Reduced Mechanism for Combustion of Ammonia and Natural Gas Mixtures
Abstract
1. Introduction
- (1)
- Higher hydrogen content relative to gasoline, diesel, and coal;
- (2)
- High adiabatic flame temperature and high laminar flame speed;
- (3)
- Well-established infrastructure;
- (4)
- Abundance of natural gas reserves in the US.
- (5)
- A worldwide increase in liquefied natural gas (LNG) plants and terminals.
2. Methodology
- The species in the reduced mechanism should be suitable for the targeted applications in turbine/engine design with CFD. In this study, the reduced mechanism should include fuel species, emission species (NO, NO2, NH3, HCN, N2O), and soot precursors (C2H2, C2H4).
- The next step is to utilize the Reaction Path Analyzer (RPA) tool in Chemkin. RPA provides a visualization of the inner relationships of the chemistry model, as shown in Figure 2.
- The final step in species selection is to apply the Reaction Rate Analysis (RRA) tool. In this Chemkin analysis, the Perfectly Stirred Reactor (PSR) model is adopted. In these PSR runs, the species with a higher rate of production or destruction are expected to be dominant in the reaction mechanism and, thus, are ranked higher. Both RPA and RRA are needed to identify important intermediate species for NH3NG to represent the essential elements of the full Modified Konnov Mechanism.
2.1. Preliminary Species Selection
2.2. Reaction Path Analyzer (RPA)
2.3. Reaction Rate Analysis (RRA)
3. Results
3.1. Comparison with the Full Mechanism
3.2. Validation with Experimental Data
3.2.1. Laminar Flame Speed
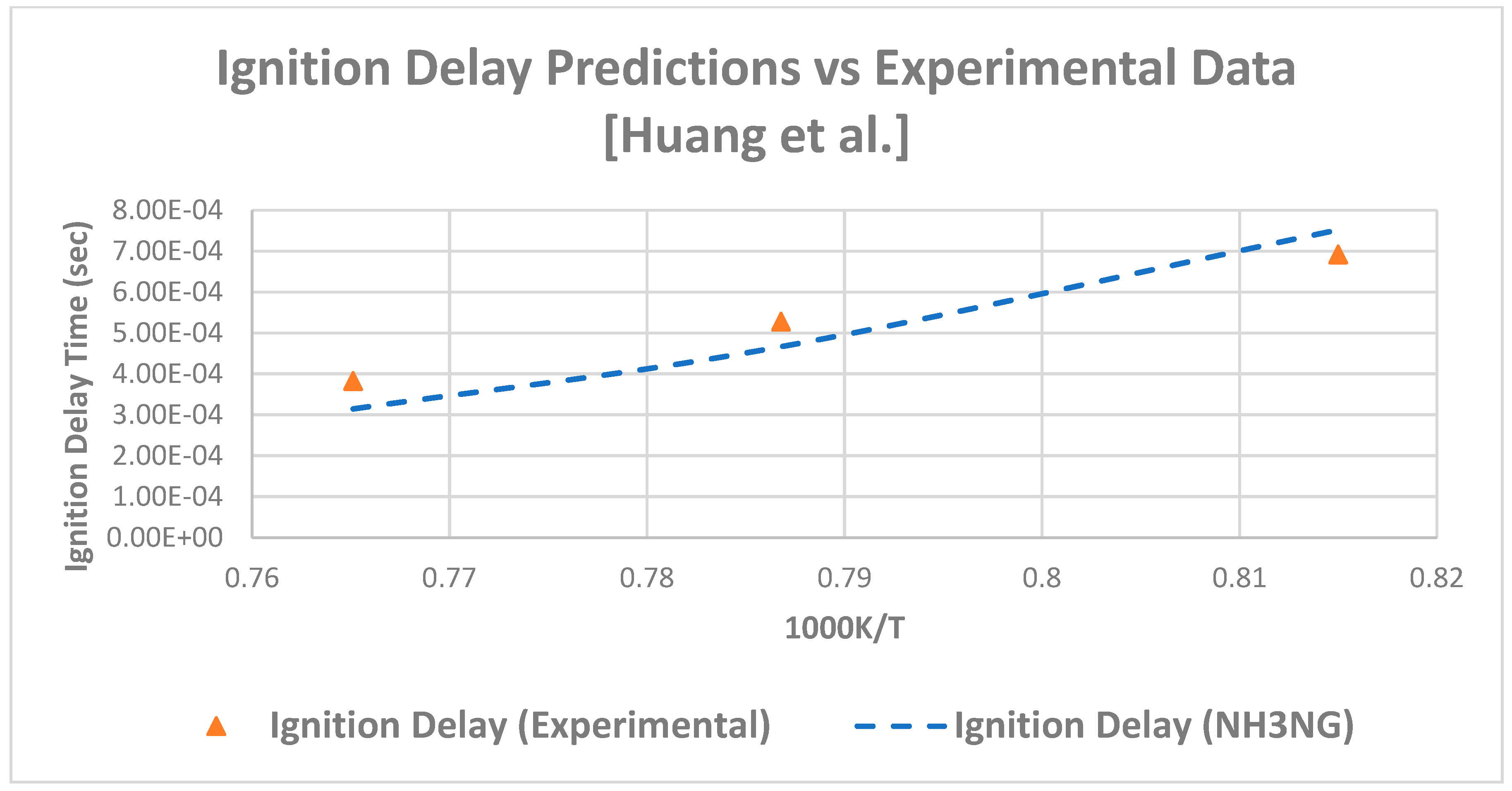
3.2.2. Ignition Delay
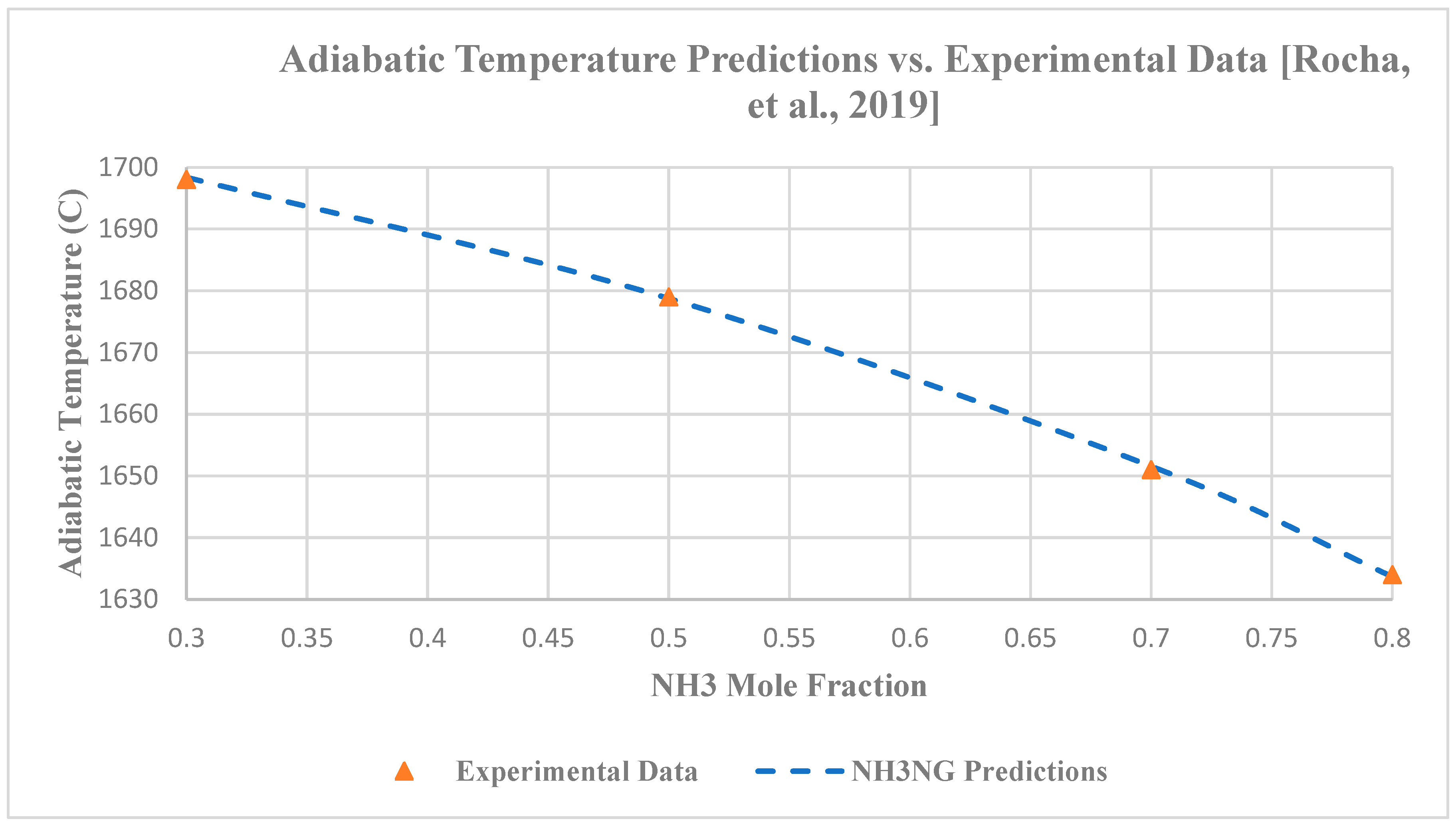
3.2.3. Adiabatic Temperature
3.2.4. NOx and CO Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| ICE | Internal Combustion Engines |
| NG | Natural Gas |
| LNG | Liquified Natural Gas |
| VOC | Volatile Organic Compound |
| CFD | Computational Fluid Dynamics |
| RPA | Reaction Path Analyzer |
| PSR | Perfectly Stirred Reactor |
References
- Trop, P.; Goricanec, D. Comparisons between energy carriers’ productions for exploiting renewable energy sources. Energy 2016, 108, 155–161. [Google Scholar] [CrossRef]
- Available online: https://www.engineeringtoolbox.com/ammonia-pressure-temperature-d_361.html (accessed on 25 February 2023).
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen Nexus in a Sustainable Energy Future. Energy Environ. Sci. 2008, 1, 79–85. [Google Scholar] [CrossRef]
- Natural Gas Specs Sheet. Available online: https://www.naesb.org/pdf2/wgq_bps100605w2.pdf (accessed on 25 February 2023).
- Loz, B. First Ammonia-Powered Jet Flight in 2023: A Roadmap to Clean Aviation; New Atlas: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Sonal, P. Ammonia Gas Turbine Combustion Has Economic Potential, GE-IHI Study Suggests. Power. 2023. Available online: https://www.powermag.com/ammonia-gas-turbine-combustion-has-economic-potential-ge-ihi-study-suggests/?oly_enc_id=1127F6602090B1F (accessed on 23 February 2023).
- Uchida, M.; Ito, S.; Suda, T.; Fujimori, T. Performance of Ammonia/Natural Gas Co-Fired Gas Turbine with 2-Stage Combustor, 367d. In Proceedings of the AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Rocha, R.; Costa, M.; Bai, X. Combustion and emission characteristics of ammonia under conditions relevant to modern gas turbines. Combust. Sci. Technol. 2021, 193, 2514–2533. [Google Scholar] [CrossRef]
- Rocha, R.; Ramos, C.; Costa, M.; Bai, X. Combustion of NH3/CH4/Air and NH3/H2/Air Mixtures in a Porous Burner: Experiments and Kinetic Modeling. Energy Fuels 2019, 33, 12767–12780. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Han, X.; Lavadera, M.L.; Konnov, A.A. An experimental and kinetic modeling study on the laminar burning velocity of NH3+N2O+air flames. Combust. Flame 2021, 228, 13–28. [Google Scholar] [CrossRef]
- Gianluca, C.; Vladimir, A.; Alekseev, A.; Konnov, A. An experimental and kinetic study of propanal oxidation. Combust. Flame 2018, 197, 11–21. [Google Scholar]
- Wang, Z.; Han, X.; He, Y.; Zhu, R.; Zhu, Y.; Zhou, Z.; Cen, K. Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in premixed flames. Combust. Flame 2021, 229, 111392. [Google Scholar] [CrossRef]
- The San Diego Mechanism: Chemical-Kinetic Mechanisms for Combustion Applications/Nitrogen Chemistry, Mechanical and Aerospace Engineering (Combustion Research), University of California, San Diego. Available online: https://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html (accessed on 25 February 2023).
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds; CERFACS Chemistry: Toulouse, France, 2007. [Google Scholar]
- CERFACS Chemistry 2023. USC II Detailed Mechanism. Available online: https://chemistry.cerfacs.fr/en/chemical-database/mechanisms-list/usc-ii-mechanism/ (accessed on 25 February 2023).
- Smith, G.P.; Tao, Y.; Wang, H. Foundational Fuel Chemistry Model Version 1.0 (FFCM-1). Available online: https://web.stanford.edu/group/haiwanglab/FFCM1/pages/FFCM1.html (accessed on 25 February 2023).
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4-NH3-air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Ramos, C.F.; Shu, B.; Fernandes, R.X.; Costa, M. Ignition Delay Times of Diluted Mixtures of Ammonia/Methane at Elevated Pressures. paper 367 c. In Proceedings of the Ammonia Energy Conference & AIChE Annual Meeting, Orlando, FL, USA, 12–14 November 2019. [Google Scholar]
- Wang, S.; Wang, Z.; He, Y.; Sun, Z.; Liu, Y.; Zhu, Y.; Costa, M. Laminar Burning Velocity and NOx Emission Measurements of NH3/CH4/Air and NH3/H2/Air Premixed Flames under Elevated Pressure; The Combustion Institute: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Shu, B.; Ramos, C.F.; He, X.; Fernandes, R.X.; Costa, M. Experimental and modeling study on the auto-ignition properties of ammonia/methane mixtures at elevated pressures. Proc. Combust. Inst. 2021, 38, 261–268. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.S.; Valera-Medina, A.; Dooley, S.; Bowen, P. Reduced Chemical Mechanisms for Ammonia/Methane Co-Firing for Gas Turbine Applications. Energy Procedia 2017, 105, 1483–1488. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.-S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Aldén, M. Structure and Laminar Flame Speed of an Ammonia/Methane/Air Premixed Flame under Varying Pressure and Equivalence Ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef] [PubMed]
- Steffen, S.; Sabrina, S.; Lena, R.; Jacqueline, H.; Franziska, S.; Lubow, M.; Olaf, D.; Kohse-Höinghaus, K. Homogeneous conversion of NOx and NH3 with CH4, CO, and C2H4 at the diluted conditions of exhaust-gases of lean operated natural gas engines. Int. J. Chem. Kinet. 2021, 53, 213–229. [Google Scholar]
- Huang, J.; Bushe, W.K. Experimental and Kinetic Study of Autoignition in Methane/Ethane/Air and Methane/Propane/Air Mixtures under Engine-Relevant Conditions. Combust. Flame 2006, 144, 74–88. [Google Scholar] [CrossRef]
- Bowman, B.R.; Pratt, D.T.; Crowe, C.T. Effects of Turbulent Mixing and Chemical Kinetics on Nitric Oxide Production in a Jet-Stirred Reactor. Symp. Int. Combust. 1973, 14, 819–830. [Google Scholar] [CrossRef]
- Kawka, L.; Juhász, G.; Papp, M.; Nagy, T.; Zsély, I.G.; Turányi, T. Comparison of detailed reaction mechanisms for homogeneous ammonia combustion. J. Z. Phys. Chem. 2020, 234, 1329–1357. [Google Scholar] [CrossRef]
- Lou, H.; Martin, C.; Chen, D.; Li, X.; Li, K.; Vaid, H.; Tula, A.; Singh, K. Validation of a Reduced Combustion Mechanism for Light Hydrocarbons. Clean Technol. Environ. Policy 2012, 14, 737–748. [Google Scholar] [CrossRef]
- Lou, H.; Chen, D.; Li, X.; Martin, C.; Richmond, P. Flare Speciation and Air Quality Modeling; TCEQ SEP Agreement No. 2009-009—Phase III, SEP Task 2-A Final Report; SEP: Austin, TX, USA, 2015. [Google Scholar]
- Damodara, V.; Chen, D.; Lou, H.; Rasel, K.; Richmond, P.; Wang, A.; Li, X. Reduced Combustion Mechanism for C1-C4 Hydrocarbons and its Application in CFD Flare Modeling. J. Air Waste Manag. Assoc. 2017, 67, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Chemkin 2019 R2. Chemistry Simulation Software. Available online: https://www.ansys.com/products/fluids/ansys-chemkin-pro (accessed on 25 February 2023).
- ANSYS. ANSYS Fluent User’s Guide Release 2021 R1; Theory Guide Release 2021 R1; ANSYS® Academic Research, Release 2021 R1; ANSYS Inc.: Canonsburg, PA, USA, 2021. [Google Scholar]
- Brookes, S.J.; Moss, J.B. Prediction of Soot and Thermal Radiation in Confined Turbulent Jet Diffusion Flames. Combust. Flame 1999, 116, 486–503. [Google Scholar] [CrossRef]
- Fenimore, C.P.; Jones, G.W. Oxidation of soot by hydroxyl radicals. J. Phys. Chem. 1967, 71, 593–597. [Google Scholar] [CrossRef]
- ANSYS. Soot Model Theory. Available online: https://www.afs.enea.it/project/neptunius/docs/fluent/html/th/node229.htm (accessed on 25 February 2023).
- Rigopoulos, S. Modelling of Soot Aerosol Dynamics in Turbulent Flow. Flow Turbul. Combust. 2019, 103, 565–604. [Google Scholar] [CrossRef]
- Reaction Design. Reaction Workbench 15131; Reaction Design: San Diego, CA, USA, 2013. [Google Scholar]
- Goodwin, H.M.D.; Speth, R. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 2.2.1. 2016. Available online: http://www.cantera.org (accessed on 10 April 2023).
- Quadrennial Technology Review 2015. Supercritical Carbon Dioxide Brayton Cycle; Technology Assessments, Chapter 4; US Department of Energy: Washington, DC, USA, 2015.
- Fernandes, D.; Wang, S.; Xu, Q.; Buss, R.; Chen, D. Process and Carbon Footprint Analyses of the Allam Cycle Power Plant Integrated with an Air Separation Unit. Clean Technol. 2019, 1, 325–340. [Google Scholar] [CrossRef]
- ANSYS. ANSYS Energico User’s Guide 2020 R1; ANSYS Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]






| Component | Typical (%) | Range (Mole) |
|---|---|---|
| Methane | 94.9 | 87.0–96.0 |
| Ethane | 2.5 | 1.8–5.1 |
| C3+ | 0.3 | 0.1–2.3 |
| Nitrogen | 1.6 | 1.3–5.6 |
| Carbon | 0.7 | 0.1–1.0 |
| 50 Species | Ar N2 H H2 O O2 OH HO2 H2O CO CO2 HCO CH3 CH4 C2H6 CH2O C2H5 CH2 CH3O CH2OH CH C2H2 C2H4 C2H3 CH3OH CH2CO HCCO C CH2HCO NH NO NCO N2O NH2 HNO NO2 NNH NH3 HONO CNN H2NO C3H6 C3H8 iC3H7 nC3H7 C3H3 C3H5 C3H4 C4H4 iC4H3 |
| Species | Konnov (Modified) | NH3NG | Absolute Error | Absolute % Error |
|---|---|---|---|---|
| CH4 | 4.85 × 10−7 | 5.34 × 10−7 | 1.10122 × 10−9 | 9.99 |
| NH3 | 1.59 × 10−6 | 1.78 × 10−6 | 1.8799 × 10−7 | 11.79 |
| CO | 5.14 × 10−6 | 5.51 × 10−6 | 3.6462 × 10−7 | 7.09 |
| CO2 | 6.06 × 10−2 | 6.06 × 10−2 | 3 × 10−7 | 0 |
| H2 | 1.37 × 10−7 | 1.46 × 10−7 | 9.101 × 10−9 | 6.65 |
| NO2 | 1.34 × 10−5 | 1.37 × 10−5 | 2.315 × 10−7 | 1.72 |
| HNO | 2.84 × 10−9 | 2.82 × 10−9 | 1.612 × 10−11 | 0.57 |
| NO | 2.48 × 10−4 | 2.34 × 10−4 | 1.41 × 10−5 | 5.69 |
| Average | 1.90 × 10−6 | 5.44 |
| Sr. No. | Pressure (Bar) | xNH3 | Equivalence Ratio | Experimental (cm/s) | NH3NG (cm/s) | Error (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 0.2 | 0.8 | 26.6 | 26.6 | 0.1% |
| 2 | 1 | 0.2 | 1 | 51.8 | 51.8 | 0.0% |
| 3 | 2 | 0.2 | 1 | 41.6 | 42.6 | 2.5% |
| 4 | 3 | 0.2 | 1 | 35.7 | 37.3 | 4.5% |
| 5 | 1 | 0.2 | 1.2 | 43.2 | 45.8 | 6.0% |
| 6 | 2 | 0.2 | 1.2 | 33.3 | 37.1 | 11.5% |
| 7 | 3 | 0.2 | 1.2 | 27.9 | 31.9 | 14.3% |
| 8 | 3 | 0.4 | 1.2 | 23.6 | 26.5 | 12.2% |
| 9 | 3 | 0.6 | 1.2 | 19.3 | 22.0 | 14.0% |
| 10 | 3 | 0.8 | 1.2 | 16.4 | 18.4 | 12.4% |
| Avg. | 7.7% |
| 8.93% CH4, 0.34% C2H6 Mixture | ||||||
|---|---|---|---|---|---|---|
| Pressure (atm) | Temp (K) | 1000/T | Ignition Time NH3NG (s) | Ignition Time Experimental (s) | Absolute Error | Absolute Error % |
| 16.3 | 1307 | 0.76511 | 3.15 × 10−4 | 3.83 × 10−4 | 6.85 × 10−5 | 18% |
| 15.8 | 1271 | 0.78678 | 4.67 × 10−4 | 5.28 × 10−4 | 6.15 × 10−5 | 12% |
| 16.2 | 1227 | 0.815 | 7.52 × 10−4 | 6.92 × 10−4 | 6.03 × 10−5 | 9% |
| Average | 13% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khade, A.R.; Damodara, V.D.; Chen, D.H. Reduced Mechanism for Combustion of Ammonia and Natural Gas Mixtures. Clean Technol. 2023, 5, 484-496. https://doi.org/10.3390/cleantechnol5020025
Khade AR, Damodara VD, Chen DH. Reduced Mechanism for Combustion of Ammonia and Natural Gas Mixtures. Clean Technologies. 2023; 5(2):484-496. https://doi.org/10.3390/cleantechnol5020025
Chicago/Turabian StyleKhade, Aniket R., Vijaya D. Damodara, and Daniel H. Chen. 2023. "Reduced Mechanism for Combustion of Ammonia and Natural Gas Mixtures" Clean Technologies 5, no. 2: 484-496. https://doi.org/10.3390/cleantechnol5020025
APA StyleKhade, A. R., Damodara, V. D., & Chen, D. H. (2023). Reduced Mechanism for Combustion of Ammonia and Natural Gas Mixtures. Clean Technologies, 5(2), 484-496. https://doi.org/10.3390/cleantechnol5020025









