Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications
Abstract
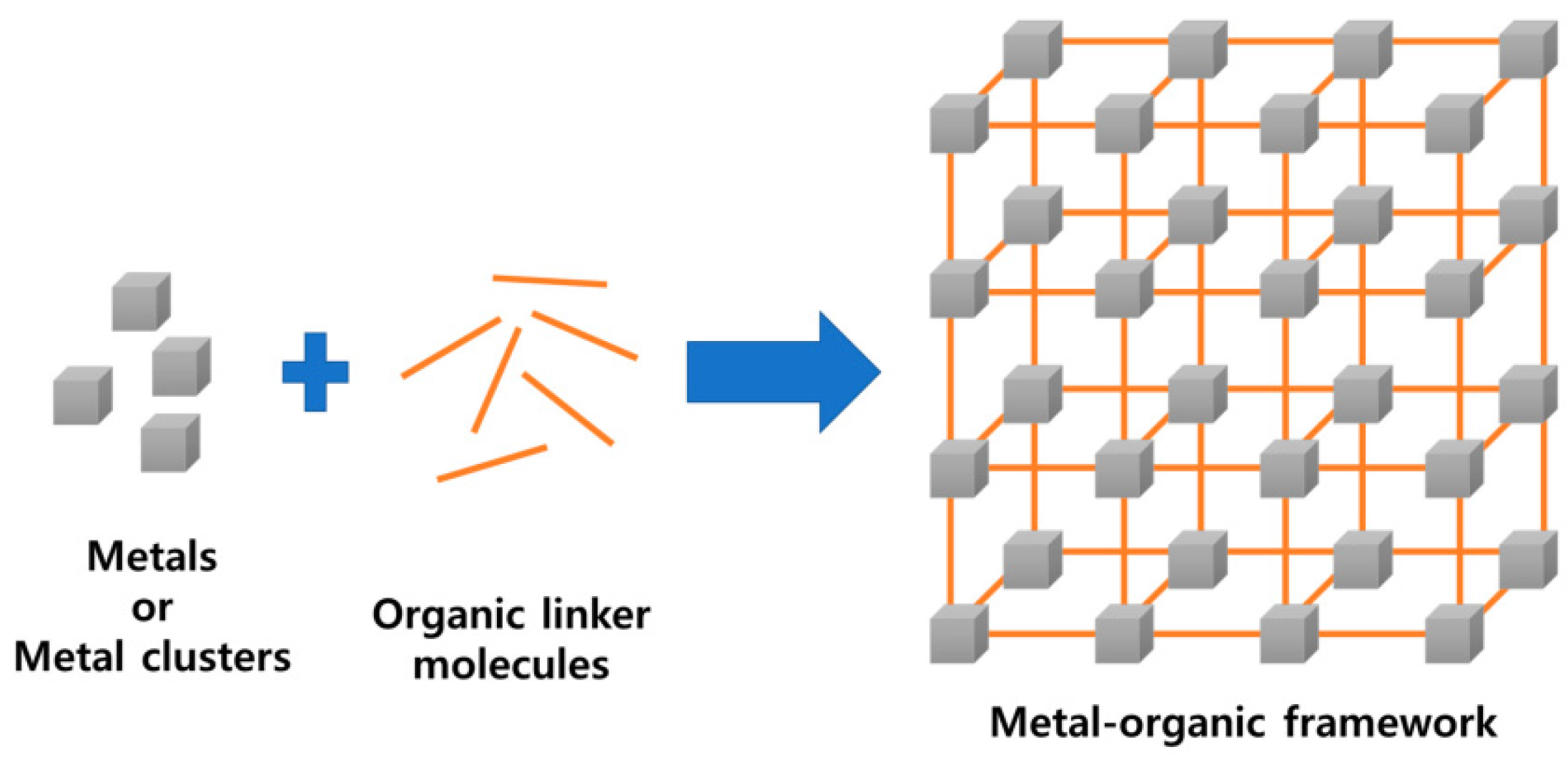
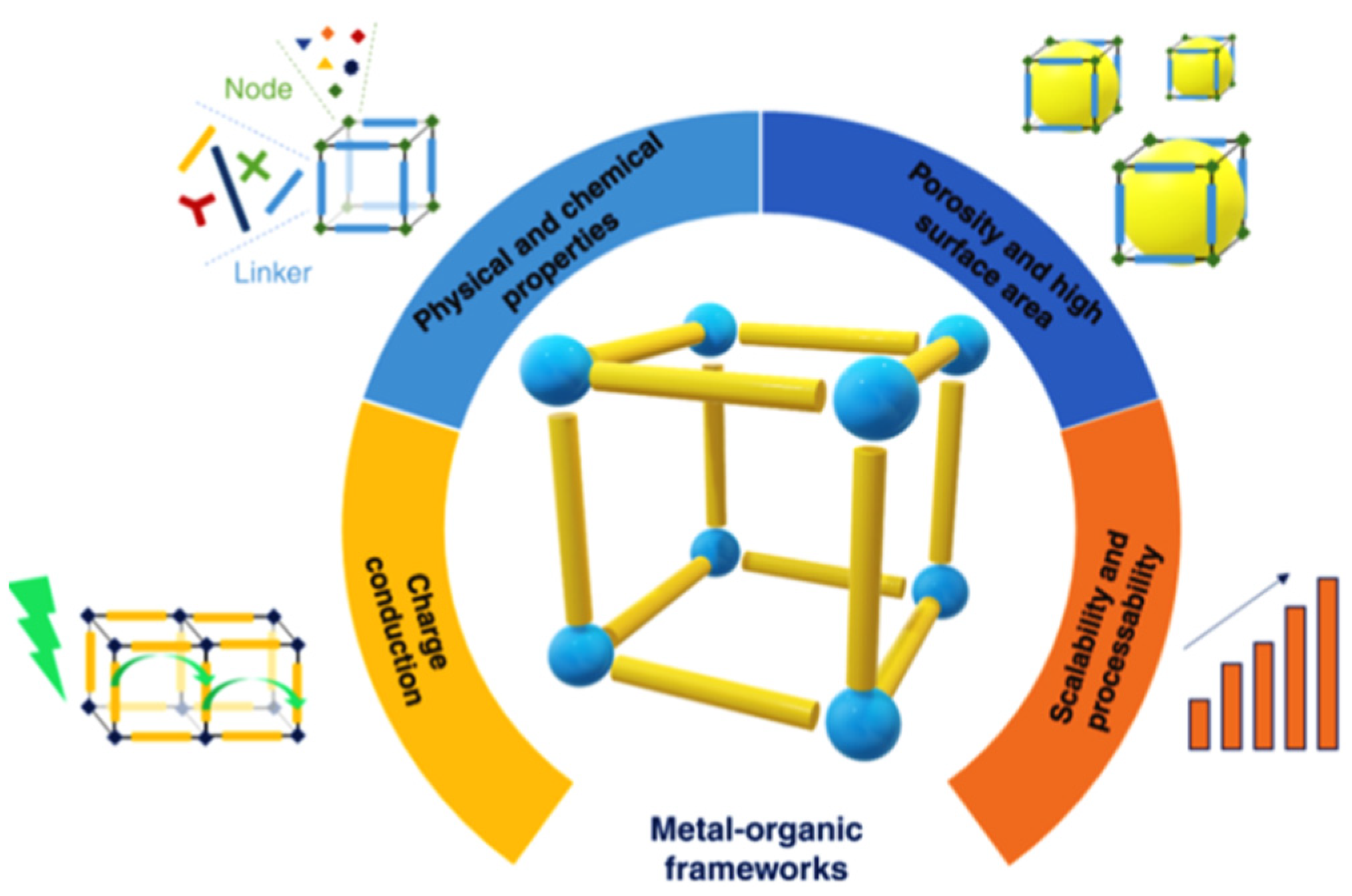
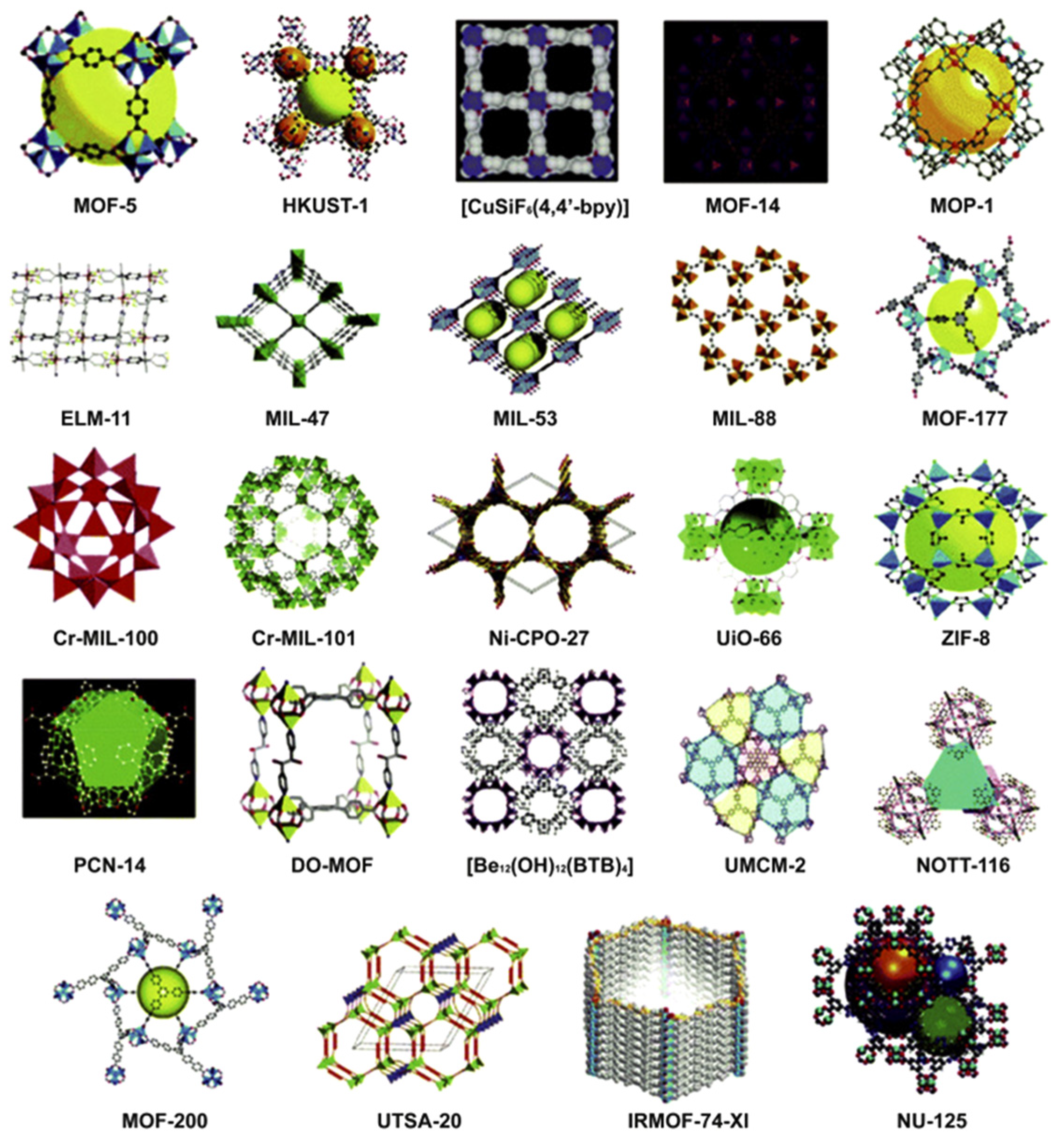
1. Introduction
2. Different Methods for Synthesis of MOFs
2.1. Microwave Assisted Methods
2.2. Sonochemical Method
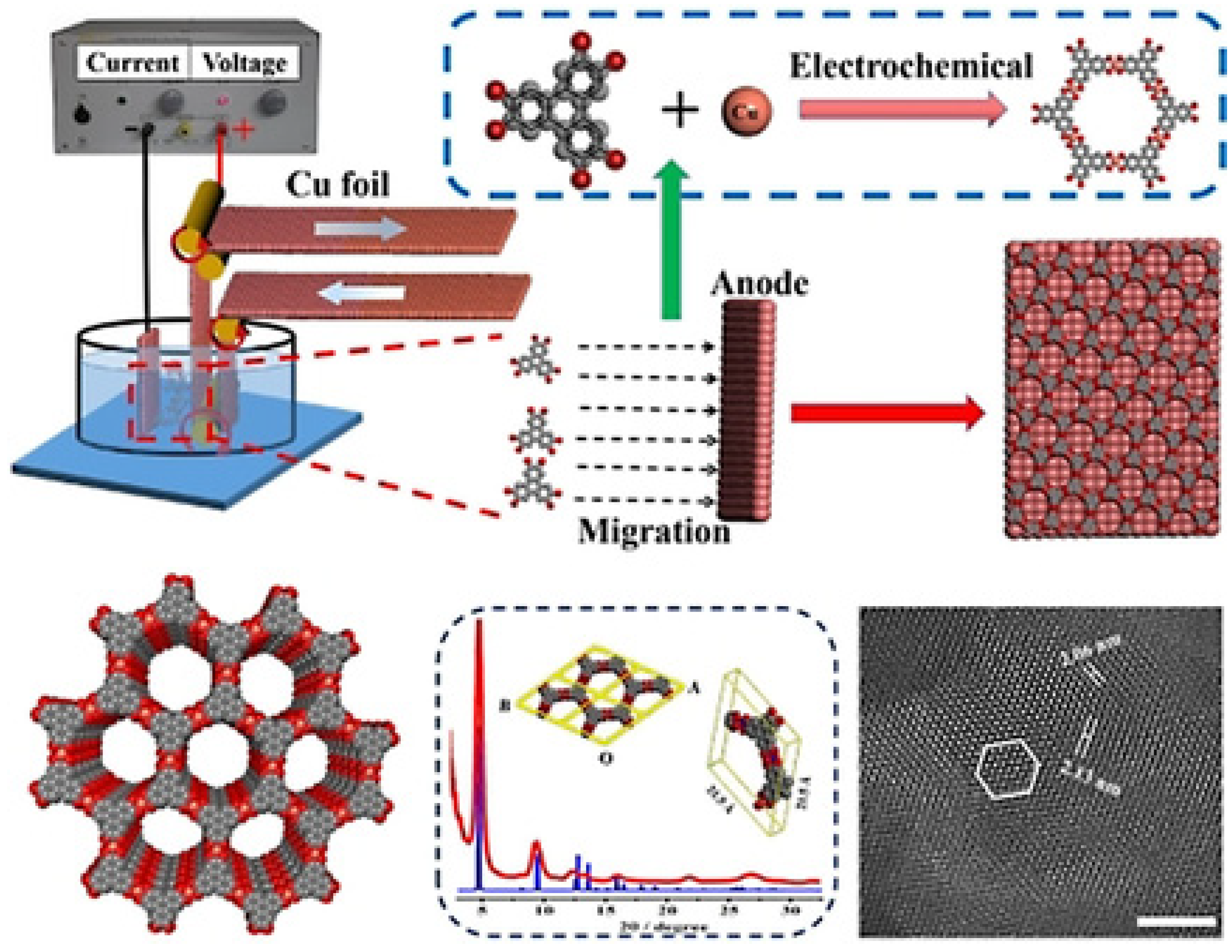
2.3. Electrochemical Method
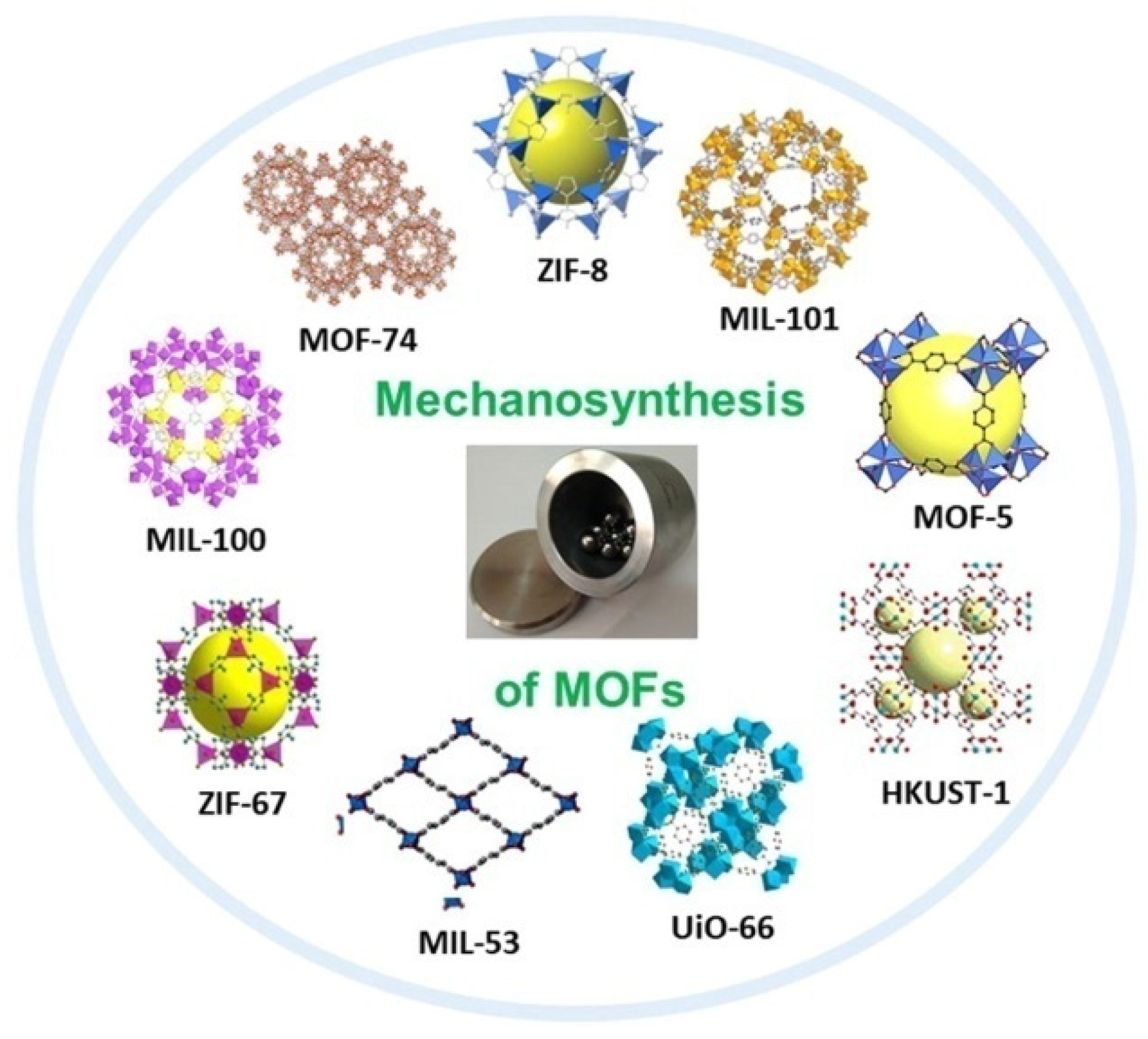
2.4. Mechanochemical Method
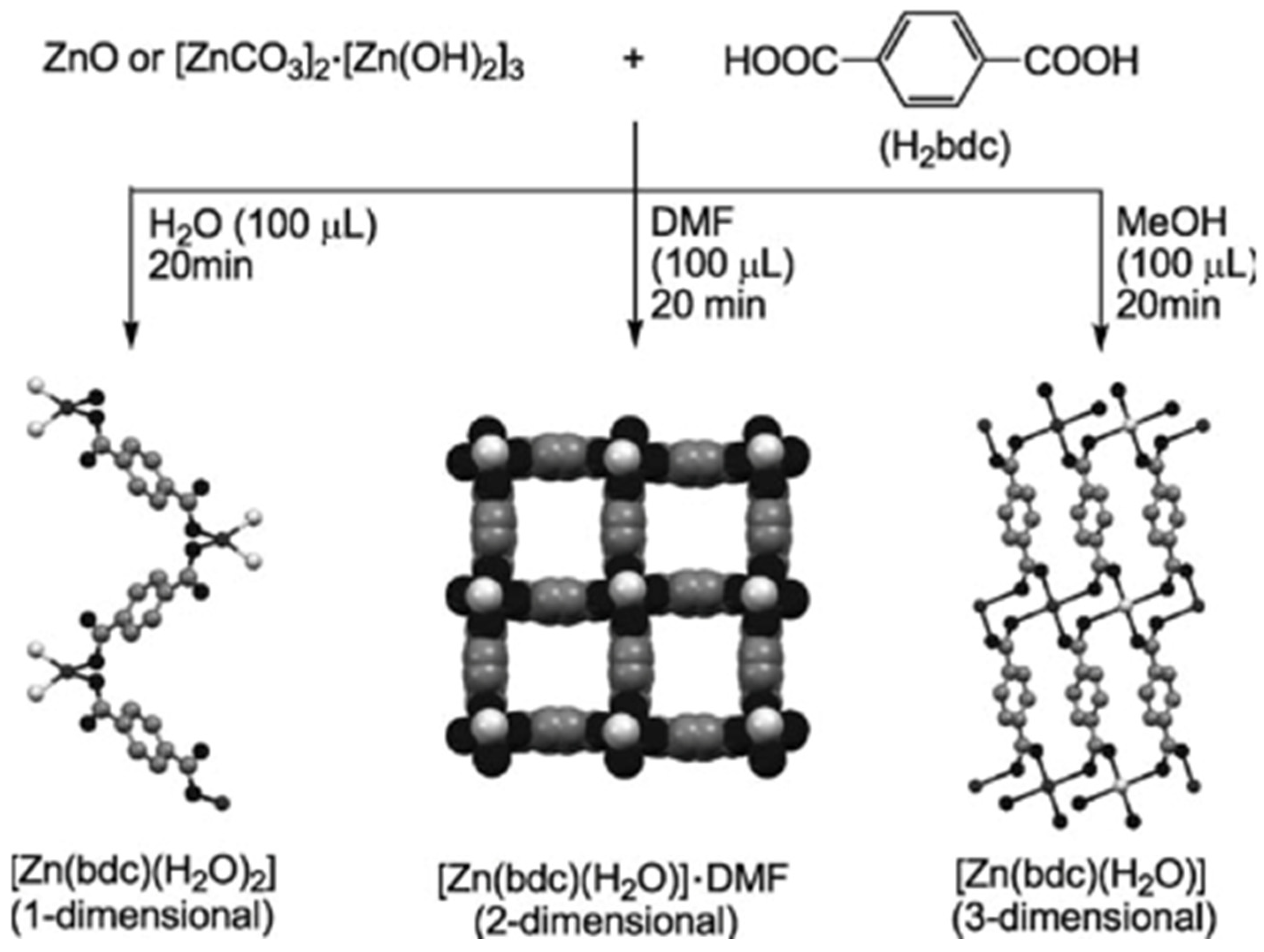
2.5. Hydrothermal Approach
2.6. Solvothermal Approach
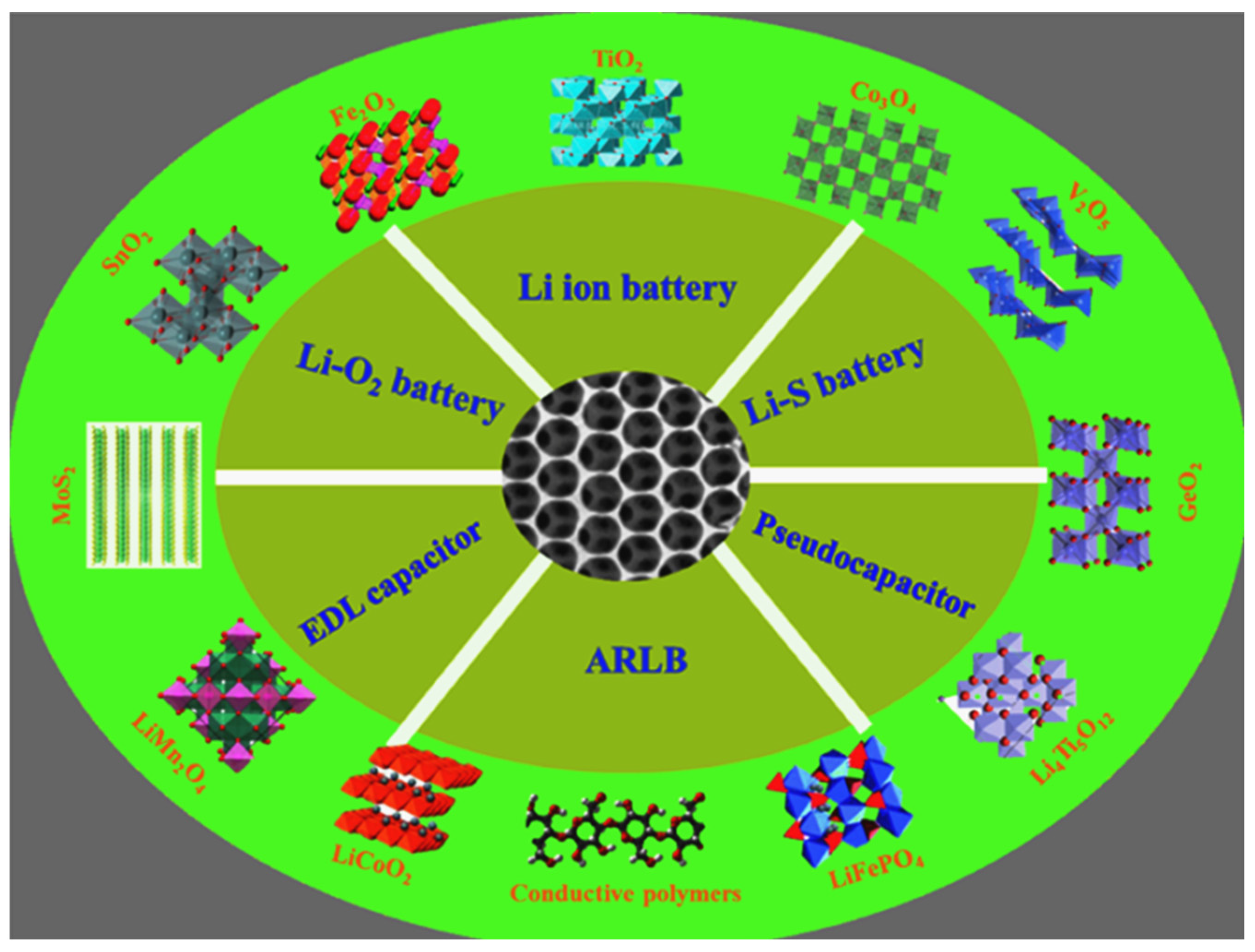
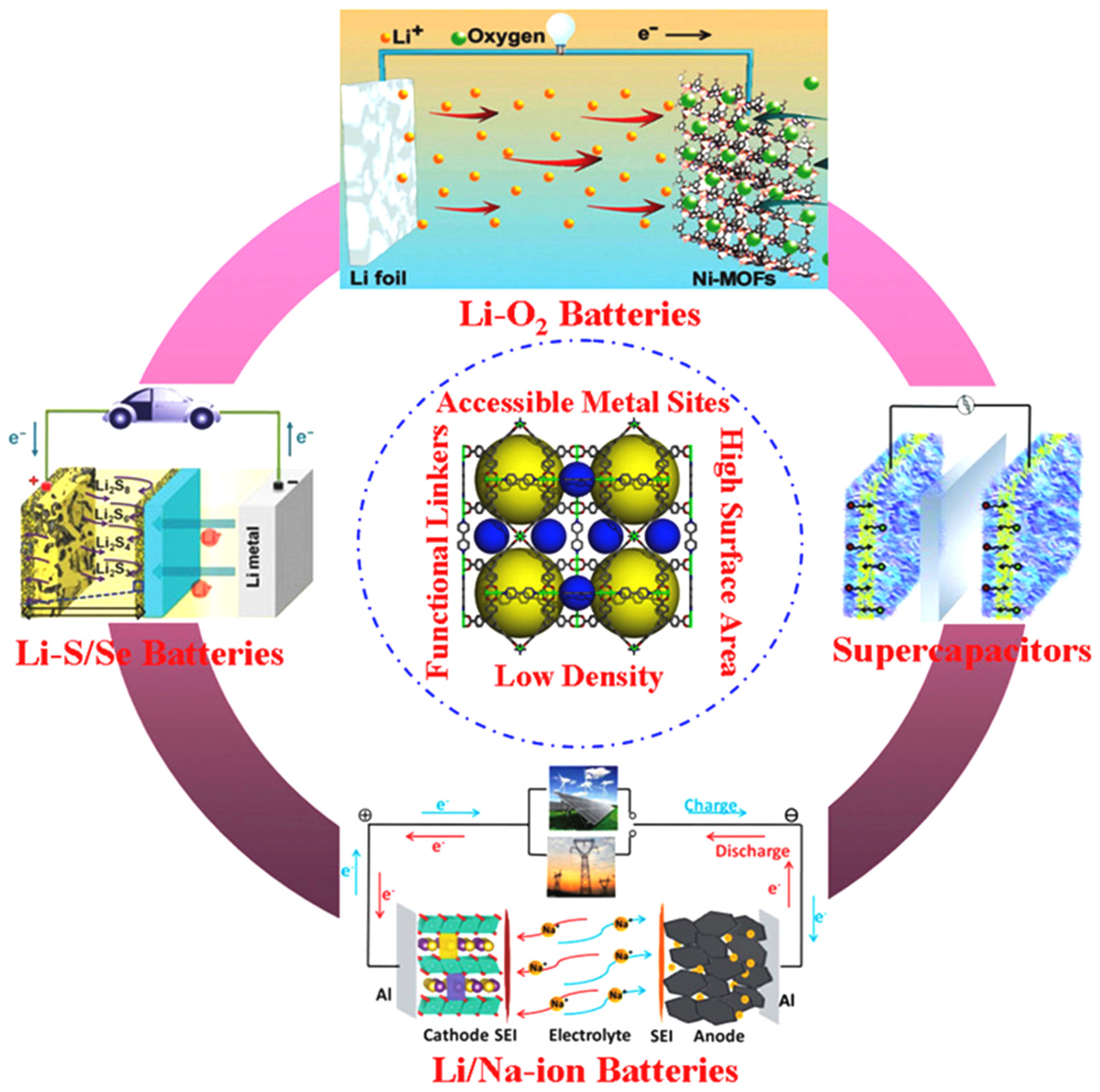
3. MOF Applications in Energy Storage Devices
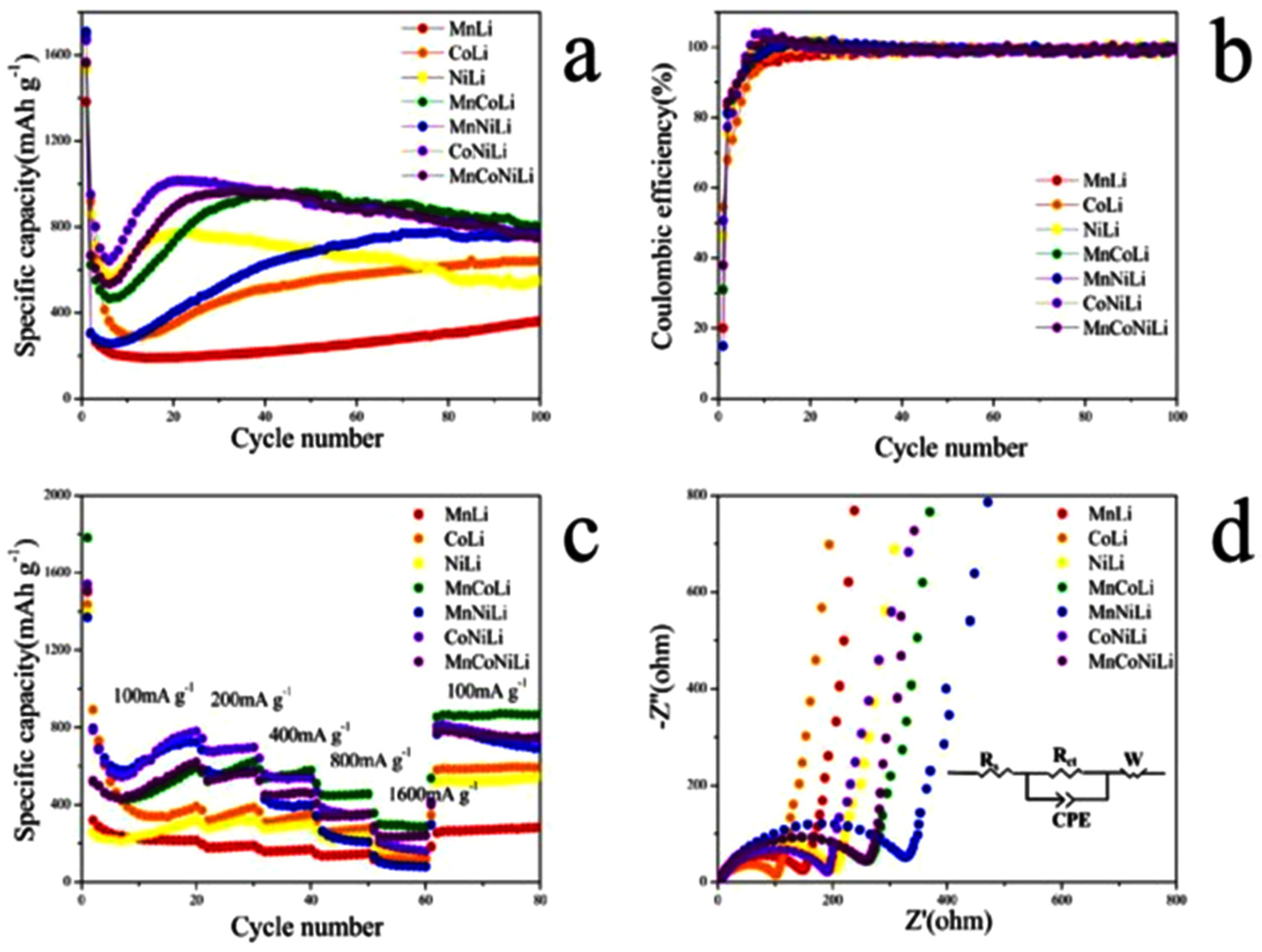
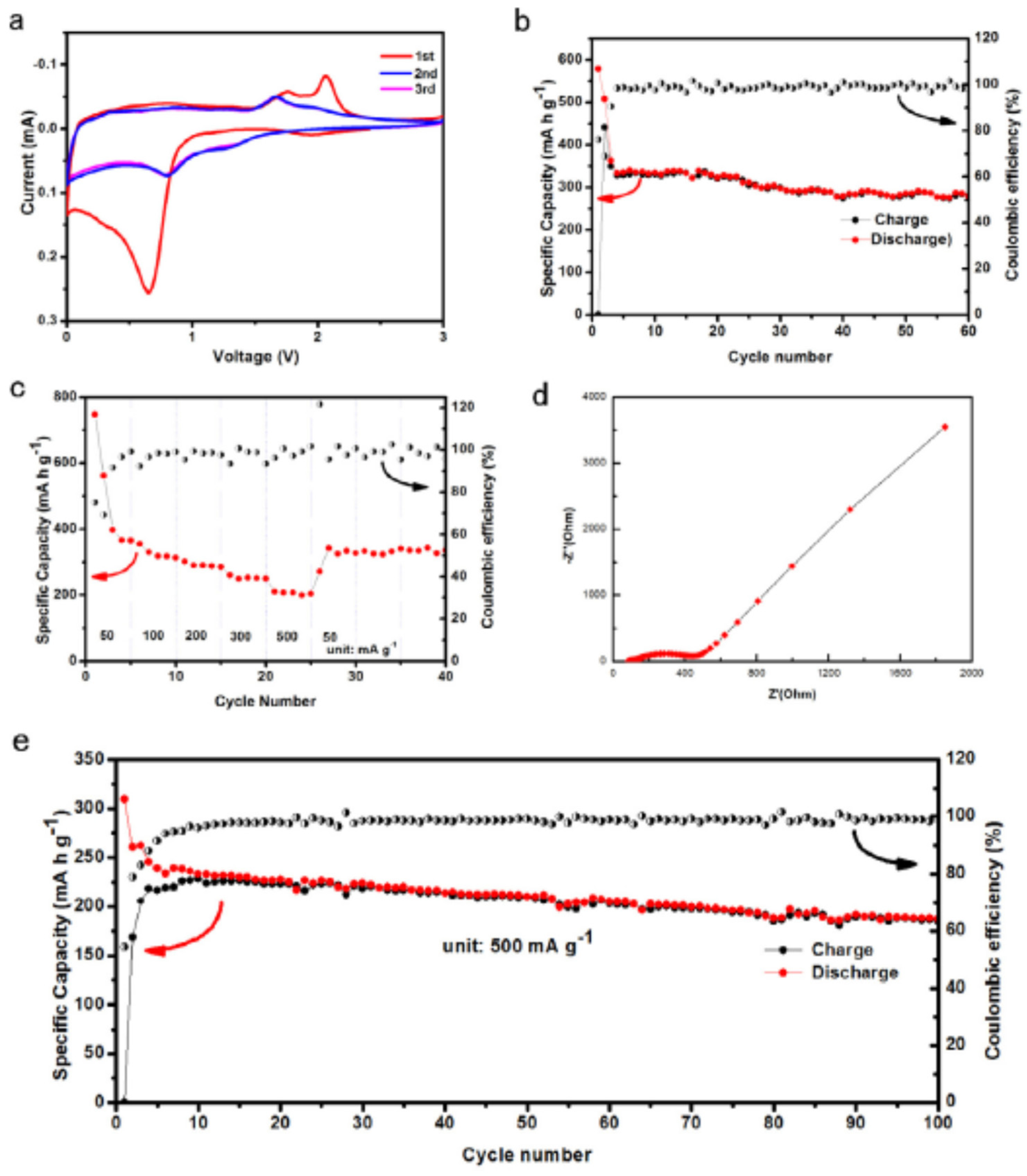
3.1. MOFs in LIBs
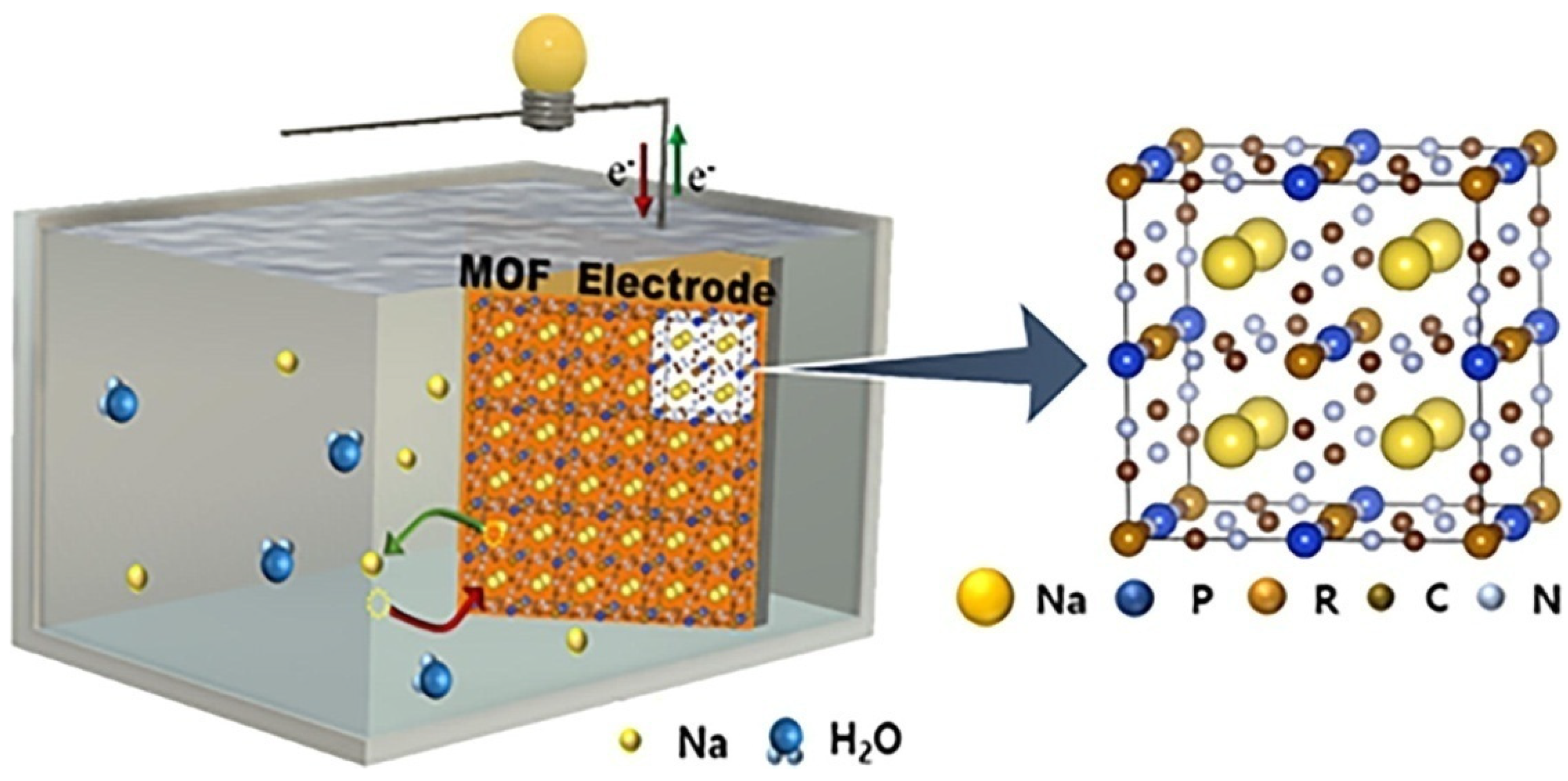
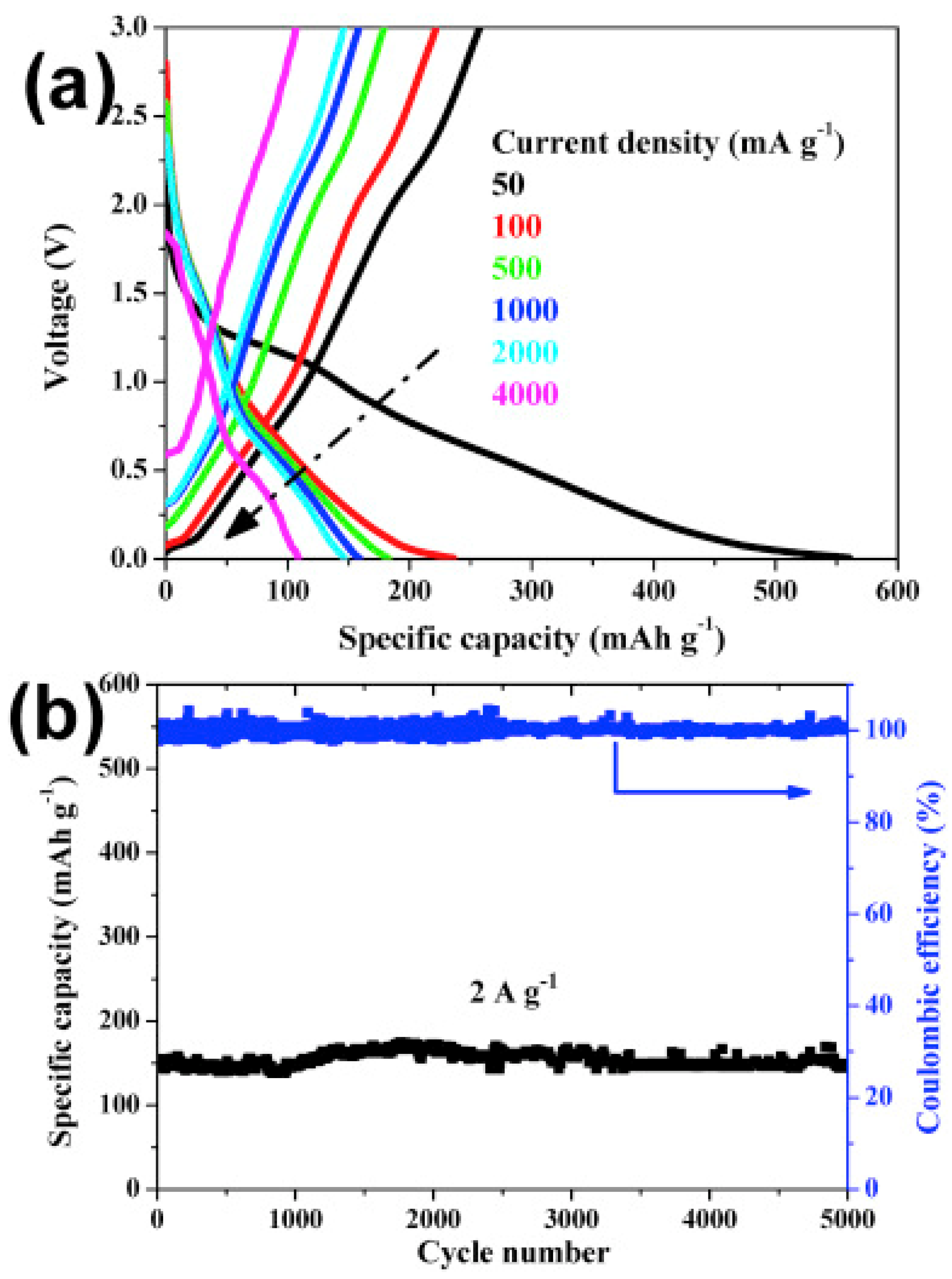
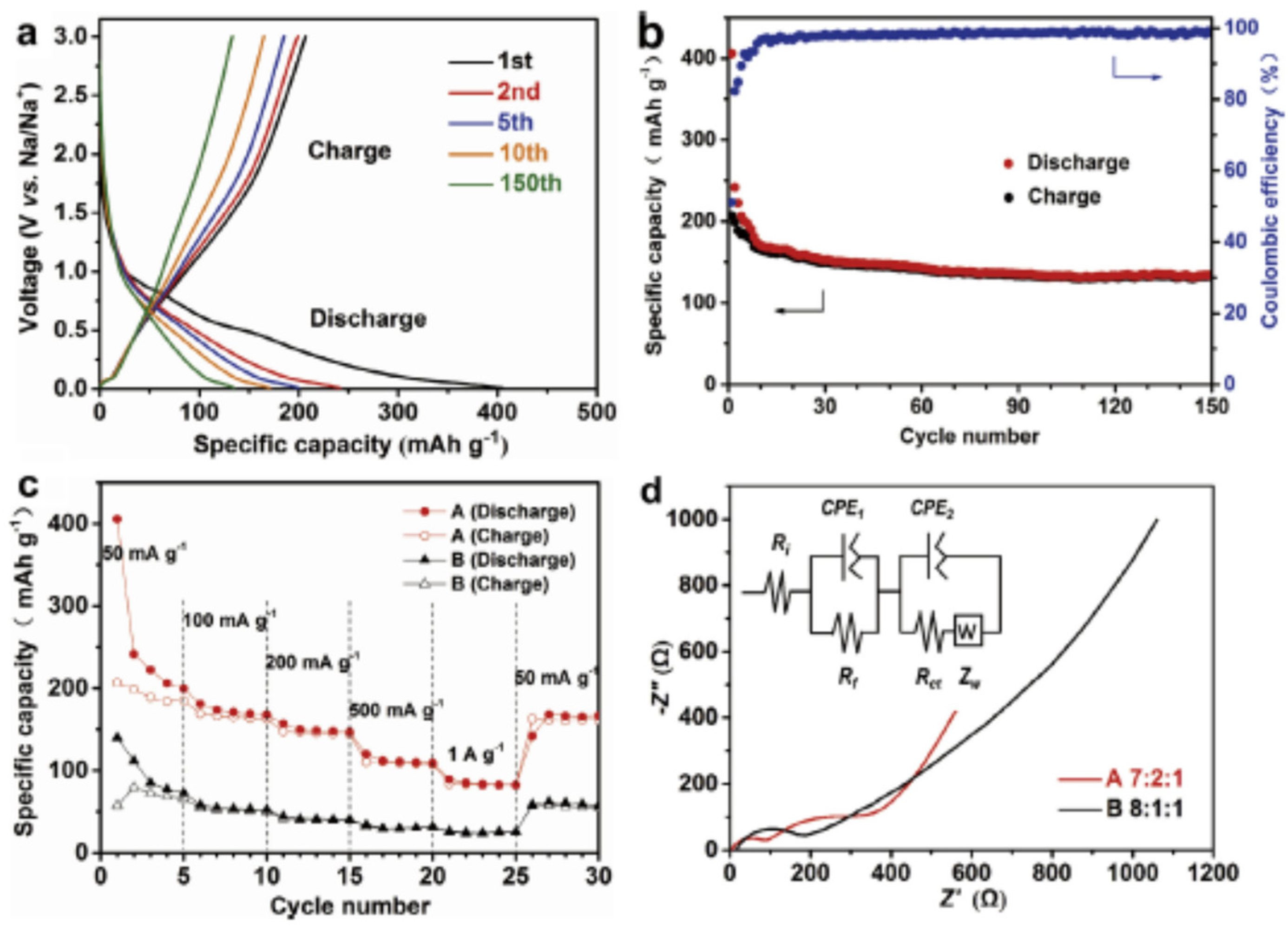
3.2. MOFs in Sodium Ion Batteries (SIBs)
3.3. MOFs in Li–O2Batteries
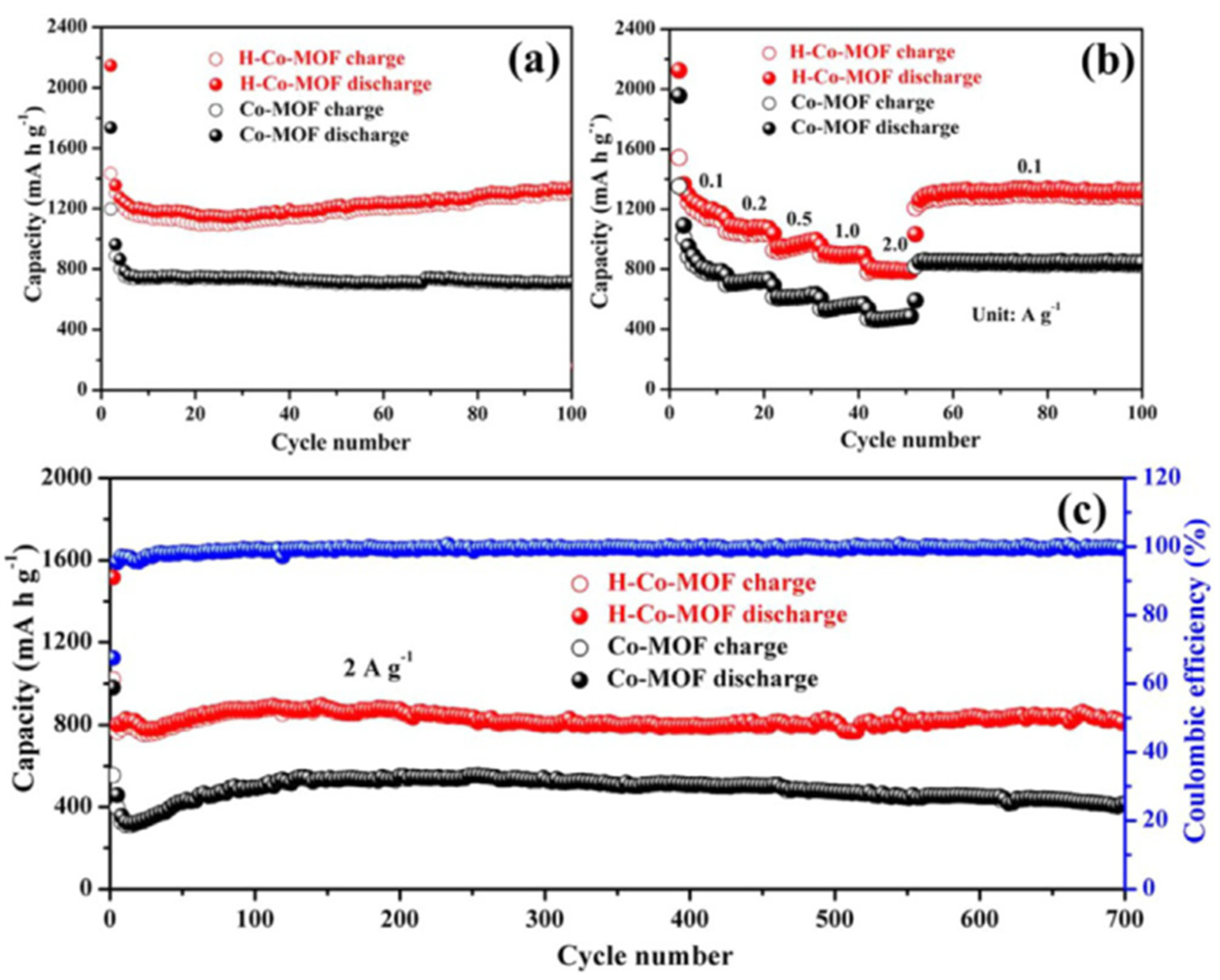
3.4. MOFs in Li–S Batteries
3.5. MOFs in Li–Se Batteries
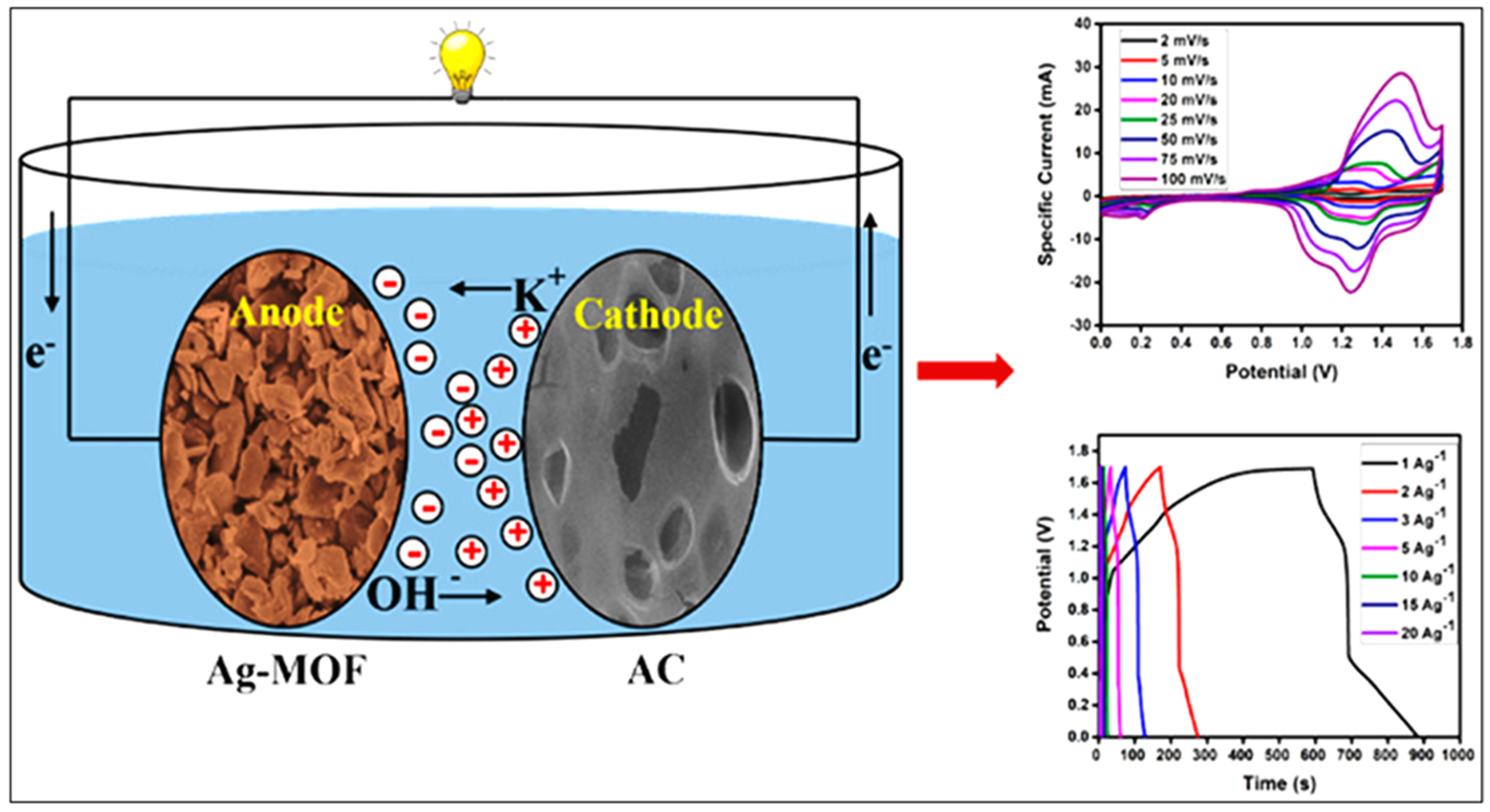
3.6. MOFs in Super Capacitors (SCs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOFs | Metal–organic frameworks |
| SCs | Super capacitors |
| LSBs | Lithium–sulfur batteries |
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| MIL101 | Metal–organic framework-101 |
| NMOF | Nanoscale metal–organic frameworks |
| Cr-BDC | Chromium-benzenedicarboxylate |
| IRMOF | Isoreticular metal framework |
| MTBS | Methyltributylammonium methyl sulfate |
| LAG | Liquid assisted grinding |
| DMF | Dimethylformamide |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| MIL-53, MIL-68, MIL-125, UiO-66, ZIF | Metal–organic frameworks |
| LIBs | Lithium-ion batteries |
| SIBs | Sulfur-ion batteries |
| ZnO | Zinc oxide |
| Li–S/Se | Lithium–sulfur/selenium batteries |
| H–Co–MOF | Co-based metal organic framework |
| EIS spectra | Electrochemical impedance spectroscopy spectra |
| SEI layer | Solid electrolyte interface layer |
| CV | Cyclic voltammetry |
| Ni–MOF | Nickel metal–organic frameworks |
| ZIF-8 | 2-Methylimidazole zinc salt |
| ZIF-C | Microporous carbon |
| CPC | Cube-shaped porous carbon |
| CNTs | Carbon nanotubes |
| Sb@PC | Antimony embedded porous carbon nanocomposite |
| 3DPC | 3D porous carbon |
| Li–O2 | Lithium–oxygen batteries |
| SCs | Super capacitors |
| EDL | Electrochemical double layer |
| EDLC | Electrochemical double-layer capacitance |
| Co8–MOF-5 | Cobalt-based metal organic framework |
| PANI-ZIF-67-CC | Polyaniline–cobalt-based MOF crystals—carbon cloth |
| LEDs | Light emitting diodes |
| FASC | Flexible-solid-state asymmetric supercapacitor |
References
- Heo, D.Y.; Do, H.H.; Ahn, S.H.; Kim, S.Y. Metal-Organic Framework Materials for Perovskite Solar Cells. Polymers 2020, 12, 2061. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 86, 2. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Chen, B.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 Adsorption in a Microporous Metal–Organic Framework with Open Metal Sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef]
- Rosseinsky, M.J. Enlightened pores. Nat. Mater. 2010, 9, 609–610. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Yap, M.H.; Fow, K.L.; Chen, G.Z. Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitorchemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Recent Progress in Supercapacitors: From Materials Design to System Construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef]
- Shi, N.; Xi, B.; Liu, J.; Zhang, Z.; Song, N.; Chen, W.; Feng, J.; Xiong, S. Dual-Functional NbN Ultrafine Nanocrystals Enabling Kinetically Boosted Lithium–Sulfur Batteries. Adv. Func. Mater. 2022, 32, 2111586. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Wang, H.; Nie, Y.; Yan, J. Porous Fe2O3 Nanoparticles as Lithium-Ion Battery Anode Materials. ACS Appl. Nano Mater. 2021, 4, 8744–8752. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Zhang, S.; Wang, J.; Huang, Q.; Yu, N.; Zhu, Y.; Fu, L.; Wang, F.; Chen, Y.; et al. Three-dimensional ordered porous electrode materials for electrochemical energy storage. NPG Asia Mater. 2011, 11, 12. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Jhung, S.H.; Lee, J.H.; Chang, J.S. Microwave synthesis of a nanoporous hybrid material, chromium trimesate. Bull. Kor. Chem. Soc. 2005, 26, 880–881. [Google Scholar]
- Taylor-Pashow, K.M.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J. Amer. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef]
- Khan, N.A.; Kang, I.J.; Seok, H.Y.; Jhung, S.H. Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem. Eng. J. 2011, 166, 1152–1157. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Gref, R. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. mat. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Qiu, L.G.; Li, Z.Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines. Chem. Comm. 2008, 31, 3642–3644. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.; Schulze, S.; Hietschold, M.; Mehring, M. Evaluation of synthetic methods for microporous metal-organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H 2O)3] and [Cu2(btc)(OH)(H2O)]. Micropo. Mesop. Mat. 2010, 132, 121–127. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal-organic frameworks-prospective industrial applications. J. Mat. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned growth of metal-organic framework coatings by electrochemical synthesis. Chem. Mat. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Ameloot, R.; Pandey, L.; Vander Auweraer, M.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned film growth of metal-organic frameworks based on galvanic displacement. Chem. Comm. 2010, 46, 3735–3737. [Google Scholar] [CrossRef]
- Friščić, T.; Fábián, L. Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG). Cryst. Eng. Comm. 2009, 11, 743–745. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Liu, M.; Bai, Y.; Chen, J.; Liu., Y. Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes. Angew. Chem. Int. Ed. 2020, 59, 1–6. [Google Scholar]
- Yuan, W.; Friščić, T.; Apperley, D.; James, S.L. High reactivity of metal–organic frameworks under grinding conditions: Parallels with organic molecular materials. AngewandteChemie Inter. Ed. 2010, 49, 3916–3919. [Google Scholar] [CrossRef]
- Giowniak, S.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Friscic, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ionandliquid-assisted grinding: Improved mechanochemical synthesis of metal-organicframeworks reveal salt inclusion and anion templating. Angew. Chem. 2010, 122, 724–727. [Google Scholar] [CrossRef]
- Loiseau, T.; Lecroq, L.; Volkringer, C.; Marrot, J.; Ferey, G.; Haouas, M.; Latroche, M. MIL-96, a porous aluminum trimesate 3D structure constructed from a hexagonalnetwork of 18-membered rings and μ 3 -oxo-centered trinuclear units. J. Amer. Chem. Soc. 2006, 128, 10223–10230. [Google Scholar] [CrossRef] [PubMed]
- Volkringer, C.; Loiseau, T. A new indium metal-organic 3D framework with 1,3,5-benzenetricarboxylate, MIL-96 (In), containing μ3-oxo-centered trinuclear units and a hexagonal 18-ring network. Mat.Res.Bull. 2006, 41, 948–954. [Google Scholar] [CrossRef]
- Volkringer, C.; Loiseau, T.; Férey, G.; Morais, C.M.; Taulelle, F.; Montouillout, V.; Massiot, D. Synthesis, crystal structure and 71Ga solid state NMR of a MOF-type gallium trimesate (MIL-96) with μ3-oxo bridged trinuclear units and a hexagonal 18-ring network. MicropoMesopo Mat. 2007, 105, 111–117. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cheng, A.L.; Yue, Q.; Sun, W.W.; Gao, E.Q. Eight coordination with bis(bidentate) bridging ligands: Zeolitic topology versus square grid networks. Chem. Comm. 2008, 7, 847–849. [Google Scholar] [CrossRef]
- Dong, B.X.; Gu, X.J.; Xu, Q. Solvent effect on the construction of two microporous yttrium-organic frameworks with high thermostability via in situ ligand hydrolysis. Dalton Trans. 2010, 39, 5683–5687. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Thornton, A.W.; Hill, M.R.; Stride, J.A. A flexible copper based microporous metal-organic framework displaying selective adsorption of hydrogen over nitrogen. Dalton Trans. 2011, 40, 3398–3401. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Férey, G.; Stock, N. High-throughput assisted rationalization of the formation of metal organic frameworks in the iron (III) aminoterephthalate solvothermal system. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]
- Ahnfeldt, T.; Guillou, N.; Gunzelmann, D.; Margiolaki, I.; Loiseau, T.; Ferey, G.; Stock, N. [Al4 (OH)2 (OCH3)4 (H2N-bdc)3]⋅xH2O: A 12-Connected Porous Metal-OrganicFramework with an Unprecedented Aluminum-Containing Brick. Angew. Chem. Intern. Edi. 2009, 48, 5163–5166. [Google Scholar] [CrossRef]
- Long, P.; Wu, H.; Zhao, Q.; Wang, Y.; Dong, J.; Li, J. Solvent effect on the synthesis of MIL-96 (Cr) and MIL-100 (Cr). Micropo. Mesopo. Mat. 2011, 142, 489–493. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.-G.; Yang, Z.; Lemmon, P.J.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Jie, X.; Xia, G.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2013, 23, 929–946. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Kubota, K.; Komaba, S. Practical issues and future perspective for Na-ion batteries. J.Electrochem. Soc. 2015, 162, A2538–A2550. [Google Scholar] [CrossRef]
- Nithya, C.; Gopukumar, S. Sodium ion batteries: A newer electrochemical storage. WIREs Energy Environ. 2015, 4, 253–278. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Singh, G.; Rojo, T. A comprehensive review of sodium layered oxides: Powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015, 8, 81–102. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar]
- Reinsch, H.; van der Veen, M.A.; Gil, B.; Marszalek, B.; Verbiest, T.; de Vos, D.; Stock, N. Structures, Sorption Characteristics, and Nonlinear Optical Properties of a New Series of Highly Stable Aluminum MOFs. Chem. Mater. 2013, 25, 17–26. [Google Scholar] [CrossRef]
- Canivet, J.; Bonnefoy, J.; Daniel, C.; Legrand, A.; Coasne, B.; Farrusseng, D. Structure–property relationships of water adsorption in metal–organic frameworks. New J. Chem. 2014, 38, 3102–3111. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Thomas, K.M.; Rosseinsky, M.J. Flexibility in metal-organic framework materials: Impact on sorption properties. J. Solid State Chem. 2005, 178, 2491–2510. [Google Scholar] [CrossRef]
- Canivet, J.; Bonnefoy, J.; Daniel, C.; Legrand, A.; Coasne, B.; Farrusseng, D. Metal–organic framework structure–property relationships for high-performance multifunctional polymer nanocomposite applications. J. Mater. Chem. A 2021, 9, 4348–4378. [Google Scholar]
- Eddaudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hendon, C.H.; Minie, M.A.; Walsh, A.; Dincă, M. Million-Fold Electrical Conductivity Enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E = S, O). J. Am. Chem. Soc. 2015, 137, 6164–6167. [Google Scholar] [CrossRef]
- Kim, S.; Joarder, B.; Hurd, J.A.; Zhang, J.; Dawson, K.W.; Gelfand, B.S.; Wong, N.E.; Shimizu, G.K.H. Achieving Superprotonic Conduction in Metal–Organic Frameworks through Iterative Design Advances. J. Am. Chem. Soc. 2018, 140, 1077–1082. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Fan, X.; Chen, Y.; Han, R.; Li, W.; Zheng, J.; Wang, B.; Li, X. Rational design of a metal–organic framework host for sulfur storage in fast, long-cycle Li–S batteries. Energy Environ. Sci. 2014, 7, 2715–2724. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Ferey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Greneche, J.-M.; Tarascon, J.-M. Mixed-valence li/fe-based metal-organic frameworks with both reversible redox and sorption properties. Angew. Chem. Int. Ed. 2007, 46, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Wang, J.; Chen, C.; Wei, M. Hierarchical Cobalt-Based Metal-Organic Framework for High-Performance Lithium-Ion Batteries. Chem.-A Eurpean J. 2018, 24, 13362–13367. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Huang, Y.; Zhu, H.; Zhang, B.; Zhu, H.; Shen, S.; Tan, G.; Wu, F.; He, H.; Lan, S.; et al. Recent progress on MOF-derived carbon materials forenergy storage. Carbon Energy 2020, 2, 176–202. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Lu, S.; Wang, H.; Zhou, J.; Wu, X.; Qin, W. Atomic layer deposition of ZnO on carbon black as nanostructured anode materials for high-performance lithium-ion batteries. Nanoscale 2017, 9, 1184–1192. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wu, J.; Fu, Y.; Zhou, R.; Chen, S.; Wang, L. Hollow metal organic frameworks-derived porous ZnO/C nanocages as anode materials for lithium-ion batteries. J. Alloy. Compd. 2017, 694, 1246–1253. [Google Scholar] [CrossRef]
- Huang, P.-B.; Tian, L.-Y.; Zhang, Y.-H.; Shi, F.-N. Facile synthesis of polymetallic Li-MOFs and their synergistic mechanism of lithium storage. Inorg. Chim. Acta 2021, 525, 120473. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Wang, Y.; Zhang, L.; Zheng, G. A flexible ligand-based wavy layered metal–organic framework for lithium-ion storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef]
- Park, G.D.; Kang, Y.C. One-Pot Synthesis of CoSex–rGO Composite Powders by Spray Pyrolysis and Their Application as Anode Material for Sodium-Ion Batteries. Chem. Eur. J. 2016, 22, 4140–4146. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Ding, L.; Zhou, Z.; Wang, Y.; Niu, S.; Liang, S.; Cao, G. Nitrogen-Doped Yolk-Shell-Structured CoSe/C Dodecahedra for High-Performance Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3624–3633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, X.; Gao, H.; Dolocan, A.; Goodenough, J.B. Elevating Energy Density for Sodium-Ion Batteries through Multielectron Reactions. Nano Lett. 2021, 21, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Jaffer, S.; Yu, H. Transition metal oxides for sodium-ion batteries. Energy Storage Materials 2016, 5, 116–131. [Google Scholar] [CrossRef]
- Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Environ. Sci. 2011, 4, 3342–3345. [Google Scholar] [CrossRef]
- Tran, V.A.; Do, H.H.; Ha, T.D.C.; Ahn, S.H.; Kim, M.-G.; Kim, Y.S.; Lee, S.-W. Metal-organic framework for lithium and sodium-ion batteries: Progress and perspectivez. Fuel 2022, 319, 123856. [Google Scholar] [CrossRef]
- Zhu, K.J.; Liu, G.; Wang, Y.J.; Liu, J.; Li, S.T.; Yang, L.Y.; Liu, S.L.; Wang, H.; Xie, T. Metal-Organic Frameworks derived novel hierarchical durian-like nickel sulfide (NiS2) as an anode material for high-performance sodium-ion batteries. Mater. Lett. 2017, 197, 180–183. [Google Scholar] [CrossRef]
- Qu, Q.; Yun, J.; Wan, Z.; Zheng, H.; Gao, T.; Shen, M.; Shao, J.; Zheng, H. MOF-derived microporous carbon as a better choice for Na-ion batteries than mesoporous CMK-3. RSC Adv. 2014, 4, 64692–64697. [Google Scholar] [CrossRef]
- Choi, D.; Lim, S.; Han, D. Advanced metal–organic frameworks for aqueous sodium-ion rechargeable batteries. J. Energy Chem. 2021, 53, 396–406. [Google Scholar] [CrossRef]
- Zou, G.; Jia, X.; Huang, Z.; Li, S.; Liao, H.; Hou, H.; Huang, L.; Ji, X. Cube-shaped Porous Carbon Derived from MOF-5 as Advanced Material for Sodium-Ion Batteries. Electro. Chim. Acta 2016, 196, 413–421. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.; Pang, G.; Zhu, Y.; Xu, G.; Qing, Y.; Dou, H.; Zhang, X. Flexible metal–organic frameworks as superior cathodes for rechargeable sodium-ion batteries. J. Mater. Chem. A 2015, 3, 16590–16597. [Google Scholar] [CrossRef]
- Yang, G.; Luo, X.-X.; Liu, Y.; Li, K.; Wu, X.-L. [Co3(μ3-O)]-Based Metal-Organic Frameworks as Advanced Anode Materials in K- and Na-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 46902–46908. [Google Scholar] [CrossRef]
- Dong, C.; Xu, L. Cobalt- and Cadmium-Based Metal-Organic Frameworks as High-Performance Anodes for Sodium Ion Batteries and Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, M.; Zhu, G.; Li, D.; Yan, D.; Lu, T.; Pan, L. Porous cake-like TiO2 derived from metal-organic frameworks as superior anode material for sodium ion batteries. Ceram. Int. 2017, 43, 2398–2402. [Google Scholar] [CrossRef]
- Yang, H.; Kruger, P.E.; Telfer, S.G. Metal–Organic Framework Nanocrystals as Sacrificial Templates for Hollow and Exceptionally Porous Titania and Composite Materials. Inorg. Chem. 2015, 54, 9483–9490. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Peng, J.; Zhang, W.; Liang, Z.; Wu, J.; Feng, J.; Li, H.; Huang, S. Metal–Organic Framework Derived Ultrafine Sb@Porous Carbon Octahedron via In Situ Substitution for High-Performance Sodium-Ion Batteries. ACS Nano 2021, 15, 15104–15113. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, G.; Zhang, X.; Hu, T. Porous carbon materials derived from in situ construction of metal-organic frameworks for high-performance sodium ions batteries. Microporous Mesoporous Mater. 2019, 273, 156–162. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Y.; Yuan, C.; Wang, C. MgFe2O4 hollow microboxes derived from metal-organic-frameworks as anode material for sodium-ion batteries. Mater. Lett. 2017, 199, 101–104. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Yuan, N.; Sun, W.; Yang, J.; Gong, X.; Liu, R. Multifunctional MOF-Based Separator Materials for Advanced Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2021, 8, 2001941. [Google Scholar] [CrossRef]
- Tian, H.; Tian, H.; Wang, S.; Chen, S.; Zhang, F.; Song, L.; Liu, H.; Liu, J.; Wang, G. High-power lithium–selenium batteries enabled by atomic cobalt electrocatalyst in hollow carbon cathode. Nat. Commun. 2020, 11, 5025. [Google Scholar] [CrossRef]
- Choi, K.M.; Jeong, H.M.; Park, J.H.; Zhang, Y.B.; Kang, J.K.; Yaghi, O.M. Supercapacitors of Nanocrystalline Metal–Organic Frameworks. ACS Nano 2014, 7, 7451−7457. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Horn, Y.S.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.R.; Kamachi, Y.; Torad, N.L.; Hwang, S.M.; Sun, Z.; Dou, S.X.; Kim, J.H.; Yamauchi, Y. Fabrication of symmetric supercapacitors based on MOF-derived nanoporous carbons. J. Mater. Chem. A 2014, 2, 19848–19854. [Google Scholar] [CrossRef]
- Shalini, S.S.; Balamurugan, R.; Velmathi, S.; Bose, A.C. Systematic Investigation on the Electrochemical Performance of Pristine Silver Metal–Organic Framework as the Efficient Electrode Material for Supercapacitor Application. Energy Fuels 2022, 36, 7104–7114. [Google Scholar] [CrossRef]
- Jiao, Y.; Pei, J.; Chen, D.; Yan, C.; Hu, Y.; Zhang, Q.; Chen, G.J. Mixed-metallic MOF based electrode materials for high performance hybrid supercapacitors. Mater. Chem. A 2017, 5, 1094–1102. [Google Scholar] [CrossRef]
- He, D.; Gao, Y.; Yao, Y.; Wu, L.; Zhang, J.; Huang, Z.H.; Wang, M.X. Asymmetric Supercapacitors Based on Hierarchically Nanoporous Carbon and ZnCo2O4 From a Single Biometallic Metal-Organic Frameworks (Zn/Co-MOF). Front. Chem. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Díaz, R.; Orcajo, M.G.; Botas, J.A.; Calleja, G.; Palma, J. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett. 2012, 68, 126–128. [Google Scholar] [CrossRef]
- Wang, L.; Feang, X.; Ren, L.; Piao, Q.; Wang, Y.; Li, H.; Wang, B. Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Lai, Z.; Li, X.; Sun, S.; Zhang, H.; Cheetam, A.K.; Wang, J. Cobalt oxide and N-doped carbon nanosheets derived from a single two-dimensional metal–organic framework precursor and their application in flexible asymmetric supercapacitors. NanoscaleHoriz 2017, 2, 99–105. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Li, S.; Ke, Q.; Li, Z.; Zhou, M. Metal-organic framework composites for energy conversion and storage. J. Semicond. 2020, 41, 091707. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Yu, L.; Liu, X.; Yang, J.; Wai, M. A new promising Ni-MOF superstructure for high-performance supercapacitors. Chem. Commun. 2020, 56, 1803–1806. [Google Scholar]
- Yue, L.; Guo, H.; Wang, X.; Sun, T.; Liu, H.; Li, Q.; Xu, M.; Yang, Y.; Yang, W. Non-metallic element modified metal-organic frameworks as high-performance electrodes for all-solid-state asymmetric supercapacitors. J. Colloid Interface Sci. 2018, 539, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Shen, L.; Luo, H.; Ding, B.; Xu, G.; Wang, J.; Zhang, X. Prussian blue analogues: A new class of anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 5852. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, S.; Shrestha, N.; Lee, S.; Ahn, H.; Han, S. Unusual energy storage and charge retention in Co-based metal–organicframeworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Gao, Y.; Wu, J.; Tang, B. Facile synthesis and supercapacitive properties of Zr-metal organic frameworks (UiO-66). RSC Adv. 2015, 5, 17601–17605. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, B.; Shi, W.; Yang, J.; Fan, Z.; Luo, Z.; Rui, X.; Chen, B.; Yan, Q.; Zhang, H. Reduced Graphene Oxide-Wrapped MoO3Composites Prepared by Using Metal-Organic Frameworks as Precursor for All-Solid-State Flexible Supercapacitors. Adv. Mater. 2015, 27, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Hsu, W.H. Nickel nanoparticles embedded in partially graphitic porous carbon fabricated by direct carbonization of nickel-organic framework for high-performance supercapacitors. J. Power Sources 2015, 274, 1055–1062. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, L.; Zhang, L.; Zhang, X. Solid-state thermolysis preparation of Co3O4 nano/micro superstructures from metal-organic framework for supercapacitors. Int. J. Electrochem. Sci. 2011, 6, 2943–2954. [Google Scholar]
- Feng, C.; Lv, C.P.; Zhao, H.; Li, Z.Q.; Xie, W.N.; Sun, L.N.; Wang, Y. Structural Elucidation and Supercapacitive Performance on a Mn(II)-Based MOF. Cryst. Growth Des. 2020, 20, 5682–5687. [Google Scholar] [CrossRef]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of Hierarchical Porous Nickel Based Metal-Organic Framework (Ni-MOF) Constructed with Nanosheets as Novel Pseudo-Capacitive Material for Asymmetric Supercapacitor. J. Colloid Interf. Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khan, I.A.; Choucair, M.; Badshah, A.; Khan, I.; Nadeem, M.A. A novel Cr2O3-carbon composite as a high performance pseudo-capacitor electrode material. Electrochimica Acta 2015, 171, 142–149. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, S.; Shen, N.; Li, D.; Li, X. MOF-Derived Porous NiO Nanoparticle Architecture for High Performance Supercapacitors. Mater. Lett. 2017, 188, 1–4. [Google Scholar] [CrossRef]
- Meng, W.; Chen, W.; Zhao, L.; Huang, Y.; Zhu, M.; Huang, Y.; Fu, Y.; Geng, F.; Yu, J.; Chen, X.; et al. Porous Fe3O4/Carbon Composite Electrode Material Prepared from Metal-Organic Framework Template and Effect of Temperature on Its Capacitance. Nano Energy 2014, 8, 133–140. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like Mesoporous α-Fe2O3 Anode Material Prepared from MOF Template for High-Rate Lithium Batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Srot, V.; Chen, S.; Zhang, M.; Aken, P.A.; Wang, Y.; Maier, J.; Yu, Y. Metal–Organic Framework-Derived Nanoconfinements of CoF2 and Mixed-Conducting Wiring for High-Performance Metal Fluoride-Lithium Battery. ACS Nano 2021, 15, 1509–1518. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Z.; Qiu, Y.; Sheng, X. Superstructure MOF as a framework to composite MoS2 with rGO for Li/Na-ion battery storage with high-performance and stability. Dalton Trans. 2022, 51, 3472–3484. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, N.; Dong, M.; Jia, P.; Ma, C.; Zhang, T.; Jiao, T. MOF-Derived MnO/C Nanocomposites for High-Performance Supercapacitors. Nanomaterials 2022, 12, 4257. [Google Scholar] [CrossRef]
- Ye, C.; Quin, Q.; Liu, J.; Mao, W.; Yan, J.; Wu, Y. Coordination derived stable Ni–Co MOFs for foldable all-solid-state supercapacitors with high specific energy. J. Mater. Chem. A 2019, 7, 4998–5008. [Google Scholar] [CrossRef]
- Yu, J.; Cai, D.; Si, J.; Zhang, H.; Wang, Q. MOF-derived NiCo2S4 and carbon hybrid hollow spheres compactly concatenated by electrospun carbon nanofibers as self-standing electrodes for aqueous alkaline Zn batteries. J. Mater. Chem. A 2022, 10, 4100–4109. [Google Scholar] [CrossRef]
- Haroon, H.; Wahid, M.; Majid, K. Structure-Activity Relationships of a Ni-MOF, a Ni-MOF-rGO, and pyrolyzed Ni/C@rGO Structures for Sodium- ion Batteries. Chem. Sel. 2022, 7, e202202011. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Zhang, X.; Huang, T.; Yu, A. Mesoporous Co–CoO@NC Micro-Disk Derived from ZIF-9 as Bifunctional Catalyst for Lithium-Oxygen Batteries. Chem. Sel. 2018, 3, 9276–9283. [Google Scholar] [CrossRef]



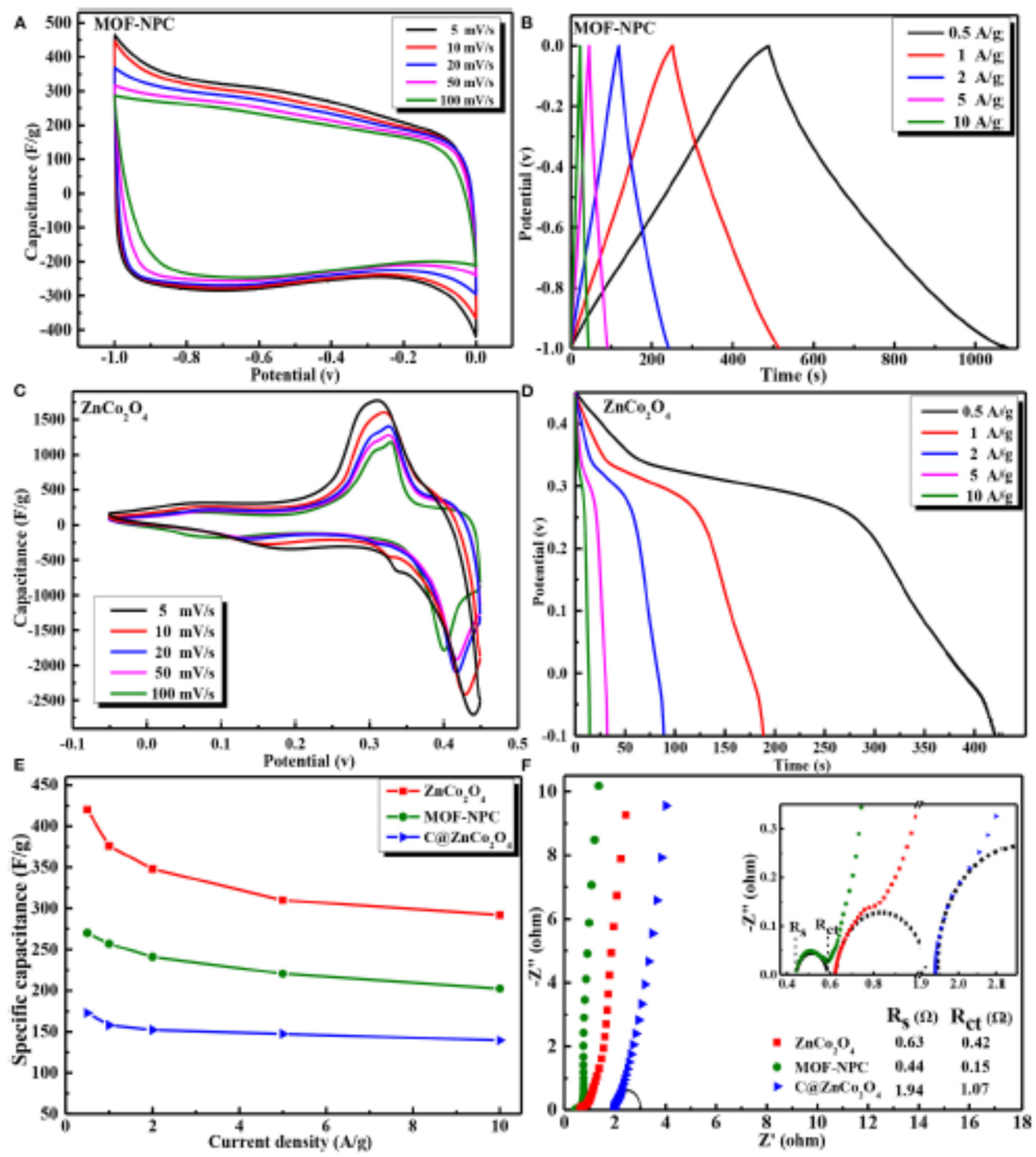
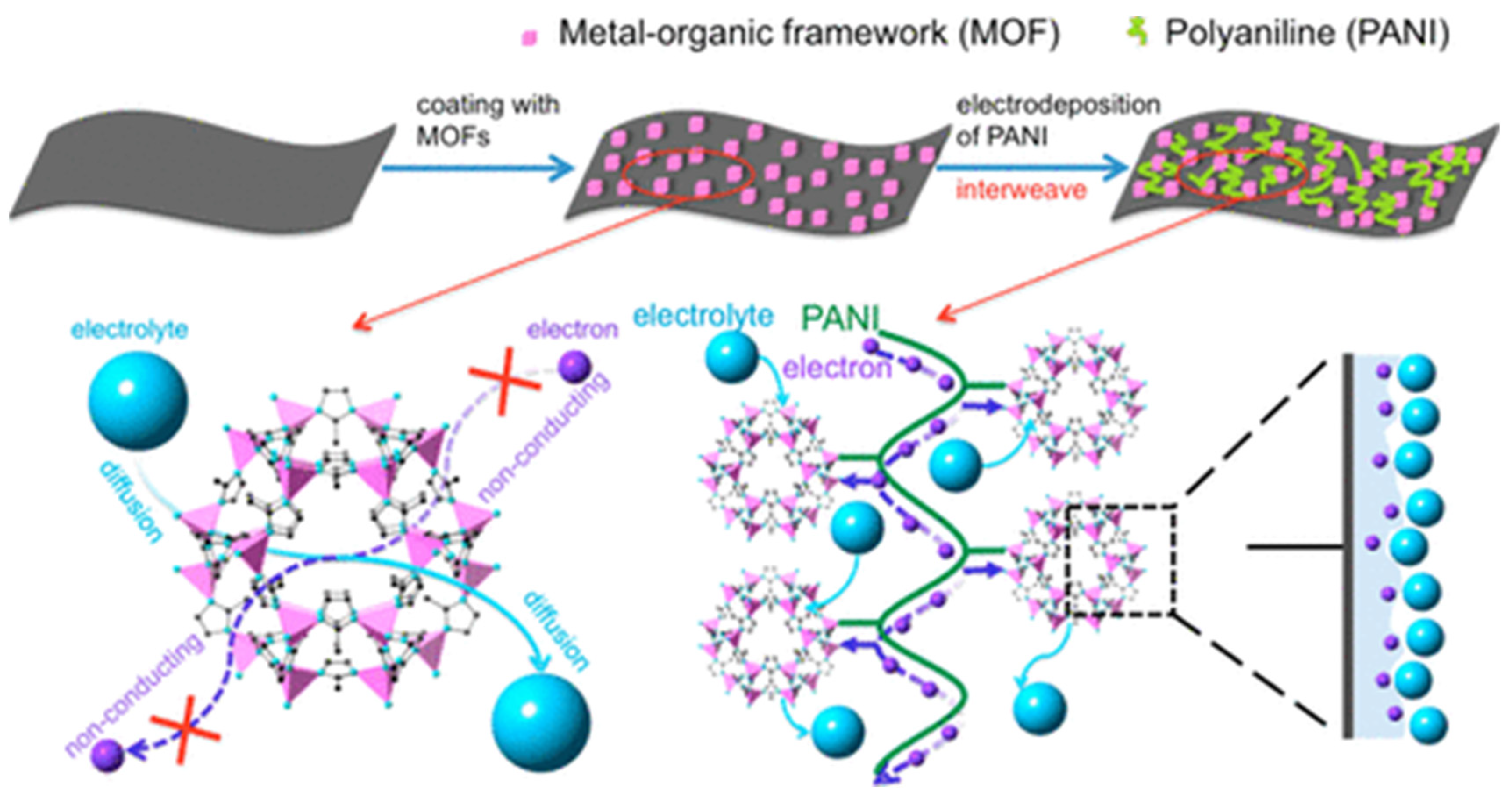


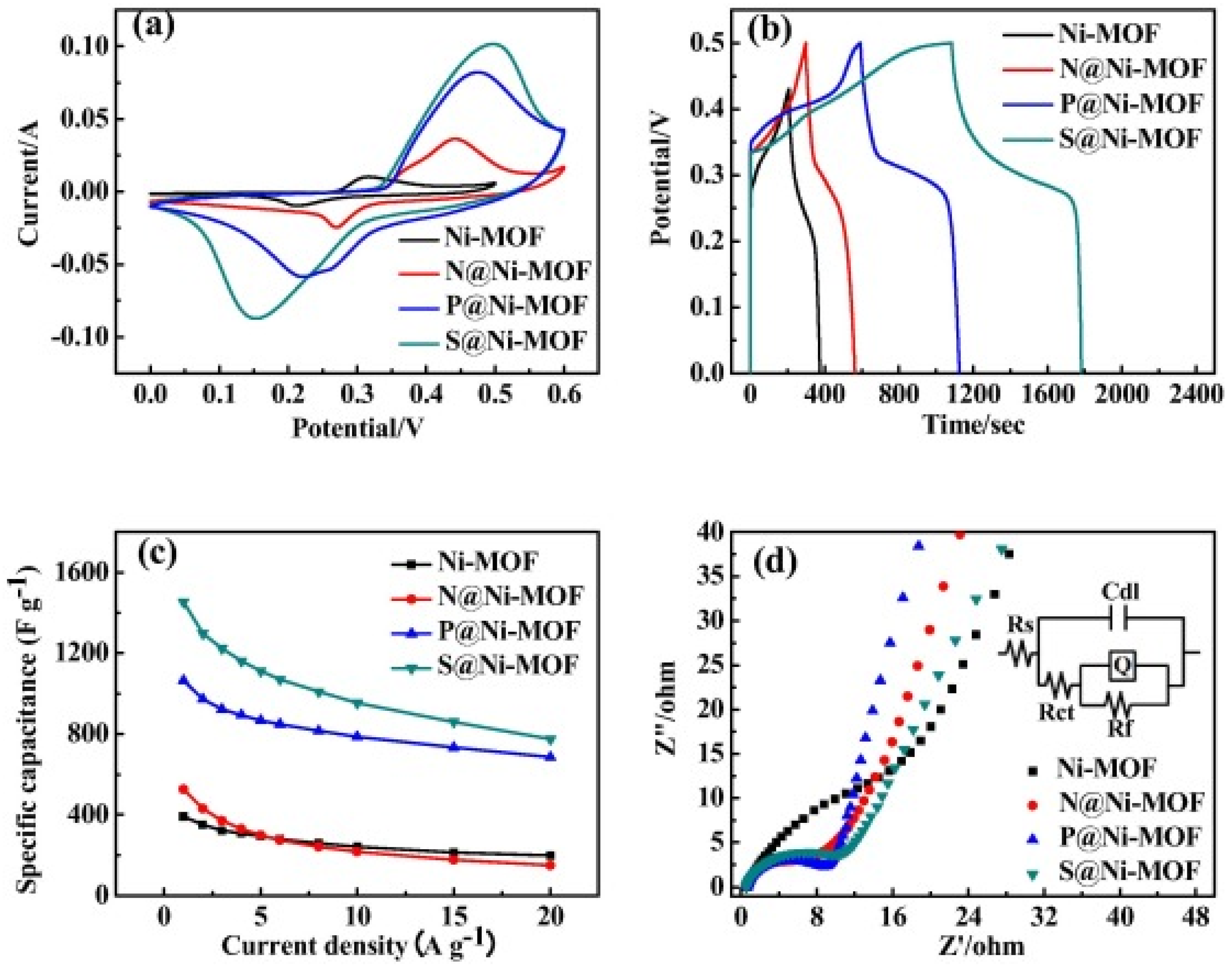
| MOF Type | Current Density/Scan Rate | Cycle Number/Electrolyte | Capacities | Ref |
|---|---|---|---|---|
| Co3[Co(CN)6]2 | 20 mA g−1 | 5 | 304 (mAh g−1) | [65] |
| MOF-177(Zn) | 50 mA g−1 | - | - | [65] |
| Co–MOF | 0.6 A g −1 | 1 M LiOH | 206.76 F g−1 | [105] |
| Zr–MOF4 | 5 mV s −1 | 6 M KOH | 207 F g−1 | [106] |
| Mo MOF-derived MoO3/rGO | 1 A g−1 | PVA−H2SO4 | 617 F g−1 | [107] |
| Ni MOF-derived nanoparticles/graphene | 1 A g−1 | 1MH2SO4 | 886 F g−1 | [108] |
| Co MOF-derived Co3O4 nano/microsuperstructures | 1 A g−1 | 6 M KOH | 208 F g−1 | [109] |
| Mn–MOF | 1 A g−1 | 6 M KOH | 443 F g−1 | [110] |
| Ni–MOF | 1 A g−1 | 3 M KOH | 1057.2 Fg−1 | [111] |
| MIL-101 | 0.25 A g−1 | 291 F g−1 | [112] | |
| Ni–MOF | 10 | 6 M KOH | 244 | [113] |
| Fe-MIL-88B NH2 | 5 | 1 M KOH | 74 | [114] |
| Porous α-Fe2O3–MOF | 50 cycles at a rate of 0.2 C | 1.1 M LiPF6in ethylene carbonate/diethylene carbonate | 424 mAh g–1 | [115] |
| Metal–organic framework-derived nanoconfinements of CoF2 | cyclic stability over 400 cycles. | modified LiPF6/FEC/EMC electrolyte | 500 mAh g–1 | [116] |
| Fe7S8–C/ZnS–C@MoS2/rGO | 5 A g−1 | 1598.3 mA h g−1 | [117] | |
| MOF-derived MnO/C nanocomposites | 0.5 A g−1 | 1 mol L−1 Na2SO4 solution | 421 F g−1 | [118] |
| Ni–Co MOFs | 0.5 A g−1 | KOH electrolyte | 172.7 Fg−1 | [119] |
| MOF-derived NiCo2S4 and carbon hybrid hollow spheres | 3.8 A g−1 | 3 M KOH and 0.02 M Zn(CH3COO)2 aqueous solution | 343.1 mAh g−1 | [120] |
| Ni–MOF–rGO | 1|A g−1 | 272|mA g−1 | [121] | |
| Co–CoO@NC/ZIF-9 | 0.05 mA cm−2 | 42 cycles | (500 mAh g−1) | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140-166. https://doi.org/10.3390/cleantechnol5010009
Dutt S, Kumar A, Singh S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technologies. 2023; 5(1):140-166. https://doi.org/10.3390/cleantechnol5010009
Chicago/Turabian StyleDutt, Sunil, Ashwani Kumar, and Shivendra Singh. 2023. "Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications" Clean Technologies 5, no. 1: 140-166. https://doi.org/10.3390/cleantechnol5010009
APA StyleDutt, S., Kumar, A., & Singh, S. (2023). Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technologies, 5(1), 140-166. https://doi.org/10.3390/cleantechnol5010009







