1. Introduction
The leather industry is a very old manufacturing sector that produces leather hides and goods all around the world with an export value exceeding
$5 billion annually. The most popular products are shoes, bags, clothing, upholstery, and so on. The leather trade value is estimated to be worth USD 70 billion per year, with an annual production of around 18 billion square feet of leather [
1].
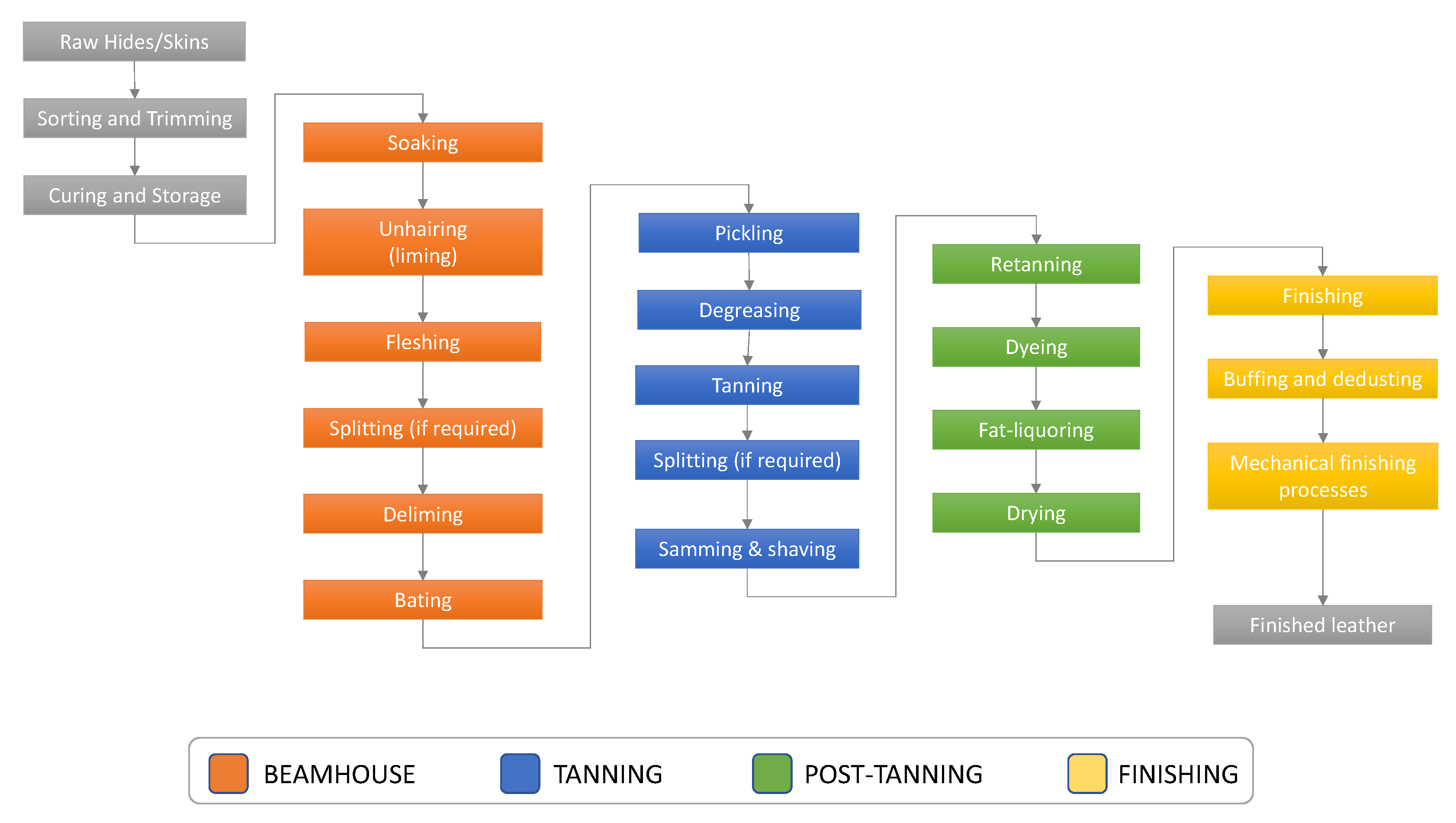
Leather tanning is defined as a process in which raw hides are converted into non-putrescible leathers with definite physical, chemical, and biological properties. This is a multistep process, involving both chemical and mechanical treatments [
2]. Chemical treatments include beamhouse, tanning and post-tanning operations (referred to as wet processes) and are typically performed in processing vessels such as drums. Following post-tanning, leather is subjected to dry finishing operations (
Figure 1).
The wastewaters generated from this process are highly loaded with hazardous chemicals including acids, alkalis, chromium salts, natural or synthetic tanning agents, solvents, auxiliaries, surfactants, dyes, sulfonated oils and salts [
3,
4,
5]. The quantity of effluent generated is about 30 L for every kilogram of hide or skin processed [
6]. In many countries, these effluents are subjected to overall legislation for industrial waste discharge rather than specific limits.
Several physical-chemical and biological treatments, including coagulation/flocculation [
7], adsorption [
8] and aerobic or anaerobic biological treatment [
9,
10], have been proposed to reduce the organic and inorganic content of tannery wastewater. These processes are based on end-pipe treatments: indeed, the aqueous streams of each exhausted bath are generally mixed together in balancing tanks before processing. However, conventional wastewater treatment processes are not able to reduce all the polluting parameters to comply with the limits required by regulations [
11]. Chemical oxygen demand (COD), chlorides, sulphates and ammonia often exceed the permissible limits. As a consequence, a great deal of sludge generated from tannery plants cannot be recycled and is forwarded into dumps even though containing primary resources which can be reused in the process.
The high concentration of pollutants with low biodegradability in tannery wastewater represents a serious technological and environmental challenge for the leather industry. In addition, the untreated release of tannery effluents containing chromium, sulfides, organics and other toxic ingredients affects flora and fauna of the ecosystem, increasing health risks of human beings [
12]. Therefore, the implementation of cleaner, economically as well as environmentally, wastewater treatment technologies is a key point for a sustainable development of this sector in agreement with a greening production [
13].
The concept of cleaner technology was introduced into leather manufacture 40 years ago. The underlying principles of this technology can be summarized as follows: (a) prevention is better than reuse, (b) reuse is better than recycling, and (c) recycling is better than disposal [
14].
In agreement with the principles of the zero-discharge approach, the recovery and recycling of primary resources from spent tanning liquor provides a better management of tanning wastewaters since these effluents contain a higher concentration of reusable substances and a lower quantity of unwanted components in comparison with the global effluent. This approach allows simplifying the treatment of the residual effluent producing solid wastes that can be used as by-products.
Pressure-driven membrane operations, such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO), have been shown to be a promising water reuse technology to achieve zero liquid discharge in the leather industry. They do not involve the use of chemicals or thermal energy. Additional advantages over conventional separation methods (i.e., distillation, adsorption, etc.) include no phase change, simplicity of operation, low energy consumption, easy scale up, low weight and space requirements, modularity and the possibility to carry out the separation continuously [
15]. These processes are based on the use of permselective barriers through which solvent fluids with permeable solutes are selectively transported under a hydrostatic pressure applied on the feed side. MF membranes are typically used to separate particles with diameters of 0.1–10 μm from a solvent and from low molecular weight compounds [
16]. UF membranes retain typically dissolved molecules or small particles not larger than 0.1 μm in diameter. They are characterized by the molecular weight cut-off (MWCO), defined as the equivalent molecular weight of the smallest species that exhibit 90% rejection. NF membranes, with pore sizes in the range of 1–10 nm, are characterized by separation capabilities between UF and RO membranes. They are essentially used to fractionate solutes based on cation or anion valence as well as to separate various organic solutes with low molecular weights [
17]. RO membranes are generally used to separate low-molecular weight compounds from a relatively pure solvent. The particles for RO applications ranging between 0.1–1 nm in size and solutes with molecular weight greater than 300 Da are separated [
18,
19].
The filtration capability of pressure-driven membrane processes along with related operating pressures is illustrated in
Figure 2.
The possibility of integrating different membrane operations in the same process or in combination with traditional separation units offers significant advantages in terms of product quality, plant compactness, environmental impact, recovery of high-added-value substances and energy consumption [
20].
Among membrane processes, membrane bioreactors (MBRs) represent technically feasible compact processes for the removal of organic substances from industrial wastewaters with low sludge production rate and reduced consumption of chemicals. They are composite systems containing units for biological degradation and physical filtration (i.e., with MF or UF membranes) for the complete retention of biomass. This leads to a high microbial concentration in the reactor and a highly efficient biological reaction process in the bioreactor with reduced sludge production [
21]. Additional advantages over conventional activated sludge technology include high effluent quality, limited space requirement, enhanced nutrient removal stability, and high filtrate flux with low energy demand [
22].
The present paper provides an overview on the potential and perspectives of membrane-based processes in the field of tanning wastewaters, highlighting developments and research efforts made in the treatment of single exhausted baths of beamhouse, tanning and post-tanning operations as well as in the treatment of global wastewaters.
2. Soaking
Raw skins are typically soaked in water and imbibing substances in order to hydrate the skin proteins, to solubilize the denatured proteins and to remove the salt used for their preservation. As a result, the exhaust bath contains organic substances, salts and chemical additives. Therefore, the recycling of fresh water and salts is considered a useful approach to reduce the volume of the global effluent and, consequently, the costs of cleaning-up treatments.
A combination of coagulation by alum and membrane separation for the treatment and recycling of exhausted soaking effluents was investigated by Das et al. [
23]. The clarified liquor, after coagulation and cloth filtration, was subjected to NF by using a thin-film composite (TFC) membrane, consisting of a thin-film polyamide (PA) skin over a polysulphone (PS) support, with a MWCO of 400 Da. The NF permeate was then treated by RO with a TFC membrane. Both membranes were supplied by Genesis Membrane Sepratech (Mumbai, India). A flowchart of the investigated process is depicted in
Figure 3.
The coagulation step with an optimal alum dosage (2%) reduced the quantity of total solids by 15% (from 12.6 to 10.8 g/L); therefore, appreciable quantities of total solids were still present in the effluent after alum treatment. The produced sludge (about 1.2 kg from 40 L of effluent) was considered suitable as fertilizer. The permeate quality in the NF step improved with an increase in transmembrane pressure and Reynolds number. The percentage decrease in COD was in the range 13–38% depending on the operating conditions. Almost all the salt present in the clarified solution permeated through the NF membrane. The subsequent treatment of the NF permeate by RO produced a permeate stream with a COD in the range of 64–128 ppm (depending on the applied operating pressure) which gave results substantially lower than the permissible limit (250 ppm). Significant amounts of the salt present in the NF permeate were retained by the membrane, making the retentate stream suitable for its reuse in the pickling process.
Multistep processes based on a sequence of screening, textile membrane filtration and UF were also proposed as optimal treatment for wastewaters of the first and second soaking stages [
24]. After membrane treatments, a decrease in the ecotoxicity was measured.
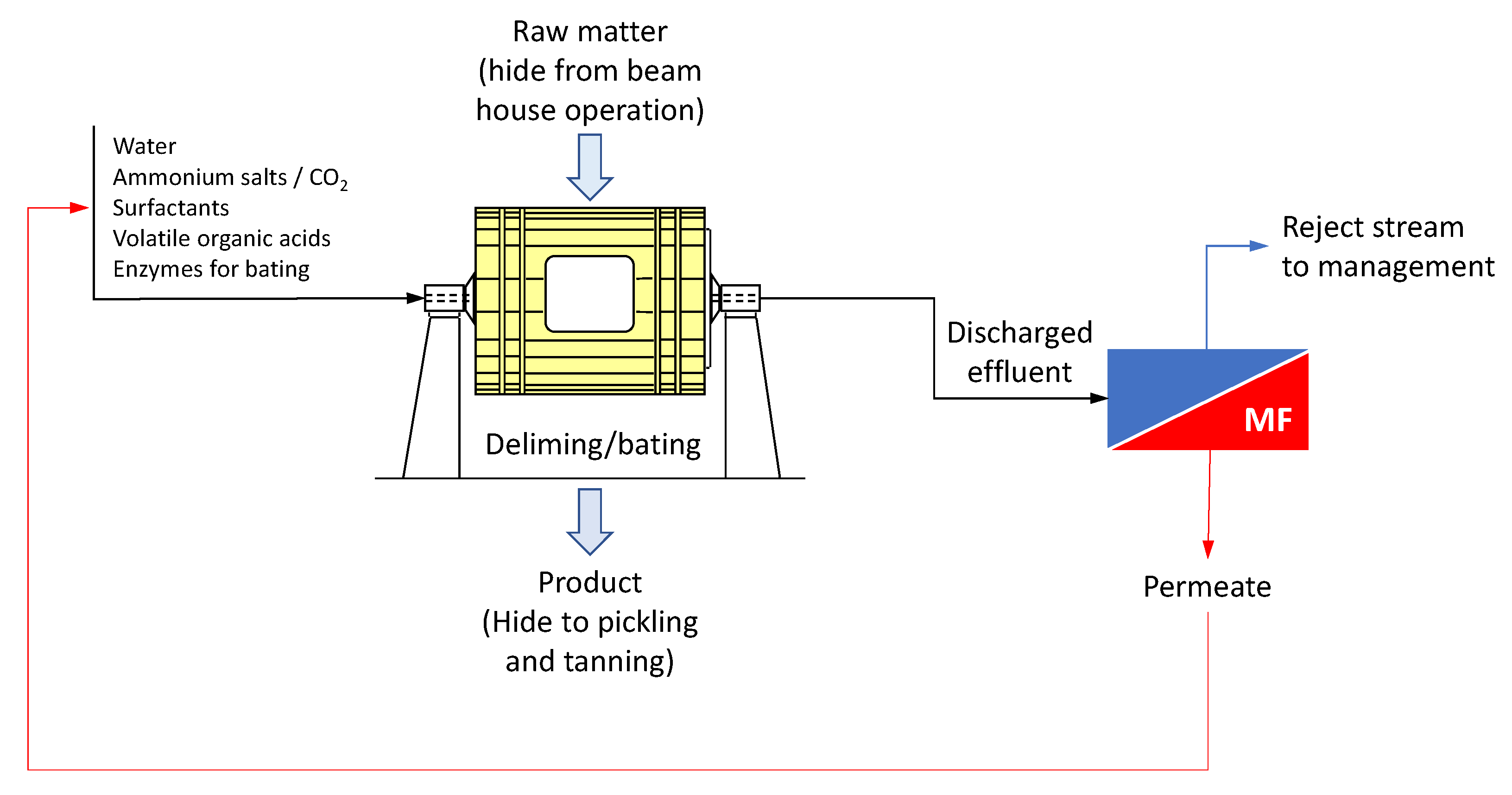
3. Liming-Unhairing
In the liming-unhairing step raw skins are treated typically with lime and sodium sulfide or sulfhydrate solutions in order to remove components that are not transformed into leather, such as superficial epidermic matter including hair and the subcutaneous adipose layer. Therefore, exhaust baths are highly polluting for the presence of sulfide, amines, by-products coming from degradation of hair and epidermis and high concentrations of alkalis. A liming unit typically employs about 15% of the total water consumed in a tannery, so recycling is required for saving water and chemicals consumption [
14].
Conventional liming/unhairing processes lead to 35–45 kg of biological oxygen demand (BOD), 100–125 kg of COD and 140–160 kg of total solids for every ton of raw skins/hides processed. As a result, more than 60% of the total pollution in the leather industry comes from the liming processes [
25].
UF is a promising technique to separate the organic matter from the exhausted bath and to recover solubilized lime and sulfides from the permeate stream, allowing its reuse in the process [
26]. Considering that 60–65% of the initial sulfide remains in the exhausted liquor and 5–10% is lost in the retentate, the quantity of sulfide that it is possible to recycle by UF is in the range of 55–60% [
27,
28].
In order to reduce the quantity of sulfide used in the unhairing process, Cassano et al. [
29,
30] investigated the use of an unhairing enzyme in combination with the UF of the exhausted bath previously screened on a coarse screen to remove the hair detached from goat skins. The UF process was performed by using a spiral-wound membrane module in PS with a MWCO of 20 kDa (MOPSL 4040 U006, Separem, Biella, Italy) and operated recycling continuously the permeate in the drum after 100 min from the beginning of the unhairing process. Organic compounds, mainly represented by products of degradation of keratin and interfibrillar proteins, were retained by the UF membrane while sodium sulfide passed through the membrane and was recycled in the drum. This approach allowed to reduce the content of sulfide from about 10% (with respect to dry skin) normally used in the traditional unhairing operation (hair degrading process) up to 1.5%. Treated skins presented chemical and physical analyses very similar to those of control skins treated according to the traditional process. In addition, the use of enzyme allowed easy recovery of the hair with consequent reduction of the polluting load and of cleaning-up costs.
Taleb-Ahmed et al. [
31] obtained a total removal of turbidity and sulfides from effluents of liming baths by using a PA/PS composite NF membrane in spiral-wound configuration (Nanomax 50, Millipore, Burlington, USA) with a MWCO of 350 Da. Conductivity and COD were also reduced of 82.52% and 95.4%, respectively. The pH of the effluent had a strong influence on the membrane selectivity: values higher than 8 produced a higher sulfur retention (up to 82.52%) in comparison to acid medium. This behaviour was attributed to the influence of pH on the membrane charge as well as on the acid-basic character of the effluent.
Brites Alves and Silva [
32] evaluated the performance of polyethersulphone (PES) UF membranes with MWCO of 10, 30 and 100 kDa for the recovery of water and sulfides from liming wastewaters. Higher permeation fluxes were detected for the 10 kDa membrane despite its lower MWCO. This behaviour was attributed to more severe concentration polarization phenomena for membranes with larger pores. Most of the fats and oils were retained by the 10 kDa membrane while sulfides and dissolved solids were totally recovered in the permeate stream.
A hybrid membrane separation process for the treatment of liming effluents was proposed by Das et al. [
33]. It was based on a pretreatment of the effluent through a combination of gravity settling, coagulation with alum and cloth filtration followed by UF and NF operations. The optimum concentration of alum for coagulation was estimated to be 2%. The produced sludge had similar properties to vermi compost, so it is appropriate for use as organic fertilizer after sulfide oxidation. The supernatant liquor, prefiltered through a fine cloth, was ultrafiltered with a 5 kDa flat-sheet membrane (Permionics Membranes Pvt. Ltd., Gorwa, Vadodara, India). Experimental results indicated that an increase in transmembrane pressure difference decreased the permeate quality in terms of COD; on the other hand, an increase of Reynolds number improved the permeate quality due to the effect of the enhanced forced convection that in turn reduced the growth of the polarized layer over the membrane surface. The salt content of the feed solution was not affected by the UF process; therefore, the permeate conductivity remained almost the same as that of the feed.
The UF permeate was submitted to a NF step performed with a 400 Da flat-sheet membrane (Genesis Membrane Sepratech Pvt. Ltd., Mumbai, India). For this membrane it was observed that the increase in transmembrane pressure difference and Reynolds number improved the permeate quality. In the range of pressures investigated, the COD analysed in the permeate stream varied from about 206 to 132 ppm; this value was much lower than the permissible limit (250 ppm).
The feasibility of unhairing liquor recovery by UF membranes at high recovery rates (90% the original volume), reasonable permeate fluxes of 35–40 L/m
2h and significant savings of 30% sulfides, 15% lime and 15% enzymes was demonstrated by Scholz and Lucas [
34]. The total annual savings in unhairing chemicals for a tannery producing 8000 hides per week was estimated to be EUR 70,400. Potential annual savings of EUR 38,400 were also estimated considering the reduction in fresh water, of the amount of discharged volume and of flocculation and coagulation agents used in primary treatment.
Mendoza-Roca et al. [
35] investigated the use of a flat-sheet UF membrane module with a MWCO of 40 kDa (IRIS 3065, from Orelis) for the purification and reuse of unhairing wastewater. Most of the organic matter was retained by the UF membrane, while the permeate stream contained the chemicals required for the unhairing process (lime and sulfur). The physical characteristics of leathers unhaired with the UF permeate were similar to those of leathers unhaired with fresh liquors and met the required quality standards. The proposed approach produces a significant saving of water, sulfide, lime and other chemicals in the unhairing step. Authors estimated a saving of 10,500 m
3 of water and 6300 kg S
2−, respectively, in a tannery processing 25 tons of cattle hide per day.
Cleaning methodologies with air pulses from the membrane permeate side or with sodium hypochlorite solution gave the best results in terms of flux recovery. In particular, flux recoveries of 92% were reached by using an NaOCl concentration of 1000 mg/L. On the other hand, cleaning procedures using surfactant or enzymes did not yield sufficient flux recovery.
Abdel-Shafy et al. [
36] investigated an effective treatment approach for the liming/unhairing effluent using the Fenton reaction followed by filtration of Fenton’s treated effluent with a MF membrane having a nominal pore size of 0.387 μm. The treatment with Fenton’s oxidation improved the biodegradability and reduced the toxicity of the wastewaters by decreasing the level of sulfides and total Kjeldahl nitrogen (TKN). Further achievement in the removal of COD, BOD, TSS, oil and grease, sulfides, TKN and total phosphates was achieved by membrane filtration. The final effluent met the standards for unrestricted water reuse.
5. Degreasing
Degreasing is most relevant in processing sheepskins, where the natural fat content is about 10–20% of the dry weight. This step aims at removing the excessive amounts of grease in the skin, which may interfere with uniform penetration of tanning substances or dyes, causing difficulties in the finishing processes and creating dark and greasy patches on the finished leather.
Methods commonly used for degreasing are based on the use of organic solvents, non-ionic surfactants and lipases. Enzymatic degreasing is a better way of carrying out degreasing than the use of solvents and detergents. Lipases are much safer and less toxic to workers and the environment. Furthermore, lipases allow for a more uniform colour, cleaner appearance, improve production of waterproof leather, and do not cause dryness in the leather. On the other hand, organic solvents produce a considerable increase of environmental pollution by emission of volatile compounds and considerable problems in biological treatment plants.
An innovative approach for the removal of fat substances from pickled sheepskins was investigated by Cassano et al. [
29]. After a preliminary treatment with glutaraldehyde the skins were degreased at 52 °C by using a commercial surfactant (Taurol Deg 51, Seici, Torino). During the process the bath was continuously ultrafiltered by using a spiral-wound PS membrane module with a MWCO of 20 kDa (MOPSL 4040 U006, from Separem, Biella, Italy) recycling the permeate in the drum. The process allowed to remove up to 55% of the initial fat content of the pickled skins, the same value obtained in a dry conventional process with tetrachloroethylene. The rejection of the UF membrane towards COD and fatty substances was about 95% and the concentration factor of oils and fats in retentate, after 300 min of bath filtration, was about 4.
A NF process was studied by Prabhavathy and De [
39] in order to reduce the COD of spent degreasing baths within the permissible limits in India (250 mg/L). The COD of the initial bath was previously reduced from 3737.3 mg/L up to 734.4 mg/L by coagulation with alum. Then a TFC PA membrane with a MWCO of 400 Da (from Genesis Membrane Sepratech Pvt. Ltd., Mumbai, India) was used to treat the collected supernatant after a clarification with a fine nylon filter cloth. The investigated process successfully reduced COD to well below the permissible limits. Transport coefficients, namely the effective osmotic coefficient, solute diffusivity and solute permeability were also estimated through a combination of film theory, solution-diffusion and osmotic pressure models.
7. Recovery of Chromium Salts
Among the various tanning systems, chrome and vegetable tanning are popular all over the world. These operations aim at preventing skin putrefaction through a process which permanently alters the protein structure of skin, making it more durable and less susceptible to decomposition and coloring.
Chrome tanning with chromium (III) salts accounts for around 85–90% percent of global leather production: 95% of shoe upper leather, 70% of leather upholstery and almost 100% of clothing leather are chrome tanned [
43]. Chrome-tanned leathers have excellent hydrothermal stability (the shrinkage temperature is usually beyond 100 °C), better dyeing characteristics and outstanding mechanical performance [
44].
Unfortunately, the uptake of chromium in the tanning process is only about 60–70% and the remaining 30–40% unfixed chromium (about 0.5 kg chromium per 1000 kg of hides tanned) is discharged as effluent [
45]. The discharged chromium, besides being a waste of primary resources, represents a serious environmental concern since it might transform into toxic and carcinogenic hexavalent chromium. Therefore, the recovery of chromium from tanning exhausted baths is of fundamental importance both for its reuse as tanning substance and for the simplification of the purification processes of the global tanning effluents.
Different methods have been proposed for the recovery of chromium from tanning processes. They include chemical precipitation, coagulation, solvent extraction process, ion exchange and adsorption methods [
46,
47,
48,
49]. The precipitation of chromium salts with NaOH followed by the dissolution of Cr(OH)
3 in sulfuric acid is one of the methods traditionally used for chromium recovery from spent tanning effluents. However, the final quality of the recovered solution is negatively affected by the presence of impurities (fat substances, metals, etc.) not completely removed during the process.
Pressure-driven membrane operations have been investigated in this field in order to produce chromium solutions to be reused in tanning and/or re-tanning processes along with purified streams to be reused in other steps of the leather tanning or discharged into the ecosystem. These processes offer significant advantages over conventional technologies such as higher removal efficiency, no pollution loads and sometimes lower energy consumption [
50,
51].
A combination of UF and NF membranes for the recovery of chromium from tanning exhausted baths was investigated by Cassano et al. [
52,
53]. Spiral-wound UF membranes in polyvinylidenefluoride (PVDF) with a MWCO of 25 kDa (411 TA from Osmonics, Minnetonka, MN) were used in the first step to remove suspended solids and fat substances from the exhaust bath. The measured rejections of these compounds were 84 and 98%, respectively. About 40% of organic nitrogen was retained by the membranes, while the rejection for chromium salts was 28%. The UF permeate was submitted to a NF process performed by using a spiral-wound NF membrane in PA with a MWCO of 150 kDa (MOCD 4040 N50 from Separem, Biella, Italy). The NF retentate, with a chromium concentration of 9.2 g/L (about 1.35% as Cr
2O
3), was reused in the re-tanning step.
A further concentration of the NF retentate by using a precipitation-dissolution method, allowed to obtain a solution (9.2% as Cr
2O
3) which was reused in chromium tanning. Tanned and re-tanned skins showed improved physical-chemical characteristics in comparison to control groups treated with conventional procedures. The high content of chlorides and absence of chromium salts in the permeate of the NF process suggested the reuse of this effluent in the pickling step with a consequent saving of water and salts. A schematic of the proposed two-step membrane process is depicted in
Figure 6. Experimental data were exploited to develop a simulation model for chromium recovery from chrome bath tanning effluents with inorganic UF and NF membranes based on the resistance model and involving material balance and energy requirements [
54]. Optimum conditions were found to be about 2 and 19.4 bar pressures for UF and NF, respectively, at corresponding recoveries of 95% and 60%. Under these conditions the total operating costs were estimated to be as low as 3.23 USD/m
3 with a net profit up to 3.46 USD/m
3. The amount of recovered chromium from the NF stage was estimated to be 5.4 kg/m
3.
A tanning solution with a chromium(III) concentration of about 10 g/L was also obtained in the treatment of a spent tanning liquor through a combination of spiral-wound UF and NF polymeric membranes (EW2540F and DK2540 F, from Osmonics Desal) with MWCO of 50–100 kDa and 150–300 Da, respectively [
55]. Physical-chemical parameters of permeate and retentate samples collected in the UF/NF process are reported in
Table 1.
The UF process provided marked reductions of suspended solids (84%) and fat substances (71%) from the spent liquor, allowing the recovery of most of the chromium in the permeate stream (a 2.1% rejection of the UF membrane towards chromium salts was measured). The UF pretreatment reduced membrane fouling of the NF membrane, thus improving the permeation flux and reducing the flux decay. According to the mass balance of the NF process, most of the chromium (98.9%) and sulfates (89.9%) were recovered in the retentate stream, whereas 58% of chlorides were recovered in the permeate. Pickled sheepskins were tanned with solutions prepared through a combination of NF retentate and basic chromium sulfate (experimental group) and their properties were compared with those of skins treated only with basic chromium sulfate (control group). Both experimental and control groups exhibited shrinkage temperature values higher than 106 °C. Skins treated with higher percentage of NF retentate in the tanning bath showed better improved grain crack and bursting strength with respect the control group. In addition, the recovered solutions provided higher uptakes of chromium (in the range of 85–89%) in comparison to the conventional tanning process (of about 78%). Accordingly, it was estimated that the use of NF retentate allowed the reduction of the pollution load of the exhaust bath of a conventional process by up to 49%.
The possibility to reuse both permeate and retentate streams from the NF treatment of spent chromium baths was also confirmed by Religa et al. [
56]. NF was performed by using negatively charged membranes at pH 4 (DL and HL membranes from GE Osmonics) with the recirculation of part of permeate. This new approach allowed the reduction of the amount of chromium in the tanning step and the consumption of salts in the pickling step, as well as to minimize the chloride concentration in global wastewaters.
Chromium retention rates of 60 and 30% were observed respectively in acid and basic medium when treating tannery effluents with a spiral-wound polymeric membrane (Nanomax 50, Millipore USA) with a negatively charged thin skin layer (0.4 μm) made of PA arylene on a PS support layer, indicating that the pH of the medium affects the ionic rejection considerably [
57].
Mert and Kestioglu [
58] evaluated the economic feasibility of an integrated membrane process based on UF, NF and RO operations for the treatment of chromium tanning wastewater with a flow rate of 200 m
3/d. The total investment and the operating costs were estimated to be EUR 228,415 and 2.77 EUR/m
3, respectively; on the other hand, for classical treatment facilities investment and process costs resulted of EUR 345,400 and 0.8 EUR/m
3, respectively. As a result, the membrane technology was found to be cheaper than classical treatment systems for both investment and the process costs.
Low pressure (7 bar) and medium pressure (16 bar) RO membranes were judged suitable for the removal of unreacted chromium from spent tanning effluents. It was estimated that the economic separation efficiency could be achieved safely when the NaCl concentration is lower than 5000 mg/L at chromium concentration less than 1000 mg/L. Under these conditions, the permeate recovery and chromium removal can be of about 45% and more than 99%, respectively [
59].
Different TFC polymeric NF membranes (all from Osmonics Desal Corporation, Minnetonka, MN, USA) were evaluated by Ortega et al. [
60] for their ability to remove chromium(III) from a solution of chromium basic sulfate (33% basicity) utilized in tanning processes. Among the investigated membranes, a polypiperazine amide on sulphonated PS with an isoelectric point of 4 displayed the highest separation factors (up to 93.5%) combined with a high dynamic permeability even at the high concentration used (30.3 mol/m
3).
The high retention of the membrane was attributed to its positive charge at pH values of the solution lower than the isoelectric point.
A combination of NF and RO was investigated by Das et al. [
61] to treat the chromium tanning effluent in a cross-flow cell. NF experiments were performed by using an organic TFC membrane consisting of a PA skin over a PS support with a MWCO of 400 Da (Genesis Membrane Sepratech, Mumbai, India). The observed chromium retention varied from about 91% to 98%. It increased with cross flow velocity and pressure in both laminar and turbulent regimes. The presence of turbulent promoters or high cross flow velocity did not affect the chromium retention to a great extent; rather, it contributed to the considerable increase in the permeate flux. The treatment of the NF permeate with a PA RO membrane allowed the reduction of the content of chromium and COD to permissible limits, producing clean water and concentrated salt solutions for reuse. The NF retentate was considered suitable for its recycling to the tanning chamber along eventually through the addition of chromium salts needed to reach the required concentration of the tanning bath.
The combination of NF with diafiltration in both constant volume diafiltration (CVD) and intermittent volume diafiltration (IVD) was investigated by Religa and Kazmierczak [
62] in order to evaluate the removal of chloride ions from the NF retentate and increase the purification degree of the chromium solution. The salt elution reached levels of 62% and 54% operating in CVD and IVD mode, respectively. Therefore, the CVD mode enabled better desalination of the chromium tannery wastewater in comparison with the IVD mode. Moreover, chromium retentions of 93% and 95% for the CVD and IVD mode were maintained.
Cellulose acetate/sulfonated poly(ether ether ketone) (CA/SPEEK) blend UF membranes were used by Arthanareeswaran et al. [
63] for separating chromium(III) ions complexed with water-soluble macroligand (PVA), in order to increase the molecular weight. Experimental results indicated that the chromium rejection improved at a pH of 6 with a macroligand concentration of 2 wt.%. Chromium rejections and product rate efficiencies of the blend membranes were also affected by the operating pressure as well as by the solute concentration in the range of investigated values (200–1000 ppm).
Recently, Zakmout et al. [
64] suggested a two-step process for the recovery of chromium(III) from tannery effluents based on a preliminary concentration of these effluents by RO, followed by treatment with a chitosan-based membrane. Real and synthetic effluents were previously concentrated by using an SW30 RO membrane (DOW Chemical Company Midland, MI, USA) which rejected more than 99% of chromium allowing for the production of a permeate stream meeting the requirements for direct discharge into natural environments. In the second step, the RO concentrate was diafiltrated with acidic water through a PES membrane coated with a thin chitosan layer. This membrane retained 97% of the total mass of chromium present in the RO concentrates from a real tannery effluent, with a selectivity of 4.2 and 5 in reference to NH
4+ and Cl
−, respectively, 12.9 and 14.6 in reference to K and Na, and >45 in reference to Mg, Ca, and S. These results were attributed to the preferential adsorption of chromium onto chitosan and by the relatively high permeability of the membrane to the other ionic species. Chromium was recovered by desorption treating the membrane with water solutions at pH 2, leading to a final solution enriched in chromium(III) which may be re-used in tannery operations.
The schematic representation of the RO-diafiltration integrated process is depicted in
Figure 7.
8. Recovery of Tannins
Vegetable tanning is the traditional method of tanning leather relying on the use of natural vegetable tannins from bark or other plant tissues (i.e., oak and spruce bark, mimosa, quebracho, chestnut, tara pods, olive leaves and rhubarb roots). In this process, the collagen chains of the skins are reticulated through hydrogen bonds between phenolic groups of tannins and NHCO groups of the collagen, preserving, strengthening and giving color to the hide.
The tanning agent is placed together with the hides in water-filled pits, resulting in a bath containing tannic acid after a few days. Skins are regularly exposed to additional baths with higher tannin concentrations. Tannins react with the skins faster than non-tannin substances (salts and other non-tannin compounds); as a result, a decrease in the tannin/non-tannin ratio (T/NT) of the tanning solution is observed. However, the concentration of tannins in the exhaust bath is still significant.
The recovery of tannins from spent vegetable baths represents a significant economic advantage for the leather industry for both its reuse as well for the simplification of cleaning-up process of global wastewaters [
65].
UF and NF processes can be used to increase the tannin/non-tannin ratio of spent vegetable tanning baths in order to promote the reuse of tanning substances. Cassano et al. [
66] evaluated the performance of different polymeric NF and RO membranes in spiral-wound configuration for the recovery of tannins from vegetable baths used in the production of sole leather. Experimental results on lab scale indicated that a better tannin/nontannin (T/NT) ratio in the retentate stream is achieved by reducing the NaCl rejection of the used membranes from 99.4 to 10%. Therefore, following experiments on semi-industrial scale were performed by using a spiral-wound membrane module (NTR-7410 S4F from Nitto-Denko, Tokyo, Japan) with a NaCl rejection of 10%. This membrane rejected more than 87% of tannin compounds, while for nontannins and salts, rejections were of 28.6% and 1.8%, respectively. Average permeate fluxes, in the selected operating conditions (operating pressure, 6.75–7-9 bar; temperature, 7–19 °C), were of about 10 L/m
2h. The NF retentate produced at a volume reduction factor of 4.26, with a density of 16 °Be, was reused for tanning tests on bovine hides. Chemical and physical parameters of the experimental skin samples were very similar to those of the control group tanned with fresh tanning solutions.
The fouling phenomena of different PA TFC membranes covering the range from RO to NF in the treatment of spent vegetable tanning baths were evaluated by Molinari et al. [
67] comparing the operating pressures required to obtain the same volume reduction factor, normalized permeate fluxes and hydraulic permeability before and after tannin concentration. The retention of tannins, which are polyphenols capable of significant hydrogen bonding, was found to be governed by the chemistry of the interactions between their complexes and the membrane material.
The performance of UF and NF polymeric membranes in the treatment of exhausted vegetable tannin liquors was evaluated by Romero-Dondiz et al. [
68]. NF membranes provided better results in comparison to the UF ones. Among the investigated membranes a NF membrane with a MWCO of 200 Da (MPF-34, from Kock Membrane Systems) showed the best separation properties in terms of increasing T/NT ratio (from 1.68 to 1.80, for a volume reduction factor of 1.04), while a PS/PA membrane with a MWCO of 150–300 Da (DK, from GE Osmonics) presented the best permeation properties and the lowest fouling, with an average permeate flux of 17.17 L/m
2h and a fouling index of 27.3%.
11. Tanning Wastewaters
Cleaning-up processes of tanning effluents usually include primary or chemical-physical treatments such as coarse screen, equalization and chemical-physical precipitation with sedimentation and sludge separation, followed by secondary or biological treatments (with partial or total recycle of sludge) and tertiary treatments (filtration, stripping, redox processes) [
9,
75,
76].
Membrane technology represents a useful approach as secondary treatment of tannery wastewater effluents. The biological treatment of tannery effluents is traditionally difficult and onerous due to the presence of high concentrations of non-biodegradable organic matter and inhibitory conditions for the nitrifying biomass; in addition, high the sludge retention times are needed to obtain a stable nitrification and a complete hydrolysis of the organic fraction [
77].
MBRs have been shown to be highly effective in the removal of organic pollutants and suspended solids from tannery effluents. In these systems, MF or UF membranes are integrated with a biological process, specifically a suspended growth bioreactor. High-molecular weight organic substances are rejected by the membranes and recycled in the bioreactor, where they can be further decomposed (
Figure 8). Therefore, MBRs operate at much higher mixed liquor concentrations, up to 30−50 g/L mixed liquor suspended solids (MLSS), than conventional biological treatment [
78].
The performance of a Zenon ZW-10 MBR equipped with hollow fiber UF membranes in the treatment of a tannery effluent having a soluble COD between 0.35 and 2 g/L was evaluated by Artiga et al. [
79]. The COD in the permeate stream was not affected by the biomass concentration and yielded results lower than 100 mg/L with removal percentages of about 86%. The biomass concentration in the reactor, in terms of volatile suspended solids (VSS), ranged between 0.5 g/L and 15 g/L. A drop in the oxygen capacity of the reactor was observed for a biomass concentration higher than 8 g/L.
Scholz et al. [
80] investigated the combination of MBR and RO processes to treat tannery effluents to an acceptable level for irrigation purposes. The MBR pilot consisted of an aerated bioreactor tank connected via a centrifugal pump to a cross-flow UF unit equipped with two tubular membrane modules in PES with a MWCO of 100 kDa. PA membrane modules in spiral-wound configurations were used for the RO treatment of the MBR permeate.
The MBR treatment allowed the reduction of COD, BOD, and ammonia concentrations of the effluent by 90–100%. COD and BOD in the permeate stream yielded results of about 344 mg/L and 20 mg/L, respectively. The MBR pretreatment improved also the overall performance of the RO unit reducing biofouling and scaling of RO membranes. The salt content of the MBR permeate was reduced up to 97.1% in the RO process. According to the experimental results a full-scale MBR and RO plant with a treatment capacity of 5000 m3 per day for mixed tannery effluents was designed.
Munz et al. [
65] evaluated the role of tannins in the treatment of vegetable tanning wastewater with conventional activated sludge processes (CASP) and MBR. The results indicated that natural and synthetic tannins constituted a relevant fraction of the organic biorefractory fraction of the treated effluent and that their removal did not greatly differ between the two treatments. In addition, the inhibition of the nitrifying biomass was attributed to the whole wastewater rather than the non-biodegradable fraction.
The use of MBR and its combination with a photoelectrooxidation process was investigated by Giacobbo et al. [
81] for the final polishing of tannery wastewater. MBR allowed the removal of the remaining BOD of treated wastewater, producing a permeate stream which can be reused in beamhouse operations. The refractory matter, quantified as COD, was removed by photoelectrooxidation providing a purified stream which could be recycled for the tanning and re-tanning steps.
Recently, Vo et al. [
82] investigated the effect of the organic loading rate on the performance and fouling behavior of a MBR in the treatment of tannery wastewater during 280 days of operation. It was found that a reduced fouling rate was achieved operating at low fluxes (of about 4.2 L/m
2h). In addition, the amount of total suspended solids together with high concentration of salts in the feed wastewater caused extremely low active biomass concentration in the MBR. Therefore, a pretreatment process aimed at reducing the content of suspended solids was suggested before the biological treatment when treating this type of wastewater.
Pressure-driven membrane processes have been also combined to chemical-physical and biological treatments in order to reduce the organic and inorganic content of tannery wastewaters [
83].
Stoller et al. [
84] proposed an integrated process based on the sequential combination of spiral-wound NF and RO membranes (models DK and SC, respectively, supplied by Osmonics) for the treatment of tanning wastewaters as alternative to the biological reactor. According to the economic analyses, a minimum total cost saving of about 21% was estimated in comparison to an external wastewater treatment service, relying on conventional biological processes. The proposed process limits the disposal of concentrates to external services to 5%, allowing the discharge of 90% of the initial wastewater volume in surface waters and the reuse of a chromium rich concentrated stream in amount equal to 5% of the initial volume. Total cost savings higher than 30% were also estimated in the treatment of tannery wastewater by NF after an initial sedimentation process [
85].
A combination of NF and RO membranes for the treatment of the effluent from the beamhouse process (except the chromium tanning) was also investigated by Purkait et al. [
86]. The effluent was previously subjected to gravity settling followed by alum treatment and cloth filtration. The supernatant liquor was treated with a PA NF membrane having a MWCO of 400 Da (from Genesis Membrane Sepratech Pvt. Ltd., Mumbai, India) and the NF permeate was finally submitted to a RO process. The analyses of the flux decline mechanisms involved in both membrane filtration processes revealed that three consecutive mechanisms, namely complete pore blocking, standard pore blocking, and cake filtration, controlled the flux decline in the NF process. During cake filtration, an almost incompressible cake was formed. Complete pore blocking and standard pore blocking were found to be the controlling flux mechanisms in RO.
Fababuj-Roger et al. [
87] proposed a combination of filtration, UF and RO for the treatment of physical-chemically treated tannery wastewater. Before UF the effluent was filtered with a 20 μm cartridge filter. Four PES membranes (Iris 3029 from Rodia Orelis) with MWCO of 3, 10, 30 and 100 kDa were tested at different transmembrane pressures. All membranes significantly removed both turbidity and color. However, removal efficiencies for COD were only around 15%. This result was attributed to the presence of hydrolyzed proteins of low molecular weight as well as of inorganic reduction agents which contribute to the high COD values of the effluent. The 30 kDa membranes exhibited the highest permeate fluxes in selected operating conditions. Clogging of membrane pores due to rejected peptidic molecules increased membrane fouling of 100 kDa membranes, producing lower permeate fluxes. The UF permeate from the 30 kDa was treated with a spiral-wound RO membrane module with an effective membrane area of 2.6 m
2 (ESPA-1 from Hydranautics). As expected, conductivity and COD rejections were higher than 98%, suggesting the reuse of the permeate stream in the tannery, even in those processes requiring the lowest salt concentrations like the dyeing one.
In the integrated process proposed by Bhattacharya et al. [
88], highly contaminated composite tannery wastewaters (COD of 5680 mg/L and BOD of 759 mg/L, respectively) were submitted to a preliminary MF treatment by using indigenously developed ceramic membranes in tubular configuration. The MF permeate was then treated with a spiral-wound RO membrane in TFC PA material. TKN and COD were significantly removed in the MF step (66% and 91%, respectively); sulfides were completely removed by the MF membrane and the turbidity of the treated water was reduced below 1 NTU. The permeate produced from RO had negligible concentrations of COD, BOD and TKN. Turbidity was about 0.025 NTU. Most of the metals such as lead, copper, chromium, zinc and nickel, etc. were also removed by the RO membrane. Cow hides tanned with the treated water sample from the RO process had better tensile strength, stitch tear strength and grain crackness properties than control hides treated with fresh water.
PES and PVDF UF membranes with MWCO of 50 kDa exhibited high removal efficiency of suspended solids, fat and proteins when used in the pretreatment of tanning wastewaters [
89] and similar filtration performance. The analyses of fouling phenomena revealed that cake formation was the main pore blocking mechanism during the process and it was independent of the operating conditions and membrane materials.
The combination of RO with conventional treatment methods like neutralization, clari-flocculation and conventional activated sludge produces also a significant reduction of COD (higher than 96%) from tannery wastewaters and the removal of the refractory inorganic compounds (chloride and sulfate) allowing the reuse up to 85% of the treated water, thus reducing groundwater consumption [
90,
91,
92].
The suitability of a RO system for the recovery and reuse of water from a secondary treated tannery effluent was investigated by Suthanthararajan et al. [
93]. A multistep pretreatment of the effluent consisting of a multistage pressure sand filter, photochemical oxidizer, softner, MF and NF was implemented to minimize the fouling of the RO membrane. The RO process was performed by using a PA membrane in spiral-wound configuration (from Hydranautics) with pore size of 0.2 Å. This membrane effectively removed more than 98% of TDS and about 94–98% of Na and Cl ions. The recovered permeate was reused for the wet finishing process in the tanneries.
Streit et al. [
94] investigated the use of UF and NF membranes in the treatment of model solutions simulating effluents from the secondary biological treatment of Brazilian tanning industries in order to evaluate their selective retention of the organics and permeation of inorganic compounds. Polymeric UF membranes with MWCO ranging from 1 to 10 kDa (ETNA01PP, ETNA 10PP and GR81PP, from Alfa Laval, Denmark) produced retentate streams enriched in organic compounds and permeate streams enriched in salts (TOC and conductivity rejections up to 67% and 24%, respectively). Low permeation fluxes were detected as the result of concentration polarization and fouling phenomena. On the other hand, PA NF membranes (NF270 and NF200, from Dow-Filmtec) exhibited lower fouling index associated with high permeation rates as well as high rejections towards organic compounds (TOC rejections up to 94%).
Recently, NF and RO membranes were tested to treat biologically treated tannery wastewater in order to eliminate refractory COD and salts [
95]. Salt compounds were completely rejected by RO membranes. For the NF membranes the salt rejection decreased by increasing the concentration factor (salt rejections were between 50–60% at the highest concentration factor). Membrane fouling for RO membranes was lower than that observed for NF membranes. The global results indicated that RO could be the most feasible technique for water reuse in the tannery industry, despite their lower permeate fluxes in comparison to NF membranes.
Solid residues, known as evaporated residues, can be obtained from concentrated streams of the RO process evaporated in solar evaporation pans or in a multiple-effect evaporator. These residues still contain high concentrations of organic compounds and other inorganic impurities along with sodium chloride. Boopathy et al. [
96] investigated the separation of sodium chloride from the evaporated residue of a RO reject stream by selective precipitation with hydrogen chloride gas. Optimum process parameters in terms of time, pH, temperature and concentration of evaporated residue were identified.
The proposed process allowed the recovery of 0.203 kg of NaCl from 1 kg of evaporated residue at an estimated cost of USD 0.058 per kg. The proposed process was considered competitive in comparison with the conventional disposal of evaporated residue onto a secured landfill. A similar approach was used to separate sulfate ions as calcium sulfate from the saturated solution of reject stream generated from the leather industry Boopathy et al. [
97]. The recovered calcium sulfate was tested for its adsorptive property toward aqueous leather dyes.
Ozonation is another approach to reduce the refractory organic pollutants of RO and NF concentrates. Ozone doses of 80 and 100 mg/min for 60 and 70 min of contact time produced 59 and 78% chemical oxygen demand (COD) reduction, respectively, in the condensate of rejected water [
98].
Single-channel tubular ceramic membranes (γ-Al
2O
3, Media and Process Technology, Inc., USA) with average pore sizes of 10, 50 and 200 nm were used to treat highly polluted tannery wastewater in order to evaluate the impact of membrane pore size and pressure on permeate flux, COD and color reduction [
99]. The results highlighted that greater than 95% color removal was consistently achieved with UF membranes (10 and 50 nm). For these membranes, COD reductions ranged between 58 and 90% and increased with an increase in the operating pressure.
13. Conclusions
Typical applications of membrane-based operations in the treatment of wastewater from the leather industry have been reviewed in the light of literature data. In particular, pressure-driven membrane operations play a key role in the recovery of water and primary resources from exhaust baths of the beam house, tanning and post-tanning operations and their reuse in the wet-end leather production process. For several applications, including chromium and tannins recovery from spent liquors, the reduction of pollution in terms of quantity and quality, leather quality improvement and cost effectiveness of the implemented processes have been clearly demonstrated.
Membrane-based operations integrated among themselves and/or with conventional physico-chemical and biological treatments provide also useful protocols for the treatment of global wastewaters with significant reduction of organic compounds and the removal of the refractory inorganic compounds allowing to reuse the treated water, thus reducing groundwater consumption.
In most cases the selection of appropriate membrane modules and membrane materials, optimization of operating and fluid-dynamic conditions and identification of a suitable pretreatment of the feed solutions appear as key factors to overcome intrinsic limitations related to fouling and the short lifespan of the membranes.
The global findings clearly highlight the key role of membrane-based technologies for the development of environment friendly processes in the leather manufacture and their significant contribution to redesign the conventional production cycle of the leather industry within the logics of the process intensification, zero-discharge and circular economy strategies.