procedure to produce bio-hydrogen.
Gasification
This process occurs at temperatures above 1000 K, enabling the conversion of biomass into gas. The particles experience partial oxidation, leading to the formation of gas and charcoal [79]. Hydrogen, carbon monoxide, carbon dioxide, and methane are generated through the reduction of charcoal. In contrast to pyrolysis, the gasification of solid biomass requires oxygen. The main goal of gasification is to produce gaseous products, while pyrolysis aims to generate both bio-oils and charcoal. The gasified biomass can be subjected to steam reforming to produce hydrogen, with the process further improved through subsequent water–gas shift reactions. However, gasification faces limitations such as low thermal efficiency, and it is crucial to minimize the moisture content as much as possible [79].
There are three primary types of reactors used for biomass gasification: fixed bed, fluidized bed, and indirect gasifier [80]. However, several crucial parameters, such as biomass type, particle size, operating temperature ranges and rates, steam-to-biomass ratio, and types of catalyst, directly influence the hydrogen yield [81].
The main challenges in biomass gasification are linked to low thermal efficiency, largely due to moisture content. The process is effective only with biomass that has a moisture content below 35% [79]. Additionally, gasification can produce tar aerosols that polymerize into complex structures, which can hinder steam reforming [82]. The formation of ash also leads to problems such as deposition, sintering, slagging, fouling, and agglomeration within the reactor [83]. Figure 9 shows the diagram of the main steps of the gasification hydrogen production process.
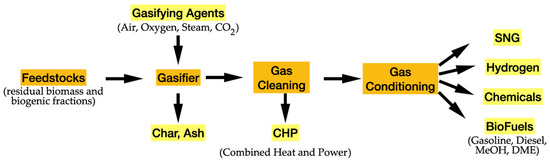
Figure 9.
Fundamental steps of hydrogen production by gasification.
Table 3 summarizes the efficiency, scalability, benefits, and disadvantages of the thermochemical processes to produce hydrogen.

Table 3.
Efficiency, scalability, benefits, and disadvantages of hydrogen-producing thermochemical processes.
2.2.2. Biological
Biological hydrogen production has garnered increasing attention from researchers, mainly due to its operation at ambient temperature and pressure, requiring less energy. Furthermore, these methods make use of renewable and sustainable energy sources, aiding waste recycling by incorporating a variety of waste materials as feedstock.
Biological processes are generally categorized into the following types: (i) direct bio-photolysis, (ii) indirect bio-photolysis, (iii) biological water–gas shift reaction, (iv) photo-fermentation, and (v) fermentation [84]. These processes are mainly driven by hydrogen-producing enzymes such as hydrogenase and nitrogenase. Nitrogenase consists of the MoFe protein and the iron protein. It can use magnesium adenosine triphosphate and electrons to reduce various protons-rich substrates, thereby generating hydrogen [85].
Most photosynthetic microorganisms contain hydrogenases, further classified as (i) uptake hydrogenases and (ii) reversible hydrogenases [80]. Considering the working conditions, reversible hydrogenases can both produce and consume hydrogen.
Photo and Dark Fermentations
Investigating solar energy in conjunction with organic acids or biomass, photosynthetic bacteria can generate hydrogen through the photo-fermentation process, which is aided by nitrogenase. During this process, light-harvesting pigments like chlorophyll capture light energy and transfer it to membrane reaction centers. Depending on the extent of sunlight conversion, water molecules are split into oxygen, electrons, and protons [82].
Furthermore, in the bio-photolysis procedure, the utilization of the nitrogenase enzyme with high energy requirements, the low solar energy-conversion efficiency, and the necessity for specific anaerobic conditions with large-area photobioreactors have emerged as significant drawbacks for this process [86]. Consequently, photo-fermentation processes are not currently considered a competitive method for hydrogen production.
Dark fermentation focuses on the fermentation process conducted by anaerobic bacteria and specific microalgae, such as green algae, using carbohydrate-rich substrates under oxygen-limited conditions to produce hydrogen. This process typically occurs at temperatures between 30 °C and 80 °C, especially in the absence of light [87]. Unlike the bio-photolysis process, where hydrogen is the sole product, the outcomes of dark fermentation include hydrogen and carbon dioxide combined with other gases, such as methane and hydrogen sulfide, depending on the reaction process and the substrate used. However, this process also yields products containing both butyrate and acetate in practice [88].
In the dark fermentation process, the quantity of produced hydrogen is influenced by factors such as pH value, hydraulic retention period, and gas partial pressure. Therefore, it is essential to maintain the partial pressure of hydrogen to prevent the formation of reduced substrates like ethanol and lactate [89], keep the pH value between 5 and 6 [90], and sustain an optimized hydraulic retention time of 0.5 days to achieve peak hydrogen production [91]. Moreover, dark hydrogen fermentation provides several benefits over other biological hydrogen production methods, such as photosynthesis and photo-fermentation. These advantages include the ability to produce hydrogen continuously without requiring light, a higher hydrogen production rate, technical simplicity, reduced net energy input, and the utilization of lower-value waste materials such as feedstocks [92]. Figure 10 schematically illustrates the main steps of the dark fermentation process.
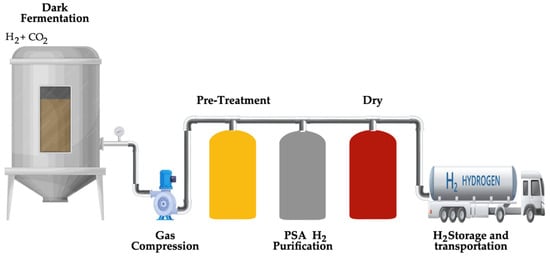
Figure 10.
Fundamental steps of the dark fermentation process.
Direct Photo-Biolysis
Direct photo-biolysis represents another form of biological process where solar energy is transformed into chemical energy by the photosynthetic systems of microalgae, leading to hydrogen production [81]. The photosynthesis process comprises two photosynthetic systems: (i) photosystem I, which generates the reductant for carbon dioxide reduction, and (ii) photosystem II, which is responsible for splitting water and evolving oxygen.
In photo-biolysis, carbon dioxide reduction can be produced from photosystem I, or hydrogen can be generated in the presence of hydrogenase using two photons of water. In contrast to green plants, where only carbon dioxide is involved, microalgae such as green algae or cyanobacteria (blue–green algae) can undergo water splitting, producing hydrogen. This is facilitated by the presence of hydrogenase in microalgae. However, when the oxygen content exceeds 0.1%, the ability of hydrogenase to produce hydrogen becomes limited. Therefore, the oxygen content must be carefully maintained [65].
As per the literature [79,92], mutants derived from microalgae exhibit a higher percentage of hydrogen production, as these mutants can tolerate oxygen. Figure 11 illustrates a typical process of hydrogen production by photo-biolysis using microalgae or cyanobacteria.
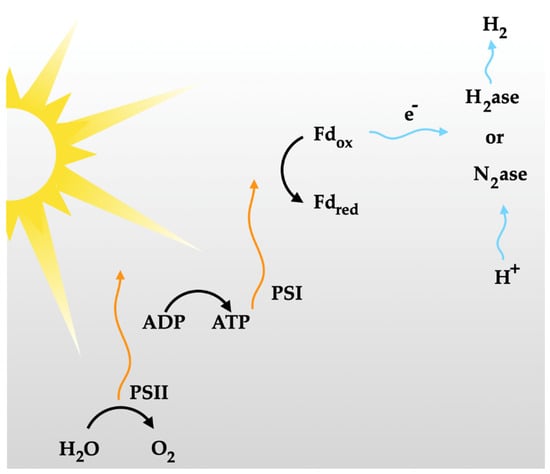
Figure 11.
Typical direct photo-biolysis for hydrogen generation using microalgae or cyanobacteria.
Indirect Photo-Biolysis
The researchers Ni et al. [81] employed an indirect photo-biolysis, involving the following steps: (i) biomass production using the photosynthesis technique, (ii) biomass concentration, (iii) aerobic dark fermentation yielding 4 mol of hydrogen/mol, and (iv) conversion of 2 mol of acetates into hydrogen. In an investigation conducted by the researchers Kerby et al. [93] on indirect bio-photolysis with the cyanobacterium Gloeocapsaalpicola, they found that optimizing hydrogen production was achieved by maintaining the pH value between 6.8 and 8.3. Additionally, there was a two-fold increase in hydrogen production when the temperature was raised from 30 °C to 40 °C. However, challenges related to indirect bio-photolysis for hydrogen production include comparatively low yield, unutilized generated waste, and the need for a considerable surface area to obtain adequate sunlight exposure.
Biological Water–Gas Shift
Certain photoheterotrophic bacteria, such as Rhodospirillum rubrum, can survive in darkness when using carbon monoxide as their sole carbon source. These bacteria produce ATP by coupling the oxidation of carbon monoxide with the reduction of hydrogen ions, H+, to hydrogen [94]. The water–gas shift reaction allows organisms like gram-negative bacteria (e.g., R. rubrum and Rubrivaxgelatinosus) and gram-positive bacteria (e.g., Carboxydo thermus hydrogenoformans) to thrive, making the process conducive for hydrogen production [95]. The primary goal of this approach is to identify an organism with high carbon monoxide and to measure the rate of hydrogen production. Soboh et al. [94] reported that under dark fermentation conditions with increased nickel, the doubling time of R. rubrum was less than 5 h. However, R. rubrum requires light for growth, and hydrogen production is inhibited if the carbon monoxide partial pressure exceeds 0.2 atm in the medium. Additionally, Wolfrum et al. [96] investigated the use of the novel chemoheterotrophic bacterium Citrobacter sp. Y19 for hydrogen production through the water–gas shift reaction. The researchers found that the hydrogen production activity of Citrobacter sp. Y19 was three times greater than that of R. rubrum. Additionally, ref. [97] conducted a detailed comparison between biological water–gas shift reactions and traditional water–gas shift processes. Their findings indicated that biological water–gas shifts are economically competitive, particularly when considering 3% methane, as opposed to thermochemical water–gas shift processes. Moreover, the expense of biological water–gas shifts is reduced because the reformer and related equipment are eliminated.
Biophotolysis
Water biophotolysis is mediated by two biochemical processes: hydrogenase and nitrogenase [98]. These enzymes function as hydrogen-producing proteins, playing a role in the metabolism of various prokaryotes and some eukaryotic organisms, including green algae [99]. Additionally, Schlegel and Barnea [99] emphasized that, as a biological process, biophotolysis consumes less energy compared to the chemical or electrochemical methods, primarily operating at ambient temperature and pressure.
Certain microalgae from the Chlorophyceae and Cyanophyceae classes can produce molecular hydrogen by decomposition water using solar energy. The bio-photolysis process begins with absorption radiation (visible, infrared, or ultraviolet), which converts light energy into chemical energy. In this context, Dincer [100] highlighted several possible approaches, such as the use of isolated cellular components or algae cultures. However, the authors note that technical and economic considerations limit practical applications to algae cultures. The only algae system proven to meet the basic requirements of biophotolysis utilizes nitrogenous cultures of heterocystous blue–green algae that fix nitrogen. Photosynthetic bacteria can also be employed in hydrogen production from waste. The practical development of biophotolysis systems is hampered by the low efficiency of photosynthesis, a dearth of fundamental scientific knowledge, and substantial economic constraints [100]. The researchers Kotay and Das [98] classified hydrogen production based on biophotolysis into three general categories: direct, indirect, and photo fermentation. In biophotolysis, specially developed photobioreactors are used as biochemical conversion devices for various photosensitive microorganisms. Among the alternative microorganisms studied in the literature, microalgae are considered the most suitable and efficient due to their ability to be cultivated and produce hydrogen in closed systems, facilitating the capture of the produced hydrogen. The key advantage of biophotolysis lies in its capacity to produce hydrogen from water under mild conditions, including moderate temperatures and pressures, as highlighted earlier. Nevertheless, biophotolysis is presently verified solely at the laboratory scale and necessitates complete commercialization before its introduction to the market [101]. Table 4 summarizes the efficiency, scalability, benefits, and disadvantages of the biological processes to produce hydrogen.

Table 4.
Efficiency, scalability, benefits, and disadvantages of hydrogen-producing biological processes.
2.3. Photocatalysis
There are several alternative methods for hydrogen production. This section focuses on two specific approaches: the use of solar energy and photocatalysis. Solar thermal energy can be harnessed through various techniques, including photovoltaic cells and bio-photolysis. Conversely, photocatalysis involves the use of semiconductors to drive chemical reactions when exposed to light, with solar energy acting as the catalyst for these reactions. This process involves capturing this energy in the form of radiation and converting it into chemical energy to produce hydrogen. Photocatalytic hydrogen production is a process that uses energy from sunlight to convert water into hydrogen and involves the use of a photosensitive material, usually a semiconductor material. When this material is exposed to sunlight, it absorbs the light energy and converts it into chemical energy, enabling water to split into hydrogen and oxygen. According to Christoforidis and Fornasiero [102], the production of hydrogen from a photocatalytic process can be achieved through two mechanisms: (I) through direct scission of water into hydrogen and oxygen, known as photocatalytic water cleavage or splitting, or by (II) the photo-reforming of organic compounds. Figure 12 illustrates the photocatalysis process. For photocatalysis to occur, the energy disparity between electrons (e−) and holes (h+) must exceed the energy needed for the desired redox reaction, and the rate of the redox reaction must surpass the recombination rate between e−/h+. In essence, it requires the absorption of light, excitation, charge separation, transport of charge carriers, and surface reactions on the catalyst.
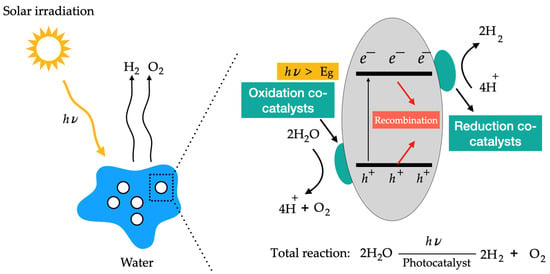
Figure 12.
Schematic illustration of the photocatalysis process.
The main challenge in developing efficient and stable catalysts for harnessing solar light lies in creating co-catalysts that do not rely on precious materials, which is crucial for scaling up and practical applications. Recent advancements in materials synthesis have led to the creation of innovative nanostructures with multi-phase compositions, unique properties, and precise nanoscale morphology control. These materials must not only absorb visible light effectively but also address key challenges, such as charge recombination. Among the various strategies employed, coupling novel or established materials to form multi-phase nanostructures through the creation of heterostructures and heterojunctions/homojunctions has proven particularly effective for enhancing hydrogen photoproduction. This approach holds great promise in advancing the field, showcasing its potential for enhancing the efficiency of solar-driven hydrogen production [103]. Davis et al. [104] emphasize that biomass holds tremendous potential as a sustainable alternative to fossil fuels for future energy production. The photocatalytic biomass conversion process not only generates valuable carbon-free energy in the form of molecular hydrogen but also opens opportunities for producing industrially relevant biomass products. This efficient and sustainable photocatalytic conversion relies on biomass as a reaction material, with inexhaustible sunlight serving as the sole energy source. While approximately 95% of hydrogen can be produced from non-renewable resources, scientists are concentrating on developing cost-effective methods for hydrogen production. One such concept is photovoltaic water electrolysis, where semiconductor materials with small band gaps are utilized. This technology has proven to be a low-cost method for hydrogen production. Alternatively, photocatalytic water splitting using titania as a photocatalyst through solar energy shows great promise for hydrogen production. This method is not only clean and environmentally friendly but also economically viable [105].
2.4. Solar Energy
Solar thermal energy can be used for hydrogen generation through various methods, including photovoltaic cells, solar thermal energy, photo-electrolysis, and bio-photolysis. Photovoltaic cells are employed in water electrolysis, where the electrical energy converted by these cells from solar energy is utilized in the electrolysis process [106]. The photo-conversion efficiency is approximately 20%, while electrolyzers exhibit around 80% efficiency. Additionally, the overall efficiency for solar energy conversion is around 16% [107]. However, challenges such as the high cost of photovoltaic cells and the need to reduce energy consumption, costs, and maintenance for large-scale production must be addressed. Improvements are also necessary in terms of energy efficiency, durability, safety, and reliability of the technology [108]. Dincer et al. [109] have successfully reduced the cost of photovoltaic cells from USD 12 in 1998 to USD 8 in 2008. For a silicon solar cell, the efficiency ranges have increased from 12% to 15% and can reach up to 25% to 30% for gallium arsenide solar cells. In the generation of hydrogen using photovoltaic cells, solar thermal hydrogen production adopts comparable procedures. However, solar energy is captured and focused to attain high-temperature heat sources surpassing 2500 K for the endothermic water decomposition reaction. In this method, water dissociates into hydrogen and oxygen in a single step [110]. In photo-electrolysis, water decomposes directly into hydrogen and oxygen by harnessing sunlight, and the semiconductor employed is akin to that of a photovoltaic cell [111]. This approach incorporates the use of a heterogeneous photocatalyst at one of the electrodes exposed to solar radiation. A photo-electrochemical cell integrates both the solar energy-absorption system and the water electrolysis system into a single unit [112]. The mechanisms of hydrogen production based on photo-electrolysis are outlined as follows [79]:
- A photon with adequate energy generates an electron-hole pair.
- Electricity is produced as electrons flow from the anode to the cathode during a chemical reaction.
- Water undergoes decomposition into H+ cations and oxygen.
- H+ cations are reduced at the cathode, leading to hydrogen production.
- The resulting gases are separated, processed, and stored. In photo-electrochemical cells, two distinct electrodes are involved: a photocathode for reduction and photoanode for oxidation of water.
In the photo-electrochemical cell, the achieved energy-conversion efficiency is primarily determined by the properties of photoelectrode materials [106]. An efficient photoelectrode necessitates great stability, inexpensive materials, low conduction bands, and enhanced absorption capability during the photon generation process in the solar spectrum. Table 5 summarizes the maturity level, economic analysis, benefits, and disadvantages of the reviewed hydrogen production technologies.

Table 5.
Maturity level, economic analysis, benefits, and disadvantages of the hydrogen production technologies.
3. Parameters That Influence the Performance of Hydrogen Production
Hydrogen production can be influenced by different parameters, which depend on the specific technology and process used. In the present section, two important physical parameters are highlighted: temperature and the concentration of the electrolytic medium. As will be demonstrated, temperature significantly impacts the ionic conductivity of water and the kinetics of electrochemical reactions. On the other hand, the concentration of ions in the solution affects electrical conductivity. Thus, proper control of these parameters can optimize the involved chemical reactions and ensure a more efficient production of hydrogen. The hydrogen production increase with increasing electrolyte temperature is consistently observed in works in the literature [113,114,115,116]. The rise in temperature enhances the electrolyte medium ionic conductivity, facilitating and intensifying the passage of the electric current. In water electrolysis, with the increasing temperature of the electrolyte, less energy is needed to reach a particular current density [115,116].
In the experiment conducted by Nikolic et al. [113], the authors observed a linear increase in hydrogen production due to the rise in temperature. The analyzed temperatures varied between 270 and 353 K and were obtained from water electrolysis. According to the authors Kothari et al. [117], an alkaline electrolysis cell operating at elevated temperature values makes it possible to increase the electrolyte ionic conductivity and enhance the electrochemical reaction rates on the surfaces of the electrodes, resulting in an increase in hydrogen production. Kothari et al. [117] conducted experimental evaluations of alkaline electrolysis using highly concentrated solutions of potassium hydroxide at temperature values ranging between 35 and 400 °C and at diverse operating pressures. The study showed a significant enhancement in the electrical energy efficiency of the cell at 400 °C and 8.7 MPa of steam partial pressure.
The researchers Badwal et al. [118] studied the temperature impact of a synthetic alkaline electrolyte on the hydrogen production rate, varying the test temperature between 10 and 80 °C. It was found that with growing electrolyte temperature, the rate of hydrogen production rate also increased, but not in the same proportion. The optimized efficiency of the system was obtained at a temperature of 50 °C using carbon electrodes. Beyond that point, the increase in the production of hydrogen efficiency was not considerable. Acids and bases are recognized for their ability to alter the non-conductive properties of water. The acids and bases significantly reduce the overvoltage of the electrolyzers [119].
The researchers Nikolic et al. [113] explained that while the addition of these substances increases the ionic conductivity, the highly corrosive behavior of the electrodes can become problematic depending on the concentration levels of these solutions. Using a graphite electrode, Nikolic et al. [113] conducted an experimental work on hydrogen electrolytic production, varying the temperature, concentration, reaction time, and applied voltage in an alkaline KOH water solution. Additionally, they compared the results with other commercially available electrodes, such as carbon rods, EN8, and 316 L stainless steel. Regarding the effect of solution concentration, their main conclusions were that hydrogen production increases significantly with the rise in electrolytic concentration, using graphite as the electrode. Graphite also proved to be the superior alternative concerning the hydrogen evolution reaction. However, for high electrolytic concentrations (above 0.025 M), long-term hydrogen production is not beneficial. Furthermore, a gradual decomposition of the anodic graphite rods was observed in such a situation [120], and an experimental study was conducted to determine the effects of the concentration of nanoparticles and the use of an external light source to heat a nanofluid composed of carbon black, surfactant sodium dodecyl sulfate, and water as base fluid during the electrolysis process. Carbon black is a material with good absorption throughout the sunlight wavelength range, which is why the researchers chose nanoparticles. The main results obtained by the researchers showed that both nanofluids and the use of external light can increase the hydrogen production rate. The maximum hydrogen production rate occurred using light and a concentration of 0.1% by weight of the nanoparticles. In the absence of light, the peak rate was reported at a concentration of 0.05% wt. At this concentration, the hydrogen production rate was enhanced by approximately 30.4% after 20 min of the electrolysis process. In general, increasing the concentration of nanoparticles results in a growing hydrogen production rate and in the electrical conductivity of the electrolyte, but this occurs up to a certain limit, after which it decreases for both situations.
4. Environmental Impact of the Hydrogen Production Technologies
The environmental impact of the Hydrogen Production technologies should be evaluated through LCA analysis. This analysis should be conducted considering the following boundaries:
- Production of energy sources and raw materials, including biomass, oil, coal, biogas, natural gas, and water.
- Inter-operational transportation of the raw materials.
- Production technologies like electrolysis and thermochemical processes.
- Purification technologies.
- Hydrogen storage in compressed tanks and geothermal reservoirs.
- Transportation of hydrogen in liquified or gaseous form via trucks, compressed gas tube trailers, and pipelines.
- Emissions during hydrogen utilization, like hydrogen-powered vehicles and trains, the generation of power employing hydrogen, and the generation of energy in refineries.
- Emissions from the waste treatment technologies to air, water, and land.
To guarantee the sustainable production of clean hydrogen, it is essential to critically evaluate the different production methods and their environmental impacts, including storage and usage options that account for seasonal variations. Hydrogen can be produced from both fossil-based and renewable sources, with each offering distinct advantages and challenges. However, the current classification of hydrogen by color is not entirely accurate—green hydrogen does not always result in lower carbon emissions compared to blue or grey hydrogen, contrary to common assumptions.
The water splitting by electrolysis has an increasing production share, but meeting a quarter of the energy demand with hydrogen in a scenario of climate change mitigation will certainly require huge quantities of extra renewable electricity generation. Under these circumstances, a huge number of watt-hours of electricity would be required to power electrolyzers. This amount would be superior to the one that is currently worldwide produced from all sources together.
Moreover, hydrogen production will require a large quantity of freshwater, and its supply is already globally seriously depleted, making it one of the main environmental concerns of our time. Consequently, the use of seawater could overcome this limitation, but seawater poses considerable challenges like the corrosion of its chloride ions to the anode metal, hindering its large-scale utilization to produce hydrogen.
Finally, processes like steam methane reforming and water–gas shift reaction release considerable quantities of carbon dioxide emissions and, hence, must consider the additional installation of carbon capture and storage equipment and systems. Also, the use of amines that can absorb a major part of the carbon dioxide emissions is an adequate manner to decrease the toxic emissions.
5. Hydrogen-Storage Technologies
Effective storage methods pose one of the major obstacles to the development of vehicles powered by hydrogen fuel cells. Gómez and Santos [121] highlighted that the lack of safe, lightweight, and energy-efficient means for onboard storage is a crucial issue to be addressed. In a recently published work, Rivard et al. [122] underscored the necessary conditions for storage tanks, emphasizing the importance of selecting materials that can withstand large tensions and deformations. Furthermore, the authors highlight that the geometry of the reservoir is crucial, with cylindrical containers being the most suitable for fitting into vehicles. Finally, they highlight the importance of the material having sufficiently high thermal conductivity to effectively manage the exothermic heat generated during the tank-filling process. During this process, compressed storage can provide a high filling rate but can also cause the release of hydrogen. Figure 13 below illustrates the main types of cylindrical high-pressure vessels used for storing compressed hydrogen.
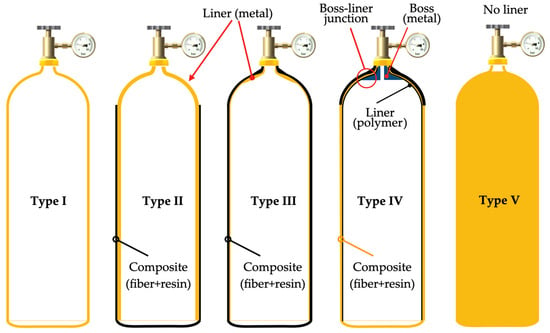
Figure 13.
Types of pressure cylinders for storing compressed hydrogen.
A brief description regarding the classification of the tanks, presented in Figure 4, is provided in the sequence:
- Type I: Designed to withstand pressures between 200 and 300 bar [123]). It has the disadvantage of providing an extremely low gravimetric energy density, around 1% by weight. As they are made of steel or aluminum alloys, the weight of the device makes it less attractive. However, it is the cheapest option on the market [124].
- Type II: Due to being constructed from a fiber and resin composite, they are around 30–40% lighter than the tanks of Type I, but, on average, are 50% more costly than those [123,124]. In summary, the Type I and Type II tanks are unsuitable for onboard applications.
- Type III: Constructed from a plastic casing reinforced with carbon fiber with a metallic coating, usually made of aluminum, as highlighted by the authors Rivard et al. [122]. They offer the advantage of hydrogen storage from 25% to 75% greater than the previous Type I and Type II tanks, respectively [124]. Gómez and Santos [121] emphasize that these tanks are durable and lightweight, but their thermal conductivity is low, which can impact the compression and hydrogen release phases during the charging process. Finally, it should be noted that they are suitable for pressures of up to 450 bar. As highlighted by Usman [123], they can also be utilized under pressure values of up to 700 bar.
- Type IV: Commonly referred to as high-pressure tanks, Type IV vessels are designed for storing hydrogen at a pressure of 700 bar [123]). According to Rivard et al. [122], they are entirely composed of composite materials like Type III. Nonetheless, the primary distinction lies in the lining material employed in this type of tank. In contrast to Type III vessels, where the coating is predominantly metallic, contributing with a minimum of 5% to the mechanical resistance, Type IV vessels mostly utilize polymeric coatings, such as high-density polyethylene, with minimal or no metallic content.
- Type V: Additionally, as noted by Rivard et al. [122] and Herdem et al. [124,125], there is a new type of tank that incorporates reinforcing space-filling skeletons to attain even greater gravimetric and volumetric hydrogen densities than Type IV. However, it is not yet commercially available.
Among all the options presented above, the Type IV tank is the most used tank in hydrogen-powered vehicles. Figure 14 represents a typical Type IV compressed hydrogen storage system, as mentioned previously.
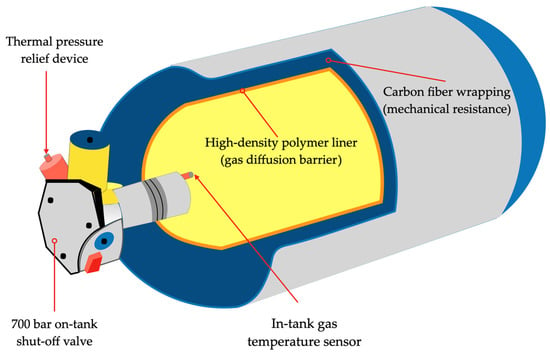
Figure 14.
Schematic drawing representing the internal and external components of a typical Type IV tank for the storage of compressed gaseous hydrogen.
The main benefits of liquid-state hydrogen compressed storage are the abundant commercial availability, elevated storage capacity, and denser hydrogen packing at low temperatures. However, it has limitations associated with the need for energy-intensive cooling systems, which affect global efficiency, and proper insulation for maintaining low temperatures, which enhances the cost and complexity of these solutions.
The following lines will briefly describe the benefits and limitations of other types of hydrogen storage methods and materials.
5.1. Solid-State Hydrogen Storage
Solid-state hydrogen storage stands out as an attractive option due to its potential for high-density storage, enhanced safety, and ease of transportation. Considering the solid-state hydrogen storage, it is worth noticing that activated carbon is one of the possible methods because of its great availability, manufacturing easiness, and cost-effectiveness. Nonetheless, this method presents merely a moderate storage capacity and its susceptibility to contamination. Another methodology applying innovative materials is the one that uses carbon nanotubes due to their lightweight, thermal stability, and mechanical robustness. Nevertheless, this solution must overcome challenges like the ones linked with the regulation of nanotube dimensions and susceptibility to structural damage. Also, the metal–organic frameworks with their open pore structure have the capability to operate under low pressures and have rapid hydrogen adsorption and desorption kinetics. But they only work at low temperatures, are sensitive to moisture sensitivity, and are less stable at high temperatures and pressure values. The last method of solid-state hydrogen storage is the one that uses metal hydrides entailing the advantageous features of increased storage capacity, safety, and efficiency in compact storage systems. Nevertheless, it is an expensive option and presents slow hydrogen absorption and desorption kinetics.
Some processes, such as catalysis, alloying with other elements, nanostructuring, and nanoconfinement, are used to improve the performance of solid-state hydrogen storage devices, as highlighted recently by [126].
Catalysis improves the kinetics of hydrogen uptake and release, primarily using transition metals and metal oxides. Ti, V, and Ni offer good performance at lower costs, while Nb2O5 enhances hydrogen storage capacity [127,128].
Alloying with transition metals such as Ti, V, and Ni also improves thermodynamic and kinetic properties [129]. Mechanical alloying through ball milling reduces particle size, creates defects, and improves hydrogen diffusion, leading to better kinetics and lower operational temperatures [130].
Nanostructuring increases surface area and creates defects, improving sorption rates [131,132]. For example, Mg nanocrystals within graphene and Ni-decorated graphene-supported LiBH4 composites exhibit lower dehydrogenation temperatures and improved cycling stability [132].
Nanoconfinement uses materials such as carbon nanotubes, providing stability and preventing agglomeration, which improves sorption reversibility [133]. For example, nanoconfined MgH2 can release hydrogen at 200 °C in 5 min instead of 400 °C in 60 min [127].
5.2. Liquid-State Hydrogen Storage
Considering now the liquid-state hydrogen storage, the liquid organic hydrogen carriers can store a high amount of hydrogen in a comparatively small space and are safe. However, they need a large amount of energy to convert hydrogen to and from the liquid organic hydrogen carriers, and this energy can be costly, particularly in large-scale applications. The ammonia solution presents elevated hydrogen storage capacity, rapid hydrogen kinetics, and cost-effectiveness.
5.3. Gaseous-State Hydrogen Storage
Finally, the gaseous-state hydrogen storage is very suitable for fuel cell vehicles, can be rapidly refueled, and can be easily integrated into the existing facilities and infrastructures. Nonetheless, this type of storage requires large volume and hydrogen embrittlement, and the used pressure vessels may present leakage issues and energy loss at high pressures, and it is a costly alternative.
6. Some Important Applications
Two important applications of hydrogen are highlighted: the production of green hydrogen and hydrogen-powered cars. These applications underscore the potential of hydrogen as a viable and sustainable alternative in key sectors, facilitating the transition to cleaner and renewable energy sources.
6.1. Production of Green Hydrogen
In the current scenario, marked by the incessant search for sustainable energy solutions, green hydrogen appears as the big bet. Due to its remarkable energy potential, green hydrogen has the power to transcend borders and become a global commodity. This promising prospect is driving collaboration between nations in the production and distribution of this resource, accelerating the search for ways to make it economically viable soon. After a careful analysis of the most recent works in the specialized literature, it is concluded that, depending on the used methods and scale, the production, storage, and transport of green hydrogen represent the biggest challenges and bottlenecks to be resolved. The production of green hydrogen requires the exploration of the electrolysis of water, which demands electricity to separate the molecules of water into hydrogen and oxygen. Consequently, the availability of clean and renewable electricity can be a limiting factor. In turn, the costs and efficiency of electrolyzers can be expensive, making production more costly and less efficient. As it is a low-density, high-volume gas, hydrogen must be compressed, liquefied, or stored in robust tanks, such as those made of metal hydride, to make it viable. Tanks require adequate safety systems as hydrogen leaks can be harmful. Transporting hydrogen may necessitate special piping or the use of liquid or gaseous carriers due to its low energy density. If transported over long distances, it can incur significant energy losses unless advanced technologies, such as transportation in liquid form, are employed.
Herdem et al. [125] conducted a brief review of the most recent research available in the literature to evaluate systems that utilize renewable energy, specifically solar and wind, to produce green hydrogen. In their approach, the researchers considered factors such as the system’s location, its connection to the electrical grid, the type of research conducted, the intended use of hydrogen, and the performance indicators adopted in these systems. Towards the end of their paper, the authors draw attention to the lack of standardization in indicators for comparing different green energy-based hydrogen production systems. Therefore, based on their review work, they suggest a set of measures to be considered. Table 6 summarizes these indicators.

Table 6.
Principal indicators suggested by [125] to analyze the systems of production of green hydrogen.
Karayel et al. [138] conducted a comprehensive review of the options from energy storage for green hydrogen. Using information obtained from data taken from official sources, researchers compared several hydrogen storage systems. They considered high-pressure tanks and storage systems based on chemical and solid-state materials. Additionally, they examined different tank materials, capacity, costs, and greenhouse gas emissions. The authors demonstrated that it is possible to augment the hydrogen storage capacity by 457.7% by changing the pressure from 100 bar to 800 bar for a tank made of carbon fiber composite material with coatings of thermoplastic polymer. Regarding greenhouse gas emissions, the impact is lower for the retention of liquid hydrogen, while emissions are higher for metal hydride storage tanks.
Recently, Tsiklios et al. [139] conducted a comprehensive analysis of green hydrogen storage and transportation technologies at a large scale. In their study, they explored the economic factors that influence current projects and government decision-making related to this emerging technology. The results presented by the researchers go beyond the exclusive consideration of the technical aspects of hydrogen’s energetic power, providing valuable insights into the current state of the technology and indicating promising paths for its future development. The automotive industry is one of the most committed sectors in developing hydrogen-powered vehicles, aiming to provide an even more environmentally friendly alternative to electrically powered transportation. This is partly due to the problems associated with electric car batteries. While electric vehicles have gained popularity owing to their lower environmental impacts in comparison to internal combustion engine vehicles, the batteries that power these cars are not without issues. Some disadvantages can be highlighted: (i) the energy storage capacity of batteries decreases over time, which can result in reduced ranges and high replacement costs; (ii) the production process, extraction, and availability of essential raw materials, like cobalt and lithium, can be harmful to the environment and have negative impacts on communities near extraction mines; (iii) improper disposal of used batteries poses a significant threat to the environment due to chemical contamination; (iv) the lack of accessible charging stations and the lengthy charging process are concerns that affect the mass adoption of electric cars. Not to mention that it is of no use for the car to be electric if the charging stations use non-renewable energy sources. In this context, hydrogen-powered vehicles stand out. They use fuel cells to generate electricity, eliminating the need for large batteries and allowing for shorter charging times. Moreover, hydrogen can be produced in a more sustainable manner using renewable energy sources. However, the large-scale transportation of hydrogen is still a considerable challenge. The most common ways to transport green hydrogen include the use of pipelines, tanker trucks, ships, and trains, and they are represented by Figure 15. To emphasize the discussions in the current work, the two most usual transportation options will be highlighted: pipelines and tanker trucks. Each of these transportation routes has benefits and limitations.
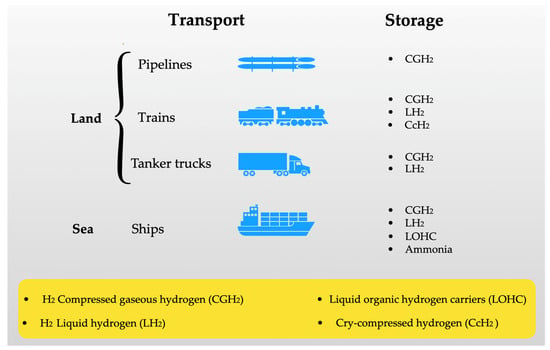
Figure 15.
Examples of ways to transport and store hydrogen.
Tsiklios et al. [139] evaluated the environmental behavior and energetic efficiency of hydrogen transportation from the pipeline large-scale networks using life cycle assessment and thermodynamic analysis. Technical specifications were based on the current design of the hydrogen compressor stations and pipelines. So, the researchers developed a thermodynamic model in Dymola that was obtained from the Modelica language. They considered a reference pipeline system having 13 GW of transportation capacity, a nominal diameter of 48″, an absolute roughness of 0.1 mm, a transport distance of 500 km, pressures of up to 100 bar, annual operation hours of 8000, and other parameters that can be checked in the work of the authors. For the compressor, a centrifugal compressor type was used as the reference, with a mechanical–electrical efficiency of 0.96, an isentropic efficiency of 0.8, a maximal pressure ratio of 1.2, annual operating hours of 8000, and a lifetime of 20 years.
The results indicated by the model presented by Weber and Perrin [140] highlight the key design and operation parameters for hydrogen pipelines. Three key measures are recommended: (1) keeping the pipelines smooth or cleaning them to reduce roughness; (2) moderately decreasing the load capacity; and (3) reducing intervals between transports using intermediate compressor stations. The goal is to minimize pressure losses to make hydrogen transmission efficient and environmentally friendly in large-scale networks.
To be capable of transporting hydrogen, especially if used over long distances, the piping materials must possess physical properties like enough strength, ductility, tenacity, and weldability, in addition to maintaining economic viability [141]. Moreover, according to Sharma and Maheshwari [141] and Cristello et al. [142], the materials must be capable of withstanding high pressure to enable hydrogen transmission. The most suitable materials for these requirements are high-strength low-alloy steels, which consist primarily of iron (98–99% by weight), with carbon (≤0.30% by weight), manganese (0.30–1.5% by weight), and small amounts of other alloying elements such as titanium, vanadium, and molybdenum. Currently, there is a trend to make use of existing pipelines to transport green hydrogen. This is because pipelines are already established infrastructure for the transportation of gases like natural gas and can be reconfigured to accommodate green hydrogen. Recently, the researchers Yang et al. [143] conducted a comprehensive study on the feasibility of mixing hydrogen to be transported in the natural gas network. The authors point out that it is pivotal to determine if the metallic materials are compatible or not with the hydrogen in the gaseous state.
The use of existing pipelines can be a more cost-effective and time-efficient alternative than building new, exclusive transportation infrastructure for hydrogen. However, it is important to emphasize that technical and safety adaptations need to be made to ensure the operational readiness of the facilities.
In the case of road transportation, hydrogen can be transported in both a compressed gaseous form and a liquid form using trucks/trailers [144]. Furthermore, according to the authors, in these cases, well-established hydrogen-storage technologies such as cryogenic liquid tanks and pressure vessels can be employed. Figure 16 summarizes all stages of production, storage, transport, and possible usage of green hydrogen in public or private vehicles.
Figure 16.
Stages of production, storage, transport, and potential use of green hydrogen in vehicles.
6.2. Hydrogen Fuel Cell Vehicles
Hydrogen fuel cell vehicles are a breakthrough technology that is poised to benefit the auto and transportation industry for years to come. Hydrogen fuel cell vehicles use a sustainable approach to propulsion. Through the fuel cell, they convert oxygen and hydrogen into electricity, obtaining water as the only by-product. The authors Lipman et al. [145] conducted a 2-year real-world study to examine the behavior of hydrogen fuel cell electric vehicles and driver acceptance. Still, according to Lipman et al. [145], despite the positive response regarding the car’s autonomy, when asked about the possibility of acquiring one of these vehicles—excluding the higher vehicle price and the reduced number of hydrogen filling stations—the main reason was the high price charged to fuel the vehicle with hydrogen. Many of the hydrogen refueling stations are composed of pre-coolers, storage tanks, dispensers, and compressors [146]. The latter, according to Jin et al. [147], is the most susceptible to failure and the most expensive investment. Figure 17 shows examples of the actual arrangement of the hydrogen tanks located inside of two commercially available vehicles.

Figure 17.
Hydrogen tanks disposition in the Toyota Mirai (left) and Honda Clarity (right).
The following lines briefly describe the general functioning of the available hydrogen vehicles. The hydrogen in the high-pressure tanks is pumped to the fuel cell stack where it is mixed with oxygen from the air intake positioned in front of the car. The reaction that occurs in the stack produces an electrical current. The voltage is strongly enhanced by a boost converter. When the car is coasting or braking, the electric motor acts as an electricity generator and the additional amount of electricity produced by the motor is stored in a so-called drive battery, which aids the motor during the acceleration process. Also, the fuel cells of hydrogen-driven vehicles can have the following general work order: the hydrogen gas enters the fuel cell anode, and it reacts with the catalyst (e.g., platinum) coating the anode, stripping the hydrogen of its electrons. Hydrogen ions pass by an electrolyte polymeric membrane to the cathode. The electrons flow around the polymeric membrane in an electrical circuit, generating driving power. At the cathode, the catalyst causes the ions and electrons to bond with the oxygen from the air to form water vapor, which is the only by-product of all processes.
Recently, Zhou et al. [148] proposed a mathematical model based on the FSI method to analyze the dynamic features of a free piston present in an ionic liquid compressor designed for use in hydrogen-filling stations. According to the authors, there has been limited research on ionic liquid compressors, and even less on the dynamic features of the free piston that interacts with the gas compression and hydraulic drive systems. According to Amin [149], the ionic liquid compressor substitutes the traditional connecting rod piston with a free piston and a liquid column. Such compressors would replace and address issues found in various types of traditional compressors utilized in refueling stations of hydrogen. Problems include leakage (reciprocating piston compressors) [150], fatigue fractures (diaphragm compressors) [151], and sealing and lubrication concerns (hydraulic fluid-driven piston) [152]. The ionic liquid can contribute to sealing and lubricating hydrogen within the compressor [153]. Additionally, during gas compression, the liquid piston can surely increase heat transport and, as a result, boost the volumetric efficiency, significantly advancing the isotropic efficiency of the compressor [154,155].
6.3. Photovoltaic Powered Hydrogen Electrolysis
The photovoltaic-based hydrogen-generation systems generally are means of hydrogen production with great effectiveness and high solar energy-to-hydrogen conversion efficiency. The major issues associated with this type of technology are the production rate, storage, safety, weather variability, and photovoltaic cell thermal management. The photovoltaic–electrolysis systems comprise photovoltaic cells that generate the electricity to power the electrolyzer. These systems are powered by the electricity generated from solar energy and produce large amounts of high-purity hydrogen without environmental impacts. Figure 18 schematically represents a typical photovoltaic–electrolysis equipment.
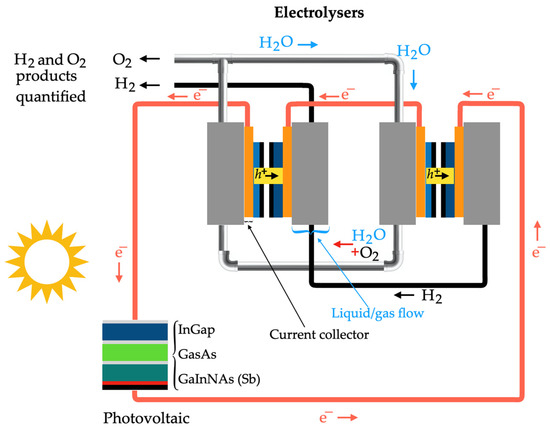
Figure 18.
Diagram of a typical photovoltaic–electrolysis equipment.
The photovoltaic–electrolysis system depicted in Figure 18 features a three-junction solar cell paired with two series-connected PEM electrolysis units. The solar cell was maintained at 25 °C through a water-cooling system and exposed to solar irradiation using an AM 1.5D sun simulator. The electrolysis units are connected in series with the photovoltaic cell. Water was supplied to the anode compartment of the first electrolysis unit, while there was no water input to its cathode. Water and oxygen from the first unit’s anode were directed to the anode of the second electrolysis unit. Hydrogen produced at the cathode of the first unit was transferred to the cathode of the second unit. Hydrogen and oxygen produced in the second electrolysis unit were collected, while the remaining water was captured in a tank and recycled back into the system. The solar photovoltaic–thermal–electrolysis hybrid systems are composed of photovoltaic cells and proton-exchange membrane electrolyzers. More specifically, the photovoltaic–thermal–electrolysis systems comprise a photovoltaic–thermal array, DC/DC converter, and electrolyzer. Figure 19 presents the diagram of a typical photovoltaic–thermal electrolysis hybrid system.
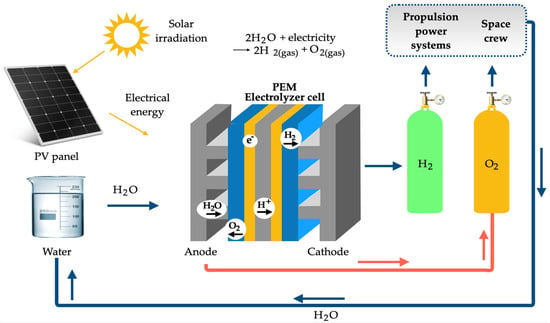
Figure 19.
Diagram of a typical photovoltaic–thermal–electrolysis hybrid system.
The performance of the photovoltaic–thermal systems has also been tested using the proton-exchange membrane electrolysis cell for hydrogen production [156]. The photovoltaic–thermal system provides the necessary current for the proton-exchange membrane electrolysis cell and preheats the feed water. An experimental study [157] investigated hydrogen production by exploring solar energy through a hybrid photovoltaic–thermal system. Electrical energy was generated to power the alkaline water electrolysis, while thermal energy was used to heat the circulating water mounted on the back of the photovoltaic panel. The hydrogen production unit was tested at various temperatures of the electrolysis water. The optimal design achieved a peak hydrogen production rate of nearly 154 milliliters per minute, with a system efficiency of approximately 21%. The daily hydrogen output was around 221 L per day. The photovoltaic–hydrogen system represents a sustainable approach to hydrogen production, leveraging photovoltaic panels to generate electricity for powering the electrolysis unit. This system is widely used for biohydrogen production due to its low cost, straightforward implementation, and improved performance. Research indicates that incorporating a photovoltaic tracking system can offer superior performance compared to traditional photovoltaic systems, although it comes at a higher cost. Additionally, using a concentrated photovoltaic system has been shown to enhance efficiency relative to conventional photovoltaic systems [158]. As was already found, the photovoltaic–hydrogen systems combined with proton-exchange membrane electrolysis have been offering ever-increasing performances. Much research focuses on enhancing the efficiency of the photovoltaic–hydrogen system and enhancing productivity while reducing the overall cost. Indeed, in 2010, the efficiency was improved and reached the value of 12.4% by using direct coupling between the photovoltaic system and the electrolyzer [159]. The productivity cost of hydrogen decreased from USD 40/kg in 2008 to USD 3.4/kg in the year 2022 [160]. This result derives from the working voltage system that is steadily available from the photovoltaic system to drive the electrolysis. Many other studies investigated the suitability between photovoltaic systems and electrolysis, and the results show that the photovoltaic–hydrogen systems are more useful when applied in remote areas, where the conventional grid is not installed due to the very high price of electricity transportation [161].
The photovoltaic–hydrogen system enables the production of hydrogen, as well as its use to provide electricity by a fuel cell that is essential for a nocturnal profile or winter seasons [162]. When solar irradiation is insufficient to supply the necessary electricity for electrolysis, the electricity generated by a fuel cell can be used to power the electrolysis unit [163]. Comparative cost analyses have shown that using grid–hydrogen for hydrogen production is more economical than employing grid–photovoltaic–hydrogen or photovoltaic–hydrogen systems [164]. Bifacial solar panels have been utilized to boost efficiency and increase hydrogen production [165]. Studies have demonstrated that bifacial panels achieve an efficiency of 13.5%, compared to 11.6% for monofacial panels, leading to a rise in hydrogen production from 3.7 g/h/m2 with monofacial panels to 4.2 g/h/m2 with bifacial panels. Incorporating batteries into a photovoltaic–hydrogen system enables hydrogen production even during nighttime, further enhancing overall hydrogen yield [166]. Additionally, the use of a high-efficiency DC/DC converter has been shown to improve overall system efficiency [167]. A photovoltaic–thermal system can provide both sufficient electricity and heat energy, which not only increases hydrogen production through electrolysis but also reduces costs. These systems, including those designed for heat and cooling applications (e.g., photovoltaic–thermal–water systems) [168], offer improved performance compared to traditional photovoltaic–hydrogen systems.
The concentrated solar power (CSP) system generates both electricity for the electrolysis unit and heat energy to produce steam for absorption cooling cycles. This system, known as CSP–hydrogen, includes a solar collector, a parabolic dish collector, and an electrolysis unit. It serves multiple functions, including hydrogen production, electricity generation, cooling, heating, and distilled water supply. The efficiency of this multi-generation system improves with increased solar radiation, which reduces the operating temperature of the electrolysis unit and enhances its current density. Studies on CSP–hydrogen systems have shown that their exergy efficiency ranges from approximately 21% to 36%, while energy efficiency varies between 34% and 72% [169].
In multi-generation systems that utilize CSP for hydrogen production, additional energy sources like geothermal energy are often incorporated to boost overall efficiency [170]. These systems can achieve a hydrogen production cost of approximately USD 2.80 per kilogram and an electricity cost of about USD 0.03 per kWh. To generate electricity directly from a solar concentrator, a Stirling engine can be positioned at the focus point to drive the electrolyzer [171]. Comparative studies of hydrogen productivity between photovoltaic–hydrogen systems and CSP–Stirling–hydrogen systems revealed that the photovoltaic–hydrogen system produced around 268 kg of hydrogen, while the CSP–Stirling–hydrogen system generated approximately 302 kg [172].
In summary, current research on various hydrogen production technologies highlights the significance of evaluating both the cost and technical viability of green hydrogen across different scenarios and assumptions. Green hydrogen, derived from renewable sources, is anticipated to become a major contributor to hydrogen production. Additionally, hydrogen facilitates the storage of renewable energy, which can be utilized for transportation, industrial heating, and electricity generation. This study examines the use of solar and wind energy as green sources to power electrolysis for hydrogen production. Photovoltaic systems are particularly beneficial in remote areas lacking a grid, providing a practical solution for generating electricity. Moreover, using solar energy to produce hydrogen allows for energy storage and conversion, enabling hydrogen to be sold, stored, or transformed into electricity via fuel cells. Renewable hydrogen that is fully converted into electricity can be fed back into the grid, supplying solar power during periods without sunlight. The cost of hydrogen production is influenced by the type of renewable energy source and the electrolysis technology used.
Electrolyzers typically require a significant amount of electricity as input, making them relatively costly due to both the high energy demands and the expense of the equipment. Electrolyzer units can be categorized into low-temperature types, such as alkaline water electrolysis, proton-exchange membrane (PEM), and anion-exchange membrane (AEM) electrolyzers, which need only a direct current (DC) electrical source for water splitting. In contrast, high-temperature electrolyzers, like solid oxide electrolyzers, necessitate both heat and power to facilitate water decomposition. Among these, proton-exchange membrane (PEM) electrolyzers are the most commonly used and effective. However, solid oxide electrolyzers are particularly well-suited for concentrated solar power–hydrogen systems due to their ability to operate at the high temperatures required for efficient performance.
The photovoltaic–hydrogen system is very suitable in remote and arid areas, requires low maintenance, and it does not need a power cycle to produce electricity. The concentrated solar power–hydrogen system needs a power cycle. The cost and efficiency of hydrogen production systems depend on the weather conditions, installation cost, productivity of hydrogen per day, energy source, and type of electrolysis. Table 7 summarizes the fundamental benefits and disadvantages of photovoltaic-assisted hydrogen production. Table 8 compares the photovoltaic and concentrated solar power hydrogen production methods.

Table 7.
Main benefits and disadvantages of the photovoltaic-assisted hydrogen production methods.

Table 8.
Fundamental benefits and performance of the PV-assisted hydrogen production systems.
7. Power Electronics
The evolution of power systems exploring hydrogen is based on an ever-increasing need for power electronics equipment. The operation at large-scale water electrolysis can be determined by a high DC current in the kiloampere and a few hundred volts ranges. Hence, the rectifier is vital for converting the AC current of the electricity grid into the DC current furnished to the electrolyzer. The power electronic converters are also taken as one of the essential components of the electrolyzers using water. Renewable forms of energy like hydrogen come from equipment and plants that are connected to medium-voltage or high-voltage AC power grids via an AC/DC/AC frequency converter and a DC/AC inverter, respectively. The obtained renewable electricity is then transmitted by the power grid to the hydrogen generation site. The converter is vital for the renewable hydrogen plant because it converts the MV AC electricity from the grid to a regulated, high-current DC power flow that is supplied to the electrolyzer for industrial-scale hydrogen generation. The generated hydrogen can be utilized or further converted to methanal and methane, which are extensively explored in many technologies, including electricity generation. The overall cost, efficiency, reliability, and power-quality level are the critical power-to-hydrogen performance metrics of the power-to-hydrogen conversion systems, being appreciably influenced by the configuration of the power-to-hydrogen converters. The thyristor AC/DC power converters are the most preferred cases for high-power AC/DC ends, which is caused by their improved characteristics, including enhanced efficiency and robustness and comparatively reduced cost [176].
A commercially available thyristor-based converter can supply 1.5–10 kA of DC current with a DC voltage of 1 kV, delivering a power peak of 10 MW [177]. Nonetheless, the high firing angle operation of thyristors may lead to strong current distortions and decreased power factors at the AC input. Therefore, passive/active power filters and reactive power compensators are indispensable, augmenting the cost of the system. In addition, a noticeable tendency in recent years is to employ a buck-type chopper as the rear stage of the diode/thyristor rectifier [178]. A commercial chopper rectifier usually supplies a peak of 10.4 kA and 1.1 kV at the DC output, delivering 10 MW of power to the electrolyzers [179]. This will provide enriched power quality and a higher power factor within a broader working range of the converter. Additionally, the emerging active front end, based on the B6 converter, is also a promising choice for power-to-hydrogen purposes [180]. It brings much fewer input current distortions by fully controlling the input AC current waveform. In this way, the active/passive power filters and the reactive power compensators can be prevented. In the work conducted by Mohamadian et al. [181], several thyristor-based AC/DC high-current rectifier configurations were evaluated and compared apart from the chopper–rectifier and active front-end possibilities. It was concluded that the insulated-gate bipolar transistor chopper rectifier was a very promising option for supplying kiloampere-scale currents.
Solanki et al. [176] observed that the diode- and thyristor-based multi-pulse rectifiers have an on-load tap changing transformer and chopper–rectifier topologies. Recommendations for further investigation trends on modular converter topologies exhibiting medium-frequency transformers and high-frequency transformers were also proposed. Also, a simplified electrical diagram of a hydrogen electrolyzer is exhibited in Figure 20, where there are indicated typical values of reverse voltage potential, capacitance, and ohmic resistance during water splitting. The ohmic resistance demonstrates a parametric enhancement with the more intense aging process of the electrolyzer.
Figure 20.
Simplified electrical diagram of a typical hydrogen electrolyzer.
The output voltage is between 640 and 1000 V because of the change in the current density and electrolyzer deterioration. The output power reaches 10 MW employing two sets of the 5 MW electrolyzer stack, presenting a rated current of up to 5 kA for each. A future tendency of the load requirements is to display a current ripple inferior to 5%. A typical MW-scale power-to-hydrogen converter is connected to an MW-scale 6.6–35 kV power grid in which a step-down line-frequency transformer is indispensable in between. It should be stated that there are many specifications for power-to-hydrogen converters as consumption devices. In the first place, converters must keep the regular operation under background frequency and voltage deviations, in the range from 47 to 63 Hz and between 90 and 110% of nominal voltage, respectively. Moreover, harmonic distortions and interference between 2 and 9 kHz should be achieved in accordance with the IEC 61000-3-6 standard [182]. The current total harmonic distortion and power factor at the connection point should be inferior to 5% and superior to 0.9%, respectively. Also, in order to accomplish the requirements of electromagnetic compatibility, the IEC 61000-6-2 [183] and IEC 61000-6-4 standards [184] stand for the power-to-hydrogen power converters. The multi-pulse thyristor rectifier is one of the most used and improved components for high-power rectification purposes. The block diagram of a 12-pulse thyristor rectifier 12-TR is represented in Figure 21.
Figure 21.
Block diagram of a 12-pulse thyristor rectifier.
A three-winding wye–delta–wye line-frequency transformer is connected to eliminate the fifth- and seventh-order harmonic currents. After that, two six-pulse thyristor rectifiers are connected to the secondary windings of the line-frequency transformer. The electrolyzer current can be controlled by regulating the firing angle αf of the dual thyristor rectifiers. A high firing angle is commonly employed in low-power operating conditions, conducting additional reactive and harmonic components. The power factor and overall demand distortion are affected by the firing angle, and a high firing angle conducts a decreased power factor and extra waveform distortions. Hence, it is pivotal to compensate for harmonic currents and reactive power at the connection point. Also, the passive trap filters adjusted to the 11th and 13th order of line frequency are normally explored in power-to-hydrogen converters using the 12-TR configuration. Furthermore, a shunt-passive and high-pass filter may also be implemented to bypass the high-order current harmonics. Another suitable configuration adopted for the hydrogen power converters consists of the 12-pulse diode rectifier having multi-phase choppers 12-DRMC [185]. The multi-phase chopper bridges can be implemented via silicon-insulated-gate bipolar transistors and freewheeling diodes, disposed of in alternate layers to reduce the current fluctuation by the electrolyzer. The electrolyzer power and current are controlled by altering the duty ratios of chopper-insulated-gate bipolar transistors. In comparison with the 12-TR, one considerable advantage of the 12-DRMC is the ameliorated power quality, concerning the comparatively low current distortion and steadily high power factor within the working range of the hydrogen converter. However, forward steps are still required to ameliorate the power quality at the connection point. The commonly used methodology uses the 11th- and 13th-adjusted shunt-passive trap filters and a shunt high-pass filter.
Currently, the technology of the high-current chopper–rectifier is mature, and its total cost has diminished as it is presently comparable with the 12-TR system. Considering the benefits and disadvantages of the aforementioned topologies, a hybrid architecture consisting of a 12-pulse thyristor rectifier and an active shunt-power filter 12-TRASPF was proposed [186]. The electrolyzer is supplied by the 12-TR because it provides a high current ability and diminished cost. The concerns about the power quality of the 12-TR are counterbalanced using a low-power shunt-active power filter. Even though the cost and losses of the 12-TRASPF system may be superior to those of the 12-TR, an increased power quality is expected. Another attractive topology choice is the active front-end rectifier [180], and its corresponding block diagram is depicted in Figure 22.
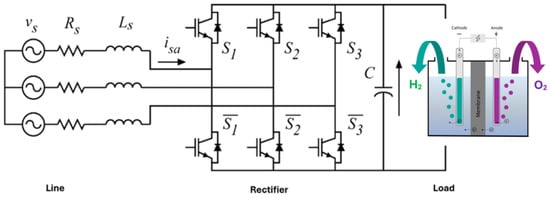
Figure 22.
Block diagram of an active front-end rectifier.
The classic B6 converter is composed of insulated-gate bipolar transistor half bridges. In the cases where a wide range of working ability is needed, an alternative B6 and chopper architecture should be employed to adjust the voltage and output current. Because of the current rating limitation of commercially available insulated-gate bipolar transistors, multiple insulated-gate bipolar transistor-based B6 converter stacks configured in parallel are imperative for a 10 MW power threshold. Three-winding line-frequency transformers employed in the 12-TR and 12-DRMC remain usable in active front-end systems to provide the intended power rating and galvanic isolation, and to attenuate the harmonics, even though such harmonics are lesser components for a B6-active front-end system. Moreover, in the active front-end rectifier system, the input-side AC current can be controlled to be sinusoidal by adjusting the duty ratio of the silicon-insulated-gate bipolar transistors so that much reduced current harmonic distortion is obtainable for such active front-end rectifier systems. In addition, the phase shift of the AC current in reference to the grid voltage can also be regulated through controlling the modulation signal of the B6 converter so that a high-power factor can be obtained at the same time. Thus, one distinguished merit of using the active front-end rectifier system is the natural increased power-quality-adjusting capacity, without the need for extra active or passive harmonic filters and VAR compensators. The active front-end rectifier system can also be implemented utilizing the arising MOSFETs made of silicon carbide acting as replacers for the silicon-insulate-gate bipolar transistors. A remarkable characteristic of the silicon carbide B6-active front-end rectifier is that the silicon carbide MOSFET can be operated at synchronous rectification regimes by the intrinsic channel-reverse conduction that presents very small losses of conduction. It was reported by Mao et al. [186] that the active front-end rectifier attains 1.2% efficiency contribution using 1200 V silicon carbide MOSFETs. Among the hydrogen-converter configurations, the three-winding line-frequency transformer is indispensable in the system. This component is the bulkiest one present in this type of power-to-hydrogen converter and entails certain limitations in its transportation and installation stages. Hence, taking away the bulky line-frequency transformers from this type of hydrogen converter system is a truly relevant investigation line. One appealing alternative is the modular multicell rectifier in which modular topologies can be used, and the converter cells are prompted by the front-end AC/DC stage, which is followed by a DC/DC stage having galvanic isolation. The greatly modularized possible configurations enable enhanced flexibility and scalability for the rectifier. Because of the cascaded topology of converter cells per phase, the 1200–1700 V silicon-insulated-gate bipolar transistors and silicon carbide MOSFET can be utilized ahead of the DC/DC galvanic isolation in each converter [187]. The conventional 12-TR can be taken as a very suitable alternative concerning the issues related to efficiency, reliability, and cost, whereas the current distortion and power factor should be properly studied, particularly when employing high firing angles. The 12-DRMC shows a high-power factor among the complete working range in respect to the 12-TR, whereas the harmonic filter is still required to decrease the input THDi. The 12-TRASPF is a hybrid conceptualization exploring the conventional 12-TR and B6 converter active-shunt power filter, in such a way that a highly improved power quality may be obtained with a minor cost increase and reduced performance comparatively to the 12-TR. The active front-end rectifier is an attractive alternative concerning improved power quality without requiring extra counterbalance measures. However, it may entail a greater cost in providing a more advanced regulating system.
8. Safety Considerations
For hydrogen to be adopted on a large scale as a sustainable, clean, and economical solution capable of meeting current energy demands, it is essential to comprehensively address safety issues across all phases of handling the resource: production, storage, and transportation. Ensuring safety at each of these stages is crucial for enabling the efficient and safe use of hydrogen.
Regarding production, handling reactive substances and processes that require high energy is critical. Safety in production involves rigorous control of temperature and pressure, as well as implementing systems to mitigate the emission of hazardous gases, per Tsiklios et al. [139]. Additionally, hydrogen’s characteristics, such as its low density, lack of odor and color, and potential for flammability with a volumetric fraction of just 4% [188], complicate its handling. These factors increase the complexity of maintaining safety during production, necessitating measures to prevent leaks, ignition, and possible explosions. For example, hydrogen gas can form explosive mixtures with atmospheric oxygen across a wide concentration range (4.0–75% for hydrogen and 18–59% for oxygen) [189]. Studies on hydrogen stations often focus on these explosion scenarios, including deflagration and detonation [189].
In terms of storage and transportation, hydrogen presents additional challenges. Storage tanks must be robust and designed to withstand high pressures or low temperatures, and regular inspections are essential to ensure container integrity. For transportation, safety is critical to prevent leaks in pipelines or tanks, which could lead to dangerous situations such as the formation of flammable and potentially explosive mixtures.
Another problem, according to Okonkwo et al. [190], is that the interaction of hydrogen with storage materials—both metallic and polymeric—is crucial. The small size of hydrogen molecules allows them to be easily absorbed by these materials, which can degrade their mechanical properties and potentially lead to unwanted hydrogen leaks and structural failures. The potential interactions of hydrogen with storage materials are relevant for fuel cell vehicles (FCVs) and other renewable energy applications [191,192].
Therefore, addressing these issues comprehensively and implementing rigorous safety measures is fundamental to ensuring that hydrogen can be used safely and efficiently, realizing its potential as a sustainable energy solution.
9. Challenges and Recommendations for Future Studies
The challenges and recommendations for further works for hydrogen production, transporting, and storage can be summarized in the following topics:
- Most of the electrolysis techniques are alkaline-based. However, proton-exchange membranes and solid oxide electrolyzer cells have already been designed and implemented. Dealing with increased working temperatures in the electrolyzer is a difficult task. The elevated temperature values in the acidic proton-exchange membrane electrolyzers intensify corrosion and membrane stability problems, making the high-temperature electrolysis process in the proton-exchange membrane unsuitable. Also, the alkaline anion exchange membranes are proton-exchange membrane alternatives with great potential [13,193]. The cross-permeation can be a strong limitation for alkaline electrolyzers to attain reliable gas purity under elevated pressures [194]. Solid oxide electrolyzer cells are the least developed cells but with the highest potential in electrical efficiency. The exploration of solid oxide electrolyzer cells poses significant challenges that hinder their large-scale exploration: difficulty associated with the functionality under pressure since the vitreous cell gaskets cannot withstand enhanced pressures, material stability, and corrosivity of the hot pure oxygen produced at the anode, causing additional complexity and heat transfer problems. These issues must be addressed to protect the metallic components [195]. The current prototypes of solid oxide electrolyzer cells have great potential for high efficiency and localized heat production [196].
- Hydrogen can offer remarkable efficiency, can be produced from a broad range of easily available resources, and emits no greenhouse gases or pollutants. Implementing hydrogen as one of the main energy carriers will require the clarification of a great number of scientific, technological, logistical, and economic challenges [197]. Such concerns should further inspire the research community to investigate new lines of study and potential ends for the hydrogen electrolyzer technology.
- According to authors Clerici and Furfari [198], the levelized cost of hydrogen for today, and for the years 2030 and 2050, considers the price and efficiency of the electrolyzers, cost of feeding, and capacity factor of the renewable energy sources. The existing hydrogen production systems using alkaline electrolyzers cost between EUR 1000 and 1500/kW with installation, whereas the proton exchange membrane electrolysis demands an investment between EUR 2000 and 3000/kW [199]. Alkaline water electrolysis is well-developed, though the corresponding production is still much reduced. Alkaline water electrolysis electrolyzer manufacturers produce low-volume electrolyzers for specific markets, enhancing the business owner’s policy costs. The overall cost reduction of alkaline electrolyzers relies on more price production, while the decrease in the proton-exchange membrane cost demands technological advances. Nonetheless, the economy of scale and installation technology can reduce the overall capital cost. Producing hydrogen through a cost-effective methodology is the fundamental obstacle for water electrolysis to facilitate green hydrogen production [196]. By the year 2030, it is expected to be a considerable decrease in the cost of fuel cell systems and electrolyzers, especially the stack cost. One approach to keep the efficiency and decrease the costs may be by enhancing the active area of the stack, decreasing the number of cells required to obtain a particular amount of hydrogen, as noted by Saygin et al. [7]. Additionally, the rising political interest in green hydrogen may provide decreases in investment costs. National and international goals may also have a great impact on the industry and economy related to hydrogen. The support of public and private investments may aid the implementation of advanced technologies, optimizing the production, construction, and installation processes and maturing the hydrogen industry [200].
- Electrolysis of water, or the splitting of its molecule into oxygen and hydrogen, began its commercialization in the year 1890. According to the authors Pastore et al. [200], a proton-exchange membrane needs 54 kW hours of power and 18 L of water to obtain 1 kg of hydrogen. Water is necessary to produce hydrogen based on the electrolysis process [199]. If all of the worldwide production of hydrogen of 70 Mt was supplied by the electrolysis of water, the water being used in the process would correspond to 1.3% of the global water use in the energy sector. For the desalination process of saltwater, reverse osmosis is an alternative with a small impact on the total cost of producing hydrogen. Presently, the incorporation of saltwater into the water electrolysis process should be accelerated [201].
- The use of water in the electrolysis process may provoke environmental concerns if the source of water is not adequately managed and evaluated [202]. The electrolysis procedure needs freshwater without contaminants like minerals and salts. In the cases where the water for the electrolysis process is not appropriately filtered and treated, it may lead to contaminated effluent discharges that are harmful to aquatic ecosystems and entail health risks for humans. The sources of energy and water for electrolysis must be accurately analyzed to decrease the associated environmental impact. It should be also noted that wind and solar power may minimize the environmental impact of the electrolysis process. Various filtration methods, such as graphene nanotubes, carbon dots, activated bentonite, and coagulation methods enhanced by electricity and diverse nanomaterials [203], can present an improved water-purification ability.
- The electrolyzer systems utilize rare materials for electrode catalysts and electrolyte additives, among others [199]. Each metal possesses specific levels of electrical resistance, corrosion resistance, durability, and activity. Cobalt and nickel are frequently used as materials for electrodes in electrolytic baths based on alkaline solutions due to their corrosion resistance and reasonable cost [204]. The corrosion resistance can be associated with the used catalysts, counter electrodes, and separate plates. Catalysts made of noble metals like platinum, iridium, and ruthenium, as well as titanium existing collectors and separator plates, are expensive, and the resources concentrated enough for profitable mining are rare [205]. The proton-exchange membrane electrodes demand the exploration of different materials for high catalytic activity and corrosion resistance. Considerable commercialization of the proton-exchange membrane electrolysis process should be conducted to infer the cost and requirements for iridium [206]. The cost decrease of the catalysts should be a priority to reduce stack costs. Advanced composite metal oxides, nanocatalysts, and support structures should be studied in-depth since they can be alternative technological solutions [199]. The research community continued to use nanoparticles of platinum supported on carbon black as standard proton-exchange membrane electrolysis catalysts [206]. Fuel cell exploration in the year 2030 in Europe will require 7% of the global platinum supply [199]. Furthermore, innovative production methodologies for new electrode systems, catalysts, and support materials are most welcome [206].
- Electrolyzer efficiency stands for the conversion rate of electricity into hydrogen by the electrolyzers. The efficiency and durability of current electrolyzer systems are still insufficient, hindering the penetration into the market of hydrogen energy systems. Limitations in efficiency caused by certain factors affect the electrical resistance of the systems. Currently, the efficiency of water electrolysis systems is in the vicinities of its optimal performance. The efficiency of a proton-exchange membrane system is 60%, with an estimated increase between 67% and 74%. The target for alkaline electrolyzers is an electrical efficiency from 70% to 80% [199]. Ionic liquids have been employed to enhance the conductivity and stability of the electrolytic solutions and constitute themselves as a promising alternative. The researchers Souza et al. [207] utilized an electrolyte solution composed of the ionic liquid 1-butyl-3-methylimidazolium-tetrafluoroborate in water at room temperature with inexpensive electrodes made of carbon steel, molybdenum, nickel, and nickel–molybdenum alloy. All electro-catalysts demonstrated an efficiency from 97% to nearly 99% [207]. The reported efficiency was above one of the industrial and commercial electrolyzers. Yet it should be noted that most electrolyzers work at current densities appreciably superior to the experimentally used values [208]. As confirmed by the authors Asghari et al. [209], a holey nanostructure and improved electrical conductivity of the electrocatalysts are needed to enhance the efficiency of the electrolysis electrode. Additionally, significant control and capacity to operate the energy system efficiently can increase the efficiency of the electrolyzers [210]. A deeper investigation should be focused on constructing an experimental setup in which the polarization curves of the thermally integrated photovoltaics and electrolyzers can be accurately matched for extra inquiry into the real efficiency and verification of the theoretical values [211]. Until now, there is a lack of a benchmark to evaluate the efficiency process of the hydrogen electrolyzers through the inclusion of heat. The European Commission defines the criteria for near-ambient temperature electrolysis of water [196].
- Most published works propose that numeric simulations of the control algorithm for hydrogen energy systems may enhance their performance and decrease their investment cost. Further research studies should consider the techno-economic analysis of the utility company and management modelling of the stations to estimate the fluctuating renewable energy output trade, the distributed power-electrolyzer functioning, and the complexities inherent to the production, storage, and delivery to the final user of hydrogen.
- It is highly recommended to update and improve the control strategy to achieve an enhanced capacity, safety, functionality, efficiency, and lifespan extension of the electrolyzers. Also, the investment cost decrease should be taken as a priority without compromising the durability and performance of the systems. Moreover, innovative techniques to considerably enhance the stability of the grid should be examined, including simulating energy-storage technology systems and backup electrolyzer infrastructures. In addition, further research works are required to implement the commercialization of systems based on the electrolysis of water using renewable energy to generate hydrogen and design cost-effective hydrogen-related processes and facilities.
- Despite the steady development of innovative methods and procedures for hydrogen production, storage, and distribution, there are still essential challenges that limit the research on the design and implementation of hydrogen infrastructures. According to the researchers Rand and Dell [212], hydrocarbon reforming is responsible for an annual production of hydrogen between 45 and 50 megatons. Nonetheless, to obtain hydrogen, the natural gas must be subjected to steam reforming, resulting in hydrogen being not economically competitive on a unit-energy basis as an energy carrier.
- Developing new techniques such as electrolysis and thermochemical processes for biomass requires significant improvements to compete with traditional hydrogen production methodologies like steam methane reforming.
- The production of hydrogen is primarily contributed to by non-renewable sources, provoking an increase in the concentration of carbon dioxide in the air. Concerning hydrogen storage, the associated challenges are fundamentally linked to cost, efficiency, volume, and weight, as well as regulations and standards [80]. Challenges arise in the hydrogen usage onboard vehicles due to the high volume, weight, and price of hydrogen, thereby limiting the feasibility of hydrogen-based vehicles. Also, the life cycle of hydrides diminishes with the ongoing refueling process, and this may be an obstacle to overall efficiency. Another drawback is the absence of adequate codes and standards for hydrogen storage systems. Considering the distribution and final delivery of hydrogen, the inherent challenges are linked to the needed facilities to distribute the hydrogen to final users since it demands completely new facilities. Additionally, production and delivery systems should be integrated to decrease the overall investment costs and benefit from site-specific advantages. The transportation, storage, and final delivery to users can be related to a lack of energy efficiency as the smallest volumetric energy density of hydrogen still does not answer to the U.S. Department of Energy’s standards. Also, the main challenge for hydrogen to smoothly transit to a hydrogen-based economy and investment cost is the limited number of proper and regulated solid-based or liquid-based hydrogen storage procedures and systems.
- Improving the safety of the hydrogen energy industry is a crucial area that deserves further attention and investigation. According to a database supported by the U.S. Department of Energy, 120 hydrogen incidents occurred between the years 1999 and 2019 [213]. Hence, safety is a pivotal issue that cannot be overlooked in the development of hydrogen technology. Due to the properties of hydrogen, it is difficult to keep elevated safety levels during production, transportation, storage, and utilization. Consequently, to ensure the safe utilization of hydrogen, its characteristics relevant to leakage and diffusion, ignition, and explosion must be further analyzed. More studies on hydrogen incident investigations before, during, and after the incidents are indeed most welcome. All these studies will furnish practical guidelines for the design and implementation of hydrogen safety-management systems.
10. Conclusions
The fundamental concluding remarks of the current overview work are summarized in the following points:
- A broad range of methods are available for hydrogen production. Steam methane reforming is presently the most cost-effective procedure, which is followed by coal gasification. Nevertheless, there is a need to design and implement alternative processes to decrease global dependence on fossil fuels. This is crucial to meet the increasing claim for hydrogen, especially in the transportation sector, driven by the anticipated future reduction in the price of this technology and the decrease in demand of fossil fuels in the next decades.
- The thermochemical pyrolysis and gasification processes are cost-effective methods and are very promising to become the main large-scale competitive routes. Near-term trends suggest a focus on reducing fuel consumption via the integration of membrane reactors and combined cycles, using alternative energy sources including concentrated solar energy and gas exhaust from gas turbines.
- It is crucial to clarify the hydrogen characteristics, adopt measures of safety in the hydrogen equipment and systems, and provide training in safe hydrogen storage and handling. With the ever-increasing green energy needs, the electrolysis of water gained considerable relevance. It is critical to review prior research, and development works for future studies and better knowledge.
- Although hydrogen storage has reached a high technological level, future research and development are required to enhance the gravimetric and volumetric density. Additionally, a better understanding of the transportation and use of hydrogen fuel is needed, along with the establishment of new safety standards for factors, including indoor and outdoor working safety distance, leakage sensing, refueling velocity control, and flammability range, among others.
- Hydrogen electrolysis technology needs to address the storage and safety issues since they are of vital importance in the design and implementation of energy equipment, systems, and plants. The safety concerns are fundamental for the well-being of the operating personnel, as well as for the surrounding regions and the public [214]. Incidents originating in hydrogen technology have already been reported. Additionally, the authors Sakamoto et al. [215] presented the hydrogen/hydrogen fueling station accident database in Japan and the United States.
- Further research and development activities regarding the production and storage of hydrogen, coupled with the establishment of standards and codes for hydrogen transportation and final use, will imply a reduction in global dependence on the imports of fossil fuel. This allows countries to obtain a significant portion of their energy from a wide range of accessible feedstocks and processes, bringing more hydrogen to the energy market.
- Hydrogen is one of the cleanest and safest energy sources, derived from different energy resources, including renewable, nuclear, and fossil. Nonetheless, despite its significant abundance, hydrogen still presents serious challenges that need to be addressed on both small and large scales. By effectively managing hydrogen-related activities and addressing problems and limitations associated with manufacturing, storage, transportation, and final use, hydrogen can become one of the most reliable energy sources.
- The effective cost of hydrogen can be determined through sufficient investigations on the development and implementation of hydrogen processing. This will aid in decreasing the working and maintenance costs of the production systems. Additionally, the synthesis of innovative materials for hydrogen storage must meet the requirements of elevated volumetric and gravimetric densities (matching the U.S. Department of Energy demands), work at low-temperature values, and enable rapid refueling. The technology and infrastructure for hydrogen production and storage demand more advancements when compared to conventional technologies and infrastructure.
- The need for hydrogen as an energy carrier is sharply increasing due to its potential as an alternative to fossil fuels. Another advantageous feature is the potential of hydrogen to play a considerable part in averting carbonization in the atmosphere.
- Green hydrogen may promote the decarbonization of several industries, including electricity production, transportation, and manufacturing. Strong efforts have been made to accelerate this process. Under these circumstances, a considerable number of countries have launched strong attempts to assess this topic. For example, South Korea intends to decrease the carbon gas emissions in the atmosphere from around 709 million tons in the year 2017 to 536 million tons by the year 2030 [216].
- Nonetheless, hydrogen can be taken as an indirect greenhouse gas [199]. The hydrogen-related technology can substitute the use of fossil fuels, which directly emit greenhouse gases, but the manufacturing, storage, and transportation emissions may conduct indirect greenhouse gas concentrations that could affect air quality [217]. The authors Rujiven et al. [217] stated that the molecular hydrogen emissions range from 0.2% to 10% in an energy system. Nevertheless, before the generalized utilization of hydrogen, it is required to better study the uncertainties associated with its effect as a greenhouse gas in energy systems. Public policies should be implemented to address the negative consequences of hydrogen exploration in the energy system and introduce regulations on molecular hydrogen emissions, air contaminants, and establish policies to promote the exploration of hydrogen energy technology.
Author Contributions
Conceptualization, J.P., J.O. and R.S.; methodology, J.P. and A.M.; software, A.M.; validation, J.P. and A.M.; formal analysis, A.M.; investigation, J.P., J.O. and R.S.; resources, A.M.; data curation, J.P., J.O. and R.S.; writing—original draft preparation, J.P., J.O. and R.S.; writing—review and editing, J.P., J.O. and R.S.; supervision, A.M.; project administration, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Fundação para a Ciência e a Tecnologia (FCT), Avenida D. Carlos I, 126, 1249–074 Lisboa, Portugal, for partially financing the Project “Estratégias interfaciais de arrefecimento para tecnologias de conversão com elevadas potências de dissipação”, (Ref. PTDC/EMETED/7801/2020). José Pereira also acknowledges FCT for his PhD Fellowship (Ref. 2021. 05830.BD). The authors are also grateful for FCT funding through 2022.03151.PTD (https://doi.org/10.54499/2022.03151.PTDC) and LA/P/0083/2020 IN +-IST-ID. Jeferson Oliveira acknowledges FUSP, Project Nº 403904. A.S. Moita acknowledges Fundação para a Ciência e Tecnologia from the support doi:10.54499/CEECINST/00043/2021/CP2797/CT0005. A.S. Moita also acknowledges FCT for partially financing her contract through CEECINST/00043/2021/CP2797/CT0005, doi:10.54499/CEECINST/00043/2021/CP2797/CT0005. The authors are grateful to Andrea Zille for providing materials and equipment, as well as for their technical support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, F.; Santos, F. Combustível “hidrogénio”. Millenium 2005, 31, 252–270. [Google Scholar]
- Davy, H. Decomposition of the Fixed Alkalies and Alkaline Earths, Simpkin, Marshall, Hamilton; Kent & Co.: London, UK, 1894. [Google Scholar]
- Volta, A. On the electricity excited by the mere contact of conducting substances of different kinds. In a letter from Mr. Alexander Volta, F.R.S. Professor of Natural Philosophy in the University of Pavia, to the Rt. Hon. Sir Joseph Banks, Bart. K.B. P. R. S. Phil. Trans. R. Soc. 1800, 90403–90431, 403–431. [Google Scholar] [CrossRef]
- Piccolino, M. The bicentennial of the Voltaic battery (1800–2000): The artificial electric organ. Trends Neurosci. 2000, 23, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Daniell, J.F. On voltaic combinations. In a letter addressed to Michael Faraday, D.C.L., F.R.S., Fullerian Prof. Chem. Royal Institution, Corr. Memb. Royal & Imp. Acadd. of Science, Paris, Petersburgh, &c. By J. F. Daniell, F.R.S., Prof. Chem. in King’s College, L. Phil. Trans. R. Soc. 1836, 126, 107–124. [Google Scholar] [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. J. CO2 Util. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Transformation of CO2 into liquid fuels and synthetic natural gas using green hydrogen: A comparative analysis. Fuel 2021, 291, 120111. [Google Scholar] [CrossRef]
- Agarwal, R. Transition to a Hydrogen-Based Economy: Possibilities and Challenges. Sustainability 2022, 14, 15975. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Frieden, F.; Leker, J. Future costs of hydrogen: A quantitative review. Sustain. Energy Fuels 2024, 8, 1806–1822. [Google Scholar] [CrossRef]
- International Renewable Energy Agency (IRENA). Making the Breakthrough: Green Hydrogen Policies and Technology Costs; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2021. [Google Scholar]
- Dubouis, N.; Aymé-Perrot, D.; Degoulange, D.; Grimaud, A.; Girault, H. Alkaline electrolyzers: Powering industries and overcoming fundamental challenges. Joule 2024, 8, 883–898. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Begum, R.A.; Hossain, M.J.; Ker, P.J.; Mahlia, T.M.I. Hydrogen electrolyser technologies and their modelling for sustainable energy production: A comprehensive review and suggestions. Int. J. Hydrogen Energy 2023, 48, 27841–27871. [Google Scholar] [CrossRef]
- Pham, C.V.; Escalera-López, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of High Performance Water Electrolyzers—From Catalyst Layer Materials to Electrode Engineering. Adv. Energy Mater. 2021, 11, 2101998. [Google Scholar] [CrossRef]
- Noh, H.; Kang, K.; Seo, Y. Environmental and Energy Efficiency Assessments of Offshore Hydrogen Supply Chains Utilizing Compressed Gaseous Hydrogen, Liquefied Hydrogen, Liquid Organic Hydrogen Carriers and Ammonia. Int. J. Hydrogen Energy 2023, 48, 7515–7532. [Google Scholar] [CrossRef]
- Salvi, B.L.; Subramanian, K.A. Sustainable Development of Road Transportation Sector Using Hydrogen Energy System. Renew. Sustain. Energy Rev. 2015, 51, 1132–1155. [Google Scholar] [CrossRef]
- Jordá-Beneyto, M.; Suárez-García, F.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen Storage on Chemically Activated Carbons and Carbon Nanomaterials at High Pressures. Carbon 2007, 45, 293–303. [Google Scholar] [CrossRef]
- Shaijumon, M.M.; Ramaprabhu, S. Studies of Yield and Nature of Carbon Nanostructures Synthesized by Pyrolysis of Ferrocene and Hydrogen Adsorption Studies of Carbon Nanotubes. Int. J. Hydrogen Energy 2005, 30, 311–317. [Google Scholar] [CrossRef]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional Hydrogen Storage Achieved by Screening Nearly Half a Million Metal-Organic Frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal Hydride Hydrogen Storage and Compression Systems for Energy Storage Technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Godula-Jopek, A. Hydrogen Production by Electrolysis; GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production via Electrolysis—A Review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Özcan, H. Comprehensive Review on the Techno-Economics of Sustainable Large-Scale Clean Hydrogen Production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Phillips, R.; Edwards, A.; Rome, B.; Jones, D.R.; Dunnill, C.W. Minimising the Ohmic Resistance of an Alkaline Electrolysis Cell through Effective Cell Design. Int. J. Hydrogen Energy 2017, 42, 23986–23994. [Google Scholar] [CrossRef]
- Tijani, A.S.; Binti Kamarudin, N.A.; Binti Mazlan, F.A. Investigation of the Effect of Charge Transfer Coefficient (CTC) on the Operating Voltage of Polymer Electrolyte Membrane (PEM) Electrolyzer. Int. J. Hydrogen Energy 2018, 43, 9119–9132. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, B.; Ning, F.; Li, Y.; Zhao, C.; He, C.; Wen, Q.; Dan, X.; Chai, Z.; Li, W.; et al. Hybrid 3D-Ordered Membrane Electrode Assembly (MEA) with Highly Stable Structure, Enlarged Interface, and Ultralow Ir Loading by Doping Nano TiO2 Nanoparticles for Water Electrolyzer. Adv. Energy Mater. 2024, 14, 2303353. [Google Scholar] [CrossRef]
- Jin, H.; Zou, S.; Wen, Q.; Li, Y.; Ning, F.; Xu, P.; Pan, S.; Zhou, X. Performance improvement of air-breathing proton exchange membrane fuel cell (PEMFC) with a condensing-tower-like curved flow field. Chin. Chem. Lett. 2023, 34, 107441. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Bockris, J.O.; Dandapani, B.; Cocke, D.; Ghoroghchian, J. On the Splitting of Water. Int. J. Hydrogen Energy 1985, 10, 179–201. [Google Scholar] [CrossRef]
- Marshall, S.L.; Blencoe, J.G. Equilibrium Analysis of Thermochemical Cycles for Hydrogen Production. Sep. Sci. Technol. 2005, 40, 483–505. [Google Scholar] [CrossRef]
- Silva, D.T.T. Tecnologias de Termólise da Água e Purificação do Hidrogénio. Master’s Thesis, Integrated Master in Chemical Engineering, University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Bidard, R.A. Thermolysis or Electrolysis? Why We Choose the Latter. Int. J. Hydrogen Energy 1977, 2, 61–68. [Google Scholar] [CrossRef]
- Perkins, C.; Weimer, A.W. Likely Near-Term Solar-Thermal Water Splitting Technologies. Int. J. Hydrogen Energy 2004, 29, 1587–1599. [Google Scholar] [CrossRef]
- Brown, L.C.; Besenbruch, G.E.; Schultz, K.R.; Showalter, S.K.; Marshall, A.C.; Pickard, P.S.; Funk, J.F. High Efficiency Generation of Hydrogen Fuels Using Thermochemical Cycles and Nuclear Power; D.O.E., General Atomics Project 30047; American Institute of Chemical Engineers: New York, NY, USA, 2002. [Google Scholar]
- Castro, A.; Gallardo, V.; Moreno, E.; Fernández, V.; Romero, M.; Marcos, M.J. Hydrogen Generation from High-Temperature Thermal Solar Energy; International Hydrogen Energy Congress and Exhibition (IHEC): Istambul, Turkey, 2005. [Google Scholar]
- Lee, J.E.; Shafiq, I.; Hussain, M.; Lam, S.S.; Rhee, G.H.; Park, Y.-K. A Review on Integrated Thermochemical Hydrogen Production from Water. Int. J. Hydrogen Energy 2022, 47, 4346–4356. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.; Lee, D.-J. Chapter 16—Bioreactor and Bioprocess Design for Biohydrogen Production. In Biomass, Biofuels, Biochemicals, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–411. [Google Scholar]
- Manish, S.; Banerjee, R. Comparison of Biohydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Pandey, A.; Mohan, S.V.; Chang, J.-S.; Hallenbeck, P.C.; Larroche, C. Biohydrogen A Volume in Biomass, Biofuels, Biochemicals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-64203-5. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable Hydrogen Production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Hankamer, B.; Lehr, F.; Rupprecht, J.; Mussgnug, J.H.; Posten, C.; Kruse, O. Photosynthetic Biomass and H2 Production by Green Algae: From Bioengineering to Bioreactor Scale-Up. Physiol. Plant. 2007, 131, 10–21. [Google Scholar] [CrossRef]
- Kovács, K.L.; Maróti, G.; Rákhely, G. A Novel Approach for Biohydrogen Production. Int. J. Hydrogen Energy 2006, 31, 1460–1468. [Google Scholar] [CrossRef]
- Sorensen, B. Hydrogen and Fuel Cells: Emerging Technologies and Applications; Elsevier Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Poudyal, R.S.; Tiwari, I.; Koirala, A.R.; Masukawa, H.; Inoue, K.; Tomo, T.; Najafpour, M.M.; Allakhverdiev, S.I.; Veziroğlu, T.N. 10—Hydrogen Production Using Photobiological Methods. In Woodhead Publishing Series in Energy; Subramani, V., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 289–317. ISBN 978-1-78242-361-4. [Google Scholar]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into Renewable Hydrogen Energy: Recent Advances and Prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Bičáková, O.; Straka, P. Production of Hydrogen from Renewable Resources and Its Effectiveness. Int. J. Hydrogen Energy 2012, 37, 11563–11578. [Google Scholar] [CrossRef]
- Hammad, A.; Ning, F.; Zou, S.; Liu, Y.; Tian, B.; He, C.; Chai, Z.; Wen, Q.; He, L.; Zhou, X. Aluminum hydrolysis for hydrogen generation enhanced by sodium hydride. Int. J. Hydrogen Energy 2024, 77, 138–148. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Chen, M.; Xin, W.; Yang, Z.; Kong, L. Hydrogen Production via Catalytic Pyrolysis of Biomass in a Two-Stage Fixed Bed Reactor System. Int. J. Hydrogen Energy 2014, 39, 13128–13135. [Google Scholar] [CrossRef]
- Singh, V.; Das, D. Chapter 3—Potential of Hydrogen Production from Biomass. In Science and Engineering of Hydrogen-Based Energy Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 123–164. ISBN 978-0-12-814251-6. [Google Scholar]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of Temperature and Residence Time in the Pyrolysis of Woody Biomass Waste in a Continuous Screw Reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Khole, P.R.; Shukla, S. Bio-oil production through biomass pyrolysis and upgrading research. Int. J. Agric. Eng. 2018, 11, 257–263. [Google Scholar] [CrossRef]
- Wang, Q. Hydrogen Production BT—Handbook of Climate Change Mitigation; Chen, W.-Y., Seiner, J., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2012; pp. 1091–1130. [Google Scholar]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Bolívar Caballero, J.J.; Zaini, I.N.; Yang, W. Reforming Processes for Syngas Production: A Mini-Review on the Current Status, Challenges, and Prospects for Biomass Conversion to Fuels. Appl. Energy Combust. Sci. 2022, 10, 100064. [Google Scholar] [CrossRef]
- Seyedeyn Azad, F.; Abedi, J.; Salehi, E.; Harding, T. Production of Hydrogen via Steam Reforming of Bio-Oil over Ni-Based Catalysts: Effect of Support. Chem. Eng. J. 2012, 180, 145–150. [Google Scholar] [CrossRef]
- Rabah, M.A.; Eldighidy, S.M. Low-Cost Hydrogen Production from Waste. Int. J. Hydrogen Energy 1989, 14, 221–227. [Google Scholar] [CrossRef]
- Zsinka, V.; Miskolczi, N.; Juzsakova, T.; Jakab, M. Pyrolysis-Gasification of Biomass Using Nickel Modified Catalysts: The Effect of the Catalyst Regeneration on the Product Properties. J. Energy Inst. 2022, 105, 16–24. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Catalytic Pyrolysis of Tyres: Influence of Catalyst Temperature. Fuel 2002, 81, 2425–2434. [Google Scholar] [CrossRef]
- Chen, G.; Andries, J.; Spliethoff, H. Catalytic Pyrolysis of Biomass for Hydrogen Rich Fuel Gas Production. Energy Convers. Manag. 2003, 44, 2289–2296. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Catalytic Conditioning of Organic Volatile Products Produced by Peat Pyrolysis. Biomass Bioenergy 2002, 23, 209–216. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic Steam Reforming of Bio-Oils for the Production of Hydrogen: Effects of Catalyst Composition. Appl. Catal. Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Ahmad, H.; Farooq, A.; Bassyouni, M.I.; Sait, H.H.; El-Wafa, M.A.; Hasan, S.W.; Ani, F.N. Pyrolysis of Saudi Arabian date palm waste: A viable option for converting waste into wealth. Life Sci. J. 2014, 11, 667–671. [Google Scholar]
- Czernik, S.; French, R.; Evans, R.; Chornet, E. Hydrogen from post-consumer residues. In Proceedings of the US DOE Hydrogen and Fuel Cells Merit Review Meeting, Berkeley, CA, USA, 18–22 May 2003; pp. 1–17. [Google Scholar]
- Onay, O.; Mete Koçkar, O. Fixed-Bed Pyrolysis of Rapeseed (Brassica napus L.). Biomass Bioenergy 2004, 26, 289–299. [Google Scholar] [CrossRef]
- French, R.; Bair, K.M.; Czernik, S.; Parent, Y.; Ritland, M.; Chornet, E. Fluidizable catalysts for hydrogen production hydrogen from steam reforming biomass pyrolysis products. ACS Fuel Chem. 2002, 47, 759–760. [Google Scholar]
- Brun, K.; Allison, T. Machinery and Energy Systems for the Hydrogen Economy; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Agrafiotis, C.; von Storch, H.; Roeb, M.; Sattler, C. Solar Thermal Reforming of Methane Feedstocks for Hydrogen and Syngas Production—A Review. Renew. Sustain. Energy Rev. 2014, 29, 656–682. [Google Scholar] [CrossRef]
- Zhai, X.; Ding, S.; Liu, Z.; Jin, Y.; Cheng, Y. Catalytic Performance of Ni Catalysts for Steam Reforming of Methane at High Space Velocity. Int. J. Hydrogen Energy 2011, 36, 482–489. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2021, Global Hydrogen Review; IEA: Paris, France, 2021. [Google Scholar] [CrossRef]
- Roh, H.-S.; Eum, I.-H.; Jeong, D.-W. Low Temperature Steam Reforming of Methane over Ni–Ce(1−x)Zr(x)O2 Catalysts under Severe Conditions. Renew. Energy 2012, 42, 212–216. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Chapter 3.1—Current state and challenges for hydrogen storage technologies. In Towards Hydrogen Infrastructure; Elsevier: Amsterdam, The Netherlands, 2024; pp. 101–132. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Liu, Y.; Qin, G.; Li, S. Oxygen promoted hydrogen production from formaldehyde reforming with oxide-derived Cu nanowires at room temperature. Chin. Chem. Lett. 2023, 34, 107905. [Google Scholar] [CrossRef]
- Ahad, M.T.; Bhuiyan, M.M.H.; Sakib, A.N.; Becerril Corral, A.; Siddique, Z. An Overview of Challenges for the Future of Hydrogen. Materials 2023, 16, 6680. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Hydrogen Production from Biomass by the Gasification Process. Energy Sources 2002, 24, 59–68. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Wornat, M.J.; Hurt, R.H.; Yang, N.Y.C.; Headley, T.J. Structural and Compositional Transformations of Biomass Chars during Combustion. Combust. Flame 1995, 100, 131–143. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen Production: Prospects and Limitations to Practical Application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological Hydrogen Production; Fundamentals and Limiting Processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Fedorov, A.S.; Tsygankov, A.A.; Rao, K.K.; Hall, D.O. Hydrogen Photoproduction by Rhodobacter Sphaeroides Immobilised on Polyurethane Foam. Biotechnol. Lett. 1998, 20, 1007–1009. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Jo, C.-H. Hydrogen Production from Sucrose Using an Anaerobic Sequencing Batch Reactor Process. J. Chem. Technol. Biotechnol. 2003, 78, 678–684. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Serebryakova, L.T.; Rao, K.K.; Hall, D.O. Acetylene Reduction and Hydrogen Photoproduction by Wild-Type and Mutant Strains of Anabaena at Different CO2 and O2 Concentrations. FEMS Microbiol. Lett. 1998, 167, 13–17. [Google Scholar] [CrossRef][Green Version]
- Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L.; Hussy, I. Sustainable fermentative hydrogen production: Challenges for process optimisation. Int. J. Hydrogen Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of PH on Hydrogen Production from Glucose by a Mixed Culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Otsuka, S.; Morimoto, M. Hydrogen Production from Industrial Wastewater by Anaerobic Microflora in Chemostat Culture. J. Ferment. Bioeng. 1996, 82, 194–197. [Google Scholar] [CrossRef]
- Ooshima, H.; Takakuwa, S.; Katsuda, T.; Okuda, M.; Shirasawa, T.; Azuma, M.; Kato, J. Production of Hydrogen by a Hydrogenase-Deficient Mutant of Rhodobacter Capsulatus. J. Ferment. Bioeng. 1998, 85, 470–475. [Google Scholar] [CrossRef]
- Troshina, O.; Serebryakova, L.; Sheremetieva, M.; Lindblad, P. Production of H2 by the Unicellular Cyanobacterium Gloeocapsa Alpicola CALU 743 during Fermentation. Int. J. Hydrogen Energy 2002, 27, 1283–1289. [Google Scholar] [CrossRef]
- Kerby, R.L.; Ludden, P.W.; Roberts, G.P. Carbon Monoxide-Dependent Growth of Rhodospirillum Rubrum. J. Bacteriol. 1995, 177, 2241–2244. [Google Scholar] [CrossRef]
- Soboh, B.; Linder, D.; Hedderich, R. Purification and Catalytic Properties of a CO-Oxidizing:H2-Evolving Enzyme Complex from Carboxydothermus Hydrogenoformans. Eur. J. Biochem. 2002, 269, 5712–5721. [Google Scholar] [CrossRef]
- Jung, G.Y.; Kim, J.R.; Park, J.-Y.; Park, S. Hydrogen Production by a New Chemoheterotrophic Bacterium Citrobacter Sp. Y19. Int. J. Hydrogen Energy 2002, 27, 601–610. [Google Scholar] [CrossRef]
- Wolfrum, P.-C.M.; Edward, J.; Vanzin, G.; Huang, J.; Watt Andrew, S.; Smolinski, S.; Biological water gas shift development. Biological Water Gas Shift Development. In DOE Hydrogen, Fuel Cell, and Infrastructure Technologies Program Review. 2003, pp. 1–7. Available online: https://www.researchgate.net/publication/228502175_Biological_water_gas_shift_development (accessed on 8 July 2024).
- Skulberg, O.M. Biophotolysis, Hydrogen Production and Algal Culture Technology BT—Hydrogen Energy System: Production and Utilization of Hydrogen and Future Aspects; Yürüm, Y., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 95–110. ISBN 978-94-011-0111-0. [Google Scholar]
- Meher Kotay, S.; Das, D. Biohydrogen as a Renewable Energy Resource—Prospects and Potentials. Int. J. Hydrogen Energy 2008, 33, 258–263. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Barnea, J. Microbial Energy Conversion: The Proceedings of a Seminar/Sponsored by the UN Institute for Training and Research (UNITAR) and the Ministry for Research and Technology of the Federal Republic of Germany, Held in Göttingen, October 1976; Edited by H.G. Schlegel and J. Barnea. 1977. Available online: https://search.lib.utexas.edu/discovery/fulldisplay?docid=alma991046935659706011&context=L&vid=01UTAU_INST:SEARCH&lang=en&search_scope=MyInst_and_CI&adaptor=Local%20Search%20Engine&tab=Everything&query=sub,contains,%20Biomass%20energy%20,AND&mode=advanced&offset=0 (accessed on 8 July 2024).
- Dincer, I. Comprehensive Energy Systems, Volume 1, Energy Fundamentals; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-814925-6. [Google Scholar]
- Chiarello, G.L.; Selli, E. 8—Photocatalytic Production of Hydrogen. In Advances in Hydrogen Production, Storage and Distribution; Basile, A., Iulianelli, A., Eds.; Woodhead Publishing: Oxford, UK, 2014; pp. 216–247. ISBN 978-0-85709-768-2. [Google Scholar]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- Davis, K.A.; Yoo, S.; Shuler, E.W.; Sherman, B.D.; Lee, S.; Leem, G. Photocatalytic Hydrogen Evolution from Biomass Conversion. Nano Converg. 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified N-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A Review on Solar-Hydrogen/Fuel Cell Hybrid Energy Systems for Stationary Applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Al-Zaharani, A.A.; Dincer, I.; Naterer, G.F. Performance Evaluation of a Geothermal Based Integrated System for Power, Hydrogen and Heat Generation. Int. J. Hydrogen Energy 2013, 38, 14505–14511. [Google Scholar] [CrossRef]
- Garrod, A.; Neda Hussain, S.; Ghosh, A.; Nahata, S.; Wynne, C.; Paver, S. An Assessment of Floating Photovoltaic Systems and Energy Storage Methods: A Comprehensive Review. Results Eng. 2024, 21, 101940. [Google Scholar] [CrossRef]
- Dincer, I. Green Methods for Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Quan, X.; Yang, S.; Ruan, X.; Zhao, H. Preparation of Titania Nanotubes and Their Environmental Applications as Electrode. Environ. Sci. Technol. 2005, 39, 3770–3775. [Google Scholar] [CrossRef]
- Yuvaraj, A.L.; Santhanaraj, D. A systematic study on electrolytic production of hydrogen gas by using graphite as electrode. Mater. Res. 2014, 17, 83–87. [Google Scholar] [CrossRef]
- Nikolić, V.M.; Žugić, D.L.; Maksić, A.D.; Šaponjić, D.P.; Marčeta Kaninski, M.P. Performance comparison of modified poly(vinyl alcohol) based membranes in alkaline fuel cells. Int. J. Hydrogen Energy 2011, 36, 11004–11010. [Google Scholar] [CrossRef]
- Udagawa, J.; Aguiar Brandon, N.P. Hydrogen production through steam electrolysis: Model-based steady state performance of a cathode-supported intermediate temperature solid oxide electrolysis cell. J. Power Sources. 2007, 166, 127–136. [Google Scholar] [CrossRef]
- Nie, J.; Chen, Y.; Boehm, R.F.; Katukota, S. A Photoelectrochemical Model of Proton Exchange Water Electrolysis for Hydrogen Production. J. Heat Transfer. 2008, 130, 042409. [Google Scholar] [CrossRef]
- Ganley, J.C. High temperature and pressure alkaline electrolysis. Int. J. Hydrogen Energy 2009, 34, 3604–3611. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Studies on the effect of temperature of the electrolytes on the rate of production of hydrogen. Int. J. Hydrogen Energy 2005, 30, 261–263. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Ciacchi, F.T. Hydrogen and oxygen generation with polymer electrolyte membrane (PEM)-based electrolytic technology. Ionics 2006, 12, 7–14. [Google Scholar] [CrossRef]
- Wei, S.; Hikmati, J.; Balakin, B.V.; Kosinski, P. Experimental study of hydrogen production using electrolyte nanofluids with a simulated light source. Int. J. Hydrogen Energy 2022, 47, 7522–7534. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Hydrogen Storage for Fuel Cell Vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Gómez, J.A.; Santos, D.M.F. The Status of On-Board Hydrogen Storage in Fuel Cell Electric Vehicles. Designs 2023, 7, 97. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.R. Hydrogen Storage Methods: Review and Current Status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Herdem, M.S.; Mazzeo, D.; Matera, N.; Baglivo, C.; Khan, N.; Afnan; Congedo, P. M.; De Giorgi, M.G. A Brief Overview of Solar and Wind-Based Green Hydrogen Production Systems: Trends and Standardization. Int. J. Hydrogen Energy 2023, 51, 340–353. [Google Scholar] [CrossRef]
- Herdem, M.S.; Mazzeo, D.; Matera, N.; Wen, J.Z.; Nathwani, J.; Hong, Z. Simulation and Modeling of a Combined Biomass Gasification-Solar Photovoltaic Hydrogen Production System for Methanol Synthesis via Carbon Dioxide Hydrogenation. Energy Convers. Manag. 2020, 219, 113045. [Google Scholar] [CrossRef]
- Abdechafik, E.H.; Ait Ousaleh, H.; Mehmood, S.; Filali Baba, Y.; Bürger, I.; Linder, M.; Faik, A. An Analytical Review of Recent Advancements on Solid-State Hydrogen Storage. Int. J. Hydrogen Energy 2024, 52, 1182–1193. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium Based Materials for Hydrogen Based Energy Storage: Past, Present and Future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal Oxides as Catalysts for Improved Hydrogen Sorption in Nanocrystalline Mg-Based Materials. J. Alloys Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Nobuki, T.; Okuzumi, Y.; Hatate, M.; Crivello, J.-C.; Cuevas, F.; Joubert, J.-M. Mechanosynthesis and Reversible Hydrogen Storage of Mg2Ni and Mg2Cu Alloys. Mater. Trans. 2019, 60, 441–449. [Google Scholar] [CrossRef]
- Rahmaninasab, M.A.; Raygan, S.; Abdizadeh, H.; Pourabdoli, M.; Mirghaderi, S.H. Properties of Activated MgH2 + Mischmetal Nanostructured Composite Produced by Ball-Milling. Mater. Renew. Sustain. Energy 2018, 7, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, X.; Yang, L.; Huang, Z.; Fang, F.; Sun, W.; Gao, M.; Pan, H. Nanostructured Light Metal Hydride: Fabrication Strategies and Hydrogen Storage Performance. Renew. Sustain. Energy Rev. 2023, 184, 113560. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative Assessment of Hydrogen Production Methods from Renewable and Non-Renewable Sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. The Role of Hydrogen in the Optimal Design of Off-Grid Hybrid Renewable Energy Systems. J. Energy Storage 2022, 46, 103893. [Google Scholar] [CrossRef]
- Hurtubia, B.; Sauma, E. Economic and Environmental Analysis of Hydrogen Production When Complementing Renewable Energy Generation with Grid Electricity. Appl. Energy 2021, 304, 117739. [Google Scholar] [CrossRef]
- Mallapragada, D.S.; Gençer, E.; Insinger, P.; Keith, D.W.; O’Sullivan, F.M. Can Industrial-Scale Solar Hydrogen Supplied from Commodity Technologies Be Cost Competitive by 2030? Cell Rep. Phys. Sci. 2020, 1, 100174. [Google Scholar] [CrossRef]
- Ma, N.; Zhao, W.; Wang, W.; Li, X.; Zhou, H. Large Scale of Green Hydrogen Storage: Opportunities and Challenges. Int. J. Hydrogen Energy 2023, 50, 379–396. [Google Scholar] [CrossRef]
- Karayel, G.K.; Javani, N.; Dincer, I. A Comprehensive Assessment of Energy Storage Options for Green Hydrogen. Energy Convers. Manag. 2023, 291, 117311. [Google Scholar] [CrossRef]
- Tsiklios, C.; Hermesmann, M.; Müller, T.E. Hydrogen Transport in Large-Scale Transmission Pipeline Networks: Thermodynamic and Environmental Assessment of Repurposed and New Pipeline Configurations. Appl. Energy 2022, 327, 120097. [Google Scholar] [CrossRef]
- Weber, M.; Perrin, J. Hydrogen Transport and Distribution BT—Hydrogen Technology: Mobile and Portable Applications; Léon, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 129–149. ISBN 978-3-540-69925-5. [Google Scholar]
- Sharma, S.K.; Maheshwari, S. A Review on Welding of High Strength Oil and Gas Pipeline Steels. J. Nat. Gas Sci. Eng. 2017, 38, 203–217. [Google Scholar] [CrossRef]
- Cristello, J.B.; Yang, J.M.; Hugo, R.; Lee, Y.; Park, S.S. Feasibility Analysis of Blending Hydrogen into Natural Gas Networks. Int. J. Hydrogen Energy 2023, 48, 17605–17629. [Google Scholar] [CrossRef]
- Yang, M.; Hunger, R.; Berrettoni, S.; Sprecher, B.; Wang, B. A Review of Hydrogen Storage and Transport Technologies. Clean Energy 2023, 7, 190–216. [Google Scholar] [CrossRef]
- Reddi, K.; Elgowainy, A.; Rustagi, N.; Gupta, E. Impact of Hydrogen Refueling Configurations and Market Parameters on the Refueling Cost of Hydrogen. Int. J. Hydrogen Energy 2017, 42, 21855–21865. [Google Scholar] [CrossRef]
- Lipman, T.E.; Elke, M.; Lidicker, J. Hydrogen Fuel Cell Electric Vehicle Performance and User-Response Assessment: Results of an Extended Driver Study. Int. J. Hydrogen Energy 2018, 43, 12442–12454. [Google Scholar] [CrossRef]
- Bhogilla, S.S.; Niyas, H. Design of a Hydrogen Compressor for Hydrogen Fueling Stations. Int. J. Hydrogen Energy 2019, 44, 29329–29337. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, Y.; Zhang, S.; Jiang, J.; Peng, X. Study on the Dynamic Characteristics of the Free Piston in the Ionic Liquid Compressor for Hydrogen Refuelling Stations by the Fluid-Structure Interaction Modelling. Int. J. Hydrogen Energy 2023, 48, 25410–25422. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, P.; Zhu, S.; Li, S.; Zhao, S.; Wang, Y. Design and Theoretical Analysis of a Liquid Piston Hydrogen Compressor. J. Energy Storage 2021, 41, 102861. [Google Scholar] [CrossRef]
- Amin, S. To the Memory of Sam Moyo. Agrar. South J. Polit. Econ. 2016, 5, 138–157. [Google Scholar] [CrossRef]
- Jiahao, C.; Xiaohan, J.; Chuang, X.; Xueyuan, P. Design and Validation of New Cavity Profiles for Diaphragm Stress Reduction in a Diaphragm Compressor. IOP Conf. Ser. Mater. Sci. Eng. 2015, 90, 12083. [Google Scholar] [CrossRef]
- Yu, W.; Dianbo, X.; Jianmei, F.; Xueyuan, P. Research on Sealing Performance and Self-Acting Valve Reliability in High-Pressure Oil-Free Hydrogen Compressors for Hydrogen Refueling Stations. Int. J. Hydrogen Energy 2010, 35, 8063–8070. [Google Scholar] [CrossRef]
- Kermani, N.A. Design and Prototyping of an Ionic Liquid Piston Compressor as a New Generation of Compressors for Hydrogen Refueling Stations; Technical University of Denmark: Lyngby, Denmark, 2017; ISBN 8774755013. [Google Scholar]
- Van de Ven, J.D.; Li, P.Y. Liquid Piston Gas Compression. Appl. Energy 2009, 86, 2183–2191. [Google Scholar] [CrossRef]
- Schober, M.; Deichsel, M.; Schlücker, E. CFD-Simulation and Experimental Validation of Heat Transfer in Liquid Piston Compressors. In Proceedings of the 12th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, HEFAT, Malaga, Spain, 11–13 July 2016; pp. 511–516. [Google Scholar]
- Xu, Z.; Wilke, V.; Chmielarz, J.J.; Tobias, M.; Atanasov, V.; Gago, A.S.; Friedrich, K.A. Novel Piperidinium-Functionalized Crosslinked Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. J. Memb. Sci. 2023, 670, 121302. [Google Scholar] [CrossRef]
- Salari, A.; Hakkaki-Fard, A.; Jalalidil, A. Hydrogen production performance of a photovoltaic thermal system coupled with a proton exchange membrane electrolysis cell. Int. J. Hydrogen Energy 2022, 47, 4472–4488. [Google Scholar] [CrossRef]
- Amori, K.E.; Salman, S.M.; Kareem, Z.H. Hydrogen Production by Hybrid Photovoltaic Thermal System. J. Eng. Sci. 2017, 20, 50–261. [Google Scholar]
- Bilgen, E. Solar hydrogen from photovoltaic-electrolyzer systems. Energy Convers. Manag. 2012, 42, 1047–1057. [Google Scholar] [CrossRef]
- Gibson, T.L.; Kelly, N.A. Optimization of solar powered hydrogen production using photovoltaic electrolysis devices. Int. J. Hydrogen Energy 2008, 33, 5931–5940. [Google Scholar] [CrossRef]
- Gibson, T.L.; Kelly, N.A. Predicting efficiency of solar powered hydrogen generation using photovoltaic-electrolysis devices. Int. J. Hydrogen Energy 2010, 35, 900–911. [Google Scholar] [CrossRef]
- Sevik, S. Techno-economic evaluation of a grid-connected PV-trigeneration-hydrogen production hybrid system on a university campus. Int. J. Hydrogen Energy 2022, 47, 23935–23956. [Google Scholar] [CrossRef]
- Hassan, A.H.; Liao, Z.; Wang, K.; Abdelsamie, M.M.; Xu, C.; Wang, Y. Exergy and Exergoeconomic Analysis for the Proton Exchange Membrane Water Electrolysis under Various Operating Conditions and Design Parameters. Energies 2022, 15, 8247. [Google Scholar] [CrossRef]
- Lagorse, J.; Simões, M.G.; Miraoui, A.; Costerg, P. Energy cost analysis of a solar-hydrogen hybrid energy system for stand-alone applications. Int. J. Hydrogen Energy 2008, 33, 2871–2879. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.; Beyza, J.; Monteiro, C. Optimal dispatch model for PV-electrolysis plants in self-consumption regime to produce green hydrogen: A Spanish case study. Int. J. Hydrogen Energy 2022, 47, 25202–25213. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, F.; Amodio, L.; Pagano, M. Hydrogen production by water electrolysis and off-grid solar PV. Int. J. Hydrogen Energy 2020, 46, 29038–29048. [Google Scholar] [CrossRef]
- Puranen, P.; Kosonen, A.; Ahola, J. Technical feasibility evaluation of a solar PV based off-grid domestic energy system with battery and hydrogen energy storage in northern climates. Sol. Energy 2020, 213, 246–259. [Google Scholar] [CrossRef]
- Gado, M.; Megahed, T.; Ookawara, S.; Nada, S.; Hassan, H. Performance assessment of photovoltaic/thermal (PVT) hybrid adsorption-vapor compression refrigeration system. J. Energy Syst. 2022, 6, 209–220. [Google Scholar] [CrossRef]
- Koleva, M.; Guerra, O.J.; Eichman, J.; Hodge, B.-M.; Kurtz, J. Optimal design of solar-driven electrolytic hydrogen production systems within electricity markets. J. Power Sources 2021, 483, 229183. [Google Scholar] [CrossRef]
- Touili, S.; Merrouni, A.A.; El Hassouani, Y.; Amrani, A.-I.; Rachidi, S. Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones. Int. J. Hydrogen Energy 2020, 45, 26785–26799. [Google Scholar] [CrossRef]
- Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Xu, J.; Chen, Y.; Li, W.; Yuan, Z.; Wan, Z.; Wang, X. Thermodynamic study of a hybrid PEMFC-solar energy multi-generation system combined with SOEC and dual Rankine cycle. Energy Convers. Manag. 2020, 226, 113512. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Lim, T.; Kim, S.K. Non-precious hydrogen evolution reaction catalysts: Stepping forward to practical polymer electrolyte membrane-based zero-gap water electrolyzers. Chem. Eng. J. 2022, 433, 133681. [Google Scholar] [CrossRef]
- Gambou, F.; Guilbert, D.; Zasadzinski, M.; Rafaralahy, H. A Comprehensive Survey of Alkaline Electrolyzer Modeling: Electrical Domain and Specific Electrolyte Conductivity. Energies 2022, 15, 3452. [Google Scholar] [CrossRef]
- Solanki, J.; Fröhleke, N.; Böcker, J. Implementation of hybrid filter for 12-pulse thyristor rectifier supplying high-current variable-voltage DC load. IEEE Trans. Ind. Electron. 2015, 62, 4691–4701. [Google Scholar] [CrossRef]
- PowerKraftTM Power Supply Solution for Hydrogen Production, KraftPowercon. Available online: https://kraftpowercon.com/ product/powerkraft (accessed on 8 July 2024).
- Koponen, J.; Ruuskanen, V.; Kosonen, A.; Niemelä, M.; Ahola, J. Effect of converter topology on the specific energy consumption of alkaline water electrolyzers. IEEE Trans. Power Electron. 2019, 34, 6171–6182. [Google Scholar] [CrossRef]
- THYROBOX DC 3 Industrial High-Power dc Power Supply, AEG Power Solutions. Available online: https://www.aegps.com/ en/products/dc-systems-industrial/thyrobox-dc-3-dc-3c/ (accessed on 8 July 2024).
- Liserre, M.; Blaabjerg, F.; Hansen, S. Design and control of an LCL-filter-based three-phase active rectifier. IEEE Trans. Ind. Appl. 2005, 41, 1281–1291. [Google Scholar] [CrossRef]
- Mohamadian, S.; Ghandehari, R.; Shoulaie, A. A comparative study of AC/DC converters used in high current applications. In Proceedings of the 2011 2nd Power Electronics, Drive Systems and Technologies Conference, Tehran, Iran, 16–17 February 2011. [Google Scholar]
- IEC 60146-1-1; Semiconductor Converters-General Requirements and Line Commutated Converters-Part 1-1: Specification of Basic Requirements. International Electrotechnical Commission: London, UK, 2016. Available online: https://webstore.iec.ch/publication/858 (accessed on 8 July 2024).
- IEC 61000-6-2; Electromagnetic Compatibility (EMC)-Part 6-2: Generic Standards-Immunity Standard for Industrial Environments. International Electrotechnical Commission: London, UK, 2016. Available online: https://webstore.iec.ch/publication/25630 (accessed on 8 July 2024).
- IEC 61000-6-4; Electromagnetic Compatibility (EMC)-Part 6-4: Generic Standards-Emission Standard for Equipment in Industrial Environments. International Electrotechnical Commission: London, UK, 2018. Available online: https://webstore.iec.ch/publication/26622 (accessed on 8 July 2024).
- Solanki, J.; Fröhleke, N.; Böcker, J.; Wallmeier, P. Comparison of thyristor-rectifier with hybrid filter and chopper rectifier for high-power, high-current application. In Proceedings of the PCIM Europe 2013, Nuremberg, Germany, 14–16 May 2013. [Google Scholar]
- Mao, S.; Wu, T.; Lu, X.; Popovic, J.; Ferreira, J.A. Three-phase active front-end rectifier efficiency improvement with silicon carbide power semiconductor devices. In Proceedings of the 2016 IEEE Energy Conversion Congress and Exposition (ECCE), Milwaukee, WI, USA, 18–22 September 2016. [Google Scholar]
- Huber, J.E.; Kolar, J.W. Optimum number of cascaded cells for high-power medium-voltage ac–dc converters. IEEE J. Emerg. Sel. Top. Power Electron. 2017, 5, 213–232. [Google Scholar] [CrossRef]
- Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 7—Hydrogen Logistics: Safety and Risks Issues; Dagdougui, H., Sacile, R., Bersani, C., Ouammi, A.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 127–148. ISBN 978-0-12-812036-1. [Google Scholar]
- Okonkwo, P.C.; Barhoumi, E.M.; Ben Belgacem, I.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A Focused Review of the Hydrogen Storage Tank Embrittlement Mechanism Process. Int. J. Hydrogen Energy 2023, 48, 12935–12948. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen Embrittlement in Different Materials: A Review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The Use of Metal Hydrides in Fuel Cell Applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Renz, S.; Lehnert, W.; Müller, M.; Carmo, M. Review—Challenges and Opportunities for Increased Current Density in Alkaline Electrolysis by Increasing the Operating Temperature. J. Electrochem. Soc. 2021, 168, 114501. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current Status of Water Electrolysis for Energy Storage, Grid Balancing and Sector Coupling via Power-to-Gas and Power-to-Liquids: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current Status, Research Trends, and Challenges in Water Electrolysis Science and Technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Abraham, S.; National Hydrogen Energy Roadmap. Based on the Results of the National Hydrogen Energy Roadmap Workshop. Washington. 2002. Available online: www.eere.energy.gov/hydrogen-andfuelcells/pdfs/national_h2_roadmap.pdf (accessed on 1 September 2024).
- Clerici, A.; Furfari, S. Challenges for Green Hydrogen Development. In Proceedings of the 2021 AEIT International Annual Conference (AEIT), Virtual, 4–8 October 2021; pp. 1–6. [Google Scholar]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Pastore, L.M.; Lo Basso, G.; Sforzini, M.; de Santoli, L. Technical, Economic and Environmental Issues Related to Electrolysers Capacity Targets According to the Italian Hydrogen Strategy: A Critical Analysis. Renew. Sustain. Energy Rev. 2022, 166, 112685. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, I.E.L.; Hinds, G.; Smith, G. Impact of Impurities on Water Electrolysis: A Review. Sustain. Energy Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.M.; Ponce de Leão, T. Water Availability and Water Usage Solutions for Electrolysis in Hydrogen Production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Zoubek, T.; Bartoš, P. Economic Impacts of Soil Fertility Degradation by Traces of Iron from Drinking Water Treatment. Environ. Dev. Sustain. 2022, 24, 4835–4844. [Google Scholar] [CrossRef]
- Wei, Z.D.; Ji, M.B.; Chen, S.G.; Liu, Y.; Sun, C.X.; Yin, G.Z.; Shen, P.K.; Chan, S.H. Water Electrolysis on Carbon Electrodes Enhanced by Surfactant. Electrochim. Acta 2007, 52, 3323–3329. [Google Scholar] [CrossRef]
- Leader, A.; Gaustad, G.; Babbitt, C. The Effect of Critical Material Prices on the Competitiveness of Clean Energy Technologies. Mater. Renew. Sustain. Energy 2019, 8, 8. [Google Scholar] [CrossRef]
- de Souza, R.F.; Loget, G.; Padilha, J.C.; Martini, E.M.A.; de Souza, M.O. Molybdenum Electrodes for Hydrogen Production by Water Electrolysis Using Ionic Liquid Electrolytes. Electrochem. Commun. 2008, 10, 1673–1675. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Asghari, E.; Abdullah, M.I.; Foroughi, F.; Lamb, J.J.; Pollet, B.G. Advances, Opportunities, and Challenges of Hydrogen and Oxygen Production from Seawater Electrolysis: An Electrocatalysis Perspective. Curr. Opin. Electrochem. 2022, 31, 100879. [Google Scholar] [CrossRef]
- Hannan, M.A.; Al-Shetwi, A.; Begum, R.A.; Young, S.E.; Hoque, M.M.; Ker, P.; Mansur, M.; Alzaareer, K. The Value of Thermal Management Control Strategies for Battery Energy Storage in Grid Decarbonization: Issues and Recommendations. J. Clean. Prod. 2020, 276, 124223. [Google Scholar] [CrossRef]
- Daneshpour, R.; Mehrpooya, M. Design and Optimization of a Combined Solar Thermophotovoltaic Power Generation and Solid Oxide Electrolyser for Hydrogen Production. Energy Convers. Manag. 2018, 176, 274–286. [Google Scholar] [CrossRef]
- Rand, D.A.J.; Dell, R.M. Hydrogen Energy: Challenges and Prospects; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on hydrogen safety issues: Incident statistics, hydrogen diffusion, and detonation process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- Mukherjee, U.; Maroufmashat, A.; Ranisau, J.; Barbouti, M.; Trainor, A.; Juthani, N.; El-Shayeb, H.; Fowler, M. Techno-Economic, Environmental, and Safety Assessment of Hydrogen Powered Community Microgrids; Case Study in Canada. Int. J. Hydrogen Energy 2017, 42, 14333–14349. [Google Scholar] [CrossRef]
- Sakamoto, J.; Sato, R.; Nakayama, J.; Kasai, N.; Shibutani, T.; Miyake, A. Leakage-Type-Based Analysis of Accidents Involving Hydrogen Fueling Stations in Japan and USA. Int. J. Hydrogen Energy 2016, 41, 21564–21570. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; Byun, M.; Lim, H. Economic and Environmental Analysis for PEM Water Electrolysis Based on Replacement Moment and Renewable Electricity Resources. Energy Convers. Manag. 2020, 224, 113477. [Google Scholar] [CrossRef]
- van Ruijven, B.; Lamarque, J.-F.; van Vuuren, D.P.; Kram, T.; Eerens, H. Emission Scenarios for a Global Hydrogen Economy and the Consequences for Global Air Pollution. Glob. Environ. Chang. 2011, 21, 983–994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).