Abstract
Sugarcane has primarily been used for sugar and ethanol production. It creates large quantities of residual lignocellulosic biomass such as sugarcane bagasse, leaves, tops, and vinasse. Biomass is a sustainable prospect for biorefineries aiming to optimize production processes. We detail recent research developments in recycling sugarcane, including energy generation and pyrolysis to obtain biofuels, for example. To produce biochar, the energy cost of operating at high temperatures and large-scale production remain as obstacles. The energy generation prospects can be enhanced by pellet production; however, it requires an improvement in quality control for long-term storage or long-distance transportation. In civil construction, the materials still need to prove their long-term efficiency and reliability. Related to adsorbent materials, the use of sugarcane bagasse has the advantage of being low-cost and environmentally friendly. Nevertheless, the extraction, functionalization, and modification of cellulose fibers, to improve their adsorption properties or even mode of operation, still challenges. The synthesis of nanostructures is still lacking high yields and the ability to scale up. Finally, controlling dispersion and orientation and avoiding fiber agglomeration could improve the mechanical response of composites using sugarcane bagasse. The different possibilities for using sugarcane and its residues reinforce the importance of this material for the industry and the global economy. Thus, the present work addresses current challenges and perspectives of different industrial processes involving sugarcane aiming to support future research on waste-derived subjects.
Keywords:
biomass; sugarcane bagasse; nanocellulose; composites; contaminants; biofuels; biosorbents; silica; dietary products; biochemicals 1. Introduction
Sugarcane is an important crop originating in Asia and currently cultivated in tropical and subtropical countries. It became an alternative source of production of ethanol biofuel for automobiles, avoiding the use of other crops commonly used for food production such as corn. Furthermore, sugarcane production is considered low-cost, employs clean technologies, and reuses waste from the production process. Thus, sugarcane is responsible for the economic development of countries and the sustainability of energy sources. Grinding sugarcane and extracting the juice is the principal target for sugar and alcohol production. In addition, sugarcane is used to produce foods and beverages. The world’s sugarcane production surpassed 1.8 billion tonnes in 2021, with 51.7% contributed by the Americas, mainly Brazil, with a production of around 715 million tonnes; 41.4% by Asia; 5.1% by Africa; and 1.8% by Oceania [1]. The national average productivity of Brazil is estimated at 72 tons per hectare, where the southeast region produces approximately 60% of the total national harvest, with 380 million tons in 2022/2023 [2]. Among Brazil’s 44.7% of internal energy offer, sugarcane biomass represents 16.4% [3].
In Brazil, the national biofuel policy is formulated by the RenovaBio Committee; this determines decarbonization targets, certifies the efficient production of biofuels, and provides decarbonization credits (CBIOs), which can be used by companies to trade on an exchange [4,5]. The amount of lignocellulosic feedstock from bagasse (135 kg per ton of sugarcane) and from tops and leaves (140 kg per ton of sugarcane) has drawn attention as a promising alternative for enhancing the production and energy optimization of the ethanol-obtaining process, primarily by the secondary generation of ethanol [6].
Sugarcane bagasse is a fibrous residue that remains after sugarcane juice extraction, and it is a significant source of cellulose and hemicellulose that could be converted into biofuels [7]. Among the products generated, sugarcane bagasse constitutes 30% of the mass of the harvested plants [8]. The annual global sugarcane bagasse production is estimated at 700 million tons [9]. Most of the sugarcane biomass waste, around 70%, is used to generate heat by combustion for sugar production boilers. The heat can be used also to produce steam to move turbines and generate electricity [10]. While sugarcane bagasse has a non-uniform and massive volume, and has been used for energy generation through combustion, pellet production has been evaluated to improve fuel handling and storage, for off-season uses [11,12].
In order to optimize energy generation, or even improve the processes of biofuels and chemicals production, the current concept of biorefineries became an interesting way by use byproducts such as biomass and mostly lignocellulosic residues [13]. Pyrolysis is a standard and efficient method for extracting bio-oil from sugarcane bagasse, for biodiesel production, which could replace petroleum-based fuels [14,15]. Hydrothermal liquefaction is a promising method for obtaining bio-oil with a high calorific value and low oxygen content [16]. Furthermore, sugarcane byproducts are used for biogas and biochar production; this reduces dependence on fossil fuels and minimizes the environmental impact of using non-renewable resources [17,18].
In addition, sugarcane bagasse is also used as a biosorbent for dyes and for absorbing heavy metals in water, to avoid their bioaccumulation and toxicity in humans [19,20]. Nitrocellulose and porous CO2 adsorbent materials are produced from sugarcane bagasse; these are examples of versatile bio-based products obtained in biorefineries [21,22].
Following energy generation, sugarcane bagasse becomes ash, which could be used as a substitute for certain civil materials [23,24] and as a source for synthesis of silica nanoparticles [25]. These possibilities are emerging as a sustainable source for the bioeconomy.
All the information cited proves the importance of sugarcane for different industry sectors. Furthermore, sugarcane bagasse, which is normally the residue of the industrial process, is also used for several purposes reducing environmental impact and increasing industry revenue. In this sense, this paper aims to provide an overview of the current reports on the utilization of sugarcane byproducts (Figure 1), for biofuel and energy generation, as feedstock for nanocellulose, silica, and carbon activate production, for the mitigation of contaminants, and as filler reinforcements in civil and polymeric composites. In addition, addressing the different uses for the sugarcane and its bagasse, this review identifies the knowledge gaps that can be worked on in future researches.
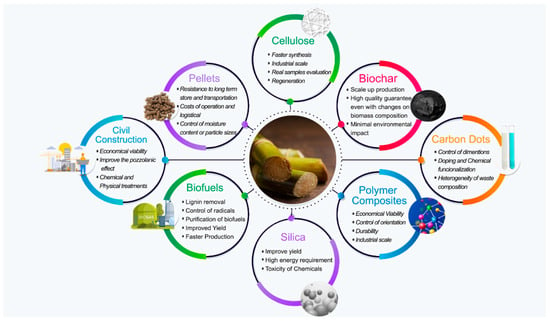
Figure 1.
Summarized applications of sugarcane bagasse and challenges of each field.
2. Biofuels from Lignocellulosic Biomass
Biofuels made from lignocellulosic biomass are renewable energy sources that can be produced from a wide variety of plant materials, such as wood [26], straw [27], and grass [28]. These materials are rich in a complex carbohydrate called cellulose, which can be broken down and converted into biofuels, such as ethanol, biohydrogen, or biodiesel [29,30]. There are several advantages in producing biofuels from lignocellulosic materials [29,31]. First, lignocellulosic materials are abundant and widely available, providing a potential source of feedstock for biofuel production. Second, lignocellulosic materials can be grown and produced in many regions worldwide, providing a more diversified energy supply than traditional biofuels, which are primarily dependent on a few crops and areas [32]. Third, compared to conventional fossil fuels, biofuels from lignocellulosic materials can lower greenhouse gas (GHG) emissions [33] throughout the fuel’s life cycle, from feedstock production to vehicle biofuel use. Notably, CO2 emissions from burning biofuels are offset by the CO2 absorbed by plants during growth [34]. Using agricultural and forestry waste as feedstock for biofuel production can reduce waste and minimize the amount of organic material in landfills [35]. In addition, biofuel production from lignocellulosic materials can provide economic benefits [36] by creating jobs in rural areas and developing new markets for agricultural products.
Lignocellulosic biofuels are more sustainable than traditional biofuels made from food crops such as corn or sugarcane because they do not compete with food production and can be grown on marginal lands [37]. However, converting lignocellulosic biomass into biofuels is currently more expensive and less efficient than the traditional methods [38]. In addition, lignin is a problem when obtaining biofuels from biomass [39] because it is difficult to break down lignin and extract cellulose and hemicellulose, which are carbohydrates that can be converted into biofuels.
Failing to remove lignin before converting cellulose to biofuels adds extra steps and costs to the process. Additionally, lignin can interfere with the efficiency of the enzymes used to break down cellulose, reducing the overall yield of biofuels [40]. Therefore, developing more efficient methods to separate and utilize lignin and enhance the breakdown of cellulose into sugars is critical to overcome these challenges. During the pretreatment process, lignin is broken down into minor compounds [41,42], including monomers (vanillin, syringaldehyde, and p-coumaryl alcohol), oligomers, aromatic compounds, and lignin-derived ash.
The compounds derived from lignin during pretreatment can vary depending on the particular pretreatment method and conditions [43,44]. Oligomers are intermediate-sized compounds that are formed when lignin is partially broken down. They can be further broken down into monomers or used as an energy source. Aromatic compounds such as guaiacol, catechol, and phenol have characteristic fragrances [45]. These compounds can be used as feedstock to produce chemicals and materials [46,47]. Finally, lignin-derived ash is a residual byproduct of the pretreatment process and is composed of inorganic compounds, such as potassium, magnesium, and calcium. Ash can be used as a source of minerals and nutrients in fertilizers [48] or as a filler in composite materials [49].
The pretreatment of sugarcane bagasse is a critical step in producing biofuels [50,51] because it helps make cellulose and hemicellulose more accessible to enzymes and microorganisms, leading to improved biofuel yields. This is typically achieved through chemical or physical treatments, such as acid hydrolysis [52], alkaline pretreatment [53], steam explosion [54], organosolv pretreatment [55], and pyrolysis [43]. Several factors, including the type of biofuel required, the availability of equipment, and cost of the process, influence the choice of technique.
Figure 2 shows the flowchart for obtaining bioethanol from lignocellulosic biomass residues. The flowchart shows the process starting from acid pretreatment and obtaining bioethanol through the fermentation of pentoses and hexoses contained in hemicellulose and cellulose hydrolysates, respectively [56].
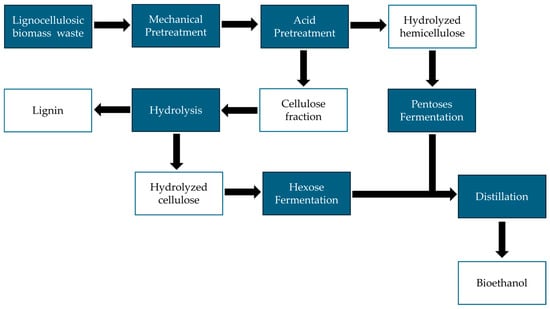
Figure 2.
A flowchart for bioethanol production from lignocellulosic biomass waste.
Biofuel Prospects
Developing new catalysts for the pretreatment of sugarcane bagasse is gaining attention. The aim is to improve the efficiency and sustainability of this process. New catalysts have been developed and studied; these include enzyme-based catalysts [57], ion-exchange resins [58], solid acid catalysts [59], supercritical fluids [60], and metal-based catalysts [61].
3. Sugarcane Bagasse Pyrolysis
Energy based on plant biomass is considered clean, sustainable, and carbon-neutral, because the amount of CO2 emitted into the atmosphere during combustion is equal to the amount captured through photosynthesis during a plant’s lifetime [62]. Therefore, the use of lignocellulosic biomass for the synthesis and production of biofuels is widely studied [43,63,64,65,66]. Thermochemical conversion plays a fundamental role in transforming lignocellulosic biomass into biofuel, offering several options such as gasification, combustion, torrefaction, pyrolysis, and hydrothermal liquefaction. However, among these methods, pyrolysis stands out as the most advantageous choice in many respects [67,68]. Pyrolysis has proven to be highly efficient in producing biofuels from lignocellulosic biomass, such as sugarcane bagasse. This technique converts the raw material into a variety of useful products, including high-quality bio-oil, biochar, and combustible gases, with significant yields compared to other approaches. Additionally, pyrolysis offers unparalleled versatility in the range of products obtained, providing flexibility and diversity of use for the resulting products [68]. Another significant advantage of pyrolysis is its ability to reduce the generation of unwanted waste. While some techniques, such as gasification and combustion, may produce ashes and other undesired by-products, pyrolysis minimizes waste production, contributing to process efficiency and reducing environmental impact [62]. Furthermore, pyrolysis can be engineered to control and minimize emissions of atmospheric pollutants, such as carbon dioxide and volatile organic compounds (VOCs), making it a more environmentally friendly option compared to other conversion methods. The superior quality of the final products, such as bio-oil and biochar, makes them highly desirable for a variety of industrial and commercial applications [69,70].
The fractionation or yield of the pyrolysis products is determined by the operational factors of the equipment and the nature of the biomass. The operational variables that most influence the conversion are the reactor type, biomass diameter, temperature, heating rate, process operating time, and inert gas flow [71]. Pyrolysis can be classified as slow, fast, or flash, depending on the operating variables, particularly the working time and heating rate [72].
3.1. Slow or Conventional Pyrolysis
The slow or conventional pyrolysis of sugarcane bagasse favors the high production of solids such as biochar and generally occurs at 5–10 °C·min−1, with an extended length of stay of more than 30 min, and particles with a diameter between 5–50 mm [9]. The transformation of lignocellulosic waste into products with high economic value is studied. Boer et al. (2021) analyzed slow pyrolysis in a fixed-bed reactor under two operating conditions. The results showed that high-quality biochar (28.97%) and high pyrolysis liquid yield (55.46%) could be achieved by processing the residue at 500 °C and 10 °C·min−1 [73]. Rodier et al. (2019) used a slow pyrolysis reactor at 200 °C, with particles between 0.4–1 mm, a heating rate of 10 °C·min−1, nitrogen (N2) flow of 2 L h−1, and residence time of 2 h, in which sugarcane bagasse biochars were produced to be incorporated into the cement base and used as an insulation material [74].
Okonkwo et al. (2021) prepared mesoporous carbon via ice-water-controlled pyrolysis and used it to improve the capacitance of the electrode of a supercapacitor. They used sugarcane bagasse particles with an average diameter of 150 µm in a quartz tube pyrolizer at 800 °C for 3 h under N2 flow at 20 mL·min−1. The incorporation of mesoporous carbon enhanced the electrochemical behavior and allowed the capacitor to attain a high energy density [75].
3.2. Fast Pyrolysis
The production of liquid biofuels through fast pyrolysis of sugarcane bagasse can be carried out by operating the reactor at an attenuation temperature (500–750 °C), with a high heating rate between 100–500 °C·s−1, a brief stay of around 1 s, and particles with a diameter of less than 1 mm [9,43]. Ghorbannezhad et al. (2018) introduced a new methodology using fast pyrolysis to produce volatile substances such as benzene, toluene, and xylene (BTX), which are widely used in the chemical industry. They evaluated the effect of pyrolysis temperature, type of catalyst, and varying proportions of different types of catalyst mixtures on the yield of BTX from sugarcane bagasse via ex situ catalytic pyrolysis. It is possible to estimate optimal operating conditions using low-cost raw materials [76]. Another approach for the extraction of volatile compounds with high commercial value and wide use in organic synthesis was proposed by Lu et al. (2018). They increased the yield and selectivity of the phenolic compound, 4-ethyl phenol (4-EP), in the fast catalytic pyrolysis of bagasse, using particles with a diameter between 0.1–0.2 mm and a hydrogen atmosphere to provide hydrogen donors. This promoted significant increases in the yield of 4-EP compared to that from using an inert atmosphere [77].
Soongprasit (2021) aimed to minimize the undesirable byproduct aromatic furan (2,3-dihydro benzofuran), the main product from bagasse lignin and commercial organosolv lignin in the pyrolysis process, to promote the production of aromatic hydrocarbons. A zeolite-based catalyst was used in the pyrolyzer at 600 °C for improving the selectivity of the desired chemical compounds. This enables developing biorefinery process strategies for target chemicals, such as BTX [78].
3.3. Flash or Ultrafast Pyrolysis
The main product from sugarcane bagasse following flash or ultra-fast pyrolysis is biofuel. The process involves a grinding step, for making the particles small enough (0.2 mm) to fluidize inside the flash reactor, before pyrolysis [79]. The operation is characterized by high heating and temperature rates, a short steam length of stay (generally less than one second), and rapid cooling of the pyrolysis steam [80]. Ayyadurai and Arunachalam (2022) analyzed the pyrolytic oil produced from the flash pyrolysis of sugarcane bagasse, performed at a pressure of 0.03 bar and an average temperature of 550 °C. The flash pyrolysis process converted all biomass residues, excluding ash, into three components: bio-oil with 70% yield, pyrolysis gas with 14% yield, and charcoal with 16% yield [81]. Flash pyrolysis can mimic gasification under certain conditions, such as at temperatures exceeding 800 °C, length of stay in the reactor less than 1 s, heating rates exceeding 1000 °C·s−1, and particles with sizes less than 0.2 mm. This process primarily generates non-condensable gases such as hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), methane (CH3), ethane (C2H6), and ethene (C2H4) [72].
3.4. Comparative Studies
Knowledge of the types of pyrolysis and the various operating parameters is often required to select the best process conditions. It is common practice to compare various methodologies or aspects of biomass production under identical circumstances to improve the process. Arni (2018) compared the results of conventional and fast pyrolysis of sugarcane bagasse to convert biomass into biogas fuel. A batch reactor was used at temperatures of 480, 580, and 680 °C. Slow pyrolysis produced the highest syngas yield with increasing temperature. The solid yield was higher for fast pyrolysis. During thermal decomposition of the lignocellulosic residue, H2 gas, CO, CO2, CH3, and some low molecular weight hydrocarbons, such as C2H4 and C2H6, were produced [82].
Pradana et al. (2019) investigated the effect of calcium oxide (CaO) and magnesium oxide (MgO) catalysts on the catalytic and non-catalytic pyrolysis of sugarcane bagasse, in a fixed-bed reactor at a temperature range of 20−480 °C. Calcium oxide exhibited the highest reaction kinetics, with the lowest reaction order in the catalytic and non-catalytic processes of MgO. Thus, the incorporation of calcium oxide (CaO) as a catalyst would improve the pyrolysis kinetics of sugarcane bagasse [83].
Hass and Lima (2018) investigated different food sources such as fresh sugarcane straw, fresh bagasse, and old bagasse for potential conversion into biochars and activated biochars for metal sorption. The pyrolysis process was evaluated at 350, 500, 650, and 800 °C. The ash content was higher in fresh sugarcane straw and old bagasse compared to that in fresh bagasse biochar. In addition, the surface area of the activated biochar was higher for that from fresh bagasse (493 m2·g−1) compared to that for biochars from old bagasse (262 m2·g−1) and fresh sugarcane straw (204 m2·g−1). Aged sugarcane bagasse produces biochar with a high sorption capacity for metals such as cadmium (Cd), lead (Pb), and copper (Cu). Sorption capacity is associated with the ash content, surface area, and composition of the biochar [84].
3.5. Pyrolysis Prospects
Pyrolysis emerges as a highly beneficial alternative in the production of biofuels from sugarcane bagasse, offering positive environmental value. This process presents several advantages, including the reduction in greenhouse gas emissions and the efficient utilization of agricultural waste, thus contributing to climate change mitigation and promoting environmental sustainability. However, despite its environmental advantages, the commercialization of biofuels produced by pyrolysis faces significant challenges. This is largely due to the high costs associated with the acquisition and operation of the equipment required to implement the pyrolysis process on a commercial scale. Additionally, the lack of policies supporting the production and consumption of sustainable biofuels can hinder the penetration of these products into the market, especially compared to traditional fossil fuels. To overcome these challenges, it is essential for governments and relevant institutions to implement policies and regulations that incentivize the production and use of biofuels derived from sugarcane bagasse pyrolysis. This may include financial incentives, subsidies, and tax incentive programs for producers and consumers. Additionally, investment in research and development of new technologies to reduce production costs and improve the efficiency of the pyrolysis process is needed. Through a comprehensive set of measures addressing both economic and environmental aspects, it is possible to enable the commercialization and widespread adoption of biofuels produced by sugarcane bagasse pyrolysis, thus contributing to the transition to a greener and more sustainable economy [62].
4. Biochar from Sugarcane
Organic solid waste from agriculture, forestry, municipal solid waste, food, and other residues are potential sources for biochar production [85]. Biochar obtained from biomass using thermal combustion in an oxygen-free environment has a high carbon content [86]. The unique properties of biochar, such as its large surface area, high porosity, functional groups, high cation exchange capacity, and stability, allow its use in various applications [87,88]. Figure 3 shows a flowchart with two paths for energy use from sugarcane bagasse. The first way is the production of pellets used as solid biofuel and the generation of thermal energy and electrical energy. The second way shows the biochar route from the pyrolysis process [9,18]. Biochar can be used to obtain syngas and used in combustion engines [89]. It has applications as a solid biofuel as well as for energy conversion and storage, such as hydrogen production and storage [90], supercapacitor electrodes [91], and lithium/sodium ion batteries [92].
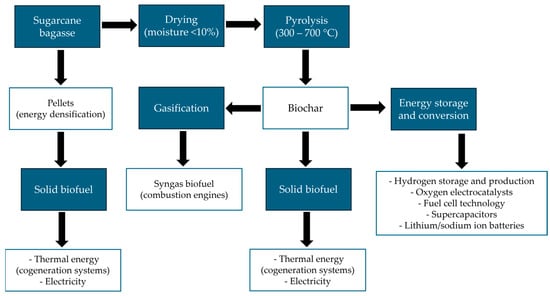
Figure 3.
A flowchart with two paths for energy use from sugarcane bagasse.
The multifunctionalities of biochar and its efficiency as a contaminant adsorbent, GHG remover, electrochemical component, and reinforcing filler in polymer composites have attracted wide attention [93,94]. The quality and yield of biochar depend on the thermal conversion process parameters, such as biomass type, temperature, residence time, heating rate, and pressure [95]. The thermochemical conversion methodology used to obtain biochar includes pyrolysis, torrefaction, hydrothermal carbonization, flash carbonization, and gasification [93]. Pyrolysis is an established alternative method for degrading organic compounds in lignocellulosic waste to produce biochar. However, this methodology requires knowledge of the reactor type, a specific operating temperature, and a limited oxygen environment [96]. Determining biochar particulars is relevant for evaluating the chemical composition, properties, and reactivity of the biochar surface [97].
The generation of large amounts of lignocellulosic waste, combined with the desire to reduce environmental impact and the search for sustainable resources, has influenced the development and optimization of the thermoconversion process for biochar. Table 1 lists the thermoconversion methods and the operating conditions. Temperature is the primary operating variable in a thermoconversion reactor; it directly influences the efficiency of the process because degradation of the biomass constituents occurs during thermochemical conversion [98,99,100,101,102,103,104,105,106,107]. According to Stegen and Kaparaju (2020), the maximum yield of biochar from sugarcane bagasse is obtained in a moderate temperature range (450 °C) in pyrolysis [108]. Temperatures above 450 °C could impair biochar yield because they favor the formation of non-condensable gases. Guida and Hannioui (2017) studied another critical parameter influencing the heating rate in a pyrolysis reactor and demonstrated that high heating rates tend to depolymerize the biomass, significantly reducing the biochar yield [109]. Other factors, such as residence time, particle diameter, catalyst, type of biomass pretreatment, and use of inert gases, such as N2, can contribute to a better biochar yield [110], being indicated for many applications, including pollutant remediation and as a sensor component.

Table 1.
Typical operating conditions and product yields for thermoconversion of lignocellulosic biomass.
4.1. Remediation of Soil Pollutants and Gas Removal
Biochar has multiple uses in agriculture, including that as a substrate to improve and increase crop yields, reduce the potential of heavy metals in soil, prevent the release of nitrous oxide (N2O) gases, and sequester carbon [111]. The outer layer of biochar contains several active chemical groups, such as carboxylic acids and ketones, among other groups with a high capacity to adsorb heavy metals from soil [112].
Heavy metal mining activities, such as that for chromium (Cr) and lead (Pb), cause damage to soil ecosystems because they are absorbed by food crops, causing environmental damage and problems for human health. Khan et al. (2020) analyzed the effectiveness of biochar from sugarcane bagasse and poplar for the remediation of contaminated soil. Biochar could extract heavy metals from the ground, reducing their absorption by plants, such as lettuce [87]. Bashir et al. (2020) showed that the combined use of biochar and elemental sulfur could alleviate the harmful effects of Cr in maize and reduce the uptake of Cr by plants [88]. Matos et al. (2021) showed that sugarcane bagasse biochar could be used for mitigating N2O (a GHG) emissions. In addition, it can be applied to soil as a reducer or sorbent for species involved in the nitrification and denitrification processes in agricultural soils [113]. Accidents caused by crude oil extraction or transportation have become a growing concern because their impacts on ecosystems are long-lasting. Wei et al. (2020) successfully produced sugarcane bagasse biochar using a rhamnolipid biosurfactant and N2; their synergistic effects could adsorb aromatic compounds and degrade aliphatic compounds. Biogas, which could replace liquefied petroleum gas, is generated through fermentation and it is composed of CH3 and CO2. The presence of CO2 decreased the calorific value of biogas [114]. Wuri et al. (2021) produced a biochar composite with natural zeolite that showed promising results for adsorbing CO2 [115].
4.2. Biochar as an Electrochemical Component
Several studies on wastewater, geothermal, and seawater treatments have been conducted worldwide [116]. Capacitive deionization via electrochemical desalination is widely used as an alternative to water treatment. Tang et al. (2020) developed a mesoporous carbon electrode with excellent electrosorption capacity from sugarcane bagasse biochar. Biochar has functional oxygen groups on its surface that allow it to interact with different species and to be used as a modified electrochemical sensor for the determination of inorganic and organic species [117]. Valenga et al. (2023) developed an electrochemical immunosensor for detecting antibodies against SARS-CoV-2 by replacing the carbon electrodes with sugarcane bagasse-based biochar. The device responded with a confidence level of 95% and remained selective, even in the presence of other drugs [118].
4.3. Biochar Prospects
Sugarcane bagasse biochar is a promising renewable source for several market segments. However, the energy cost of operating at high temperatures remains an obstacle. Continuous large-scale production for industrial applications could make the method viable. Scaling up biochar production, by using biomass from plants with short life cycles, maintaining high quality even with changes in feedstock properties, and minimizing environmental impact are challenges in the way of market acceptance of biochar.
5. Boosting Energy Efficiency Using Sugarcane Bagasse Pellets
Biomass, as a renewable resource, has significant environmental and socioeconomic benefits in waste utilization and mitigation of environmental impacts [119]. Various types of biomasses and their residues (sugarcane bagasse, palm tree residues, coconut fiber, rice husk, wheat straw, and tree pruning) [120,121,122], can be used as feedstock for modern bioenergy production chains by generating electricity, heat, and other benefits for the bioeconomy [123]. However, lignocellulosic biomass residues have substantial limitations, such as large volumes and low apparent density associated with transportation and storage, and low energy efficiency compared with petroleum-derived fuels [124]. These limitations in biomass characteristics demonstrate the need for planning logistics supply chains. However, the diversity of biomass residue species and their idleness instigate the need for a more dynamic product design to supply production and consumption cycles [125]. Densification methods, such as pelletization, could be an alternative to reduce the costs associated with the logistics of transport, storage, and handling of biomass and to increase energy efficiency [126].
The global market for solid fuels based on lignocellulosic residues has grown in recent years, with a focus on the consumption of pellets and briquettes in rising economies [124]. Albashabsheh and Stamm (2021) reported that the cost of logistics from the field to the energy plants represents about 35 to 65% of the total cost of biofuel production [126]. It is recommended that the logistical prices of the biomass supply chain be at most 25% of the total output. Strategies such as raw material densification and transportation are essential for minimizing total supply chain cost [127]. Among the densification techniques, pelletization is considered the most advantageous.
Pelletization generally increases the density by about 70 kg·m−3 up to 1000 kg·m−3 and reduces the moisture content to less than 10% (weight basis) [128]. A high density also reduces the costs associated with transporting and storing biomass. The particles produced from agricultural or agro-forestry waste, compacted under high pressure, have the shape of a small cylinder between 6.3–6.5 mm in diameter and 6–20 mm in length; these are called pellets, and they can be made available mainly to local industries to feed boilers for heat generation, thermoelectric plants for conversion of heat into electricity, and to traders and households as a source of thermal energy [129].
The European Union remains the global leader in wood pellet production, followed by the United States (US) and Canada. Pellet consumption in the European Union reached 24.5 million tons in 2021, with the residential and commercial sectors accounting for two-thirds of this consumption, and industry and utilities for the remaining [130].
Large sugar and ethanol producers, such as Brazil, use sugarcane bagasse to heat the boilers at the plants; this is called energy cogeneration [131]. However, the energy gain from direct burning was only 26% [132]. Therefore, there is a need to focus on the densification of lignocellulosic waste, emphasizing pellet production [133].
5.1. Parameters Influencing Sugarcane Bagasse Pellets
The densification process generally includes biomass selection and collection, drying, particle size reduction, pretreatment, pelleting, densification, and bulk storage. They can be characterized by evaluating the physicochemical, mechanical, and fuel properties of biomass pellets [134]. Chen et al. (2021) assessed some of these parameters, such as pellet density, specific energy consumption, and maximum radial pressure of bagasse pellets, and how they were affected by temperature, moisture content, particle size, and waiting time. At the optimal temperature, it is possible to achieve 1172 kg·m−3 pellet density, 7.1 kJ·kg−1 specific energy consumption, and 2539.4 N maximum radial pressure for sugarcane bagasse pellets [135].
Silva et al. (2020) evaluated the physical, chemical, and energy characteristics of pellets produced from mixtures of lignocellulosic biomass, such as elephant grass, eucalyptus wood, and sugarcane bagasse, to generate bioenergy. There was an increase in calorific value and energy density, and a reduction in moisture content. In addition, the pellets produced with biomass mixtures exhibited better energy and mechanical performance compared to those made with only one type of biomass [136].
The resistance of the pellets can be increased using additives called binders. Akbar et al. (2021) analyzed the effect of bovine manure and molasses in pellet formation. Under ideal conditions, the pellet had a calorific value of 16.43 MJ·kg−1 with a resistance index of 84.2%. During biomass torrefaction, heat treatment improved the energy content of the biomass and slightly increased the durability of the pellets [137].
5.2. Sugarcane Bagasse Pellets as Energy Sources
Excess ash after biomass burning hinders the combustion efficiency in boilers. Therefore, it is necessary to develop alternatives for the proper combustion of biomass. A study by Moreira et al. (2024) used an automated pelletizer at 200 MPa and 125 °C to co-press sugarcane bagasse with pine sawdust or peanut shells in the typical 50/50 ratio to create pellets. The ash content decreased in the biomass mixture, making them highly energetic. In addition, the concentrations of volatile compounds and permanent toxic gases (nitrogen oxides (NOX), sulfur dioxide (SO2), CO, CO2) decreased [138].
Scatolino et al. (2018) studied biomass mixtures for pellet production using soybean waste, sugarcane bagasse, and sawdust from eucalyptus wood, focusing on thermal energy generation. The hybrid pellets showed a higher calorific value, mechanical resistance, and hardness, and lower amounts of fines and ash, compared to that of those composed only of soybean residues [133].
Santana et al. (2021) manufactured pellets from soybean, sorghum, pine needles, rice powder, eucalyptus sawdust, and small coal particles. In addition, pure pellets made from soybean leftovers, sugarcane bagasse, and pinewood were tested. Pellets made from soybean waste have high N2 and ash levels, and limited mechanical durability, making it difficult to sell them for industrial use. In contrast, the sugarcane bagasse pellets presented a net calorific value, mechanical durability, and N2 and ash contents suitable for industrial energy generation [139].
Using agro-industrial residues is an exciting possibility for generating energy and heat in homes. Cardozo and Malmquist (2019) compare the performances of wood pellets and sugarcane bagasse in a micro-cogeneration system using a Stirling engine. The sugarcane bagasse and wood pellets had similar Stirling engine hot-end temperatures, power outputs, and emission levels. However, the Stirling heat exchanger fouling factors increased after shorter operating times when bagasse pellets were used compared to that when wood pellets were used [11].
5.3. Other Applications of Sugarcane Bagasse Pallets
Sugarcane bagasse pellets could be used as low-cost adsorbents for removing heavy metals such as Ni2+ ions from an aqueous solution in batch and fixed-bed column operation modes [140]. Fly ash was used from sugarcane bagasse as a matrix to produce pellets with fertilizers (ammonium sulfate and potassium chloride), for the slow release of fertilizers into the soil. Moreover, the application of a sintering process to sugarcane bagasse pellets was investigated for lightweight aggregate production intended for use in the civil engineering field, thus maximizing their potential applications [141].
5.4. Pellets Prospects
Pelletizing improves energy efficiency, transport, produces lightweight aggregates, and results in easier storage of biomass; in addition, it results in the emission of fewer GHGs than petroleum-derived fuels [142]. However, the high concentration of inorganics in the ash and the low mechanical resistance are the most significant limitations for the energy applications of pellets produced from agro-industrial residues [6].
Controlling the quality of pellets, during long-term storage or long-distance transportation, remains a challenge. This can be achieved by torrefaction; however, this increases the pelletization cost. Therefore, operating and logistical costs are the key barriers; some of these costs can be reduced by automating the pelletizing and densification. No-wood pellets should have optimal values for parameters, such as moisture content and particle size, and should offer a high calorific value to reach market acceptance.
6. Second-Generation Ethanol (2G)
The high consumption of ethanol has enabled the development of several works searching for new methods and raw materials for production of this fuel production [143]. However, the sugar-cane industrial process continues to produce a large amount of lignocellulosic residues, which is one of the main sources having great potential for the production of second-generation ethanol (2G) [56,144]. Ethanol 2G is a bioethanol produced from biomass, mainly lignocellulosic fractions of sugar cane (bagasse and leaves), which has a high market potential as a fuel for motor vehicles [145], but can be also used to other application, such as hand sanitizers [146].
The lignocellulosic biomass (LCB) can be found in various agricultural residues being abundant, easily available, and cheap feedstock for ethanol 2G production [147]. Chemically, LCBs consists mainly of lignin (10–25%), cellulose (40–60%), and hemicellulose (20–40%), which bind tightly together forming a heterogeneous recalcitrant matrix, resistant to chemical and biochemical degradation [148].
There are several factors that involve the process of recalcitrance to lignocellulosic hydrolysis to produce 2G ethanol, such as cellulose crystallinity, degree of polymerization, surface area, pore size, and volume. Transforming LCB into 2G ethanol is challenging when compared to the transformation required into 1G ethanol. This fact is mainly due to the resistance of the biomass matrix, the high lignin content, and the inefficient breakdown of the complete carbohydrate-lignin structure [148].
According to Vieira et al. (2020), despite the great efforts made by researchers, the use of sugarcane bagasse to produce 2G ethanol has not yet reached commercial scales due to the high costs involved in the pretreatment processes that enable hydrolysis to be carried out for production of 2G ethanol [149].
In this sense, there are authors researching different techniques to improve the 2G ethanol production process. For example, Prajapati et al. (2020) presented a saccharification of sugarcane bagasse using an Aspergillus tubingensis enzymatic cocktail. The enzymatic cocktail used by the authors performed hydrolysis efficiently on different residues and, therefore, was considered promising for achieving greater cellulase production and saccharification of sugarcane bagasse to produce 2G ethanol [150]. For Vanmarcke et al. (2021), it is extremely important to evaluate the ethanol produced using different techniques, as well as analyze yeast inhibitors to improve the economy of industrial processes [151].
7. Sugarcane Bagasse: The Potential Cellulose Source for Biosorbents
Sugarcane bagasse is mainly composed of cellulose (42–58.2%), hemicellulose (9.2–25%), and lignin (13.4–20%) [8,152], a potential residue to obtain these biopolymers to apply in different applications. Considering its renewability, biocompatibility, chemical stability, biodegradability, and excellent mechanical properties, cellulose has been extensively applied in various scientific fields [153,154]. It comprises repeated units of D-glucose linked through β-1,4 glycosidic linkages; it is mainly composed of carbon, hydrogen, and oxygen [155], a natural polymer abundant worldwide with annual yields of approximately 1.5 × 1012 tons. Massive intra- and inter-molecular hydrogen bonds between the hydroxyl groups, originating from elementary fibrils, form microfibers with diameters of approximately 5–50 nm; these are responsible for the high cohesiveness [154,155]. Nanostructured cellulose adsorbents with surface modifications through functionalization enable decontamination of wastewater and air through an adsorption mechanism.
7.1. Removal of Contaminants from Wastewater
The most important resource for human survival is water, which is threatened by unprecedented pollution. Global industrialization has improved society; however, it has resulted in the production of several wastewater and pollutant gases from complex substances, such as organic pollutants and toxic metals. The persistence of these compounds is caused by inappropriate treatment; therefore, drinking water is subject to contamination, posing a risk to human health. This makes it necessary to develop new treatment types, such as adsorption-based methods, that are more efficient and can be widely used owing to their low cost, simplicity, and efficient application [154,156]. The development of biosorbents based on cellulose nanofibers (CNFs), hydrogels, aerogels, and xerogel nanostructures is promising [154].
To increase the affinity between the biosorbent and contaminants, chemical modifications of the cellulose structure, including sulfonation, oxidation, amidation, carboxylation, cationization, etherification, phosphorylation, and esterification are studied [157].
7.2. Heavy Metal Removal Using Sugarcane Bagasse
Various toxic metals are considered the most hazardous compounds that pose a risk to human and animal health owing to their toxicity and bioaccumulation. These metals are pollutants in soil, marine environments, and in untreated industrial wastewater; developing methods for their efficient removal is necessary [158]. Negatively charged functional groups are efficiently utilized for heavy metal removal, such as Cr6+ removal. Polypyrrole/sugarcane bagasse, a material obtained through facile treatment of bagasse with pyrrole monomer under room temperature, has an adsorption maximum capacity (qm) of 156 mg·g−1 [159]. Sugarcane bagasse was used for the synthesis of hybrid cellulose nanofibers doped with MgS/FeS, with an adsorption capacity of 142.8 mg·g−1; these were used for Cr6+ removal [160].
Guleria et al. (2020) studied sugarcane cellulose nanofibers that form a copolymer with a binary monomer, acrylamide/acrylic acid, resulting in metal adsorption capacities of 101.73 (Cd2+), 61.84 (Cu2+), 209.64 (Pb2+), and 55.04 mg·g−1 (Zn2+), under pH 5.5–6.0, with a contact time of 49.7 min, and at room temperature [161]. Different treatments with sugarcane bagasse showed promising results for the adsorption of other heavy metals. Sugarcane treated with ferric chloride (FeCl3) presented an adsorption capacity of 20 mg·g−1 for As3+ removal (at pH 4, contact time of 3 h, and 22 °C) [162]. Sugarcane bagasse-treated alkaline and nitric acid solutions were applied as a Pb2+ adsorbent with a maximum adsorption capacity of 200.3 mg·g−1 (at pH 5, contact time of 2h, and 25 °C). The use of trimellitic anhydride to promote the esterification of sugarcane bagasse (STA) resulted in adsorption capacities of 1.140, 1.197, and 1.563 mmol·g−1 (at pH 5.5 to 5.75) for Co2+, Cu2+, and Ni2+, respectively [158].
Modification or functionalization of the biochar surface can improve its properties and adsorption capacities. The adsorption of arsenic using raw biochar without modification and with chemical modification using iron coating was investigated. Biochars coated with Fe3+ were more efficient in removing arsenic, and the adsorption capacity was influenced by the type of biomass and pyrolysis temperature [162]. Optimized biochars derived from rotten sugarcane bagasse demonstrated a higher Pb2+ adsorption capacity than biochars prepared from fresh bagasse. Raw sugarcane bagasse is highly efficient for treating effluents containing more than one metal ion [163]. A work by Jiang et al. (2022) produced an iron sulfide composite with sugarcane bagasse biochar modified with zinc chloride for the adsorption of Cr6+ ions [164]. Zhu et al. (2021) developed three-dimensional nickel nanoparticles embedded in N2-doped carbon nanotubes supported on the surface of porous biochar [165].
This study contributes to the consideration of sugarcane bagasse under soft conditions and as natural raw fibers by modifying the hydroxyl groups, making it a potential adsorbent for treating wastewater polluted by toxic metal ions.
7.3. Dye Removal by Sugarcane Bagasse
The levels of emerging contaminants, such as dyes, have increased drastically in water sources; they are released mainly from the textile industries [166]. These compounds can exist freely, in metabolic form, or in combination with other environmental organic pollutants. The persistence of dyes in the environment is linked to damage to marine species and in humans, causing numerous diseases, such as allergies, skin flushing, and carcinogenesis, justifying the importance of their remediation.
Kamran et al. (2022) applied pyrrole to the surface of sugarcane bagasse to generate a polypyrrole biocomposite (Ppy-SB), which can be used to remove acid red 1 dye. At pH 2, 30 °C, and a contact time of 75 min, Ppy-SB had an adsorption capacity of 205.1 mg·g−1, while untreated sugarcane bagasse had an adsorption capacity of 143.4 mg·g−1 [166]. Studies on the behaviors of different composites based on sugarcane bagasse coupled to chitosan, starch, polyaniline, and polypyrrole have determined that polypyrrole/sugarcane bagasse (Ppy-SB) is the most efficient, with an adsorption capacity of 100 mg·g−1 for acid black dye (at pH 3, 60 min) [167]. Sugarcane bagasse functionalized with β-cyclodextrin (SB-β-CD) was used to remove the cationic dye. Generation of carboxylic groups and the presence of hydroxyl groups on SB promote effective adsorption at room temperature, in 240–300 min, at 292.8 and 193.1 mg·g−1 for methylene blue and neutral red, respectively. All these treatments demonstrate the possible applications of sugarcane bagasse in dyeing wastewater remediation [168].
7.4. Organic Compound Removal
In addition to dyes, other organic compounds are emerging as contaminants, generating huge concerns about water pollution worldwide. Pesticides [169], pharmaceutical and personal care products (PPCPs) [170], and veterinary medicines [171] are listed among the primary water contaminants. To remove these contaminants, composites, nanocomposites, and treatments have been applied to sugarcane bagasse, promoting properties that allow for a higher removal efficiency.
Magnetic nanocomposites were produced from sugarcane bagasse (N/S-MCA) to obtain a highly porous material for the removal of bisphenol A (BPA) from water. The maximum reduction obtained for this organic contaminant was approximately 98–99% at pH 7 and a temperature of 70 °C. Therefore, N/S-MCA is a highly efficient alternative material for adsorbing environmental pollutants [172]. In addition, a composite of sugarcane bagasse (BG) and carboxymethyl cellulose (CMC) was modified using micelles of cetyltrimethylammonium bromide (CTAB) (CMCBG-CTAB) by [173]. This was applied for the simultaneous removal of methylene blue (MB), bisphenol A (BPA), and Cr6+ ions, reaching removal capacities of 100%, 95%, and 70%, respectively.
Other phenolic compounds (vanillin and tannic acid) generated during the pretreatment of sugarcane in liquid hot water (LHW) were removed using sugarcane bagasse fly ash. In addition, fly ash as an adsorbent removed 80% of the phenolic compounds from the LHW liquor, similar to the performance obtained using commercial activated carbon [174].
A graft copolymerization process (PBCF) was used to create a sugarcane bagasse-based flocculant to remove humic acid (HA) from an aqueous solution. The removal effectiveness was up to 90.6% in synthetic water and 91.6% in lake water [175]. Komal et al. (2020) functionalized cellulose nanofibers (CNFs) and modified graphene oxide (GO) using mechanical and chemical processes to remove drug species from aqueous solutions. For ciprofloxacin and ofloxacin, maximal removal capacities of 45.04 mg·g−1 and 85.30 mg·g−1, respectively, were observed [176].
Hydrothermal carbonization (HTC) and sodium hydroxide (NaOH) activation was used to produce biochar from sugarcane bagasse to remove sulfamethoxazole, an antibiotic, from water. Isotherm studies showed the Freundlich model well described the isotherm of a heterogeneous and spontaneously interacting compound with a maximum adsorption capacity of 400 mg·g−1 [177]. Diclofenac sodium (DFC), an anti-inflammatory drug, can be adsorbed on to sugarcane bagasse. Naga et al. produced a porous activated carbon from sugarcane bagasse (SCB-AC) for high DFC removal; this reached an adsorption capacity of 315.0 mg·g−1 with 92.4% removal after five cycles [178].
Perfluorooctane sulphonate (PFOS), a persistent bioaccumulative and toxic compound, was removed from water. Magnetic biochar (MBC) was synthesized by modifying sugarcane bagasse (SB) through contact with hematite nanoparticles for PFOS adsorption [179]. The maximum adsorption capacity was 120.44 ± 12.37 mg·g−1. Silanized cellulose nanofibers (SCNFs) are derived from sugarcane bagasse with cadmium sulfide (CdS) nanoparticles; this is used as an adsorbent to remove chlorpyrifos, an insecticide widely used in agriculture, from wastewater. The adsorption shows a removal capacity of 93.16% for chlorpyrifos (initial concentration of 8 × 10−5 M and pH 3) [176].
Simpler treatments such as acid and alkaline treatments have been explored. Sulfuric acid modification of sugarcane bagasse (SBAC) exhibits an efficient adsorption capacity of 771.1 mg·g−1 for toluene (30 °C), which is three times higher than that for the commercial activated carbons [180]. Alkaline treatment with NaOH was used to functionalize sugarcane bagasse fibers (SBNaOH) for the removal of glyphosate from an aqueous medium. Chemical treatment increased the surface area of the fiber. Sugarcane bagasse (SB) and SBNaOH are promising as adsorbents for removing herbicides, with efficiencies greater than 65% [181].
7.5. Cellulose Hydrogel, Aerogel, and Xerogel
Hydrogels, aerogels, and xerogels are three-dimensional materials that can be classified according to their fabrication method [182]. The aerogels develop large pores during freeze-drying once the ice crystal is formed, followed by sublimation into its 3D structure [183]. Evaporation drying results in the conventional collapse of pores owing to the high capillary pressure, giving rise to xerogels with high density and low porosity [182]. Hydrogels encapsulate water molecules inside the network and present a synergistic action between the preserved pores and functional groups chosen to compound the structure [182].
Ahamad et al. (2019) produced a magnetic carbon aerogel based on sugarcane bagasse for BPA removal; removal of 98–99% of the initial concentration of 100 ppm was achieved. A comparison with other materials with higher porosities was presented. However, BPA adsorption onto this organic compound was superior, even though it was a natural product source derivative [172]. Xerogel porosity depends on the solvent used for drying; Ganesan et al. (2016) showed that using isopropanol and ethanol resulted in 80% and 70% porosity, respectively [182]. The properties of the composite obtained using isopropanol was better with a surface area of 100 m2·g−1, with more pores, and fewer agglomeration nanofibers, as elucidated by SEM; this has potential for application in adsorption. A hydrogel biosorbent was developed based on cellulose from sugarcane bagasse and modified with thiourea groups; this exhibited the maximum adsorption capacities for methylene blue and crystal violet, which reached 632.9 and 574.7 mg·g−1, respectively. In addition, it was amenable to efficient regeneration that enabled reuse in the ion-exchange process. This new production of structures based on cellulose from sugarcane bagasse opens possibilities for their application in other natural polymeric systems to improve the material’s biodegradability, efficiency, and toxicity [184].
7.6. Gas Adsorption by Sugarcane Bagasse
Sugarcane bagasse adsorbents have been applied in CO2 adsorption. A selective CO2 adsorbent from sugarcane bagasse was produced, exhibiting the highest CO2 removal of 4.8 mmol·g−1 (pressure of 1 bar and 25 °C). The produced active carbon showed excellent CO2/N2 selectivity, guaranteeing high application efficiency [175]. Gou et al. (2020) studied the production of porous activated carbons from sugarcane bagasse. This study investigates different activating agents (H3PO4, CO2, NaOH, and air). Carbon activation using NaOH (CAC-S) with a large pore volume and a high specific surface area showed CO2 removal efficiencies of 5.50 and 4.28 mmol·g−1 (1 bar, 0 °C, and 25 °C, respectively) [21]. Porous carbon was modified with N2 by varying the ratio of carbon–melamine from sugarcane bagasse to solid-state melamine to investigate alternatives for CO2 adsorption. The modified carbon–melamine demonstrates the highest CO2 removal of 3.34 mmol·g−1 (ambient pressure and temperature) [185]. All sugarcane bagasse-based adsorbents were comparable to similar materials reported in the literature.
7.7. Adsorption Prospects
Adsorbents from sugarcane bagasse have the advantage of being low-cost and eco-friendly materials that can contribute to a circular economy. However, despite the promising findings, the development of new biosorbents from sugarcane bagasse has several challenges. Table 2 summarizes the results obtained using sugarcane bagasse byproducts as adsorbents, comparing the adsorption capacity and removal efficiency. Its effects on different contaminants were tested, revealing the importance of sugarcane bagasse in this field. Adsorbent tests should consider the temperature, pH, dosage, and contact time. The extraction, functionalization, and modification of cellulose fibers to enhance their adsorption properties is challenging. Simple extraction can yield nano- and micro-cellulose from cellulosic fibers, such as sugarcane bagasse residue. However, most of these modifications are based on complex syntheses and treatments. Therefore, finding a cleaner, cheaper, and more accessible treatment is a priority [186,187]. In addition, the operation mode, the application to real samples, and the regeneration of the biosorbent are concerns pertaining to the application of these materials. Factors such as the pH of the wastewater, contaminant concentration, temperature, adsorbent dosage, and various interfering agents can hinder the interaction between the target contaminant and the biosorbent material, reducing its treatment efficiency [188].

Table 2.
Several adsorbents, their respective experimental parameters, and the removal capacity.
However, large-scale application, which is rarely reported, is the main challenge for this type of adsorbent. Dotto et al. (2020) reported that less than 10% of the reported studies covered fixed-bed column studies, which can be efficiently applied to large-scale adsorption systems [189]. The development of cellulose nanocomposite membranes has gained attention because of the synergistic effects of filtration and adsorption. However, developing adsorptive membranes with high interaction and biosorbent stability in the membrane matrix is a challenge that makes large-scale applications difficult [190].
To develop sugarcane bagasse-based biosorbents as potential substitutes for decontaminating wastewater and removing various types of emerging contaminants, new materials, extraction techniques, analysis methodologies, adsorption mechanisms, and adaptation of natural systems have been studied.
8. Sugarcane Bagasse in Civil Construction
The issue of sustainability in civil construction is being addressed continuously. This sector accounts for 36% of global final energy consumption and 37% of energy-related CO2 emissions. The use of materials is expected to more than double by 2060, with one-third of this increase attributable to raw materials used in construction [191]. In addition, the uncontrolled extraction of natural resources triggered during the industrial revolution and intensified by the industrial actions of the 20th and 21st centuries is a significant cause of environmental instability [192].
To overcome these issues, new eco-friendly solutions were studied and developed, to minimize the environmental impact. Agro-industrial residues from the production of sugarcane and alcohol are promising as alternative materials for sustainable civil construction, to obtain eco-efficient resources [23,193]. Byproducts such as sugarcane bagasse have a high moisture content and are used as a mill fuel source [194,195]. Furthermore, bagasse burned in boilers yields ash rich in silica, which has the potential for pozzolanic reactivity and filling effects in concrete and other civil construction combinations [196].
Sugarcane bagasse is an organic material that contains water, lignin, and other components; therefore, treatments that improve interfacial adhesion and its physical and mechanical properties, are important. Santos et al. (2022) compared composites reinforced with sugarcane bagasse fibers with and without chemical treatment. Fiber treatment significantly influences most of the physical and mechanical properties; it increases the strength and tenacity, water absorption, and porosity with an increase in the amount of fiber, making it difficult to treat and use them [197]. Hernández-Olivares et al. (2020) studied the effects of pretreatment of sugarcane fibers; pretreatment proved to be practical, cheap, and straightforward, increasing the hardness of the composites following carbonation; this offered excellent acoustic and thermal insulation [198].
The potential applications of this residue as a thermal and acoustic insulating material have been studied. Boards with sugarcane bagasse, polyvinyl alcohol, and polymeric compounds with fibers from empty clusters of sugarcane bagasse and palm oil proved to be efficient, significantly reducing excess energy and costs [199,200].
Mortar was compared with sugarcane bagasse fibers and ash. Tests showed that, with up to 2% bagasse and 5% ash, the mechanical properties exceeded the expected resistance following curing [195].
Owing to its composition, several researchers have studied sugarcane bagasse ash; it is mainly applied in cementitious compounds; 10% ash is ideal to improve performance [23,195,201,202]. However, 20% is ideal for improving the transition zone by thinning and closing the gap between aggregates and folders, decreasing the porosity of the samples by 35%, improving the diffusion resistance of chlorides by 10 times, and increasing the compressive strength of the mortars by up to 62% [203]. A high ash composition was studied; it showed poor workability in the dough. However, it exhibited 50% better performance in terms of compressive strength and rapid permeability to chloride ions [204]. The application of ash in asphalt mixtures was studied. A 5% replacement of Portland cement showed superior resistance to fatigue cracking owing to increased stiffness and cohesion between the bitumen and the filler [205].
In a study on the pozzolanic effect, sugarcane bagasse ash stood out from bamboo cortex ash and performed better than pure cement [206]. In addition, a comparison was made between the cement with slag and bagasse ash; the ash showed better performance with 20% replacement and superior pozzolanic performance owing to its greater specific surface area and resistance gain [207].
Compounds with other byproducts, including concrete with nano-eggshell powder and sugarcane bagasse ash, were examined. The ideal mixture included 15% ash; the concrete presented a dense shape without pores and cracks and consequently better resistance [208]. A geopolymer composed of fly ash and bagasse was developed, and the percentage of 10% was considered ideal, as the increase in SCBA led to a decrease in mechanical properties. The main reason is that, due to the highly crystalline quartz phase of SCBA, which is very stable and non-reactive, the bagasse did not dissolve in the activated alkaline solution and did not participate in the geopolymerization process (Figure 4).
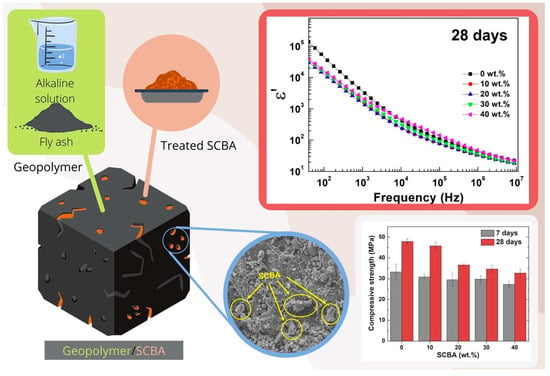
Figure 4.
Representation of the geopolymer with fly ash and sugar cane bagasse obtained through alkaline treatment and graphic demonstration of the results obtained from compression and dielectric constant [209].
In addition, geopolymeric compounds with metakaolin and sugarcane bagasse ash were studied for various parameters such as the Si/Al ratio, number of alkaline activators in each remnant, and propylene filaments incorporated into the ash. In both cases, rigid polymeric structures were obtained with an increase of 60% in resistance [210,211].
Prospects for Sugarcane Bagasse in Civil Construction
The substitution of civil construction materials enables developing products with remarkable mechanical performance and durability. Many researchers have attempted to use these residues in different ways. Few studies have compared the economic and environmental effects of applications such as sieving, burning, grinding, and chemical treatment, and the strategies to enhance the pozzolanic effect and reduce the water-absorption modulus of elasticity. However, these studies have focused solely on technical performance. Therefore, many related parameters, such as total added value, profit, pollution, and soil depletion, among others, need further studies.
9. Fiber-Based Filler in Polymer Composites
Sugarcane bagasse fibers have applications in polymer composites [212,213,214,215]; they are emerging as an alternative to new technological materials, for example, in thermal insulation and sound-absorbing building materials [200] (Figure 5), and in multilayered armor with aligned fibers, with a ballistic performance similar to that of commercial products [216]. Natural fibers are biodegradable, have a low density, are nontoxic, have proven heat and noise insulation properties, and are inexpensive. Therefore, their application in polymeric composites has several advantages, such as low manufacturing costs, high-quality end materials, easy obtainability, and large-volume byproducts. However, the use of natural fibers as reinforcement in polymer composites still needs to overcome the limitation of poor interfacial adhesion between the fibers and the polymeric matrix. The commonly used treatments include chemical processes with acids or alkalis through delignification and mercerization, because of their low cost. These help remove lignin and other components from the fiber cell, improving its solubility and compatibility with polymers. Long fibers tend to agglomerate and reduce the mechanical properties. To overcome this, sugarcane bagasse fibers are treated using an alkaline route [212], alkaline treatment and acetylation [217,218], silane [213], and steam treatments [219].

Figure 5.
Different preparation stages of sugarcane bagasse waste samples for thermal insulation and sound-absorbing building materials. Reproduced from Mehrzad et al. (2022) with permission from Elsevier [200].
Sugarcane bagasse fiber reinforcement in various polymer matrices (natural and synthetic) and biodegradable polymer materials has been studied. Fiber loadings of 30, 35, and 40 wt.% untreated and treated (5% NaOH, 30 min, 30 °C, liquor ratio of 20:1) reinforcing epoxy resin matrix were analyzed [212]. Alkaline treatment improved the mechanical properties (elongation percentage, flexural and tensile strength, and modulus), with the best results obtained at 40 wt.% of sugarcane bagasse fiber. SEM showed that NaOH treatment partially removed hemicellulose, lignin, and other soluble substances, thereby promoting significant interfacial fiber/matrix interactions. Finally, the optimal mixture of 40 wt.% was used to make a dashboard for automotive applications, reducing the car’s weight and influencing the increase in fuel economy [212].
For applications using thermal insulation materials, made a composite panel with phenolic resin and sugarcane bagasse fibers treated with silane (2% v/v) and hydrogen peroxide (H2O2) (4% v/v) [199,213]. Silane-treated composites exhibited improved compressive, flexural, and tensile strengths. In addition, owing to the better interfacial bonding between the fiber and the phenolic matrix with silane treatment, the composites showed lower water absorption and thickness swelling, suggesting future studies on thermal and acoustic properties for applications as sustainable wall-building materials [213]. Ramlee et al. (2019) evaluated the thermal stability of sugarcane bagasse fibers treated with silane and H2O2. Only silane treatment increased the thermal stability of the fiber, possibly because of the breakdown of the molecular structure and the linkages between the cell wall molecules, such as hemicellulose and lignin [220]. Abedom et al. (2021) evaluated alkaline treatment (10 wt.% NaOH, 3 h) of sugarcane bagasse fiber mixed with bamboo charcoal, using a biodegradable polyurethane resin as a matrix, for making panels for automotive thermal applications. The mixed composites with sugarcane bagasse fiber/bamboo (30:70) resulted in lower thermal conductivity (0.084 W·(mK)−1). The combined use of sugarcane bagasse fiber and bamboo charcoal combines the advantages of thermal insulation of vegetable fiber with that of bamboo charcoal, which provides a low density to the composite, resulting in an engineering material with a high potential [221].
Owing to the long time required for the degradation of polymeric composites and synthetic fibers, biodegradable materials with environmental benefits have been developed. For example, Guna et al. (2019) developed biodegradable tiles using raw sugarcane bagasse fibers (80 wt.%) as reinforcement and wheat gluten (20 wt.%) as the polymer matrix. Compared to the ceiling tiles, the bagasse–gluten composites increased flexural strength by 63%, reduced the maximum water absolved, increased thermal stability up to 250 °C, and exhibited similar flammability to ceiling tiles. In addition, the bagasse–gluten compound exhibited a higher heat transmission than the ceiling tiles because the fabrication reduced the weight, resulting in greater porosity. The higher porosity also resulted in the lower acoustic absorption of the bagasse–gluten compound [214]. Santos et al. (2018) studied biodegradable thermoplastic starch composites (TPSs) with sugarcane bagasse fibers. The fibers were immersed in deionized water for 36 h to remove impurities, ground in a knife mill, and retained in 28, 48, and 60 mesh sizes. To the TPS, 10 wt.% of the fibers was added. Adding different fiber size ranges influences the increase in the mechanical properties of Young’s modulus (660%) and tensile strength (100%) compared to that in the TPS control. The optimal size range was 297–595 µm (mesh 30) [222].
Due to its ease of processing, low density, and low cost, natural rubber has become a promising alternative for preparing biocomposites. Paiva et al. (2019) manufactured a prototype using sand with natural rubber reinforced with sugarcane bagasse fibers (Figure 6). The hydrophilic characteristics of natural fibers, owing to strongly polarized hydroxyl groups, require alkaline treatment (NaOH, 24 h) to promote the interfacial adhesion of the fiber/matrix. The fiber roughness increased after treatment, possibly owing to reduced surface impurities and lignin/hemicellulose. The increased contact area and better dispersion of the treated filler increased the abrasion and mechanical resistances. This allowed its application in sandals according to the guidelines of the Testing and Research Institute for Footwear Production [215,223].

Figure 6.
Sandals prepared using 10 phr of SCB residues. Reproduced from Paiva et al. with permission from Springer [215].
Nakanishi et al. (2018) studied sugarcane bagasse with natural latex for particleboard production. Particleboard was produced with 28, 42, and 56% latex in its composition, and molded using thermal pressure at 160 °C for 10 min. The density was not affected by the increasing latex concentration. However, mechanical properties (modulus of rupture and elasticity) were better with 42% latex/bagasse, with values of 6.32 and 1275 MPa, respectively. Samples with 50 mm diameter were evaluated for thermal conductivity. The results varied between 0.158 and 0.174 W·(mK)−1; materials for thermal insulation should have thermal conductivity lower than 0.25 W·(mK)−1 [224].
Composites with resin and sugarcane bagasse fiber are usually evaluated for particleboard panels because of the porosity of natural fibers; the internal voids provide high thermal resistance and excellent acoustic absorption, in addition to environmental comfort. Ribeiro et al. (2020) evaluated urea-formaldehyde adhesives and sugarcane bagasse particles for application in particleboard panels. Sugarcane bagasse was heat-treated at 170, 200, and 230 °C to provide superior dimensional stability and resistance to xylophage attacks. The water absorption (24 h) of the panels produced with the fibers treated at 200 and 230 °C was lower. The decrease in hygroscopicity could be attributed to a reduction in the accessibility of free hydroxyl groups and the formation of furfural polymers due to the degradation of hemicellulose, which is less hygroscopic. In addition, the rupture modulus was negatively affected when heat-treated at 170 and 200 °C; this could be associated with a lower internal adhesion rate due to the inhibition of adhesive curing [195]. Yano et al. (2020) studied the incorporation of sugarcane bagasse (0, 10, 20, 30, 40, and 50%) and timber waste into a castor-oil-based bicomponent polyurethane resin for application in particleboards. The best result was obtained with 50% bagasse and a 2 to 4 mm particle size. Using sugarcane bagasse fiber with timber waste contributed to greater dimensional stability, reduced moisture content, and met the physical-mechanical requirements for non-structural indoor use and dry conditions.
Liu et al. (2019) investigated the effect of fiber orientation on the mechanical properties, using three-dimensional printing of poly (lactic acid) with cellulose fibers isolated from sugarcane bagasse. The mechanical response improved as the fibers and molecular chains were oriented parallel to the direction of the loading stress [225].
The substitution of synthetic fibers with eco-friendly natural fibers is studied extensively; however, the requirement of fiber treatments and reduction in particle size or fiber length, consequently, introduces further necessity for economic viability studies. Sugarcane bagasse is a natural biodegradable material; its easy degradation poses a challenge to the durability of the developed materials. Controlling dispersion and orientation and avoiding fiber agglomeration could improve the mechanical response. In addition, production methods must be enhanced to reach the industrial scale.
9.1. Sugarcane Bagasse Ash as Filler in Polymer Composites
Sugarcane bagasse ash is mainly composed of silica with different structural characteristics, such as amorphous or crystalline forms, based on the firing temperature of the bagasse [226]. Sugarcane bagasse ash with particle sizes between 45 and 150 µm and concentrations of 15, 30, and 45 phr (per hundred of rubber) are used in natural rubber (NR)-based composites [227]. Adding sugarcane bagasse ash did not affect the curing time; however, inclusion of higher proportions increased the hardness of the composites and, consequently, the rheometric torque. In addition, the tensile strength is low owing to particle aggregation and poor dispersion. Therefore, sugarcane bagasse ash is recommended as an alternative filler to silica in NR at concentrations of up to 15 phr.
Sugarcane bagasse ash (SCBA) could improve the physical and mechanical properties of polymer matrices. The reinforcement characteristics are mainly attributed to the higher concentration of ash in silica [228]. The incorporation of sugarcane bagasse ash was evaluated with silane as a coupling agent to improve the waste/matrix interactions. First, sugarcane bagasse was burned at 650 °C and then sieved through sieves with diameter ranges between 44–125 µm (mesh 115–400). Sugarcane bagasse ash of 125 µm had more thermal stability, with 95.4% residue in thermogravimetric analysis and following treatment at 750 °C. The coupling agent (silane) contributed to the greater homogenization of ash in natural rubber, enabling better physical and mechanical properties; this is a promising ecological alternative to inorganic additives such as carbon black and conventional silica.
Chandrika et al. (2022) studied hybrid biocomposites with sugarcane bagasse ash and fabricated fiber-reinforced epoxy-based composites. The samples were molded into layers through compression: three layers of epoxy and two layers of fiber and ash in different proportions. The ideal composition was 28% and 7% of madar fiber and ash, respectively, which exhibited maximum flexural strength of 147 MPa and 17.5 kJ·m−2, respectively, in the impact test [229]. Balachandran et al. (2021) reported optimal mechanical strength when 7% ash was incorporated into silicone rubber. In addition, this biocomposite was studied for its application as an electrical insulator; an electric breakdown maximum of 108.65 × 105 V·m−1 was obtained, which enabled better interaction with the matrix molecules [230].
Purification of the silica present in sugarcane bagasse ash was carried out by Huabcharoen et al. (2017); they evaluated natural rubber composites with ash, without treatment, and with two treatments: hydrochloric acid (HCl) and HCl/ammonium fluoride (HCl/NH4F). The silica in the untreated ash was 77.2%; this increased to 90.6% and 97% when treated with HCl and HCl/NH4F, respectively. In addition, in natural rubber, the treated ash underwent a second treatment process with silane to improve surface adhesion. Increasing the purity of silica increased the curing time. This could be associated with the fewer metallic oxides in the purer silica, which could act as co-activators to reduce the curing time [231].
In contrast, the mechanical strength was only optimized when treated with HCl and up to 15 phr ash. There was better dispersion and strong silica/rubber interactions; higher amounts resulted in particle aggregation and strong filler/filler interactions. Boonmee et al. (2020) synthesized silica nanoparticles from sugarcane bagasse and used them as a filler in rubber composites, reaching sizes of approximately 90 nm, and increasing the tensile strength resistance from approximately 10 MPa for natural rubber without filler to approximately 20 MPa with 5 phr filler incorporation [232].
9.2. Prospects for Sugarcane Bagasse Ash
Challenges related to sugarcane bagasse ash include the search for sustainable methods of sugarcane bagasse burning. The pyrolysis process represents an alternative approach to obtain value-added products from biomass, with controlled parameters, which are economical and environmentally friendly. Energy production from sugarcane biomass shifts the focus to biogas production, which is an eco-friendly procedure that is easy to commercialize and can reach high calorific power; in addition, the residues could be used as fertilizers in sugarcane production.
10. Sugarcane-Based Silica
Sugarcane-based silica is obtained from biomass through acid leaching or calcination (ash) followed by pyrolysis, sol-gel, or precipitation methods [233,234], yielding micro- [235] and nano-sized particles [25]. The reduction of silica to obtain silicon is performed using different techniques such as metallothermic, carbothermic, and electrochemical reduction processes [236].
Silica from sugarcane bagasse is used for its antibacterial activities [237], photocatalysis ability against dyes [238,239] and heavy metals [152], used in agriculture [240], and has applications in hydrophobic coatings [241], insect control [242], membranes for wastewater purification [243,244], and fillers in polymeric composites [232].
Hikmah et al. (2021) developed Fe3+-doped SiO2/TiO2 composites using silica from sugarcane bagasse ash as an adsorption site to TiO2 particles and promoted 98.18% degradation of Congo red dye through photocatalysis [238]. Goswami and Mathur (2022) evaluated the use of silica nanoparticles obtained from sugarcane bagasse in sustainable agriculture. Seed germination and growth were measured; silica improved plant biometrics and physiology. In addition, it served as an antifungal agent, with 73.42% inhibition of mycelia growth [240]. Saed et al. (2021) tested the use of local diatomaceous earth with silica from sugarcane bagasse against insect pests. The addition of silica enhanced the insecticidal activity, with 95.5% mortality over galvanized steel surfaces for Rhyzopertha dominica specimens [242].
Prospects for Sugarcane Bagasse Silica
To reach an industrial level, the synthesis of silica from sugarcane bagasse must overcome challenges such as the toxicity of chemicals and the high energy required for production while maintaining a high yield.
11. Carbon Dot Synthesis
Carbon dot synthesis based on sugarcane bagasse is a renewable source alternative. This class of nanomaterials is usually photoluminescent, has low toxicity, and can be easily functionalized or solubilized. They are mainly obtained through hydrothermal methods, carbonization, or chemical oxidation [60,245]. Carbon dots are evaluated for ion and heavy metal detection [246,247], medical [248] and gas [249] sensors, antimicrobial/bacterial activity, drug delivery [250,251], and the removal of pollutants such as dyes [252] and polycyclic aromatic hydrocarbons from water [253].
Kasinathan et al. (2022) prepared carbon quantum dots from sugarcane bagasse using a hydrothermal method (Figure 7). Waste is dried in the sun before stirring in citric acid to obtain carbon dots with sizes ranging from 2 to 8 nm. The photoluminescence of quantum dots is used for Hg2+ ion sensing because the formation of complexes between the dots and Hg2+ causes luminescence quenching [246]. Alfi et al. (2022) synthesized carbon dot nanoparticles with an average size of 5–8 nm from sugarcane bagasse using a hydrothermal method and embedded them in a cellulose paper assay for use in a platform for tetracycline antibiotic sensors. The results showed high reversibility and stability with a low detection limit of 0.01 mM [248].
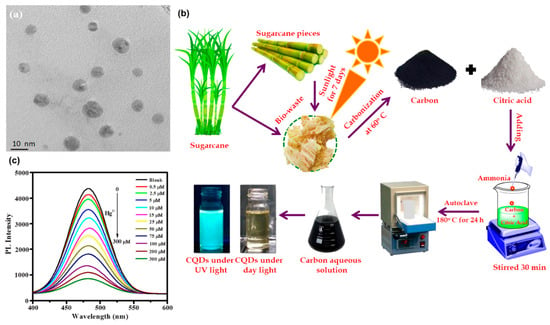
Figure 7.
(a) HR-TEM images of CQDs at 10 nm of magnifications (b) Fluorescent quenching of CQDs in the presence of Hg2+ ions concentration varying from 0 to 300 μM (c) Schematic representation of the synthesis of carbon quantum dots by hydrothermal method. Reproduced from Kasinathan et al. (2022) with permission from Elsevier (Color figure can be viewed at sciencedirect.com, accessed on 27 March 2024) [246].
Nugraha et al. (2021) produced tungsten oxide-functionalized carbon quantum dot composites (WO3/N-CQDs) from sugarcane bagasse. They evaluated the photocatalytic performance of the composites in removing methylene blue dye from an aqueous solution; the composite had 96.86% removal efficiency, making it a potential alternative for future wastewater treatment [254].
Prospects for Carbon Dots
Biomass is a renewable source; however, to improve the synthesis of carbon dots, developing synthesis methods for large-scale (over gram scale of yields) and low-cost production is essential. In addition, controlling nanoparticle dimensions, doping, and chemical functionalization, to suit the heterogeneity of waste sources, is a challenge.
12. Dietary Products
In dietary contexts, diverse applications are found using sugarcane bagasse, showcasing its potential for enhancing nutritional quality and serving as a valuable resource in the animal feed and food industries. In the study by So et al. (2020), additives like Lactobacillus, cellulase, and molasses are employed to improve sugarcane bagasse fermentation, positively influencing key nutritional parameters for optimizing its role as ruminant feed [255].
In aquaculture, the positive effects of sugarcane bagasse powder as a feed additive in Nile tilapia were demonstrated, enhancing growth performance, immune response, and acting as a prebiotic source [256]. Afrazeh et al. (2021) explore sugarcane bagasse dietary fiber, emphasizing its higher content and superior properties compared to date seed dietary fiber, offering prebiotic effects and antioxidant properties [257]. Additionally, Gil-López et al. (2019) focus on sustainable dietary products, highlighting the benefits of sugarcane bagasse and tops in improving the nutritional quality of food products [258]. Moreover, sugarcane bagasse treated with additives was used as a feed source for Thai native steers, showcasing improved feed utilization, digestibility, and nutrient utilization [259].
Molavian et al. (2020) assess sugarcane bagasse as a low-cost alternative for mid-lactation dairy cows, revealing its potential as a fiber source without significant impacts on milk production [260]. Finally, Hesam et al. (2023) explore the production of xylooligosaccharides from sugarcane bagasse, demonstrating their prebiotic function in synbiotic pomegranate juice, offering a promising solution for preserving probiotic bacteria in acidic environments [252].
13. Source of Biochemicals
Sugarcane bagasse takes center stage in various biochemical applications, showcasing its versatility and potential. In the study by Munagala et al. (2021), the focus is on lactic acid (LA) production, with a detailed exploration of sugarcane bagasse’s viability through life cycle assessment (LCA) and techno-economic analysis (TEA). Another work highlights sugarcane bagasse as a key feedstock for isopropanol, butanol, and ethanol (IBE) production through clostridia, emphasizing its role in sustainable and economically viable biochemical processes. Both studies underscore the crucial importance of sugarcane bagasse in advancing diverse applications within the realm of biochemical products [261].
Sugarcane bagasse appears as a versatile feedstock for xylitol production, presenting various innovative approaches across different studies. Antunes et al. (2021) conduct a multi-scale study, exploring integrated developments for utilizing sugarcane bagasse carbohydrates in sustainable processes for both ethanol and xylitol production [262]. Ahuja et al. (2022) adopt a microbial fermentation approach with Pseudomonas gessardii VXlt-16, designing a cost-effective process for xylitol production from untreated sugarcane bagasse hydrolysate [263]. Narisetty et al. (2021) leverage hemicellulosic sugars from sugarcane bagasse and olive pits, demonstrating high-level xylitol production with Pichia fermentans [264]. Queiroz et al. (2023) focus on co-production systems, evaluating xylitol and ethanol co-production by Candida tropicalis using sugarcane bagasse and straw hemicellulosic hydrolysate supplemented with molasses. These studies collectively highlight the potential of sugarcane bagasse in diverse pathways for xylitol production, providing insights into sustainable and economically viable approaches, setting the stage for a comprehensive review on the subject [265].
Sugarcane bagasse emerges as a valuable resource for furfural production, offering diverse pathways for sustainable and economically viable processes. One approach involves the co-production of furfural and ethanol from bagasse, demonstrating the challenges of maximizing yields for both products [266]. Li et al. (2021) innovatively employs coconut shell activated carbon for sulfonated Al-CSAC catalysis, achieving high yields of furfural from KOH-pretreated sugarcane bagasse [202]. Trung et al. (2020) introduce magnetic sulfonated graphene oxide as a catalyst, showcasing efficient furfural synthesis from sugarcane bagasse [267]. Silva et al. (2023) focus on the production of sulfonated activated carbons from sugarcane bagasse for furfural synthesis in aqueous media, highlighting the importance of Lewis and Brønsted acid sites for selective xylose dehydration [268]. These studies collectively underscore the potential of sugarcane bagasse as a versatile and renewable feedstock for furfural production, emphasizing the need for further exploration and optimization in this promising field.
14. Packaging
Sugarcane bagasse has also been used in the manufacture of packages for various purposes. Gupta et al. (2019), for example, use sugarcane bagasse, together with rice husk, to produce food packaging material. To this end, an alkaline treatment is carried out on the sugarcane bagasse before carrying out acid hydrolysis to remove lignin and other impurities [269]. It is also possible to use bleached bagasse pulp as raw material to produce hydrogel as a colorimetric freshness indicator for smart food packaging [270] and other applications, such as sugarcane bagasse in the production of food packages in agroindustry [244,271,272] and as packing material in filters for hydrogen sulfide removal [141].
15. Conclusions
Environmental harm due to the use of fossil fuels has been a concern over the years. The use of biomass as source of energy appears as an environmentally friendly alternative. Currently, especially sugarcane biomass, even after the extraction of sugar and ethanol, or even the burning of bagasse, presents several possibilities for using waste to produce new engineering materials or new biofuels. Here, we present the recent developments and challenges in recycling sugarcane, including such areas as energy generation and pyrolysis to obtain biofuels, producing biochar or pellets. Furthermore, we state the use of bagasse in construction contributing to the broader field of sustainable and ecological construction materials. The obtaining of cellulose fibers and the synthesis of nanostructures are also addressed, as they have emerged as a distinct new field in the reuse of sugarcane waste. The definition of challenges related the use of sugarcane bagasse supports future research on waste-derived subjects and can contribute significant breakthrough in the recycling methods.
Author Contributions
Conceptualization, C.T.H., R.J.d.S. and F.C.C.; methodology, C.T.H., A.S.G., F.F.G.P., G.R.T., L.L.P. and G.D.; validation, C.T.H. and R.J.d.S.; formal analysis, H.P.C., G.P.C. and F.C.C.; investigation, C.T.H., A.S.G., F.F.G.P., G.R.T., L.L.P., G.D., H.P.C. and F.C.C.; writing—original draft presentation, C.T.H., A.S.G., F.F.G.P., G.R.T., L.L.P., G.D., H.P.C. and F.C.C.; writing—review and editing, H.P.C., G.P.C. and F.C.C.; visualization, L.L.P. and F.C.C., supervision, F.C.C.; project-administration, G.P.C. and F.C.C., funding acquisition, H.P.C. and R.J.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was supported by UNESP PROPG through the EDITAL PROPG 16/2024.
Acknowledgments
The authors thank the UNESP PROPG to support the APC.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO—Food and Agriculture Organization of the United Nations Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 March 2024).
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento Da Safra Brasileira de Cana-de-Açucar, 3rd ed.; CONAB—Companhia Nacional de Abastecimento: Brasília, DF, Brazil, 2022; Volume 9. [Google Scholar]
- MME—Ministério de Minas e Energia; EPE—Empresa de Pesquisa Energética. BEN: Balanço Energético Nacional—Relatório Síntese 2022: Ano Base 2021; MME—Ministério de Minas e Energia: Brasília, Brazil; EPE—Empresa de Pesquisa Energética: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Federal Government of Brazil. LEI No 13.576. Available online: https://www2.camara.leg.br/legin/fed/lei/2017/lei-13576-26-dezembro-2017-786013-publicacaooriginal-154631-pl.html (accessed on 6 December 2023).
- Federal Government of Brazil. DECRETO No 9.888. Available online: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2019/decreto/d9888.htm (accessed on 6 December 2023).
- Negrão, D.R.; Grandis, A.; Buckeridge, M.S.; Rocha, G.J.M.; Leal, M.R.L.V.; Driemeier, C. Inorganics in Sugarcane Bagasse and Straw and Their Impacts for Bioenergy and Biorefining: A Review. Renew. Sustain. Energy Rev. 2021, 148, 111268. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Haldar, D.; Dey, P.; Patel, A.K.; Singhania, R.R.; Dong, C.-D.; Purkait, M.K. Sugarcane Bagasse into Value-Added Products: A Review. Environ. Sci. Pollut. Res. 2022, 29, 62785–62806. [Google Scholar] [CrossRef]
- Khatri, P.; Pandit, A.B. Systematic Review of Life Cycle Assessments Applied to Sugarcane Bagasse Utilization Alternatives. Biomass Bioenergy 2022, 158, 106365. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Chizitere Emenike, E.; Ighalo, J.O.; Omoarukhe, F.O.; Omuku, P.E.; George Adeniyi, A. A Review on the Thermochemical Conversion of Sugarcane Bagasse into Biochar. Clean. Mater. 2022, 6, 100162. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of Nanocellulose from Sugarcane Bagasse and Its Characterization for Potential Applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
- Cardozo, E.; Malmquist, A. Performance Comparison between the Use of Wood and Sugarcane Bagasse Pellets in a Stirling Engine Micro-CHP System. Appl. Therm. Eng. 2019, 159, 113945. [Google Scholar] [CrossRef]
- de Palma, K.R.; García-Hernando, N.; Silva, M.A.; Tomaz, E.; Soria-Verdugo, A. Pyrolysis and Combustion Kinetic Study and Complementary Study of Ash Fusibility Behavior of Sugarcane Bagasse, Sugarcane Straw, and Their Pellets—Case Study of Agro-Industrial Residues. Energy Fuels 2019, 33, 3227–3238. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.-J. Enhanced Bioelectricity Generation in Thermophilic Microbial Fuel Cell with Lignocellulose as an Electron Donor by Resazurin-Mediated Electron Transfer. Bioresour. Technol. 2023, 388, 129764. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.K.; Ali, U.F.M.; Isa, K.M.; Gopinath, S.C.B. Study on Characterization of Bio-Oil Derived from Sugarcane Bagasse (Saccharum barberi) for Application as Biofuel. Clean Energy 2022, 6, 297–304. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Gouda, N.; Singh, R.K. Investigation on Thermokinetic Study and Optimization of Sugarcane Bagasse Thermal Pyrolysis. Sugar Tech 2023, 25, 198–209. [Google Scholar] [CrossRef]
- Yerrayya, A.; Nikunj, A.; Prashanth, P.F.; Chakravarthy, S.R.; Natarajan, U.; Vinu, R. Optimization of Bio-Crude Yield and Its Calorific Value from Hydrothermal Liquefaction of Bagasse Using Methanol as Co-Solvent. Energy 2022, 244, 123192. [Google Scholar] [CrossRef]
- Agarwal, N.K.; Kumar, M.; Ghosh, P.; Kumar, S.S.; Singh, L.; Vijay, V.K.; Kumar, V. Anaerobic Digestion of Sugarcane Bagasse for Biogas Production and Digestate Valorization. Chemosphere 2022, 295, 133893. [Google Scholar] [CrossRef]
- de Almeida, S.G.C.; Tarelho, L.A.C.; Hauschild, T.; Costa, M.A.M.; Dussán, K.J. Biochar Production from Sugarcane Biomass Using Slow Pyrolysis: Characterization of the Solid Fraction. Chem. Eng. Process.—Process Intensif. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A Review on Modified Sugarcane Bagasse Biosorbent for Removal of Dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Ighalo, J.O.; Eshiemogie, S.; Omuku, P.E.; Adeniyi, A.G. Valorization of Sugar Industry’s By-Products: A Perspective. Sugar Tech 2022, 24, 1052–1078. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous Activated Carbons Derived from Waste Sugarcane Bagasse for CO2 Adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Multi-Product Biorefinery with Sugarcane Bagasse: Process Development for Nanocellulose, Lignin and Biohydrogen Production and Lifecycle Analysis. Chem. Eng. J. 2022, 446, 137233. [Google Scholar] [CrossRef]
- Khalil, M.J.; Aslam, M.; Ahmad, S. Utilization of Sugarcane Bagasse Ash as Cement Replacement for the Production of Sustainable Concrete—A Review. Constr. Build. Mater. 2021, 270, 121371. [Google Scholar] [CrossRef]
- Neto, J.d.S.A.; de França, M.J.S.; de Amorim Júnior, N.S.; Ribeiro, D.V. Effects of Adding Sugarcane Bagasse Ash on the Properties and Durability of Concrete. Constr. Build. Mater. 2021, 266, 120959. [Google Scholar] [CrossRef]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; de Oliveira, A.P.N. Synthesis of Silica Nanoparticles from Sugarcane Bagasse Ash and Nano-Silicon via Magnesiothermic Reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
- Paone, E.; Mauriello, F. Sustainable Production of Textile Fibers, Biofuels, and Chemicals from Poplar Wood. Trends Chem. 2023, 5, 1–2. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-Oligosaccharides, Fermentable Sugars, and Bioenergy Production from Sugarcane Straw Using Steam Explosion Pretreatment at Pilot-Scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Crow, S.E.; Khanal, S.K.; Turn, S.Q. Lignin Chemical Controls on Bioconversion of Tropically Grown C4 Bioenergy Grasses to Biofuels and Biobased Products. Bioresour. Technol. Rep. 2022, 18, 101015. [Google Scholar] [CrossRef]
- Bhatia, L.; Sarangi, P.K.; Singh, A.K.; Prakash, A.; Shadangi, K.P. Lignocellulosic Waste Biomass for Biohydrogen Production: Future Challenges and Bio-economic Perspectives. Biofuels Bioprod. Biorefin. 2022, 16, 838–858. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent Advances and Sustainable Development of Biofuels Production from Lignocellulosic Biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts—A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Muys, B.; Hermy, M. Lignocellulosic Biomass for Bioenergy beyond Intensive Cropland and Forests. Renew. Sustain. Energy Rev. 2019, 102, 139–149. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Valorisation of Lignocellulosic Biomass to Value-Added Products: Paving the Pathway towards Low-Carbon Footprint. Fuel 2022, 313, 122678. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lu, C. Development Perspectives of Promising Lignocellulose Feedstocks for Production of Advanced Generation Biofuels: A Review. Renew. Sustain. Energy Rev. 2021, 136, 110445. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of Waste Biomass for Sustainable Product Development and Minimizing Environmental Impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef]
- Ingle, A.P.; Ingle, P.; Gupta, I.; Rai, M. Socioeconomic Impacts of Biofuel Production from Lignocellulosic Biomass. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–366. [Google Scholar]
- Sarwer, A.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Rafiq, S.; Khurram, M.S.; Ul-Haq, N.; Shah, N.S.; Din, A.A.; Ahmad, I.; et al. Suitability of Biofuels Production on Commercial Scale from Various Feedstocks: A Critical Review. ChemBioEng Rev. 2022, 9, 423–441. [Google Scholar] [CrossRef]
- Sidana, A.; Yadav, S.K. Recent Developments in Lignocellulosic Biomass Pretreatment with a Focus on Eco-Friendly, Non-Conventional Methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- de Moraes Dutenkefer, R.; Machado, P.G.; de Oliveira Ribeiro, C. Lignin Chemical Derivatives in Brazilian Sugarcane Sector: An Alternative to Make 2G Ethanol Viable? J. Clean. Prod. 2022, 369, 133286. [Google Scholar] [CrossRef]
- Curran, L.M.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of Advances in the Development of Laccases for the Valorization of Lignin to Enable the Production of Lignocellulosic Biofuels and Bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Romanenko, I.; Kurz, F.; Baumgarten, R.; Jevtovikj, I.; Lindner, J.-P.; Kundu, A.; Kindler, A.; Schunk, S.A. Lignin Depolymerization in the Presence of Base, Hydrogenation Catalysts, and Ethanol. Catalysts 2022, 12, 158. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Zeilerbauer, L.; Lindorfer, J.; Süss, R.; Kamm, B. Techno-economic and Life-cycle Assessment of a Wood Chips-based Organosolv Biorefinery Concept for Production of Lignin Monomers and Oligomers by Base-catalyzed Depolymerization. Biofuels Bioprod. Biorefin. 2022, 16, 370–388. [Google Scholar] [CrossRef]
- Brown, E.E. Minireview: Recent Efforts toward Upgrading Lignin-Derived Phenols in Continuous Flow. J. Flow Chem. 2023, 13, 91–102. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valoriz. 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Mota, I.F.; da Silva Burgal, J.; Antunes, F.; Pintado, M.E.; Costa, P.S. High Value-Added Lignin Extracts from Sugarcane by-Products. Int. J. Biol. Macromol. 2023, 230, 123144. [Google Scholar] [CrossRef]
- Silva, J.C.d.A.; Gonçalves, E.P.; Viana, J.S.; Souza, C.M.P.G.; Borges, J.P.G.d.S.; Cavalcante, W.F. Growth and Physiology of Two Sunflower Cultivars Fertilized with Sugarcane Bagasse Ash. Acta Sci. Agron. 2022, 44, e54392. [Google Scholar] [CrossRef]
- Jayamani, E.; Rahman, M.R.; Benhur, D.A.; Bakri, M.K.B.; Kakar, A.; Khan, A. Comparative Study of Fly Ash/Sugarcane Fiber Reinforced Polymer Composites Properties. Bioresources 2020, 15, 5514–5531. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of Sugarcane Bagasse Using Pretreatment Strategies for Bioethanol Production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Tantayotai, P.; Gundupalli, M.P.; Katam, K.; Rattanaporn, K.; Cheenkachorn, K.; Sriariyanun, M. In-Depth Investigation of the Bioethanol and Biogas Production from Organic and Mineral Acid Pretreated Sugarcane Bagasse: Comparative and Optimization Studies. Biocatal. Agric. Biotechnol. 2022, 45, 102499. [Google Scholar] [CrossRef]
- Gomes, M.G.; Paranhos, A.G.d.O.; Camargos, A.B.; Baêta, B.E.L.; Baffi, M.A.; Gurgel, L.V.A.; Pasquini, D. Pretreatment of Sugarcane Bagasse with Dilute Citric Acid and Enzymatic Hydrolysis: Use of Black Liquor and Solid Fraction for Biogas Production. Renew. Energy 2022, 191, 428–438. [Google Scholar] [CrossRef]
- Alino, J.H.L.; Bastos, J.A.; Remor, P.V.; Frare, L.M.; Orssatto, F.; Damaceno, F.M.; Edwiges, T. Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane 2022, 1, 189–200. [Google Scholar] [CrossRef]
- Monroy Vázquez, F.J.; González Uribe, E.E.; García Enríquez, S.; Fernández-Escamilla, V.V.A.; González Núñez, R.; Canche Escamilla, G.; Moscoso Sanchez, F.J. Influence of Pretreated Sugarcane Bagasse Fiber by Steam Explosion, Soaking with Caustic Soda, and Addition of Coupling Agent into Polylactic Acid Biocomposites. J. Compos. Mater. 2022, 56, 4621–4633. [Google Scholar] [CrossRef]
- Hermsdorff, G.B.; Escobar, E.L.N.; da Silva, T.A.; Filho, A.Z.; Corazza, M.L.; Ramos, L.P. Ethanol Organosolv Pretreatment of Sugarcane Bagasse Assisted by Organic Acids and Supercritical Carbon Dioxide. Carbohydr. Polym. 2023, 300, 120263. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated Lignocellulosic Biorefinery: Gateway for Production of Second Generation Ethanol and Value Added Products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Gan, J.; Iqbal, H.M.N.; Show, P.L.; Rahdar, A.; Bilal, M. Upgrading Recalcitrant Lignocellulosic Biomass Hydrolysis by Immobilized Cellulolytic Enzyme–Based Nanobiocatalytic Systems: A Review. Biomass Convers. Biorefin. 2024, 14, 4485–4509. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of Detoxified Sugarcane Bagasse Hydrolysate by Atmospheric Cold Plasma for Bacterial Cellulose Production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.P.; Philippini, R.R.; da Silva, S.S. Pretreatment of Sugarcane Bagasse Using Two Different Acid-Functionalized Magnetic Nanoparticles: A Novel Approach for High Sugar Recovery. Renew. Energy 2020, 150, 957–964. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; da Silva Abreu, F.O.M. Carbon Quantum Dots Synthesis from Waste and By-Products: Perspectives and Challenges. Mater. Lett. 2021, 282, 128764. [Google Scholar] [CrossRef]
- Zhuang, J.; Kim, K.H.; Jia, L.; Meng, X.; Kumar, D.; Leem, G.; Kang, S.B.; Li, Y.; Ragauskas, A.J.; Hou, Y.; et al. Ferric Chloride Aided Peracetic Acid Pretreatment for Effective Utilization of Sugarcane Bagasse. Fuel 2022, 319, 123739. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yu, H.-Q. Thermochemical Conversion of Lignocellulosic Biomass into Mass-Producible Fuels: Emerging Technology Progress and Environmental Sustainability Evaluation. ACS Environ. Au 2022, 2, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Ullah, Z.; Taqvi, S.A.A.; Khan, M.N.A.; Farooq, W.; Mehran, M.T.; Juchelková; D; Štěpanec, L. Applications of Machine Learning in Thermochemical Conversion of Biomass—A Review. Fuel 2023, 332, 126055. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen Production from the Thermochemical Conversion of Biomass: Issues and Challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass Gasification as an Industrial Process with Effective Proof-of-Concept: A Comprehensive Review on Technologies, Processes and Future Developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.A.S.; Schneider, J.K.; Farrapeira, R.O.; Andrade, Y.B.; Krause, L.C.; Bjerk, T.R.; Caramão, E.B. Recovery of Waste Biomass: Pyrolysis and Characterization of Sugarcane Residues and Their Bio-Oils. Biofuels 2022, 13, 843–852. [Google Scholar] [CrossRef]
- Saif, A.G.H.; Wahid, S.S.; Ali, M.R.O. Pyrolysis of Sugarcane Bagasse: The Effects of Process Parameters on the Product Yields. Mater. Sci. Forum 2020, 1008, 159–167. [Google Scholar] [CrossRef]
- Miranda, N.T.; Motta, I.L.; Filho, R.M.; Maciel, M.R.W. Sugarcane Bagasse Pyrolysis: A Review of Operating Conditions and Products Properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Boer, F.D.; Valette, J.; Commandré, J.M.; Fournier, M.; Thévenon, M.F. Slow Pyrolysis of Sugarcane Bagasse for the Production of Char and the Potential of Its By-Product for Wood Protection. J. Renew. Mater. 2021, 9, 97–117. [Google Scholar] [CrossRef]
- Rodier, L.; Bilba, K.; Onésippe, C.; Arsène, M.-A. Utilization of Bio-Chars from Sugarcane Bagasse Pyrolysis in Cement-Based Composites. Ind. Crops Prod. 2019, 141, 111731. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Menkiti, M.C.; Obiora-Okafo, I.A.; Ezenwa, O.N. Controlled Pyrolysis of Sugarcane Bagasse Enhanced Mesoporous Carbon for Improving Capacitance of Supercapacitor Electrode. Biomass Bioenergy 2021, 146, 105996. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Firouzabadi, M.D.; Ghasemian, A.; de Wild, P.J.; Heeres, H.J. Sugarcane Bagasse Ex-Situ Catalytic Fast Pyrolysis for the Production of Benzene, Toluene and Xylenes (BTX). J. Anal. Appl. Pyrolysis 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, X.; Zhang, Z.; Wang, Z.; Cui, M.; Yang, Y. Catalytic Fast Pyrolysis of Sugarcane Bagasse Using Activated Carbon Catalyst in a Hydrogen Atmosphere to Selectively Produce 4-Ethyl Phenol. J. Anal. Appl. Pyrolysis 2018, 136, 125–131. [Google Scholar] [CrossRef]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Selective Aromatic Production from Fast Pyrolysis of Sugarcane Bagasse Lignin over ZSM-5 Catalyst. Energy Rep. 2021, 7, 830–843. [Google Scholar] [CrossRef]
- Inocente, J.M.; Elyseu, F.; Nieves, L.J.J.; Jiusti, J.; Cargnin, M.; Peterson, M. Production and Characterization of High-Reactivity Metakaolins Calcined in Flash Reactor. Appl. Clay Sci. 2021, 213, 106247. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, O.C.; Igwegbe, C.A. Flash Pyrolysis of Biomass: A Review of Recent Advances. Clean Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Arunachalam, K.D. Experimental Investigations on Sugarcane Bagasse Pyrolytic Oil Production from Flash Pyrolysis Using a Rotary Screw Reactor. Biofuels Bioprod. Biorefin. 2022, 16, 576–586. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Daniyanto; Hartono, M.; Prasakti, L.; Budiman, A. Effect of Calcium and Magnesium Catalyst on Pyrolysis Kinetic of Indonesian Sugarcane Bagasse for Biofuel Production. Energy Procedia 2019, 158, 431–439. [Google Scholar] [CrossRef]
- Hass, A.; Lima, I.M. Effect of Feed Source and Pyrolysis Conditions on Properties and Metal Sorption by Sugarcane Biochar. Environ. Technol. Innov. 2018, 10, 16–26. [Google Scholar] [CrossRef]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green Remediation of Pharmaceutical Wastes Using Biochar: A Review. Environ. Chem. Lett. 2022, 20, 681–704. [Google Scholar] [CrossRef]
- Jacob, M.M.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Adsorptive Decontamination of Organophosphate Pesticide Chlorpyrifos from Aqueous Systems Using Bagasse-Derived Biochar Alginate Beads: Thermodynamic, Equilibrium, and Kinetic Studies. Chem. Eng. Res. Des. 2022, 186, 241–251. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, S.; Ayaz, T.; Brusseau, M.L.; Khan, M.A.; Nawab, J.; Muhammad, S. Popular Wood and Sugarcane Bagasse Biochars Reduced Uptake of Chromium and Lead by Lettuce from Mine-Contaminated Soil. Environ. Pollut. 2020, 263, 114446. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined Application of Biochar and Sulfur Regulated Growth, Physiological, Antioxidant Responses and Cr Removal Capacity of Maize (Zea mays L.) in Tannery Polluted Soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Ighalo, J.O.; Bamigbola, J.O.; Omonayin, E.O.; Ojo, H.T.; Adeleke, J.; Adeniyi, A.G. Advancing the Circular Economy through the Thermochemical Conversion of Waste to Biochar: A Review on Sawdust Waste-Derived Fuel. Biofuels 2024, 15, 433–447. [Google Scholar] [CrossRef]
- Deng, C.; Peng, L.; Ling, X.; Wang, T.; Xu, R.; Zhu, Y.; Wang, C.; Qian, X.; Wang, L.; Wu, Y.; et al. Construction of S-Scheme Zn0.2Cd0.8S/Biochar Aerogel Architectures for Boosting Photocatalytic Hydrogen Production under Sunlight Irradiation. J. Clean. Prod. 2023, 414, 137616. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Gupta, S.; Lee, C.-Y.; Tai, N.-H. Effects of Physical and Chemical Activations on the Performance of Biochar Applied in Supercapacitors. Appl. Surf. Sci. 2023, 610, 155560. [Google Scholar] [CrossRef]
- Sang, J.; Sun, C.; Pan, J.; Gao, C.; Zhang, R.; Jia, F.; Wang, F.; Wang, Q. Seaweed—Modification of Si by Natural Nitrogen-Doped Porous Biochar for High-Efficiency Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 11389–11399. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A Review on Biochar Production Techniques and Biochar Based Catalyst for Biofuel Production from Algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cai, L.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R. An Overview on Biochar Production, Its Implications, and Mechanisms of Biochar-Induced Amelioration of Soil and Plant Characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Khapre, A.; Bordoloi, N.; Kumar, S. Sorption of Volatile Organic Compounds on Non-Activated Biochar. Bioresour. Technol. 2020, 297, 122469. [Google Scholar] [CrossRef]
- Moharm, A.E.; El Naeem, G.A.; Soliman, H.M.A.; Abd-Elhamid, A.I.; El-Bardan, A.A.; Kassem, T.S.; Nayl, A.A.; Bräse, S. Fabrication and Characterization of Effective Biochar Biosorbent Derived from Agricultural Waste to Remove Cationic Dyes from Wastewater. Polymers 2022, 14, 2587. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, E. Technologies for Converting Biomass to Useful Energy: Combustion, Gasification, Pyrolysis, Torrefaction and Fermentation; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- dos Santos, J.V.; Fregolente, L.G.; Laranja, M.J.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Hydrothermal Carbonization of Sugarcane Industry By-Products and Process Water Reuse: Structural, Morphological, and Fuel Properties of Hydrochars. Biomass Convers. Biorefin. 2022, 12, 153–161. [Google Scholar] [CrossRef]
- Briongos, J.V.; Taramona, S.; Gómez-Hernández, J.; Mulone, V.; Santana, D. Solar and Biomass Hybridization through Hydrothermal Carbonization. Renew. Energy 2021, 177, 268–279. [Google Scholar] [CrossRef]
- Raheem, A.; Zhao, M.; Dastyar, W.; Channa, A.Q.; Ji, G.; Zhang, Y. Parametric Gasification Process of Sugarcane Bagasse for Syngas Production. Int. J. Hydrogen Energy 2019, 44, 16234–16247. [Google Scholar] [CrossRef]
- Cao, W.; Guo, L.; Yan, X.; Zhang, D.; Yao, X. Assessment of Sugarcane Bagasse Gasification in Supercritical Water for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 13711–13719. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M.; Higman, C.; van der Burgt, M. Chapter 3—The Kinetics of Gasification and Reactor Theory. In Gasification; Gulf Professional Publishing: Burlington, NJ, USA, 2008; pp. 33–45. [Google Scholar]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass Torrefaction: Properties, Applications, Challenges, and Economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of Agriculture Residue To Enhance Combustible Properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Stegen, S.; Kaparaju, P. Effect of Temperature on Oil Quality Obtained through Pyrolysis of Sugarcane Bagasse. Fuel 2020, 276, 118112. [Google Scholar] [CrossRef]
- Guida, M.Y.; Hannioui, A. Properties of Bio-Oil and Bio-Char Produced by Sugar Cane Bagasse Pyrolysis in a Stainless Steel Tubular Reactor. Prog. Agric. Eng. Sci. 2017, 13, 13–33. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass Pyrolysis Technologies for Value-Added Products: A State-of-the-Art Review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef] [PubMed]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of Biochar Application to the Environment and Economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Matos, T.T.S.; Fornari, M.R.; Mangrich, A.S.; Schultz, J.; Cardoso Batista, E.M.C.; Ribeiro, R.O.C.; Romão, L.P.C.; Yamamoto, C.I.; Grasel, F.S.; Bayer, C.; et al. Low Temperature Production of Biochars from Different Biomasses: Effect of Static and Rotary Lab Reactors and Application as Soil Conditioners. J. Environ. Chem. Eng. 2021, 9, 105472. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Gaston, L.A.; Li, J.; Fultz, L.M.; DeLaune, R.D.; Dodla, S.K. Remediation of Crude Oil-Contaminated Coastal Marsh Soil: Integrated Effect of Biochar, Rhamnolipid Biosurfactant and Nitrogen Application. J. Hazard. Mater. 2020, 396, 122595. [Google Scholar] [CrossRef] [PubMed]
- Wuri, M.A.; Pertiwiningrum, A.; Budiarto, R.; Gozan, M.; Harto, A.W. The Waste Recycling of Sugarcane Bagasse-Based Biochar for Biogas Purification. IOP Conf. Ser. Earth Environ. Sci. 2021, 940, 012029. [Google Scholar] [CrossRef]
- Bundschuh, J.; Kaczmarczyk, M.; Ghaffour, N.; Tomaszewska, B. State-of-the-Art of Renewable Energy Sources Used in Water Desalination: Present and Future Prospects. Desalination 2021, 508, 115035. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Liu, S.-H.; Tsang, D.C.W. Microwave-Assisted Production of CO2-Activated Biochar from Sugarcane Bagasse for Electrochemical Desalination. J. Hazard. Mater. 2020, 383, 121192. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Martins, G.; Martins, T.A.C.; Didek, L.K.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar: An Environmentally Friendly Platform for Construction of a SARS-CoV-2 Electrochemical Immunosensor. Sci. Total Environ. 2023, 858, 159797. [Google Scholar] [CrossRef]
- Iftikhar, M.; Asghar, A.; Ramzan, N.; Sajjadi, B.; Chen, W. Biomass Densification: Effect of Cow Dung on the Physicochemical Properties of Wheat Straw and Rice Husk Based Biomass Pellets. Biomass Bioenergy 2019, 122, 1–16. [Google Scholar] [CrossRef]
- Bot, B.V.; Sosso, O.T.; Tamba, J.G.; Lekane, E.; Bikai, J.; Ndame, M.K. Preparation and Characterization of Biomass Briquettes Made from Banana Peels, Sugarcane Bagasse, Coconut Shells and Rattan Waste. Biomass Convers. Biorefin. 2023, 13, 7937–7946. [Google Scholar] [CrossRef]
- Gautam, N.; Chaurasia, A. Study on Kinetics and Bio-Oil Production from Rice Husk, Rice Straw, Bamboo, Sugarcane Bagasse and Neem Bark in a Fixed-Bed Pyrolysis Process. Energy 2020, 190, 116434. [Google Scholar] [CrossRef]
- Sagani, A.; Hagidimitriou, M.; Dedoussis, V. Perennial Tree Pruning Biomass Waste Exploitation for Electricity Generation: The Perspective of Greece. Sustain. Energy Technol. Assess. 2019, 31, 77–85. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Silva, D.A.L.; Filleti, R.A.P.; Musule, R.; Matheus, T.T.; Freire, F. A Systematic Review and Life Cycle Assessment of Biomass Pellets and Briquettes Production in Latin America. Renew. Sustain. Energy Rev. 2022, 157, 112042. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for Energy: A Review on Supply Chain Management Models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Albashabsheh, N.T.; Heier Stamm, J.L. Optimization of Lignocellulosic Biomass-to-Biofuel Supply Chains with Densification: Literature Review. Biomass Bioenergy 2021, 144, 105888. [Google Scholar] [CrossRef]
- Martinez-Valencia, L.; Camenzind, D.; Wigmosta, M.; Garcia-Perez, M.; Wolcott, M. Biomass Supply Chain Equipment for Renewable Fuels Production: A Review. Biomass Bioenergy 2021, 148, 106054. [Google Scholar] [CrossRef]
- Varshney, D.; Mandade, P.; Shastri, Y. Multi-Objective Optimization of Sugarcane Bagasse Utilization in an Indian Sugar Mill. Sustain. Prod. Consum. 2019, 18, 96–114. [Google Scholar] [CrossRef]
- Lee, J.S.; Sokhansanj, S.; Lau, A.K.; Lim, C.J. Heats of Wetting and Sorption for Wood Pellets. Biomass Bioenergy 2020, 142, 105791. [Google Scholar] [CrossRef]
- Alan Sherrard Pellets Remain a Competitive Renewable Alternative. Available online: https://bioenergyinternational.com/pellets-remain-a-competitive-renewable-alternative/ (accessed on 2 March 2024).
- Setter, C.; Costa, K.L.S.; de Oliveira, T.J.P.; Mendes, R.F. The Effects of Kraft Lignin on the Physicomechanical Quality of Briquettes Produced with Sugarcane Bagasse and on the Characteristics of the Bio-Oil Obtained via Slow Pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of Sugarcane Bagasse in Semi Batch Reactor: Effects of Process Parameters on Product Yields and Characterization of Products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Neto, L.F.C.; de Paula Protásio, T.; de Cássia Oliveira Carneiro, A.; Andrade, C.R.; Guimarães Júnior, J.B.; Mendes, L.M. Options for Generation of Sustainable Energy: Production of Pellets Based on Combinations Between Lignocellulosic Biomasses. Waste Biomass Valoriz. 2018, 9, 479–489. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Meda, V.; Dalai, A.K. Densification of Waste Biomass for Manufacturing Solid Biofuel Pellets: A Review. Environ. Chem. Lett. 2023, 21, 231–264. [Google Scholar] [CrossRef]
- Chen, X.; Liang, J.; Liao, P.; Huang, W.; He, J.; Chen, J. Effect of Process Parameters and Raw Material Characteristics on the Physical and Mechanical Quality of Sugarcane Bagasse Pellets. Biomass Bioenergy 2021, 154, 106242. [Google Scholar] [CrossRef]
- da Silva, S.B.; Arantes, M.D.C.; de Andrade, J.K.B.; Andrade, C.R.; Carneiro, A.d.C.O.; Protásio, T.d.P. Influence of Physical and Chemical Compositions on the Properties and Energy Use of Lignocellulosic Biomass Pellets in Brazil. Renew. Energy 2020, 147, 1870–1879. [Google Scholar] [CrossRef]
- Akbar, A.; Aslam, U.; Asghar, A.; Aslam, Z. Effect of Binding Materials on Physical and Fuel Characteristics of Bagasse Based Pellets. Biomass Bioenergy 2021, 150, 106118. [Google Scholar] [CrossRef]
- de Almeida Moreira, B.R.; Barbosa Júnior, M.R.; de Brito Filho, A.L.; da Silva, R.P. Production of High-Quality Biogenic Fuels by Co-Pelletization of Sugarcane Bagasse with Pinewood Sawdust and Peanut Shell. Biomass Convers. Biorefin. 2024, 14, 6797–6820. [Google Scholar] [CrossRef]
- Santana, D.A.R.; Scatolino, M.V.; Lima, M.D.R.; de Oliveira Barros Junior, U.; Garcia, D.P.; Andrade, C.R.; de Cássia Oliveira Carneiro, A.; Trugilho, P.F.; de Paula Protásio, T. Pelletizing of Lignocellulosic Wastes as an Environmentally Friendly Solution for the Energy Supply: Insights on the Properties of Pellets from Brazilian Biomasses. Environ. Sci. Pollut. Res. 2021, 28, 11598–11617. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Dhanyashree, J.K.; Varma, E.; Sirivibha, S.P. Batch and Continuous Packed Bed Column Studies on Biosorption of Nickel (II) by Sugarcane Bagasse. Results Chem. 2022, 4, 100328. [Google Scholar] [CrossRef]
- Promnuan, K.; Sittijunda, S.; Reungsang, A. Evaluation of Commercial Moving Bed Media and Sugarcane Bagasse as Packing Material in Biotrickling Filter for Hydrogen Sulfide Removal. Bioresour. Technol. 2023, 388, 129788. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A Comprehensive Review of Biomass Based Thermochemical Conversion Technologies Integrated with CO2 Capture and Utilisation within BECCS Networks. Resour Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Awan, A.T.; Tsukamoto, J.; Tasic, L. Orange Waste as a Biomass for 2G-Ethanol Production Using Low Cost Enzymes and Co-Culture Fermentation. RSC Adv. 2013, 3, 25071. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G Ethanol from the Whole Sugarcane Lignocellulosic Biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef]
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of First- and Second-Generation Ethanol for Use in Alcohol-Based Hand Sanitizers and Disinfectants in India. Biomass Convers. Biorefin. 2023, 13, 7423–7440. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent Developments and Key Barriers to Advanced Biofuels: A Short Review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.-J.; Bhatia, S.K.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent Advances in Commercial Biorefineries for Lignocellulosic Ethanol Production: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Vieira, S.; Barros, M.V.; Sydney, A.C.N.; Piekarski, C.M.; de Francisco, A.C.; Vandenberghe, L.P.d.S.; Sydney, E.B. Sustainability of Sugarcane Lignocellulosic Biomass Pretreatment for the Production of Bioethanol. Bioresour. Technol. 2020, 299, 122635. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Jana, U.K.; Suryawanshi, R.K.; Kango, N. Sugarcane Bagasse Saccharification Using Aspergillus tubingensis Enzymatic Cocktail for 2G Bio-Ethanol Production. Renew. Energy 2020, 152, 653–663. [Google Scholar] [CrossRef]
- Vanmarcke, G.; Demeke, M.M.; Foulquié-Moreno, M.R.; Thevelein, J.M. Identification of the Major Fermentation Inhibitors of Recombinant 2G Yeasts in Diverse Lignocellulose Hydrolysates. Biotechnol. Biofuels 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental Impact of Lignocellulosic Wastes and Their Effective Exploitation as Smart Carriers—A Drive towards Greener and Eco-Friendlier Biocatalytic Systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent Advances in the Application of Cellulose Derivatives for Removal of Contaminants from Aquatic Environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-Based Nanomaterials for Water and Wastewater Treatments: A Review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the Recent Developments in All-Cellulose Nanocomposites: Properties and Applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ullah, M.W.; Ali, J.; Aziz, K.; Javed, M.A.; Shi, Z.; Manan, S.; Ul-Islam, M.; Nazar, M.; Yang, G. The Versatility of Nanocellulose, Modification Strategies, and Its Current Progress in Wastewater Treatment and Environmental Remediation. Sci. Total Environ. 2023, 858, 159937. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.N.d.C.; Xavier, A.L.P.; Teodoro, F.S.; Elias, M.M.C.; Gonçalves, F.J.; Gil, L.F.; de Freitas, R.P.; Gurgel, L.V.A. Modeling Mono- and Multi-Component Adsorption of Cobalt(II), Copper(II), and Nickel(II) Metal Ions from Aqueous Solution onto a New Carboxylated Sugarcane Bagasse. Part I: Batch Adsorption Study. Ind. Crops Prod. 2015, 74, 357–371. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, K. Enhanced Removal of Cr(VI) via in-Situ Synergistic Reduction and Fixation by Polypyrrole/Sugarcane Bagasse Composites. Chemosphere 2021, 272, 129606. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Shankhwar, A.; Chauhan, D. Mechanistic Insights on Immobilization and Decontamination of Hexavalent Chromium onto Nano MgS/FeS Doped Cellulose Nanofibres. Chemosphere 2019, 228, 390–397. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-Poly-(Acrylamide-Co-Acrylic Acid) Polymeric Bioadsorbent for the Removal of Toxic Inorganic Pollutants from Wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef]
- Montero, J.I.Z.; Monteiro, A.S.C.; Gontijo, E.S.J.; Bueno, C.C.; de Moraes, M.A.; Rosa, A.H. High Efficiency Removal of As(III) from Waters Using a New and Friendly Adsorbent Based on Sugarcane Bagasse and Corncob Husk Fe-Coated Biochars. Ecotoxicol. Environ. Saf. 2018, 162, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, M.; Niu, B.; Zhang, W.; Wang, X.; Wang, J.; Wu, D.; Wang, L.; Jiang, K. Rotten Sugarcane Bagasse Derived Biochars with Rich Mineral Residues for Effective Pb (II) Removal in Wastewater and the Tech-Economic Analysis. J. Taiwan Inst. Chem. Eng. 2022, 132, 104231. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Li, C.; Yue, F.; Zheng, L. Properties and Mechanism of Cr(VI) Removal by a ZnCl2-Modified Sugarcane Bagasse Biochar–Supported Nanoscale Iron Sulfide Composite. Environ. Sci. Pollut. Res. 2022, 30, 26889–26900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shao, J.; Li, Z.; Yang, H.; Zhang, S.; Chen, H. Nano Nickel Embedded in N-Doped CNTs-Supported Porous Biochar for Adsorption-Reduction of Hexavalent Chromium. J. Hazard. Mater. 2021, 416, 125693. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically Modified Sugarcane Bagasse-Based Biocomposites for Efficient Removal of Acid Red 1 Dye: Kinetics, Isotherms, Thermodynamics, and Desorption Studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Iqbal, M.; Hussain, F.; Sarim, F.M. Chitosan, Starch, Polyaniline and Polypyrrole Biocomposite with Sugarcane Bagasse for the Efficient Removal of Acid Black Dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Guo, Q.; Dovi, E.; Qu, L.; Li, Z.; Han, R. Uptake of Micropollutant-Bisphenol A, Methylene Blue and Neutral Red onto a Novel Bagasse-β-Cyclodextrin Polymer by Adsorption Process. Chemosphere 2020, 259, 127439. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and Personal Care Product (PPCP) Contamination—A Global Discharge Inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.-F.; Le Bot, B. Veterinary Pharmaceutical Residues from Natural Water to Tap Water: Sales, Occurrence and Fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ruksana; Alhabarah, A.N.; Alshehri, S.M. N/S Doped Highly Porous Magnetic Carbon Aerogel Derived from Sugarcane Bagasse Cellulose for the Removal of Bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Meneses, I.P.; Novaes, S.D.; Dezotti, R.S.; Oliveira, P.V.; Petri, D.F.S. CTAB-Modified Carboxymethyl Cellulose/Bagasse Cryogels for the Efficient Removal of Bisphenol A, Methylene Blue and Cr(VI) Ions: Batch and Column Adsorption Studies. J. Hazard. Mater. 2022, 421, 126804. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Farinas, C.S. Sugarcane Bagasse Fly Ash as a No-Cost Adsorbent for Removal of Phenolic Inhibitors and Improvement of Biomass Saccharification. ACS Sustain. Chem. Eng. 2017, 5, 11727–11736. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-Doped Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Komal; Gupta, K.; Kumar, V.; Tikoo, K.B.; Kaushik, A.; Singhal, S. Encrustation of Cadmium Sulfide Nanoparticles into the Matrix of Biomass Derived Silanized Cellulose Nanofibers for Adsorptive Detoxification of Pesticide and Textile Waste. Chem. Eng. J. 2020, 385, 123700. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Abo El Naga, A.O.; El Saied, M.; Shaban, S.A.; El Kady, F.Y. Fast Removal of Diclofenac Sodium from Aqueous Solution Using Sugar Cane Bagasse-Derived Activated Carbon. J. Mol. Liq. 2019, 285, 9–19. [Google Scholar] [CrossRef]
- Hassan, M.; Du, J.; Liu, Y.; Naidu, R.; Zhang, J.; Ahsan, M.A.; Qi, F. Magnetic Biochar for Removal of Perfluorooctane Sulphonate (PFOS): Interfacial Interaction and Adsorption Mechanism. Environ. Technol. Innov. 2022, 28, 102593. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, L.; Chen, Y.; Sun, S.; Wang, Y.; Guo, L. Efficient Toluene Adsorption/Desorption on Biochar Derived from in Situ Acid-Treated Sugarcane Bagasse. Environ. Sci. Pollut. Res. 2021, 28, 62616–62627. [Google Scholar] [CrossRef]
- Bezerra, W.F.d.P.; Dognani, G.; Alencar, L.N.d.; Parizi, M.P.S.; Boina, R.F.; Cabrera, F.C.; Job, A.E. Chemical Treatment of Sugarcane Bagasse and Its Influence on Glyphosate Adsorption. Matéria 2022, 27, e13142. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of Aerogels, Cryogels and Xerogels of Cellulose with Hierarchical Porous Structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin Hydrogels, Aerogels, Cryogels and Xerogels: Influence of Drying on Structural and Release Properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, X.; Cai, P.; Guo, T.; Tong, Z.; Xiao, H. Dye Removal from Single and Binary Systems Using Gel-like Bioadsorbent Based on Functional-Modified Cellulose. Cellulose 2018, 25, 2559–2575. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and Efficient Nitrogen Modified Porous Carbon Derived from Sugarcane Bagasse for CO2 Capture: Experimental and DFT Investigation of Nitrogen Atoms on Carbon Frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar] [CrossRef]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and Hemicellulosic Fractions of Sugarcane Bagasse: Potential, Challenges and Future Perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An Insight into Nanocellulose as Soft Condensed Matter: Challenge and Future Prospective toward Environmental Sustainability. Sci. Total Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Syeda, H.I.; Yap, P.-S. A Review on Three-Dimensional Cellulose-Based Aerogels for the Removal of Heavy Metals from Water. Sci. Total Environ. 2022, 807, 150606. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current Scenario and Challenges in Adsorption for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive Nanocomposite Membranes for Heavy Metal Remediation: Recent Progresses and Challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Hamilton, I.; Rapf, O.; Kockat, D.J.; Zuhaib, D.S.; Abergel, T.; Oppermann, M.; Otto, M.; Loran, S.; Fagotto, I.; Steurer, N.; et al. Global Status Report for Buildings and Construction; United Nations Environmental Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Molin Filho, R.G.D.; Longhi, D.A.; de Souza, R.C.T.; Ferrer, M.M.; Vanderlei, R.D.; Paraíso, P.R.; Jorge, L.M.d.M. Self-Compacting Mortar with Sugarcane Bagasse Ash: Development of a Sustainable Alternative for Brazilian Civil Construction. Environ. Dev. Sustain. 2019, 21, 2125–2143. [Google Scholar] [CrossRef]
- Nguyen, H.; Jamali Moghadam, M.; Moayedi, H. Agricultural Wastes Preparation, Management, and Applications in Civil Engineering: A Review. J. Mater. Cycles Waste Manag. 2019, 21, 1039–1051. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary Cementitious Materials: New Sources, Characterization, and Performance Insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Ribeiro, B.; Yamashiki, Y.; Yamamoto, T. A Study on Mechanical Properties of Mortar with Sugarcane Bagasse Fiber and Bagasse Ash. J. Mater. Cycles Waste Manag. 2020, 22, 1844–1851. [Google Scholar] [CrossRef]
- Torres, S.M.; de Lima, V.E.; de Azevedo Basto, P.; de Araújo Júnior, N.T.; de Melo Neto, A.A. Assessing the Pozzolanic Activity of Sugarcane Bagasse Ash Using X-Ray Diffraction. Constr. Build. Mater. 2020, 264, 120684. [Google Scholar] [CrossRef]
- Cláudia dos Santos, A.; Cardoso, F.G.; da Silva, R.J.; de Fátima Gorgulho, H.; Panzera, T.H. Modification of Short Sugarcane Bagasse Fibres for Application in Cementitious Composites: A Statistical Approach to Mechanical and Physical Properties. Constr. Build. Mater. 2022, 353, 129072. [Google Scholar] [CrossRef]
- Hernández-Olivares, F.; Medina-Alvarado, R.E.; Burneo-Valdivieso, X.E.; Zúñiga-Suárez, A.R. Short Sugarcane Bagasse Fibers Cementitious Composites for Building Construction. Constr. Build. Mater. 2020, 247, 118451. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Naveen, J.; Jawaid, M. Potential of Oil Palm Empty Fruit Bunch (OPEFB) and Sugarcane Bagasse Fibers for Thermal Insulation Application—A Review. Constr. Build. Mater. 2021, 271, 121519. [Google Scholar] [CrossRef]
- Mehrzad, S.; Taban, E.; Soltani, P.; Samaei, S.E.; Khavanin, A. Sugarcane Bagasse Waste Fibers as Novel Thermal Insulation and Sound-Absorbing Materials for Application in Sustainable Buildings. Build. Environ. 2022, 211, 108753. [Google Scholar] [CrossRef]
- Quedou, P.G.; Wirquin, E.; Bokhoree, C. Sustainable Concrete: Potency of Sugarcane Bagasse Ash as a Cementitious Material in the Construction Industry. Case Stud. Constr. Mater. 2021, 14, e00545. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.-L.; Zhang, P.-Q.; Li, Y.-Y.; Zhu, X.; He, Y.-C. Effective Conversion of Sugarcane Bagasse to Furfural by Coconut Shell Activated Carbon-Based Solid Acid for Enhancing Whole-Cell Biosynthesis of Furfurylamine. Ind. Crops Prod. 2021, 160, 113169. [Google Scholar] [CrossRef]
- de Sande, V.T.; Sadique, M.; Bras, A.; Pineda, P. Activated Sugarcane Bagasse Ash as Efficient Admixture in Cement-Based Mortars: Mechanical and Durability Improvements. J. Build. Eng. 2022, 59, 105082. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Kroehong, W.; Damrongwiriyanupap, N.; Suriyo, W.; Jaturapitakkul, C. Mechanical Properties, Chloride Resistance and Microstructure of Portland Fly Ash Cement Concrete Containing High Volume Bagasse Ash. J. Build. Eng. 2020, 31, 101415. [Google Scholar] [CrossRef]
- Akarsh, P.K.; Ganesh, G.O.; Marathe, S.; Rai, R. Incorporation of Sugarcane Bagasse Ash to Investigate the Mechanical Behavior of Stone Mastic Asphalt. Constr. Build. Mater. 2022, 353, 129089. [Google Scholar] [CrossRef]
- Perez-Diaz, E.D.; Reyes-Araiza, J.L.; Millán-Malo, B.M.; Londoño-Restrepo, S.M.; Rodríguez-Garcia, M.E. Evaluation of Bamboo Cortex Ash as Supplementary Cementitious Material: Comparative Analysis with Sugarcane Bagasse Ash and Natural Pozzolan. J. Build. Eng. 2023, 66, 105846. [Google Scholar] [CrossRef]
- Minnu, S.N.; Bahurudeen, A.; Athira, G. Comparison of Sugarcane Bagasse Ash with Fly Ash and Slag: An Approach towards Industrial Acceptance of Sugar Industry Waste in Cleaner Production of Cement. J. Clean. Prod. 2021, 285, 124836. [Google Scholar] [CrossRef]
- Amin, M.; Attia, M.M.; Agwa, I.S.; Elsakhawy, Y.; El-hassan, K.A.; Abdelsalam, B.A. Effects of Sugarcane Bagasse Ash and Nano Eggshell Powder on High-Strength Concrete Properties. Case Stud. Constr. Mater. 2022, 17, e01528. [Google Scholar] [CrossRef]
- Chuewangkam, N.; Nachaithong, T.; Chanlek, N.; Thongbai, P.; Pinitsoontorn, S. Mechanical and Dielectric Properties of Fly Ash Geopolymer/Sugarcane Bagasse Ash Composites. Polymers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and Characterization of Geopolymer from Metakaolin and Sugarcane Bagasse Ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Akbar, A.; Farooq, F.; Shafique, M.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Sugarcane Bagasse Ash-Based Engineered Geopolymer Mortar Incorporating Propylene Fibers. J. Build. Eng. 2021, 33, 101492. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Manimaran, P.; Verma, A.; Sanjay, M.R.; Siengchin, S.; Kandakodeeswaran, K.; Geetha, M. A Novel Palm Sheath and Sugarcane Bagasse Fiber Based Hybrid Composites for Automotive Applications: An Experimental Approach. Polym. Compos. 2021, 42, 512–521. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Yamani, S.A.K.; Zainudin, E.S.; Alamery, S. Effect of Surface Treatment on Mechanical, Physical and Morphological Properties of Oil Palm/Bagasse Fiber Reinforced Phenolic Hybrid Composites for Wall Thermal Insulation Application. Constr. Build. Mater. 2021, 276, 122239. [Google Scholar] [CrossRef]
- Guna, V.; Ilangovan, M.; Hu, C.; Venkatesh, K.; Reddy, N. Valorization of Sugarcane Bagasse by Developing Completely Biodegradable Composites for Industrial Applications. Ind. Crops Prod. 2019, 131, 25–31. [Google Scholar] [CrossRef]
- de Paiva, F.F.G.; de Maria, V.P.K.; Torres, G.B.; Dognani, G.; dos Santos, R.J.; Cabrera, F.C.; Job, A.E. Sugarcane Bagasse Fiber as Semi-Reinforcement Filler in Natural Rubber Composite Sandals. J. Mater. Cycles Waste Manag. 2019, 21, 326–335. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Candido, V.S.; Braga, F.O.; Bolzan, L.T.; Weber, R.P.; Drelich, J.W. Sugarcane Bagasse Waste in Composites for Multilayered Armor. Eur. Polym. J. 2016, 78, 173–185. [Google Scholar] [CrossRef]
- Bam, S.A.; Gundu, D.T.; Onu, F.A. The Effect of Chemical Treatments on the Mechanical and Physical Properties of Bagasse Filler Reinforced Low Density Polyethylene Composite. Am. J. Eng. Res. 2019, 8, 95–98. [Google Scholar]
- EL-Zayat, M.M.; Abdel-Hakim, A.; Mohamed, M.A. Effect of Gamma Radiation on the Physico Mechanical Properties of Recycled HDPE/Modified Sugarcane Bagasse Composite. J. Macromol. Sci. Part A 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Sumarji; Sholahuddin, I.; Laksana, D.D.; Syuhri, A.; Aditya, Y. Effect of Steam Pre-Treatment of Bagasse as Fiber Reinforcement SCG Composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012042. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Zainudin, E.S.; Yamani, S.A.K. Modification of Oil Palm Empty Fruit Bunch and Sugarcane Bagasse Biomass as Potential Reinforcement for Composites Panel and Thermal Insulation Materials. J. Bionic Eng. 2019, 16, 175–188. [Google Scholar] [CrossRef]
- Abedom, F.; Sakthivel, S.; Asfaw, D.; Melese, B.; Solomon, E.; Kumar, S.S. Development of Natural Fiber Hybrid Composites Using Sugarcane Bagasse and Bamboo Charcoal for Automotive Thermal Insulation Materials. Adv. Mater. Sci. Eng. 2021, 2021, 2508840. [Google Scholar] [CrossRef]
- dos Santos, B.H.; de Souza do Prado, K.; Jacinto, A.A.; da Silva Spinacé, M.A. Influence of Sugarcane Bagasse Fiber Size on Biodegradable Composites of Thermoplastic Starch. J. Renew. Mater. 2018, 6, 176–182. [Google Scholar] [CrossRef]
- Fischer, W.N. Manual Do PFI—Testing and Research Institute for Footwear Production; PFI: Pirmasens, Germany, 1987. [Google Scholar]
- Nakanishi, E.Y.; Cabral, M.R.; Gonçalves, P.d.S.; dos Santos, V.; Savastano Junior, H. Formaldehyde-Free Particleboards Using Natural Latex as the Polymeric Binder. J. Clean. Prod. 2018, 195, 1259–1269. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Peng, X.; Huang, B.; Li, J. Three-dimensional Printing of Poly(Lactic Acid) Bio-based Composites with Sugarcane Bagasse Fiber: Effect of Printing Orientation on Tensile Performance. Polym. Adv. Technol. 2019, 30, 910–922. [Google Scholar] [CrossRef]
- de Soares, M.M.N.S.; Garcia, D.C.S.; Figueiredo, R.B.; Aguilar, M.T.P.; Cetlin, P.R. Comparing the Pozzolanic Behavior of Sugar Cane Bagasse Ash to Amorphous and Crystalline SiO2. Cem. Concr. Compos. 2016, 71, 20–25. [Google Scholar] [CrossRef]
- Kanking, S.; Niltui, P.; Wimolmala, E.; Sombatsompop, N. Use of Bagasse Fiber Ash as Secondary Filler in Silica or Carbon Black Filled Natural Rubber Compound. Mater. Des. 2012, 41, 74–82. [Google Scholar] [CrossRef]
- dos Santos, R.J.; Agostini, D.L.d.S.; Cabrera, F.C.; dos Reis, E.A.P.; Ruiz, M.R.; Budemberg, E.R.; Teixeira, S.R.; Job, A.E. Sugarcane Bagasse Ash: New Filler to Natural Rubber Composite. Polímeros 2014, 24, 646–653. [Google Scholar] [CrossRef]
- Chandrika, V.S.; Anamika, A.; Jeeva, C.; Perumal, B.; Kumar, S.S.; Roseline, J.F.; Raghavan, I.K. Natural Fiber Incorporated Polymer Matrix Composites for Electronic Circuit Board Applications. Adv. Mater. Sci. Eng. 2022, 2022, 3035169. [Google Scholar] [CrossRef]
- Balachandran, G.B.; David, P.W.; Alexander, A.B.; Mariappan, R.K.; Balasundar, P.; Parrthipan, B.K.; Saravanakumar, S.S.; Kannan, P.S. Saccharum barberi Grass Bagasse Ash-Based Silicone Rubber Composites for Electrical Insulator Applications. Iran. Polym. J. 2021, 30, 1285–1296. [Google Scholar] [CrossRef]
- Huabcharoen, P.; Wimolmala, E.; Markpin, T.; Sombatsompop, N. Purification and Characterization of Silica from Sugarcane Bagasse Ash as a Reinforcing Filler in Natural Rubber Composites. Bioresources 2017, 12, 1228–1245. [Google Scholar] [CrossRef]
- Boonmee, A.; Jarukumjorn, K. Preparation and Characterization of Silica Nanoparticles from Sugarcane Bagasse Ash for Using as a Filler in Natural Rubber Composites. Polym. Bull. 2020, 77, 3457–3472. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Kaushal, R. An Insight into the Green Synthesis of SiO2 Nanostructures as a Novel Adsorbent for Removal of Toxic Water Pollutants. Environ. Res. 2022, 212, 113328. [Google Scholar] [CrossRef]
- Seroka, N.S.; Taziwa, R.T.; Khotseng, L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Appl. Sci. 2022, 12, 2310. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Itua, A.B.; Maliki, M.; Ize-Iyamu, C.O.; Omorogbe, S.O.; Aigbodion, A.I.; Ikhuoria, E.U. The Removal of Nickel and Lead Ions from Aqueous Solutions Using Green Synthesized Silica Microparticles. Heliyon 2020, 6, e04907. [Google Scholar] [CrossRef]
- September, L.A.; Kheswa, N.; Seroka, N.S.; Khotseng, L. Green Synthesis of Silica and Silicon from Agricultural Residue Sugarcane Bagasse Ash—A Mini Review. RSC Adv. 2023, 13, 1370–1380. [Google Scholar] [CrossRef]
- Negash, Y.; Tolosa, B.; Bantewesen, T.; Bekele, G. ASTU Antibacterial Activities of the CuO/ZnO Nanocomposite Grown on Silica Extracted from Bagasse Ash. Ethiop. J. Sci. Sustain. Dev. 2020, 8, 2021. [Google Scholar] [CrossRef]
- Hikmah, N.; Agustiningsih, D.; Nuryono, N.; Kunarti, E.S. Preparation of Iron-Doped SiO2/TiO2 Using Silica from Sugarcane Bagasse Ash for Visible Light Degradation of Congo Red. Indones. J. Chem. 2021, 22, 402. [Google Scholar] [CrossRef]
- Hendra Saputera, W.; Egiyawati, C.; Setyani Putrie, A.; Fathoni Amri, A.; Rizkiana, J.; Sasongko, D. Titania Modified Silica from Sugarcane Bagasse Waste for Photocatalytic Wastewater Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012073. [Google Scholar] [CrossRef]
- Goswami, P.; Mathur, J. Application of Agro-Waste-Mediated Silica Nanoparticles to Sustainable Agriculture. Bioresour Bioprocess 2022, 9, 9. [Google Scholar] [CrossRef]
- Natarajan, S.; Subramaniyam, S.T.; Kumaravel, V. Fabrication of Hydrophobic Coatings Using Sugarcane Bagasse Waste Ash as Silica Source. Appl. Sci. 2019, 9, 190. [Google Scholar] [CrossRef]
- Saed, B.; Ziaee, M.; Kiasat, A.; Jafari Nasab, M. Evaluation of Iranian Diatomaceous Earth in Combination with Nanosilica from Sugarcane Bagasse Ash Applied on Three Different Storage Surfaces against Two Insect Pests of Stored Products. Int. J. Trop. Insect Sci. 2021, 41, 1747–1752. [Google Scholar] [CrossRef]
- Mulyati, S.; Muchtar, S.; Yusuf, M.; Arahman, N.; Sofyana, S.; Rosnelly, C.M.; Fathanah, U.; Takagi, R.; Matsuyama, H.; Shamsuddin, N.; et al. Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes 2020, 10, 17. [Google Scholar] [CrossRef]
- Singh, R.V.; Sharma, P.; Sambyal, K. Application of Sugarcane Bagasse in Chemicals and Food Packaging Industry: Potential and Challenges. Circ. Econ. Sustain. 2022, 2, 1479–1500. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent Trends in the Use of Green Sources for Carbon Dot Synthesis–A Short Review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Kasinathan, K.; Samayanan, S.; Marimuthu, K.; Yim, J.-H. Green Synthesis of Multicolour Fluorescence Carbon Quantum Dots from Sugarcane Waste: Investigation of Mercury (II) Ion Sensing, and Bio-Imaging Applications. Appl. Surf. Sci. 2022, 601, 154266. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, D.; Li, Y.; Ma, X.; Li, J. Green Carbon Quantum Dots from Sustainable Lignocellulosic Biomass and Its Application in the Detection of Fe3+. Cellulose 2022, 29, 367–378. [Google Scholar] [CrossRef]
- Alfi, A.A.; Alamrani, N.A.; Azher, O.A.; Snari, R.M.; Abumelha, H.M.; Al-Ahmed, Z.A.; El-Metwaly, N.M. Development of Carbon Dots Sensor Dipstick from Sugarcane Bagasse Agricultural Waste toward All-Cellulose-Derived Tetracycline Sensor. J. Mater. Res. Technol. 2022, 19, 4697–4707. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, B.; Shen, X.; Yu, Y.; Ji, S.; Wen, C.; Liang, H. Selective Probing of Gaseous Ammonia Using Red-Emitting Carbon Dots Based on an Interfacial Response Mechanism. Chem. Eur. J. 2015, 21, 18993–18999. [Google Scholar] [CrossRef]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible Carbon Quantum Dots Derived from Sugarcane Industrial Wastes for Effective Nonlinear Optical Behavior and Antimicrobial Activity Applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef]
- Chung, H.K.; Wongso, V.; Sambudi, N.S. Isnaeni Biowaste-Derived Carbon Dots/Hydroxyapatite Nanocomposite as Drug Delivery Vehicle for Acetaminophen. J. Solgel Sci. Technol. 2020, 93, 214–223. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Valorization of Sugarcane Bagasse to High Value-Added Xylooligosaccharides and Evaluation of Their Prebiotic Function in a Synbiotic Pomegranate Juice. Biomass Convers. Biorefin. 2023, 13, 787–799. [Google Scholar] [CrossRef]
- Eslami, A.; Borghei, S.M.; Rashidi, A.; Takdastan, A. Preparation of Activated Carbon Dots from Sugarcane Bagasse for Naphthalene Removal from Aqueous Solutions. Sep. Sci. Technol. 2018, 53, 2536–2549. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Zainal Abidin, N.H.; Supandi; Sambudi, N.S. Synthesis of Tungsten Oxide/ Amino-Functionalized Sugarcane Bagasse Derived-Carbon Quantum Dots (WO3/N-CQDs) Composites for Methylene Blue Removal. Chemosphere 2021, 277, 130300. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Cherdthong, A.; Wanapat, M. Improving Sugarcane Bagasse Quality as Ruminant Feed with Lactobacillus, Cellulase, and Molasses. J. Anim. Sci. Technol. 2020, 62, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Lumsangkul, C.; Tapingkae, W.; Sringarm, K.; Jaturasitha, S.; Le Xuan, C.; Wannavijit, S.; Outama, P.; Van Doan, H. Effect of Dietary Sugarcane Bagasse Supplementation on Growth Performance, Immune Response, and Immune and Antioxidant-Related Gene Expressions of Nile Tilapia (Oreochromis niloticus) Cultured under Biofloc System. Animals 2021, 11, 2035. [Google Scholar] [CrossRef] [PubMed]
- Afrazeh, M.; Tadayoni, M.; Abbasi, H.; Sheikhi, A. Extraction of Dietary Fibers from Bagasse and Date Seed, and Evaluation of Their Technological Properties and Antioxidant and Prebiotic Activity. J. Food Meas. Charact. 2021, 15, 1949–1959. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of Dietary Fibers from Sugarcane Bagasse and Sugarcane Tops Using Microwave-Assisted Alkaline Treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Growth Performances, Nutrient Digestibility, Ruminal Fermentation and Energy Partition of Thai Native Steers Fed Exclusive Rice Straw and Fermented Sugarcane Bagasse with Lactobacillus , Cellulase and Molasses. J. Anim. Physiol. Anim. Nutr. 2022, 106, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Molavian, M.; Ghorbani, G.R.; Rafiee, H.; Beauchemin, K.A. Substitution of Wheat Straw with Sugarcane Bagasse in Low-Forage Diets Fed to Mid-Lactation Dairy Cows: Milk Production, Digestibility, and Chewing Behavior. J. Dairy Sci. 2020, 103, 8034–8047. [Google Scholar] [CrossRef] [PubMed]
- Dantas, E.R.S.; Bonhivers, J.-C.; Maciel Filho, R.; Mariano, A.P. Biochemical Conversion of Sugarcane Bagasse into the Alcohol Fuel Mixture of Isopropanol-Butanol-Ethanol (IBE): Is It Economically Competitive with Cellulosic Ethanol? Bioresour. Technol. 2020, 314, 123712. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.A.F.; Thomé, L.C.; Santos, J.C.; Ingle, A.P.; Costa, C.B.; Dos Anjos, V.; Bell, M.J.V.; Rosa, C.A.; Silva, S.S.D. Multi-Scale Study of the Integrated Use of the Carbohydrate Fractions of Sugarcane Bagasse for Ethanol and Xylitol Production. Renew. Energy 2021, 163, 1343–1355. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Mehta, S.; Sharma, V.; Rathour, R.K. Sheetal Xylitol Production by Pseudomonas Gessardii VXlt-16 from Sugarcane Bagasse Hydrolysate and Cost Analysis. Bioprocess Biosyst. Eng. 2022, 45, 1019–1031. [Google Scholar] [CrossRef]
- Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Jacob, S.; Kumar, D.; Awasthi, M.K.; Pant, K.K.; Parameswaran, B.; Kumar, V. High Level Xylitol Production by Pichia Fermentans Using Non-Detoxified Xylose-Rich Sugarcane Bagasse and Olive Pits Hydrolysates. Bioresour. Technol. 2021, 342, 126005. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.d.S.; Jofre, F.M.; dos Santos, H.A.; Hernández-Pérez, A.F.; Felipe, M.d.G.d.A. Xylitol and Ethanol Co-Production from Sugarcane Bagasse and Straw Hemicellulosic Hydrolysate Supplemented with Molasses. Biomass Convers. Biorefin. 2023, 13, 3143–3152. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Farzad, S.; Görgens, J.F. Furfural Production from Sugarcane Bagasse along with Co-Production of Ethanol from Furfural Residues. Biomass Convers. Biorefin. 2022, 12, 5257–5267. [Google Scholar] [CrossRef]
- Trung, T.Q.; Thinh, D.B.; Anh, T.N.M.; Nguyet, D.M.; Quan, T.H.; Viet, N.Q.; Tuan, T.T.; Dat, N.M.; Nam, H.M.; Hieu, N.H.; et al. Synthesis of Furfural from Sugarcane Bagasse by Hydrolysis Method Using Magnetic Sulfonated Graphene Oxide Catalyst. Vietnam. J. Chem. 2020, 58, 245–250. [Google Scholar] [CrossRef]
- Silva, T.A.L.; da Silva, A.C.; Pasquini, D. Synthesis and Characterization of Acid-Activated Carbon Prepared from Sugarcane Bagasse for Furfural Production in Aqueous Media. Catalysts 2023, 13, 1372. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, H.; Kumar, M.; Gehlaut, A.K.; Gaur, A.; Sachan, S.; Park, J.-W. Synthesis of Biodegradable Films Obtained from Rice Husk and Sugarcane Bagasse to Be Used as Food Packaging Material. Environ. Eng. Res. 2019, 25, 506–514. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of Sugarcane Bagasse Nanocellulose Hydrogel as a Colourimetric Freshness Indicator for Intelligent Food Packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.A.; Rodrigues, J.S.; Gonçalves, M.P.; Borsagli, F.G. Ecofriendly Bio-Packing Based on Sugarcane Bagasse Fiber for Potential Application in Agroindustry. Sustain. Chem. Eng. 2022, 3, 66–74. [Google Scholar] [CrossRef]
- Nida, S.; Moses, J.A.; Anandharamakrishnan, C. 3D Printed Food Package Casings from Sugarcane Bagasse: A Waste Valorization Study. Biomass Convers. Biorefin. 2021, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).