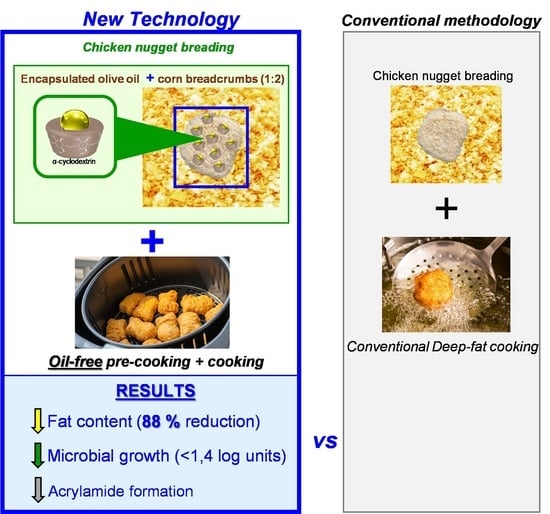
Encapsulated EVOO Improves Food Safety and Shelf Life of Refrigerated Pre-Cooked Chicken Nuggets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
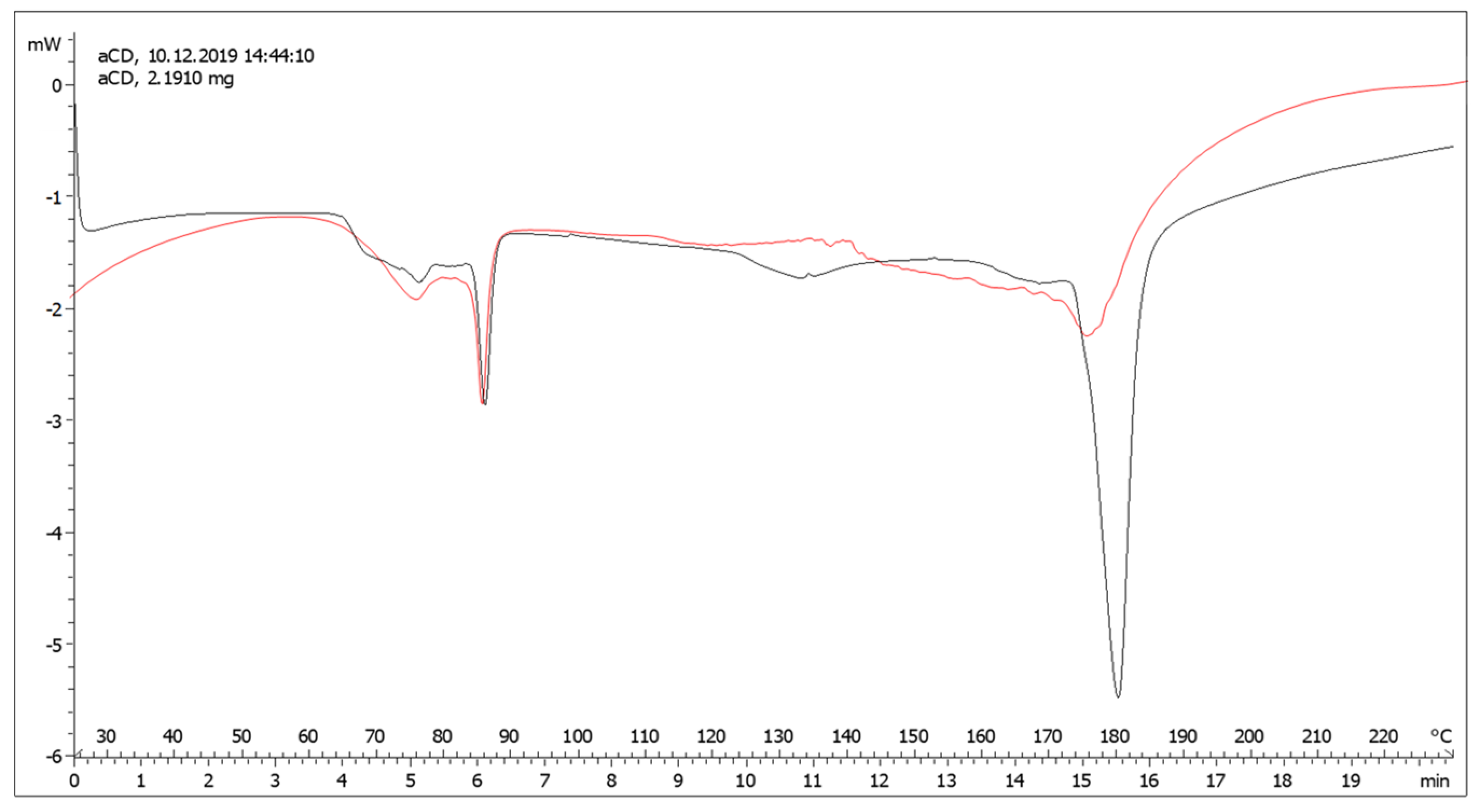
2.2. Preparation of the EVOO−αCD Inclusion Complex and Characterization
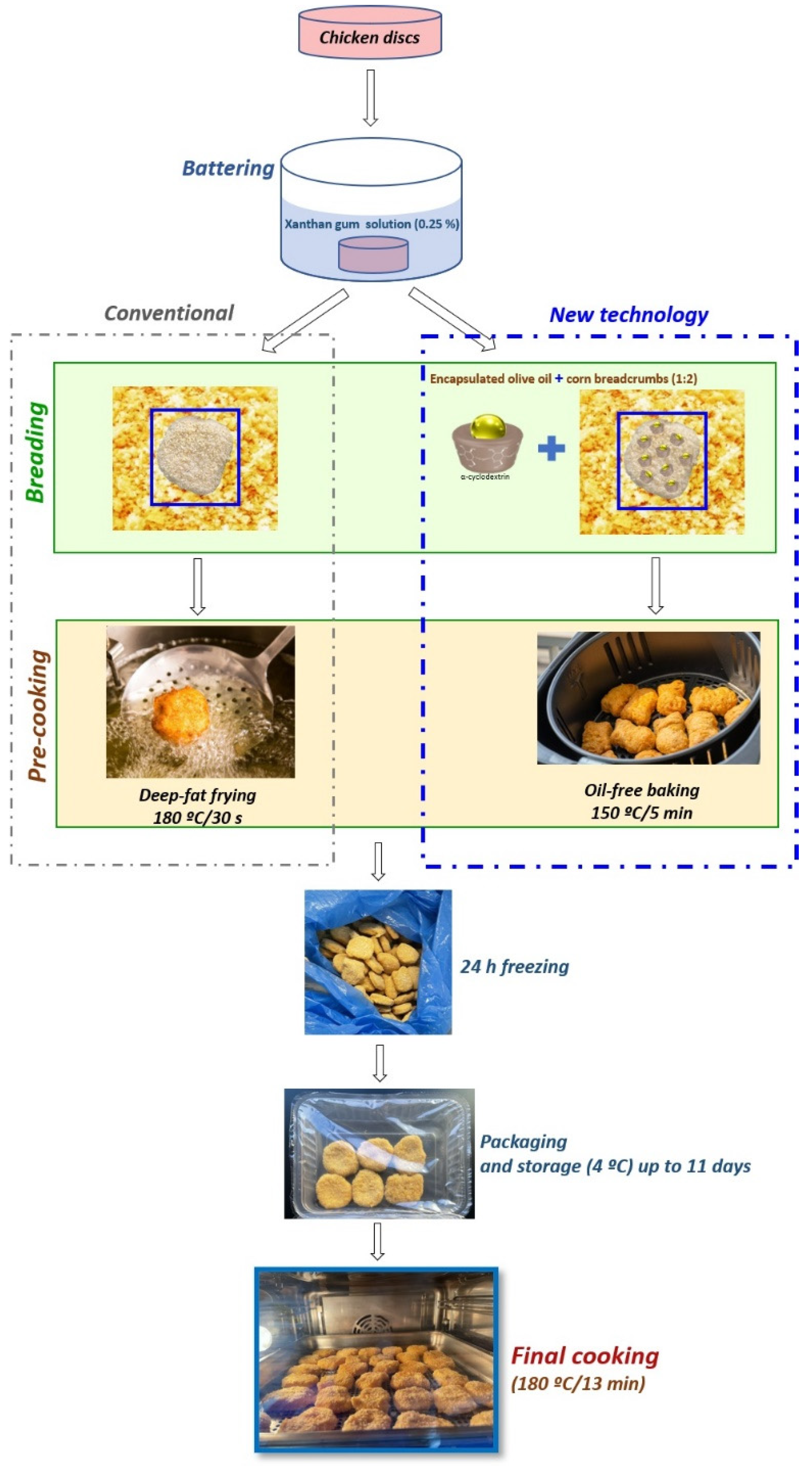
2.3. Chicken Nugget Preparation, Cooking Treatments, Packaging, and Storage Conditions
2.4. Analyses and Determinations
2.4.1. Fat Content
2.4.2. Color
2.4.3. Sensory Analyses
2.4.4. Acrylamide Contents
2.4.5. Microbial Analyses
2.5. Statistical Analyses
3. Results and Discussion
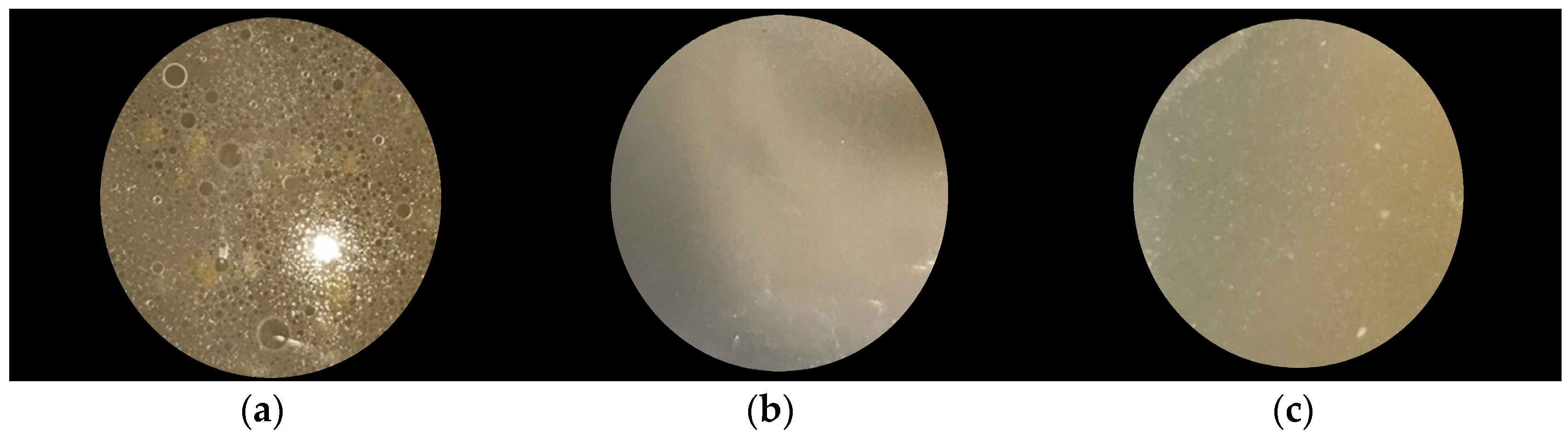
3.1. Preparation and Characterization of the EVOO:αCD Inclusion Complex
3.2. Fat Content
3.3. Colour
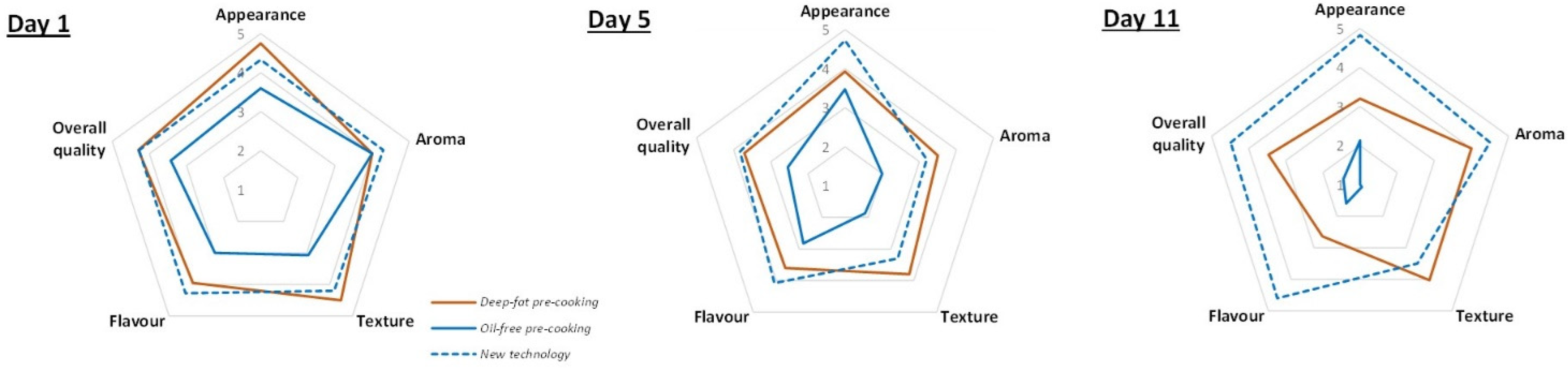
3.4. Sensory Analyses
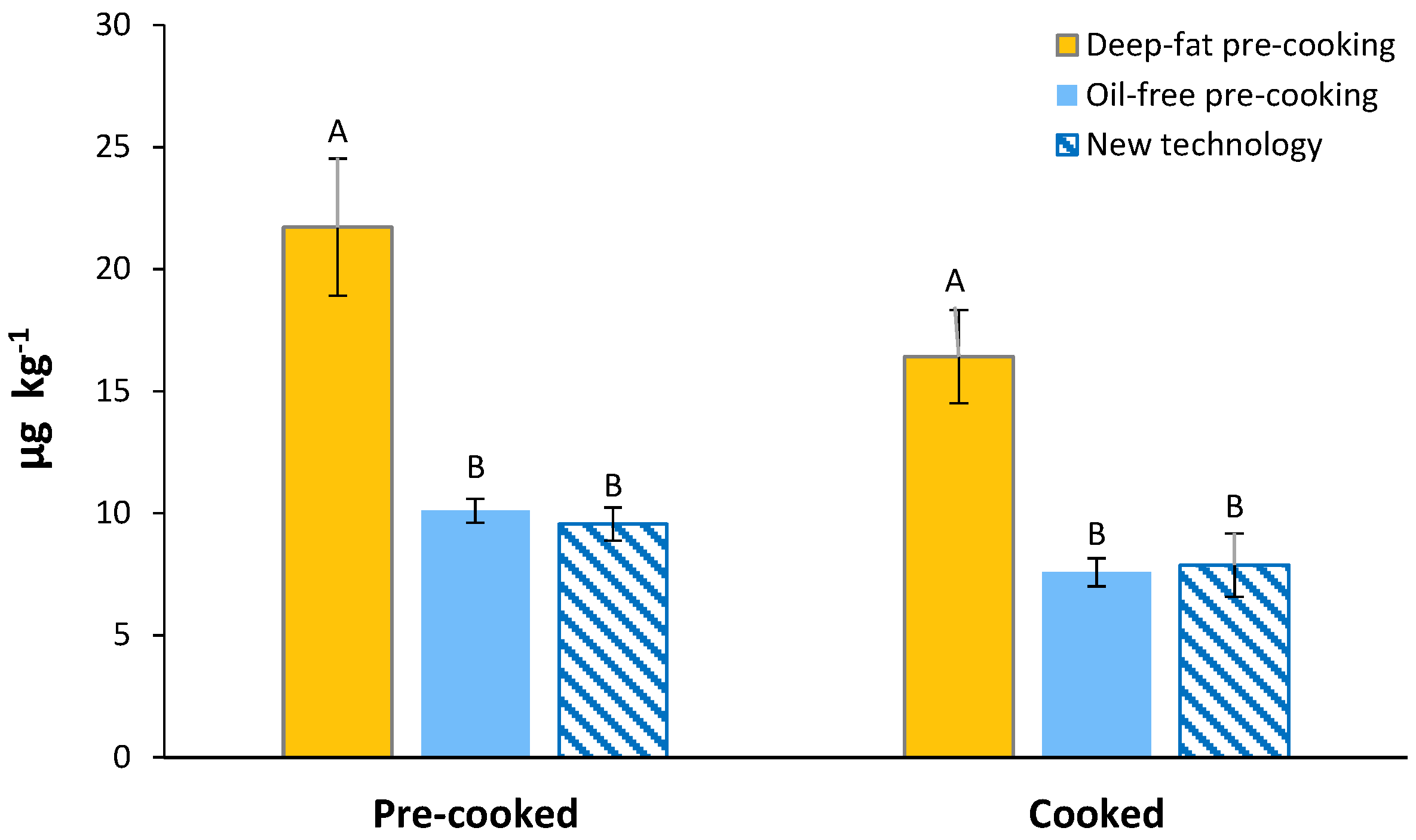
3.5. Acrylamide Content
3.6. Microbial Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rossell, J.B. Frying: Improving Quality; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9781855735569. [Google Scholar]
- Soto-Jover, S.; Boluda-Aguilar, M.; Esnoz-Nicuesa, A.; Iguaz-Gainza, A.; López-Gómez, A. Texture, oil adsorption and safety of the european style croquettes manufactured at industrial scale. Food Eng. Rev. 2015, 8, 181–200. [Google Scholar] [CrossRef]
- Fiszman, S.M.; Salvador, A. Recent developments in coating batters. Trends Food Sci. Technol. 2003, 14, 399–407. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 3 May 2000 replacing Decision 94/3/EC establishing a list of wastes pursuant to Article 1(a) of Council Directive 75/442/EEC on waste and Council Decision 94/904/EC establishing a list of hazardous waste pursuant to Article 1(4) of Council Directive 91/689/EEC on hazardous waste (notified under document number C(2000) 1147) (Text with EEA relevance) (2000/532/EC). Off. J. Eur. Communities 2000, 6, 3–24. [Google Scholar]
- Mannu, A.; Garroni, S.; Porras, J.I.; Mele, A. Available technologies and materials for waste cooking oil recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Fiszman, S.M.; Salvador, A.; Sanz, T.; Lluch, M.A.; Castellano, J.V.; Camps, J.L.; Gamero, M. Proceso para la Preparación de un Alimento Rebozado y Congelado. Patent Application No. 200,201,276, 2002. [Google Scholar]
- López-Gómez, A.; Soto-Jover, S.; Boluda-Aguilar, M. Composition and Method of Making Breaded Foods with Low Oil Absorption during Frying. Spanish Patent 2 440 092 B1, 14 August 2014. [Google Scholar]
- do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and its uses in food science and technology: A Review. In Microencapsulation—Processes, Technologies and Industrial Applications; Salaün, F., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83881-870-8. [Google Scholar]
- Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Variables affecting lipid oxidation in dried microencapsulated oils. Grasas y Aceites 2003, 54, 304–314. [Google Scholar] [CrossRef]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to microencapsulation and controlled delivery in foods. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; pp. 3–12. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main applications of cyclodextrins in the food industry as the compounds of choice to form host–guest complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Szejtli, J. Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 1997, 7, 575–587. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, e04628. [Google Scholar] [CrossRef]
- Furune, T.; Terao, K. Complexation of ingredients in foods by alpha-cyclodextrin to improve their functions. In Functionality of Cyclodextrins in Encapsulation for Food Applications; Ho, T.M., Yoshii, H., Terao, K., Bhandari, B.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 277–297. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to alpha cyclodextrin and reduction of post prandial glycaemic responses (ID 2926, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2713. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stab. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Hadaruga, D.I.; Hadaruga, N.G.; Hermenean, A.; Rivis, A.; Paslaru, V.; Codina, G. Bionanomaterials: Thermal stability of the oleic acid/α- and β-cyclodextrin complexes. Rev. Chim. 2008, 59, 994–998. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef]
- Chikamoto, K.; Terao, K. Alpha-cyclodextrin functions as a dietary fiber. In Functionality of Cyclodextrins in Encapsulation for Food Applications; Ho, T.M., Yoshii, H., Terao, K., Bhandari, B.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 255–276. [Google Scholar]
- Manolikar, M.; Sawant, M. Study of solubility of isoproturon by its complexation with β-cyclodextrin. Chemosphere 2003, 51, 811–816. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- ASTM. Physical Requirement Guidelines for Sensory Evaluation Laboratories; Eggert, J., Zook, K., Eds.; ASTM International: Philadelphia, PA, USA, 1986; ISBN 0803109245. [Google Scholar]
- Jiménez-Martín, E.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Enrichment of chicken nuggets with microencapsulated omega-3 fish oil: Effect of frozen storage time on oxidative stability and sensory quality. Food Bioprocess. Technol. 2016, 9, 285–297. [Google Scholar] [CrossRef]
- International Organization for Standardization. Sensory Análisis—General Guidance for the Design of Test Rooms; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Ho, B.T.; Joyce, D.C.; Bhandari, B.R. Encapsulation of ethylene gas into α-cyclodextrin and characterisation of the inclusion complexes. Food Chem. 2011, 127, 572–580. [Google Scholar] [CrossRef]
- Brannan, R.G.; Mah, E.; Schott, M.; Yuan, S.; Casher, K.L.; Myers, A.; Herrick, C. Influence of ingredients that reduce oil absorption during immersion frying of battered and breaded foods. Eur. J. Lipid Sci. Technol. 2014, 116, 240–254. [Google Scholar] [CrossRef]
- Gerrish, T.; Higgins, C.; Kresl, K. Method of Making Battered and Breaded Food Compositions Using Calcium Pectins. U.S. Patent US5601861A, 11 February 1997. [Google Scholar]
- Purlis, E. Browning development in bakery products—A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Mariscal, M.; Bouchon, P. Comparison between atmospheric and vacuum frying of apple slices. Food Chem. 2008, 107, 1561–1569. [Google Scholar] [CrossRef]
- Teruel, M.R.; García-Segovia, P.; Martínez-Monzó, J.; Linares, M.B.; Garrido, M.D. Use of vacuum-frying in chicken nugget processing. Innov. Food Sci. Emerg. Technol. 2014, 26, 482–489. [Google Scholar] [CrossRef]
- Ngadi, M.; Li, Y.; Oluka, S. Quality changes in chicken nuggets fried in oils with different degrees of hydrogenatation. LWT Food Sci. Technol. 2007, 40, 1784–1791. [Google Scholar] [CrossRef]
- Mallikarjunan, P.K.; Ngadi, M.O.; Chinnan, M.S. Breaded Fried Foods; CRC Press: Boca Raton, FL, USA, 2010; p. 179. [Google Scholar]
- Salvador, A.; Hough, G.; Fiszman, S.M. Acceptability of batter-coated squid rings prepared without industrial pre-frying. Eur. Food Res. Technol. 2005, 221, 36–40. [Google Scholar] [CrossRef]
- Carere, A. Genotoxicity and carcinogenicity of acrylamide: A critical review. Ann. Ist Super Sanita 2006, 42, 144–155. [Google Scholar]
- Matoso, V.; Bargi-Souza, P.; Ivanski, F.; Romano, M.A.; Romano, R.M. Acrylamide: A review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019, 283, 422–430. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Cortés, P.; Dueik, V.; Bouchon, P.; Pedreschi, F. Application of vacuum frying as a furan and acrylamide mitigation technology in potato chips. Food Bioprocess. Technol. 2017, 10, 2092–2099. [Google Scholar] [CrossRef]
- Yavas, E.; Bilgin, B. Effect of calcium lactate, sodium diacetate and sodium chloride mixture on the microbiological, chemical and sensory properties of chicken nuggets stored in refrigeration and under modified atmospheres. Int. J. Poult. Sci. 2010, 9, 66–71. [Google Scholar] [CrossRef]
- Corry, J.E.L.; Atabay, H.I. Poultry as a source of Campylobacter and related organisms. Symp. Ser. Soc. Appl. Microbiol. 2001, 90, 96S–114S. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. Modern Food Microbiology; Chapman & Hall: London, UK, 1996; ISBN 0412076918. [Google Scholar]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Shahrezaee, M.; Soleimanian-Zad, S.; Soltanizadeh, N.; Akbari-Alavijeh, S. Use of Aloe vera gel powder to enhance the shelf life of chicken nugget during refrigeration storage. LWT 2018, 95, 380–386. [Google Scholar] [CrossRef]

| Parameter | Deep-Fat Pre-Cooking | Oil-Free Pre-Cooking | New Technology * | |||
|---|---|---|---|---|---|---|
| Pre-Cooked | Cooked | Pre-Cooked | Cooked | Pre-Cooked | Cooked | |
| Fat content | 19.9 ± 7.6 a | 17.9 ± 3.5 a | 0.92 ± 0.52 b | 1.33 ± 0.60 b | 2.29 ± 0.49 b | 4.37 ± 1.42 b |
| L * | 57.6 ± 3.9 c | 52.9 ± 6.3 d | 68.2 ± 2.6 b | 65.6 ± 2.6 b | 75 ± 1.6 a | 68.5 ± 2.3 b |
| a * | 4.43 ± 2.04 b | 7.58 ± 2.15 a | 1.10 ± 0.63 cd | 2.32 ± 0.84 c | 0.21 ± 0.56 d | 1.70 ± 0.90 d |
| b * | 42.3 ± 3.1 c | 42.4 ± 5.5 c | 46.5 ± 2.5 b | 50.5 ± 1.6 a | 40.9 ± 2.6 c | 49.8 ± 1.7 ab |
| Hue | 1.46 ± 0.05 a | 1.39 ± 0.06 a | 1.55 ± 0.01 a | 1.52 ± 0.01 a | 0.51 ± 1.54 b | 1.54 ± 0.01 a |
| Chroma | 42.6 ± 2.9 d | 43.2 ± 5.3 de | 46.5 ± 2.5 bce | 50.6 ± 1.56 a | 42.8 ± 3.45 cd | 49.9 ± 1.65 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barón-Yusty, M.; Martínez-Hernández, G.B.; Ros-Chumillas, M.; Navarro-Segura, L.; López-Gómez, A. Encapsulated EVOO Improves Food Safety and Shelf Life of Refrigerated Pre-Cooked Chicken Nuggets. Clean Technol. 2022, 4, 53-66. https://doi.org/10.3390/cleantechnol4010005
Barón-Yusty M, Martínez-Hernández GB, Ros-Chumillas M, Navarro-Segura L, López-Gómez A. Encapsulated EVOO Improves Food Safety and Shelf Life of Refrigerated Pre-Cooked Chicken Nuggets. Clean Technologies. 2022; 4(1):53-66. https://doi.org/10.3390/cleantechnol4010005
Chicago/Turabian StyleBarón-Yusty, Marta, Ginés Benito Martínez-Hernández, María Ros-Chumillas, Laura Navarro-Segura, and Antonio López-Gómez. 2022. "Encapsulated EVOO Improves Food Safety and Shelf Life of Refrigerated Pre-Cooked Chicken Nuggets" Clean Technologies 4, no. 1: 53-66. https://doi.org/10.3390/cleantechnol4010005
APA StyleBarón-Yusty, M., Martínez-Hernández, G. B., Ros-Chumillas, M., Navarro-Segura, L., & López-Gómez, A. (2022). Encapsulated EVOO Improves Food Safety and Shelf Life of Refrigerated Pre-Cooked Chicken Nuggets. Clean Technologies, 4(1), 53-66. https://doi.org/10.3390/cleantechnol4010005