Effects of MgO and Fe2O3 Addition for Upgrading the Refractory Characteristics of Magnesite Ore Mining Waste/By-Products
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
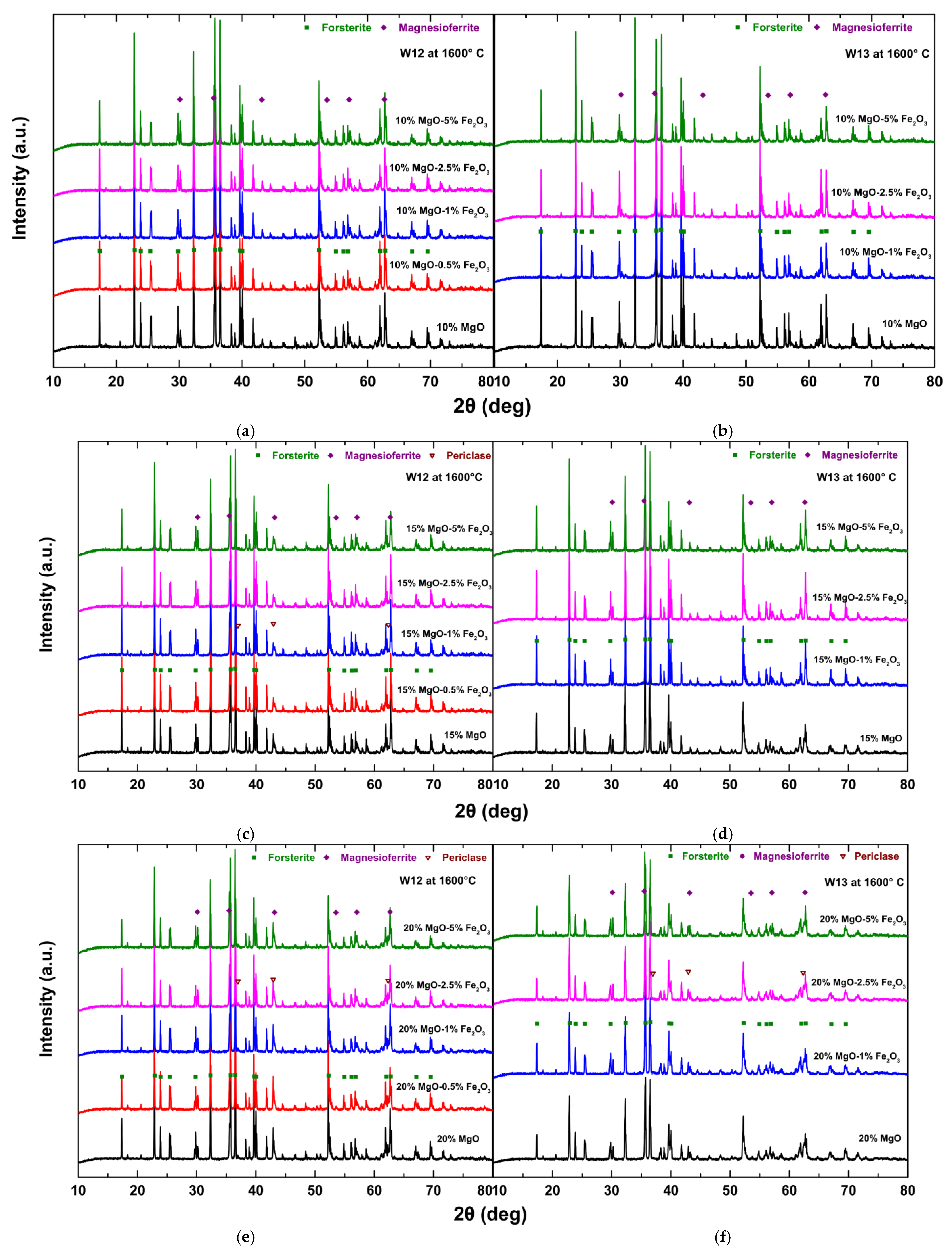
3.1. XRD Analysis of Crystal Phases of the Samples after Application of Thermal Treatment at 1300 °C and 1600 °C
- The raw mining waste samples (i.e., without additives) after the thermal treatment at 1300 °C for 120 min consist mainly of the desired crystal phase of olivine (forsterite) and pyroxene (enstatite) mineral phases.
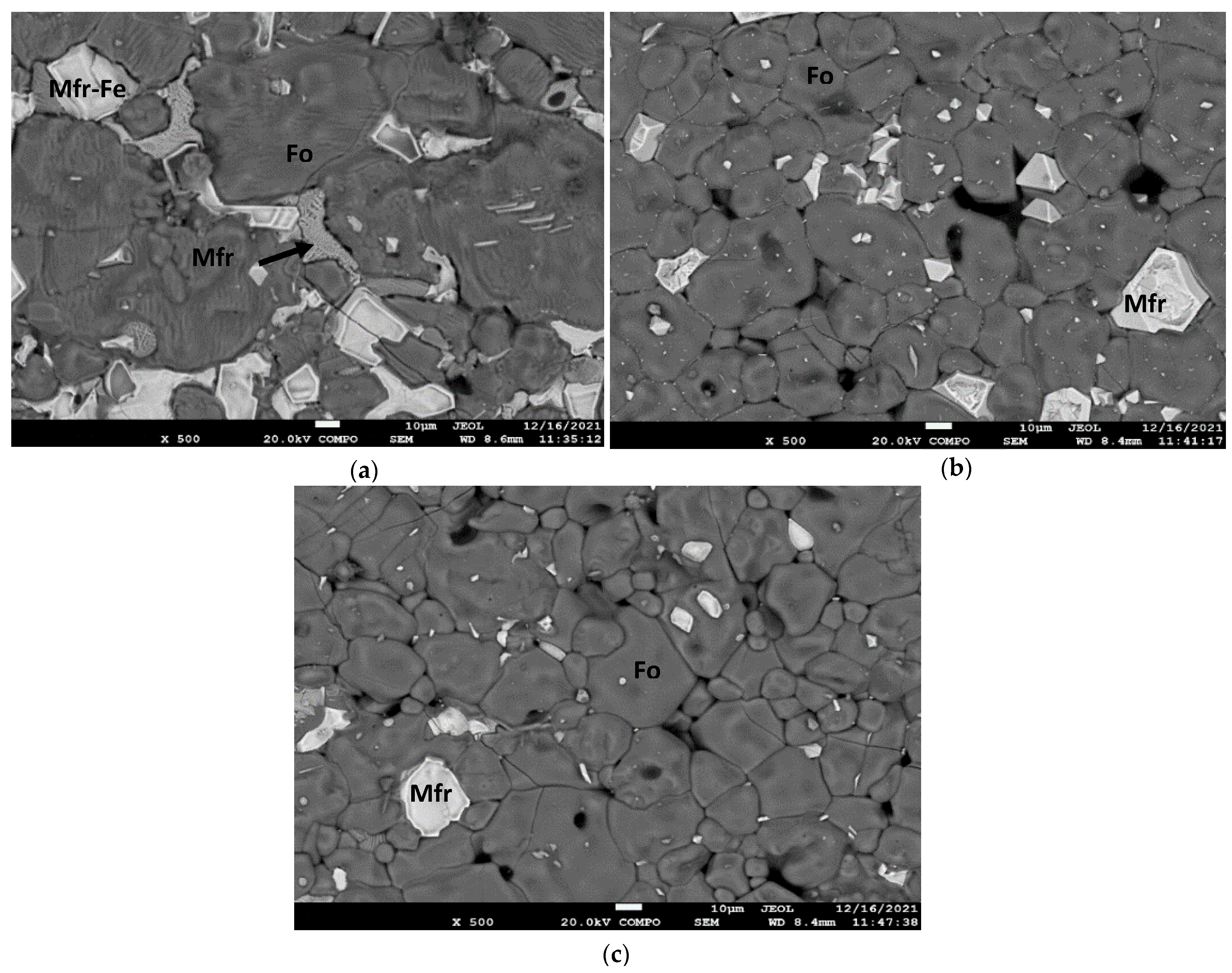
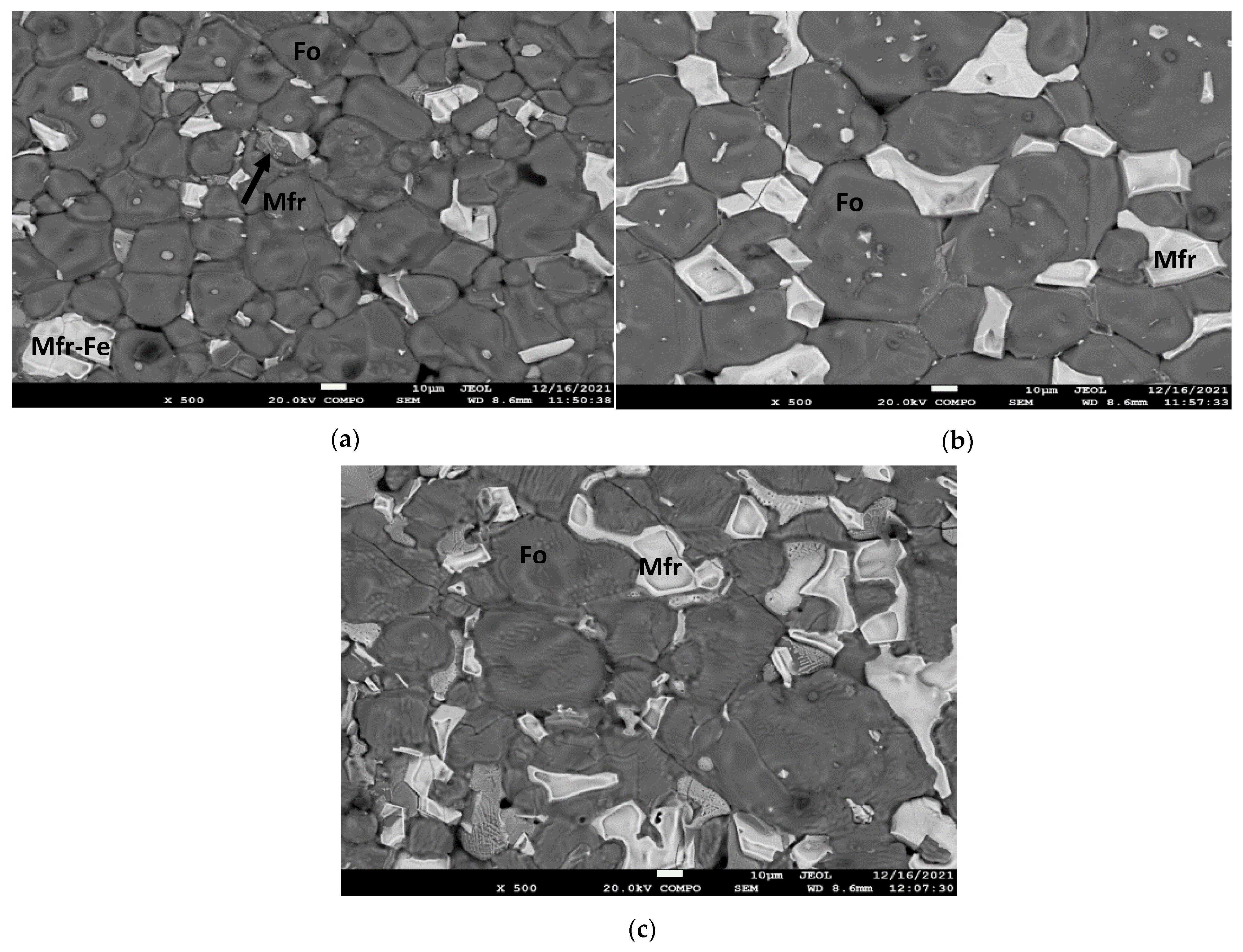
- The formation of magnesioferrite in the products produced by the addition of MgO is associated with the oxidative atmosphere, where olivine (forsterite) particles decompose to form magnesioferrite crystals and vitreous silica. The oxidation of olivine (Mg, Fe)2SiO4 results in the formation of a major oxide phase, i.e., ferriolivine, magnesioferrite, and a minor oxide phase, i.e., magnetite [36]. Magnesioferrite mineral phase formation is higher with the combination of the additives MgO and Fe2O3 and increases by increasing the percentage of Fe2O3.
- The increasing addition of MgO resulted in a decrease in the percentage of unwanted pyroxenes (mainly enstatite), while an increase in the percentage of the desirable forsterite is observed, especially for sample W13, which presents the highest percentage of forsterite with 15 wt.% addition, as the optimum dose for olivine formation for sample W13 is 15.6 wt.%. Maximization of the formation of olivine in the product is reported with the increase of MgO addition after applying the thermal treatment at 1300 °C. On the other hand, sample W12 (optimum dose for sample W12 is 9.4 wt.%) has a maximum percentage of forsterite of 10 wt.% MgO addition and this percentage decreased (~2 wt.%) with increasing magnesioferrite formation.
- Periclase phase formation in the product is attributed to the higher quantities of MgO (wt.%) added, compared to the respective optimum dose calculated (see Table 3), especially for the sample W12, where the optimum wt.% MgO dose was determined as 9.4%.
- The main mineral phases for the products of the raw mining waste samples, i.e., without additives, after the application of thermal treatments, are olivine (forsterite), pyroxenes (clinoenstatite), and magnesioferrite. The oxidative atmosphere, inducing the olivine (forsterite) particles to decompose and form magnesioferrite, is the main reason for the formation of magnesioferrite in the products [37]. The percentage of magnesioferrite increased by increasing Fe2O3 addition.
- The percentage of unwanted pyroxenes (clinoenstatite) decreased and was eventually eliminated as the pyroxenes (enstatite) phases melted at 1557 °C [26,37]. Clinoenstatite is one of the enstatite polymorphs. The rate of metastatic inversion of protoenstatite to clinoenstatite during the cooling step is overly sensitive to the initial properties of the examined sample. It depends on the particle and crystallite size, the thermal treatment temperature, and the degree of compaction of the sample [38].
- Periclase formation is attributed to the excess of wt.% MgO in terms of the optimum dose required for the formation of olivine for samples W12 and W13, which is 9.4 and 15.6 wt.%, respectively (Table 3).
- The reported percentages for the desired crystal phase of olivine are higher than 88% as well as the relative percentage of the olivine (forsterite) phase obtained at 1300 °C (+~5wt.%), and of the unwanted crystal phase of pyroxenes is absent at 1600 °C according to the data presented in Tables S1–S4 (Supplementary Material), however, the quantification of mineral phases at 1600 °C cannot lead to safe results, due to the formation of glassy phase at this temperature in high percentages.
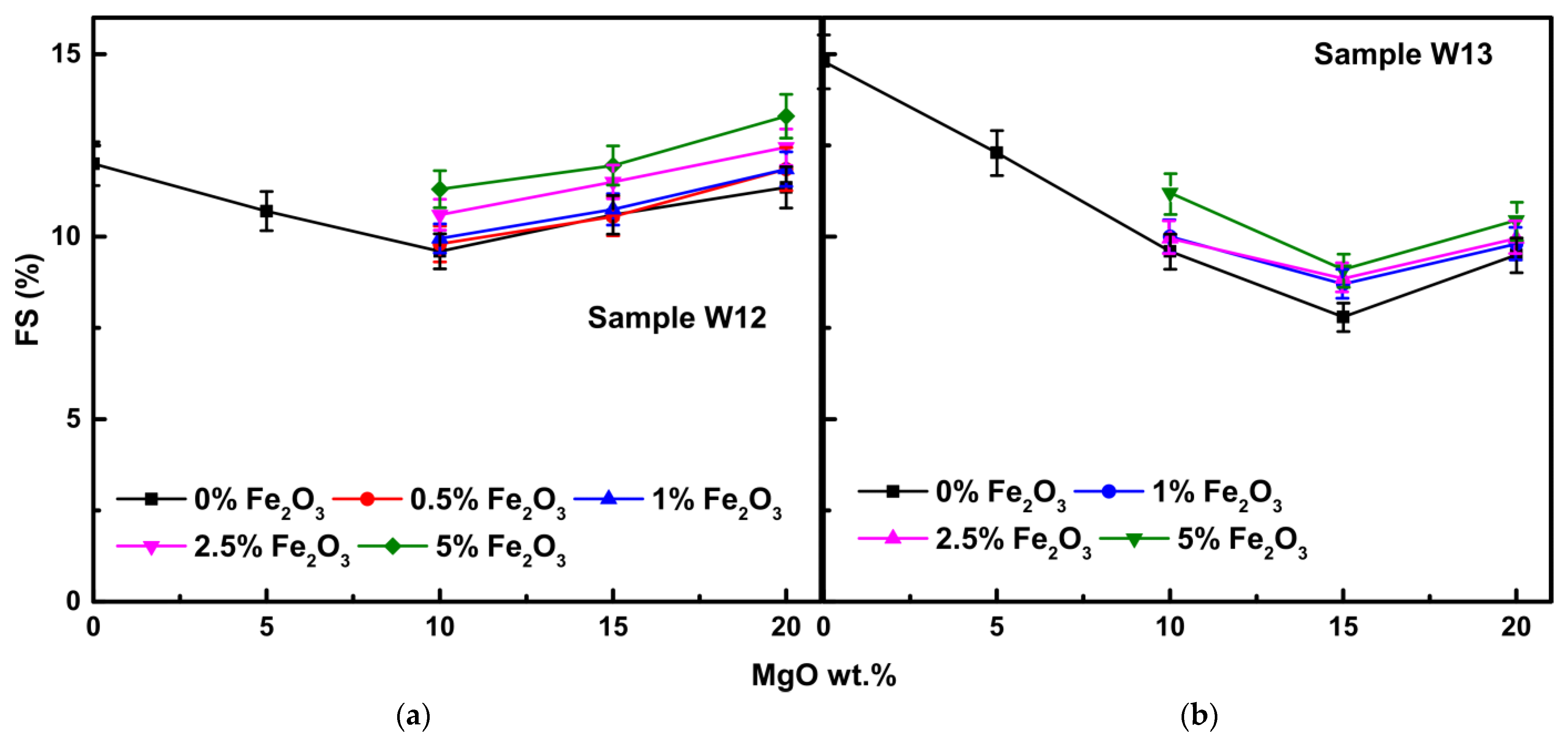
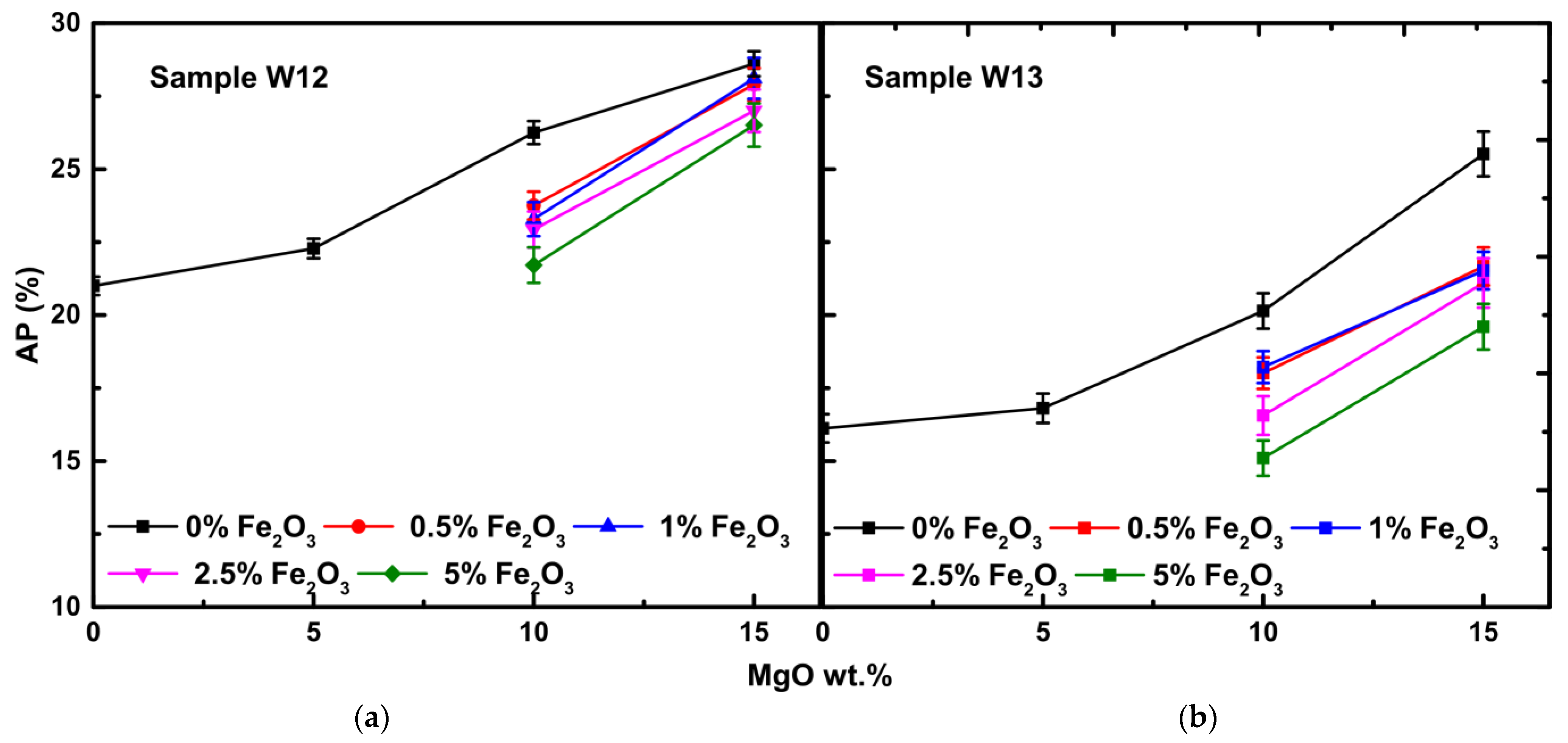
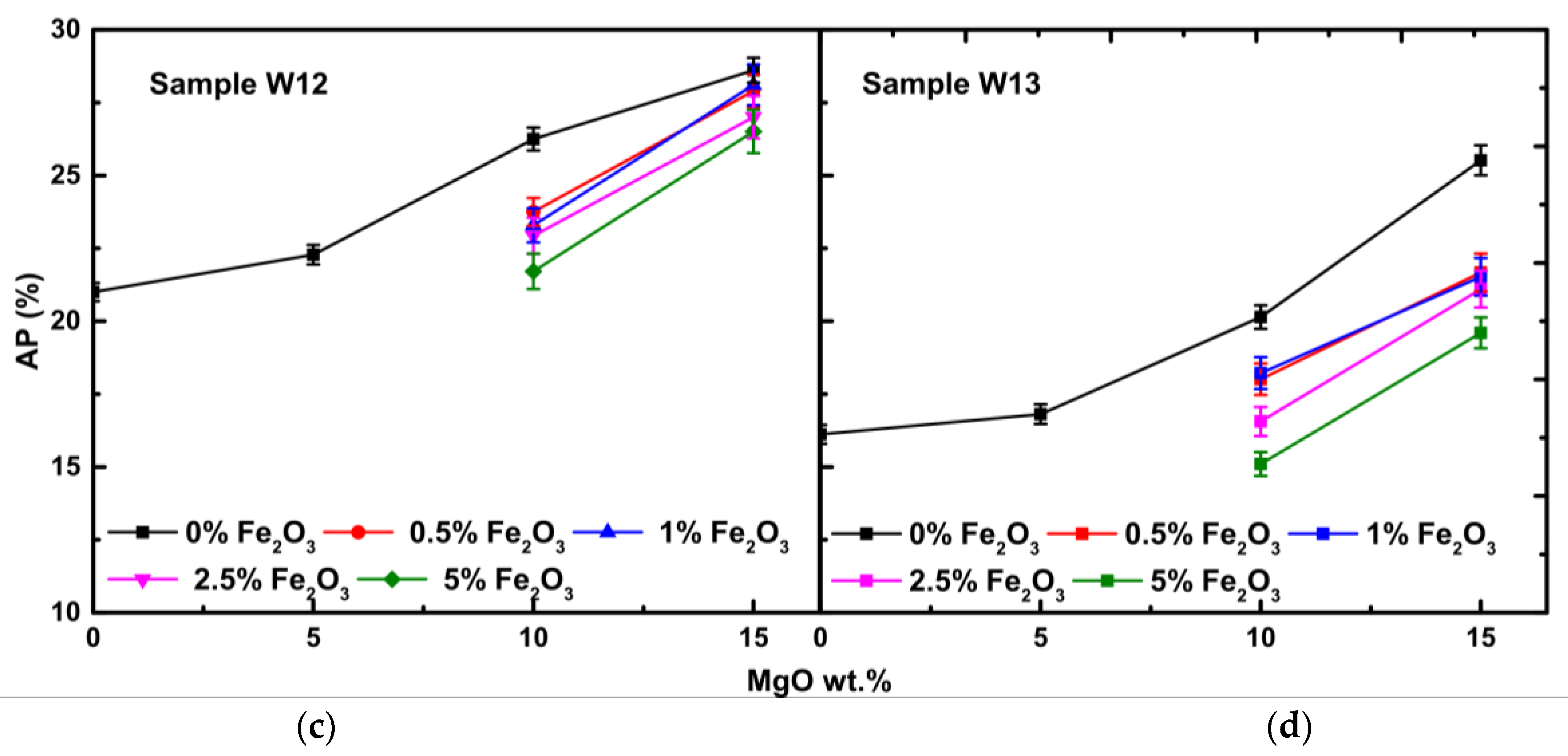
3.2. Physical Properties
3.2.1. Firing Shrinkage
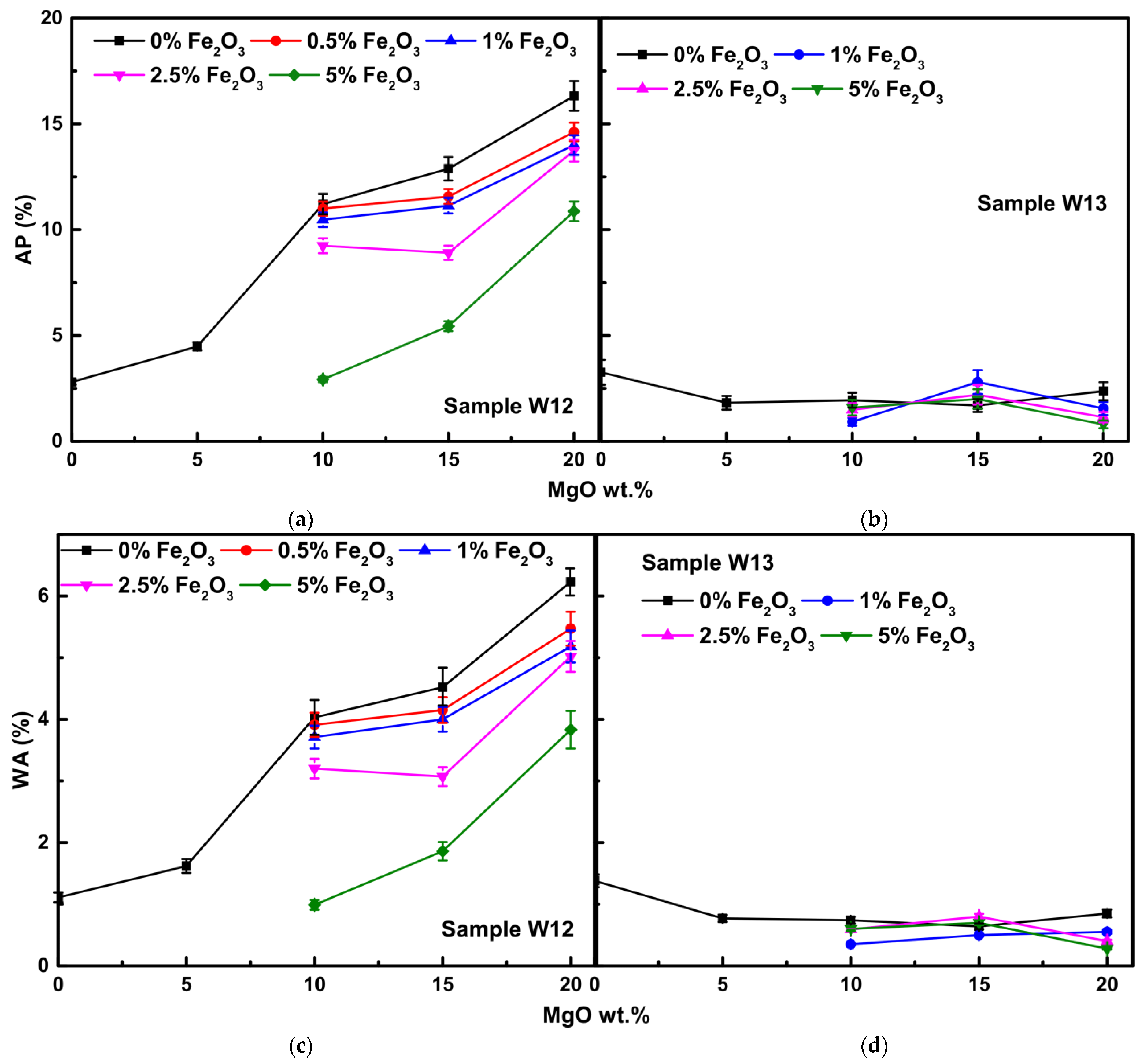
3.2.2. Water Absorption and Apparent Porosity
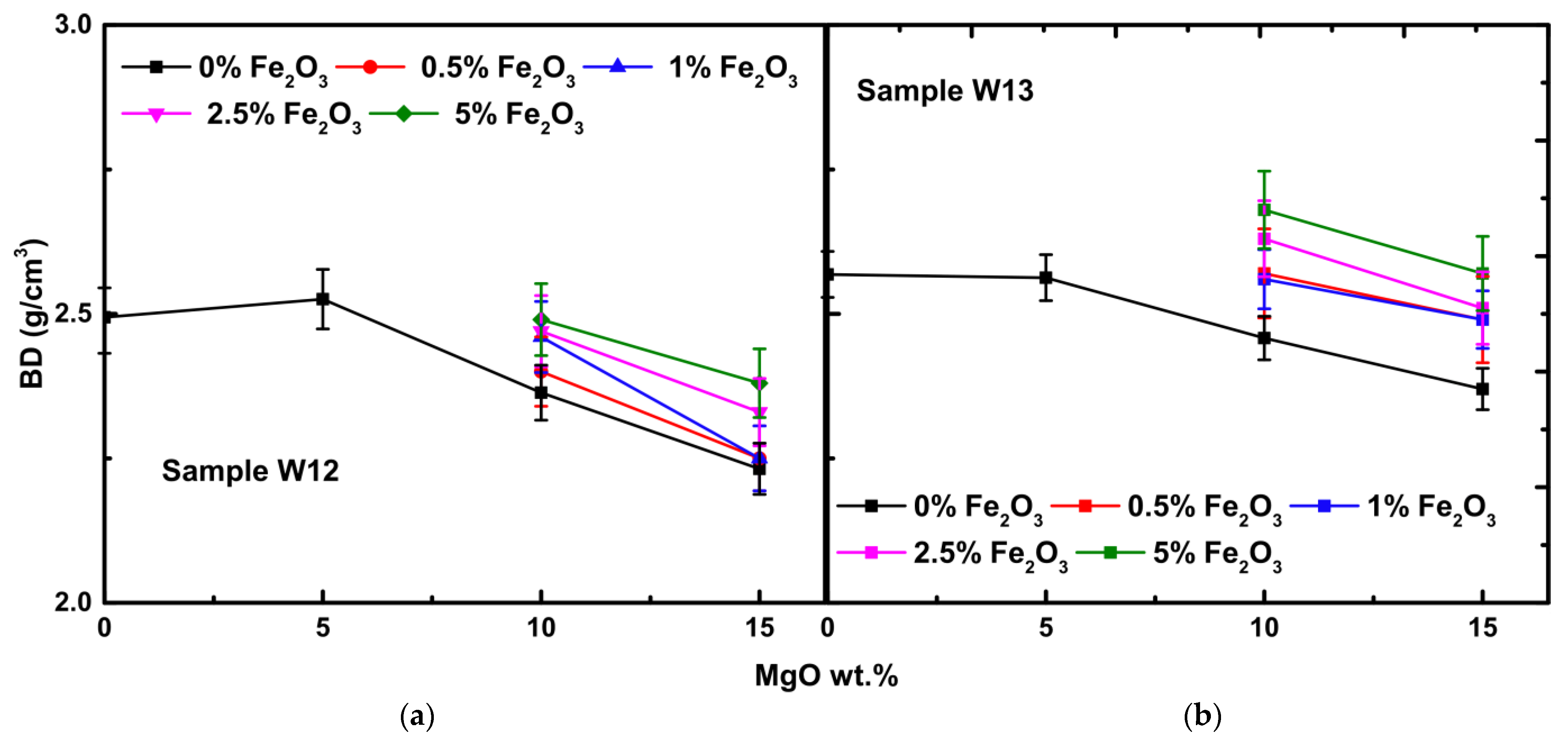
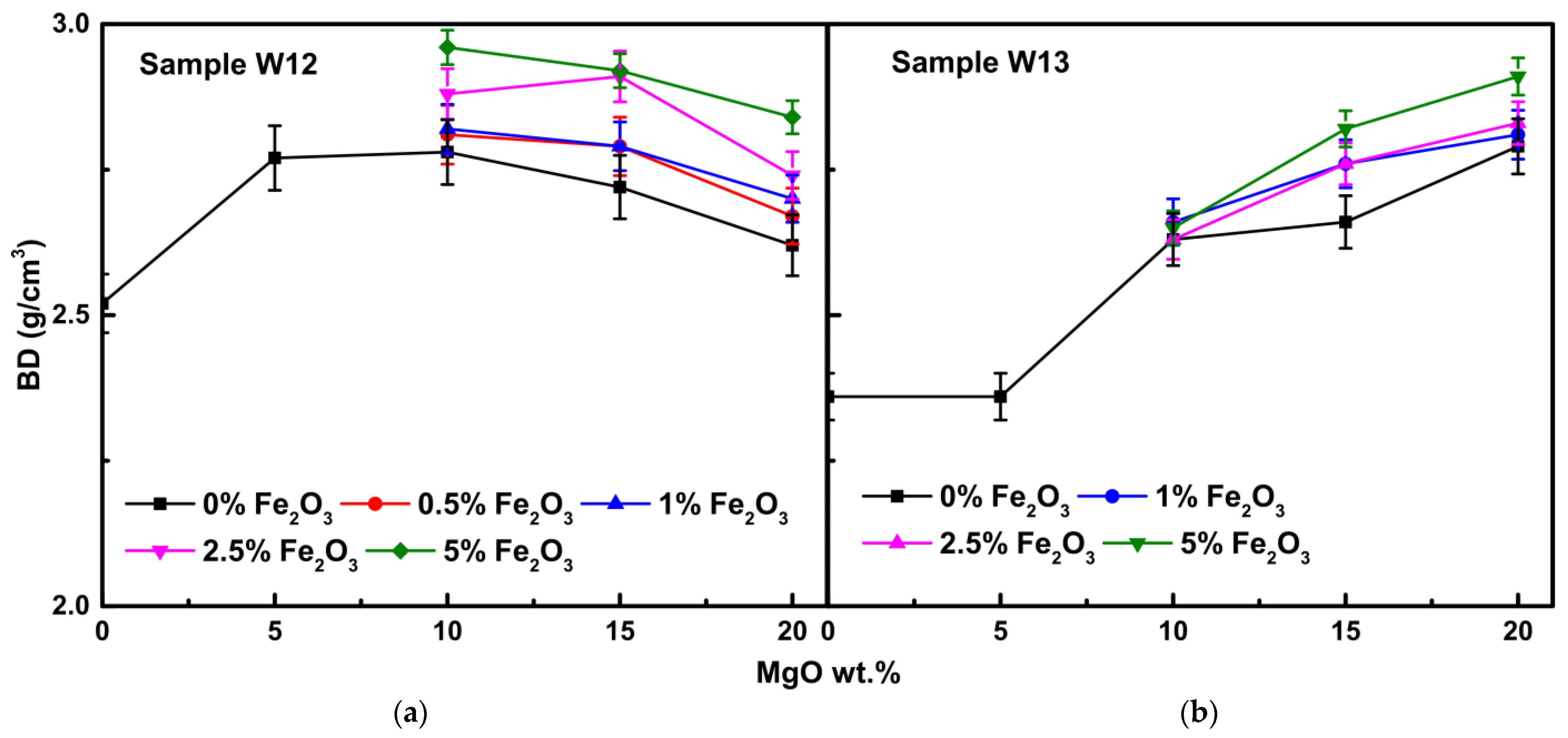
3.2.3. Bulk Density
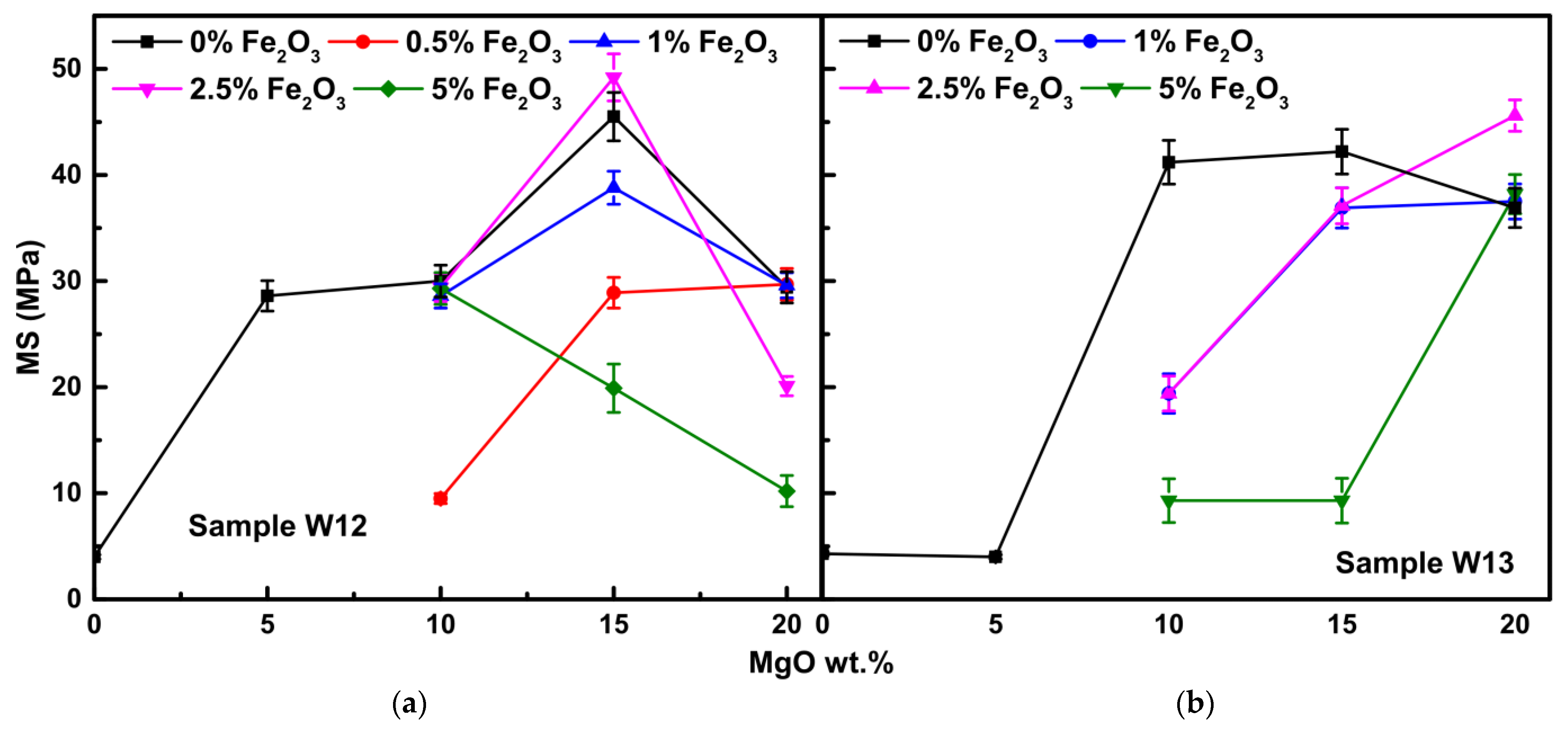
3.2.4. Mechanical Strength
3.3. SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through and Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Pactwa, K.; Woźniak, J.; Dudek, M. Coal mining waste in Poland in reference to circular economy principles. Fuel 2020, 270, 117493. [Google Scholar] [CrossRef]
- Haibin, L.; Zhenling, L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- Borja, W.; El Boudour El Idrissi, H.; Mossaab, M.; Sbi, S.; Daafi, Y.; Tamraoui, Y.; Alami, J. Phosphate waste rocks recycling in ceramic wall tiles: Technical performances. Ceram. Int. 2022, 48, 30031–30040. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Garzón, E.; Pérez-Villarejo, L.; Angelopoulos, G.N.; Eliche-Quesada, D. Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals 2021, 11, 232. [Google Scholar] [CrossRef]
- Aznar-Sánchez, J.A.; García-Gómez, J.J.; Velasco-Muñoz, J.F.; Carretero-Gómez, A. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Dino, G.A.; Rossetti, P.; Perotti, L.; Alberto, W.; Sarkka, H.; Coulon, F.; Wagland, S.; Griffiths, Z.; Rodeghiero, F. Landfill mining from extractive waste facilities: The importance of a correct site characterization and evaluation of the potentialities. A case study from Italy. Resourc. Policy 2018, 59, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.K.; Roy, P.K. Sustainable ceramics derived from solid wastes: A review. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Shaheen, F.; Lodhi, M.S.; Rosak-Szyrocka, J.; Zaman, K.; Awan, U.; Asif, M.; Ahmed, W.; Siddique, M. Cleaner Technology and Natural Resource Management: An Environmental Sustainability Perspective from China. Clean Technol. 2022, 4, 584–606. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Todaro, F.; Notarnicola, M. Environmental Comparison of Different Mechanical–Biological Treatment Plants by Combining Life Cycle Assessment and Material Flow Analysis. Clean Technol. 2022, 4, 380–394. [Google Scholar] [CrossRef]
- Diamantis, K.; Exarhakos, G.; Migiros, G.; Gartzos, E. Evaluating the Triaxial Characteristics of Ultamafic Rocks from Central Greece Using the Physical, Dynamic and Mechanical Properties. OALib 2016, 03, 2333–9705. [Google Scholar] [CrossRef]
- Cheng, T.W.; Ding, Y.C.; Chiu, J.P. A study of synthetic forsterite refractory materials using waste serpentine cutting. Min. Eng. 2002, 15, 271–275. [Google Scholar] [CrossRef]
- Emrullahoglu Abi, C.B.; Gürel, S.B.; Kılınç, D.; Emrullahoglu, Ö.F. Production of forsterite from serpentine—Effects of magnesium chloride hexahydrate addition. Adv. Powder Technol. 2015, 26, 947–953. [Google Scholar] [CrossRef]
- Elmaghraby, M.S.; Ismail, A.I.M.; Abd El Ghaffar, N.I. Atalla Egyptian serpentinite for producing forsterite and its thermos-mechanical behavior. Int. J. Res. Stud. Sci. Eng. Technol. 2015, 2, 137–146. [Google Scholar]
- Ramezani, A.; Emami, S.M.; Nemat, S. Effect of waste serpentine on the properties of basic insulating refractories. Ceram. Int. 2018, 44, 9269–9275. [Google Scholar] [CrossRef]
- Emami, S.M.; Ramezani, A.; Nemat, S. Sintering behavior of waste serpentine from Abdasht chromite mines and Kaolin blends. Ceram. Int. 2017, 43, 15189–15193. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L.Y.; Hong, D.; Qian, G.R.; Zhou, M. A study of making synthetic oxy-fluoride construction material using waste serpentine and kaolin mining tailings. Int. J. Min. Process. 2012, 104–105, 31–36. [Google Scholar] [CrossRef]
- Abdelwahab, W.; Mekky, H.; Khalil, A.; Belal, Z. Production of spinel forsterite refractories using sheared serpentinized ultramafic rocks Um Seleimat, Egypt. J. Eng. Appl. Sci. 2019, 14, 3386–3400. [Google Scholar]
- Mymrin, V.; Presotto, P.; Alekseev, K.; Avanci, A.M.; Rolim, P.H.B.; Petukhov, V.; Taskin, A.; Gidarakos, E.; Valouma, A.; Yu, G. Application of hazardous serpentine rocks’ extraction wastes in composites with glass waste and clay-sand mix to produce environmentally clean construction materials. Constr. Build. Mater. 2020, 234, 117319. [Google Scholar] [CrossRef]
- Ramezani, A.; Emami, S.M.; Nemat, S. Reuse of spent FCC catalyst, waste serpentine and kiln rollers waste for synthesis of cordierite and cordierite-mullite ceramics. J. Hazard. Mater. 2017, 338, 177–185. [Google Scholar] [CrossRef]
- IlIna, V.P. Ceramic heat-insulation materials based on serpentinites. Glas. Ceram. (Engl. Transl. Steklo I Keram.) 2013, 70, 12–15. [Google Scholar] [CrossRef]
- Zhao, J.; Hao, X.; Wang, S.; Zhang, X.; Wang, Z.; Luo, X.; Xie, Z.; Luo, J. Sintering behavior and thermal shock resistance of aluminum titanate (Al2TiO5)-toughened MgO-based ceramics. Ceram. Int. 2021, 47, 26643–26650. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Wojsa, J.; Psiuk, B.; Wala, T. Influence of zirconia addition on the properties of magnesia refractories. Ceram. Int. 2016, 42, 11373–11386. [Google Scholar] [CrossRef]
- Kucuk, I.; Boyraz, T.; Gökçe, H.; Öveçoğlu, M.L. Thermomechanical properties of aluminum titanate (Al2TiO5)-reinforced forsterite (Mg2SiO4) ceramic composites. Ceram. Int. 2018, 44, 8277–8282. [Google Scholar] [CrossRef]
- Rauta, P.R.; Sahoo, N. Properties enhancement of refractory bricks by incorporation of nano materials. In Proceedings of the 2015 International Conference on Nascent Technologies in the Engineering Field (ICNTE), Navi Mumbai, India, 9–10 January 2015. [Google Scholar] [CrossRef]
- Nemat, S.; Ramezani, A.; Emami, S.M. Recycling of waste serpentine for the production of forsterite refractories: The effects of various parameters on the sintering behavior. J. Aust. Ceram. Soc. 2019, 55, 425–431. [Google Scholar] [CrossRef]
- Nemat, S.; Ramezani, A.; Emani, S.M. Possible use of waste serpentine from Abdasht chromite mines into refractory and ceramic industries. Ceram. Int. 2016, 42, 18479–18483. [Google Scholar] [CrossRef]
- Cheng, T.W.; Hsu, C.W. A study of silicon carbide synthesis from waste serpentine. Chemosphere 2006, 64, 510–514. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Vinokurov, S.E.; Khamizov, R.K.; Vlasovskikh, N.S.; Belova, K.Y.; Dzhenloda, R.K.; Konov, M.A.; Myasoedov, B.F. The use of MgO obtained from serpentinite in the synthesis of a magnesium potassium phosphate matrix for radioactive waste immobilization. Appl. Sci. 2021, 11, 220. [Google Scholar] [CrossRef]
- Pagona, E.; Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Estimation and addition of MgO dose for upgrading the refractory characteristics of magnesite ore mining wastes/by-products. Waste Biomass Valor. 2022, 13, 4057–4072. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Pagona, E.; Stratigousis, P.; Ntampou, X.; Zaspalis, V.; Zouboulis, A.; Mitrakas, M. Hematite nanoparticles addition to serpentine/pyroxenes by-products of magnesite mining enrichment process for the production of refractories. Appl. Sci. 2022, 12, 2094. [Google Scholar] [CrossRef]
- Sadik, C.; Moudden, O.; El Bouari, A.; El Amrani, I. Review on the elaboration and characterization of ceramics refractories based on magnesite and dolomite. J. Asian Ceram. Soc. 2016, 4, 219–233. [Google Scholar] [CrossRef]
- Rodríguez Gomez, C.; Das Roy, T.K.; Shaji, S.; Castillo Rodríguez, G.A.; García Quiñonez, L.; Rodríguez, E.; González, J.O.; Aguilar-Martínez, J.A. Effect of addition of Al2O3 and Fe2O3 nanoparticles on the microstructural and physicochemical evolution of dense magnesia composite. Ceram. Int. 2015, 41, 7751–7758. [Google Scholar] [CrossRef]
- Pagona, E.; Tzamos, E.; Grieco, G.; Zouboulis, A.; Mitrakas, M. Characterization and evaluation of magnesite ore mining by-products of Gerakini mines (Chalkidiki, N. Greece). Sci. Total Environ. 2020, 732, 139279. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard C20; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Biswas, S.; Sarkar, D. Introduction to Refractories for Iron- and Steelmaking; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kadziolka-Gawel, M.; Adamczyk, Z.; Kalinowski, L. Mossbauer study of changes in olivine after heating in air. Can. Mineral. 2019, 57, 105–115. [Google Scholar] [CrossRef]
- Kanzaki, M.; Xue, X. Protoenstatite in MgSiO3 samples prepared by conventional solid state reaction. J. Mineral. Petrol. Sci. 2017, 112, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Katsavou, I.D.; Krokida, M.K.; Ziomas, I.C. Effect of production conditions on structural properties of refractory materials. Mater. Res. Innov. 2011, 15, 47–52. [Google Scholar] [CrossRef]
- Park, S.H.; Jeon, S.; Jang, J. Characteristic behavior of hydration of magnesium oxide. In Geotechnics for Sustainable Infrastructure Development; Lecture Notes in Civil Engineering 62; Duc Long, P., Dung, N., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- dos Santos, T.; Pinola, F.G.; Luz, A.P.; Pagliosa, C.; Pandolfelli, V.C. Al2O3-MgO refractory castables with enhanced explosion resistance due to in situ formation of phases with lamellar structure. Ceram. Int. 2018, 44, 8048–8056. [Google Scholar] [CrossRef]
- Wang, W.B.; Shi, Z.M.; Wang, X.G.; Wang, Z.X.; Cao, Z.; Fan, W. The synthesis and properties of high-quality forsterite ceramics using desert drift sands to replace traditional raw materials. J. Ceram. Soc. Japan 2017, 125, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.P. High-Temperature Properties of Magnesia-Refractory Brick Treated with Oxide and Salt Solutions; Pergamon Press: Oxford, UK, 1985. [Google Scholar]
- Routschka, G.; Wuthnow, H. Handbook of Refractory Materials: Design, Properties, Testing; Vulkan-Verlag: Essen, Germany, 2012. [Google Scholar]
- Surendranathan, A.O. An Introduction to Ceramics and Refractories, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Nguyen, M.; Sokolář, R. Corrosion Resistance of Novel Fly Ash–Based Forsterite–Spinel Refractory Ceramics. Materials 2022, 15, 1363. [Google Scholar] [CrossRef]
- Golestani Fard, F.; Talimian, A. Improving corrosion behavior of magnesia-chrome refractories by addition of nanoparticles. Refract. Worldforum 2014, 6, 93. [Google Scholar]
- Fernandes, D.; Wang, S.; Xu, Q.; Buss, R.; Chen, D. Process and Carbon Footprint Analyses of the Allam Cycle Power Plant Integrated with an Air Separation Unit. Clean Technol. 2019, 1, 325–340. [Google Scholar] [CrossRef]





| Sample | W12 | W13 |
|---|---|---|
| wt.% | ||
| MgO | 42.9 | 40.8 |
| FeO | 7.3 | 7.2 |
| Al2O3 | 0.50 | 1.4 |
| CaO | 0.35 | 1.6 |
| Na2O | 0.30 | 0.30 |
| SiO2 | 41.5 | 44.5 |
| LOI | 6.7 | 4.1 |
| mg/kg | ||
| K2O | 650 | 730 |
| MnO2 | 1460 | 1510 |
| NiO | 3110 | 2870 |
| Cr2O3 | 450 | 850 |
| Sample (Temperature, °C) | Olivine | Pyroxenes | Serpentine |
|---|---|---|---|
| W12 | 65.1 ± 4.5 | 8.4 ± 1.3 | 26.4 ± 1.8 |
| W12 (650) | 79.0 ± 5.7 | 13.1 ± 2.1 | 7.9 ± 0.7 |
| W12 (850) | 80.9 ± 6.0 | 19.1 ± 2.3 | --- |
| W12 (1300) | 66.0 ± 4.8 | 34.0 ± 3.5 | --- |
| W13 | 52.9 ± 3.3 | 19.6 ± 1.7 | 27.5 ± 2.0 |
| W13 (650) | 64.8 ± 4.5 | 26.8 ± 3.0 | 8.3 ± 1.0 |
| W13 (850) | 76.3 ± 5.2 | 23.7 ± 2.3 | --- |
| W13 (1300) | 55.0 ± 3.8 | 45.0 ± 3.6 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagona, E.; Kalaitzidou, K.; Zaspalis, V.; Zouboulis, A.; Mitrakas, M. Effects of MgO and Fe2O3 Addition for Upgrading the Refractory Characteristics of Magnesite Ore Mining Waste/By-Products. Clean Technol. 2022, 4, 1103-1126. https://doi.org/10.3390/cleantechnol4040067
Pagona E, Kalaitzidou K, Zaspalis V, Zouboulis A, Mitrakas M. Effects of MgO and Fe2O3 Addition for Upgrading the Refractory Characteristics of Magnesite Ore Mining Waste/By-Products. Clean Technologies. 2022; 4(4):1103-1126. https://doi.org/10.3390/cleantechnol4040067
Chicago/Turabian StylePagona, Evangelia, Kyriaki Kalaitzidou, Vasileios Zaspalis, Anastasios Zouboulis, and Manassis Mitrakas. 2022. "Effects of MgO and Fe2O3 Addition for Upgrading the Refractory Characteristics of Magnesite Ore Mining Waste/By-Products" Clean Technologies 4, no. 4: 1103-1126. https://doi.org/10.3390/cleantechnol4040067
APA StylePagona, E., Kalaitzidou, K., Zaspalis, V., Zouboulis, A., & Mitrakas, M. (2022). Effects of MgO and Fe2O3 Addition for Upgrading the Refractory Characteristics of Magnesite Ore Mining Waste/By-Products. Clean Technologies, 4(4), 1103-1126. https://doi.org/10.3390/cleantechnol4040067








